Abstract
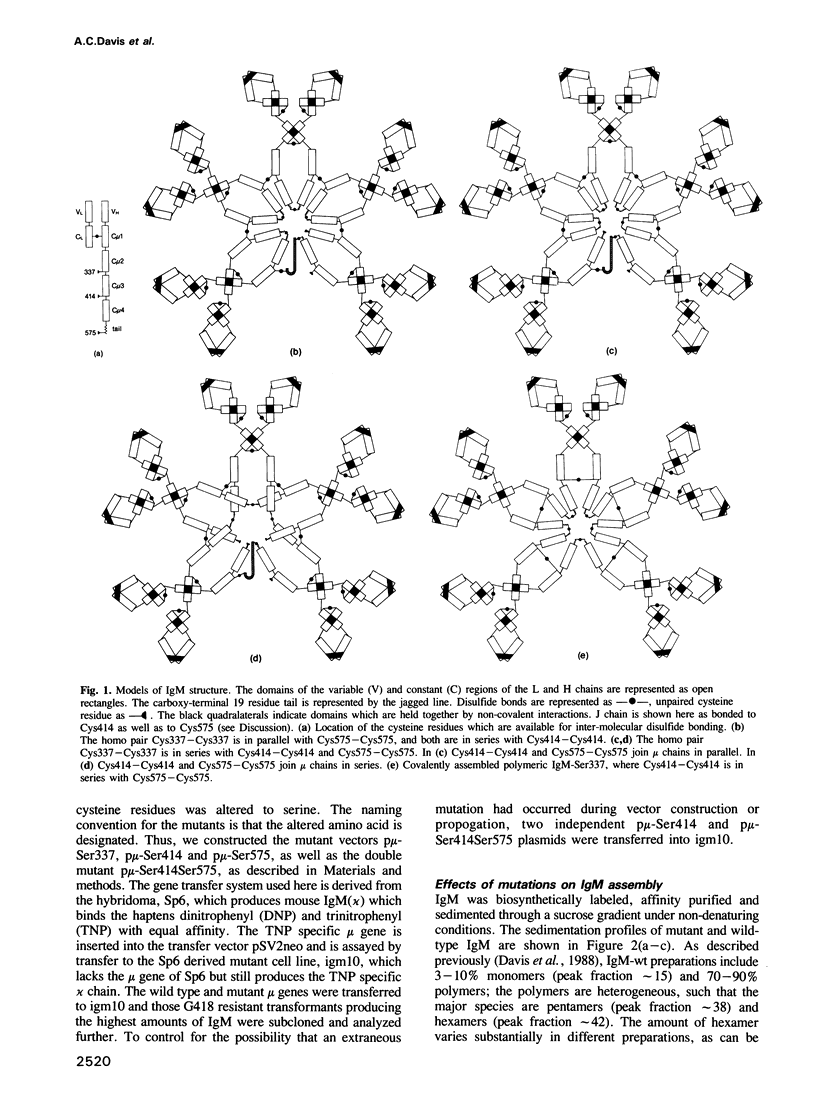
The conventional model of polymeric IgM depicts a unique structure in which the mu heavy chains and J chain are joined by well defined disulfide bonds involving cysteine residues at positions 337, 414 and 575 of the mu chain. To test this model, we have used site directed mutagenesis to produce IgM in which these cysteines have been replaced by serine. In each case the single mutants were able to assemble polymeric IgM, which was analyzed for its size, morphology, J chain content and activity in complement dependent cytolysis. Whereas normal polymeric IgM is composed predominantly of pentameric and hexameric molecules, the mutant IgM-Ser414 is covalently assembled as pentamers and smaller forms; IgM-Ser575 is assembled as covalent hexamers. IgM-Ser337 appears to include the same pentameric and hexameric forms as normal IgM except that, unlike normal polymeric IgM, most pentameric/hexameric IgM-Ser337 is not covalently assembled. J chain is present in polymeric IgM-Ser337 but absent in polymeric IgM-Ser414 and IgM-Ser575. IgM-Ser414 is defective in activating the classical pathway of complement dependent cytolysis. Our observations are consistent with models in which the covalent linkages between mu chains are mediated by disulfide bonded Cys337-Cys337, Cys414-Cys414 and Cys575-Cys575 but indicate that the arrangement of these Cys-Cys pairs in series and in parallel varies among and within IgM molecules.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale D., Feinstein A. Structure and function of the constant regions of immunoglobulins. Q Rev Biophys. 1976 May;9(2):135–180. doi: 10.1017/s0033583500002390. [DOI] [PubMed] [Google Scholar]
