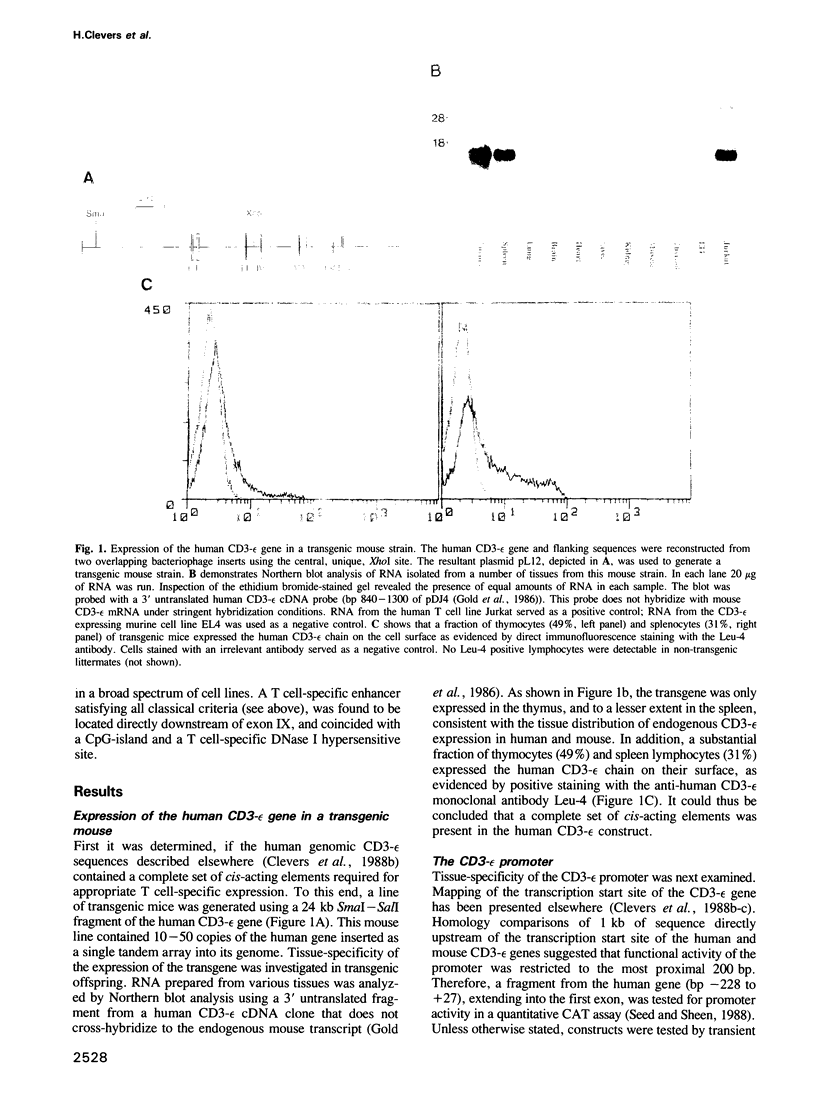