- Beale D., Feinstein A. Studies on the reduction of a human 19S immunoglobulin M. Biochem J. 1969 Apr;112(2):187–194. doi: 10.1042/bj1120187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A., Neuberger M. S. Polymeric immunoglobulin M is secreted by transfectants of non-lymphoid cells in the absence of immunoglobulin J chain. EMBO J. 1987 Sep;6(9):2753–2758. doi: 10.1002/j.1460-2075.1987.tb02569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis R. M., Koshland M. E. Mechanism of IgM polymerization. Proc Natl Acad Sci U S A. 1974 Mar;71(3):657–661. doi: 10.1073/pnas.71.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. C., Collins C., Shulman M. J. Differential glycosylation of polymeric and monomeric IgM. Mol Immunol. 1989 Feb;26(2):147–152. doi: 10.1016/0161-5890(89)90096-5. [DOI] [PubMed] [Google Scholar]
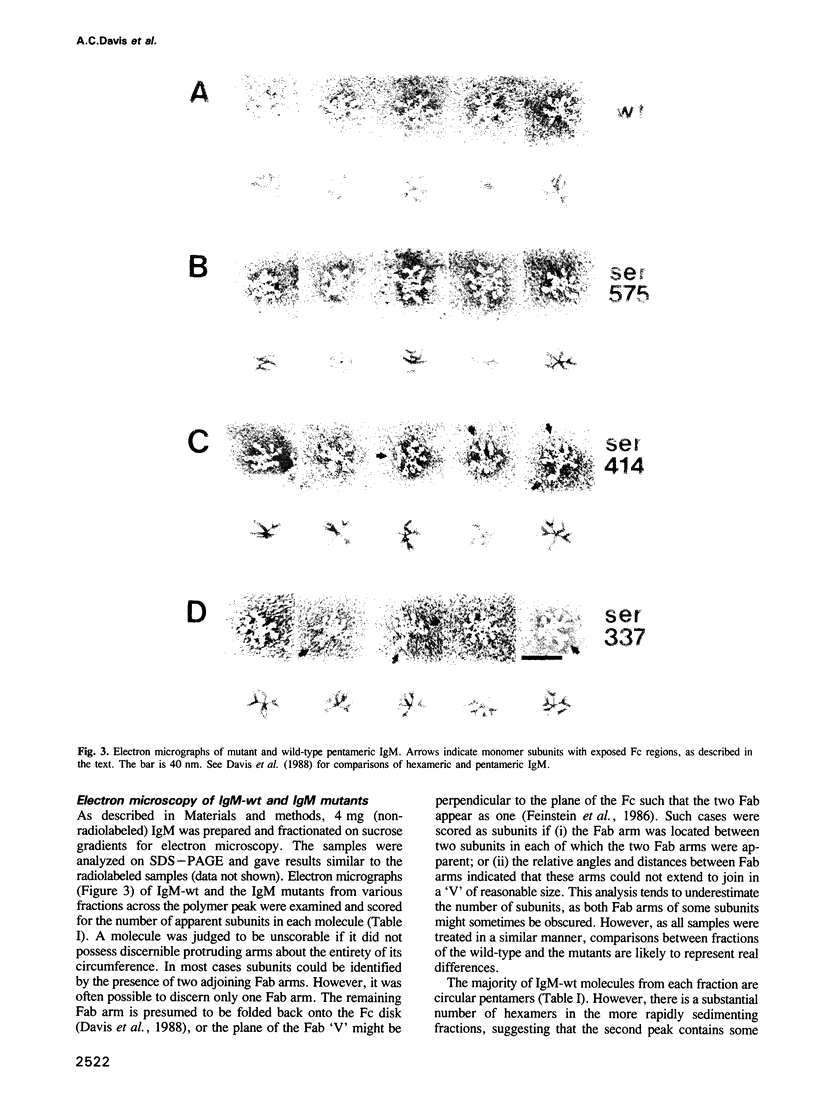
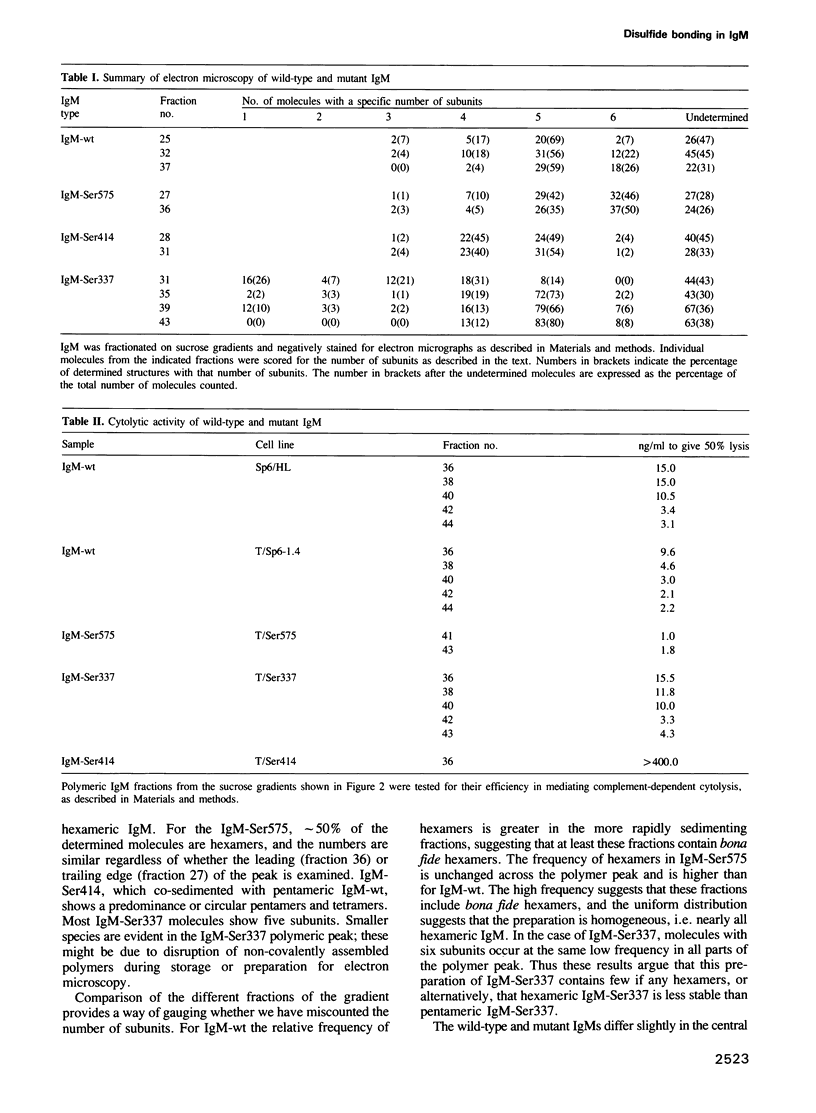
- Davis A. C., Roux K. H., Shulman M. J. On the structure of polymeric IgM. Eur J Immunol. 1988 Jul;18(7):1001–1008. doi: 10.1002/eji.1830180705. [DOI] [PubMed] [Google Scholar]
- Davis A. C., Shulman M. J. IgM--molecular requirements for its assembly and function. Immunol Today. 1989 Apr;10(4):118–128. doi: 10.1016/0167-5699(89)90244-2. [DOI] [PubMed] [Google Scholar]
- Della Corte E., Parkhouse R. M. Biosynthesis of immunoglobulin A (IgA). Secretion and addition of carbohydrate to monomer and polymer forms of a mouse myeloma protein. Biochem J. 1973 Nov;136(3):589–596. doi: 10.1042/bj1360589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolder F. Occurrence, isolation and interchain bridges of natural 7-S immunoglobulin M in human serum. Biochim Biophys Acta. 1971 Jun 29;236(3):675–685. [PubMed] [Google Scholar]
- Eskeland T., Christensen T. B. IgM molecules with and without J chain in serum and after purification, studied by ultracentrifugation, electrophoresis, and electron microscopy. Scand J Immunol. 1975;4(3):217–228. doi: 10.1111/j.1365-3083.1975.tb02620.x. [DOI] [PubMed] [Google Scholar]
- Eskeland T., Harboe M. 7S immunoglobulin M (IgM) from serum purified by anti-mu-chain immunoadsorbent: comparison of the reassociation properties of 7S IgM and various 19S IgM subunits. Scand J Immunol. 1973;2(5):511–522. doi: 10.1111/j.1365-3083.1973.tb02057.x. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., Koshland M. E. The stoichiometry of J chain in human secretory IgA. J Immunol. 1973 Dec;111(6):1653–1660. [PubMed] [Google Scholar]
- Kownatzki E. Reassociation of IgM subunits in the presence and absence of J chain. Immunol Commun. 1973;2(1):105–113. doi: 10.3109/08820137309022886. [DOI] [PubMed] [Google Scholar]
- Köhler G., Hengartner H., Shulman M. J. Immunoglobulin production by lymphocyte hybridomas. Eur J Immunol. 1978 Feb;8(2):82–88. doi: 10.1002/eji.1830080203. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Schrohenloher R. E. Site of attachment of J chain to human immunoglobulin M. Nature. 1974 Jun 14;249(458):650–652. doi: 10.1038/249650a0. [DOI] [PubMed] [Google Scholar]
- Milstein C. P., Richardson N. E., Dieverson E. V., Feinstein A. Interchain disulphide bridges of mouse immunoglobulin M. Biochem J. 1975 Dec;151(3):615–624. doi: 10.1042/bj1510615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi A., Hawley R. G., Shulman M. J., Hozumi N. Transfer of a cloned immunoglobulin light-chain gene to mutant hybridoma cells restores specific antibody production. Nature. 1983 Mar 24;302(5906):340–342. doi: 10.1038/302340a0. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Askonas B. A., Dourmashkin R. R. Electron microscopic studies of mouse immunoglobulin M; structure and reconstitution following reduction. Immunology. 1970 Apr;18(4):575–584. [PMC free article] [PubMed] [Google Scholar]
- Reisfeld R. A., Small P. A., Jr Electrophoretic heterogeneity of polypeptide chains of specific antibodies. Science. 1966 May 27;152(3726):1253–1255. doi: 10.1126/science.152.3726.1253. [DOI] [PubMed] [Google Scholar]
- Roux K. H., Monafo W. J., Davie J. M., Greenspan N. S. Construction of an extended three-dimensional idiotope map by electron microscopic analysis of idiotope-anti-idiotope complexes. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4984–4988. doi: 10.1073/pnas.84.14.4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M. J., Collins C., Pennell N., Hozumi N. Complement activation by IgM: evidence for the importance of the third constant domain of the mu heavy chain. Eur J Immunol. 1987 Apr;17(4):549–554. doi: 10.1002/eji.1830170418. [DOI] [PubMed] [Google Scholar]
- Shulman M. J., Heusser C., Filkin C., Köhler G. Mutations affecting the structure and function of immunoglobulin M. Mol Cell Biol. 1982 Sep;2(9):1033–1043. doi: 10.1128/mcb.2.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M. J., Pennell N., Collins C., Hozumi N. Activation of complement by immunoglobulin M is impaired by the substitution serine-406----asparagine in the immunoglobulin mu heavy chain. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7678–7682. doi: 10.1073/pnas.83.20.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson S. M., Dombrink-Kurtzman M. A., Voss E. W., Jr C1q binding by a high affinity anti-fluorescein murine monoclonal IgM antibody and monomeric subunits. Mol Immunol. 1988 Jun;25(6):545–554. doi: 10.1016/0161-5890(88)90076-4. [DOI] [PubMed] [Google Scholar]
- Wright J. F., Shulman M. J., Isenman D. E., Painter R. H. C1 binding by murine IgM. The effect of a Pro-to-Ser exchange at residue 436 of the mu-chain. J Biol Chem. 1988 Aug 15;263(23):11221–11226. [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]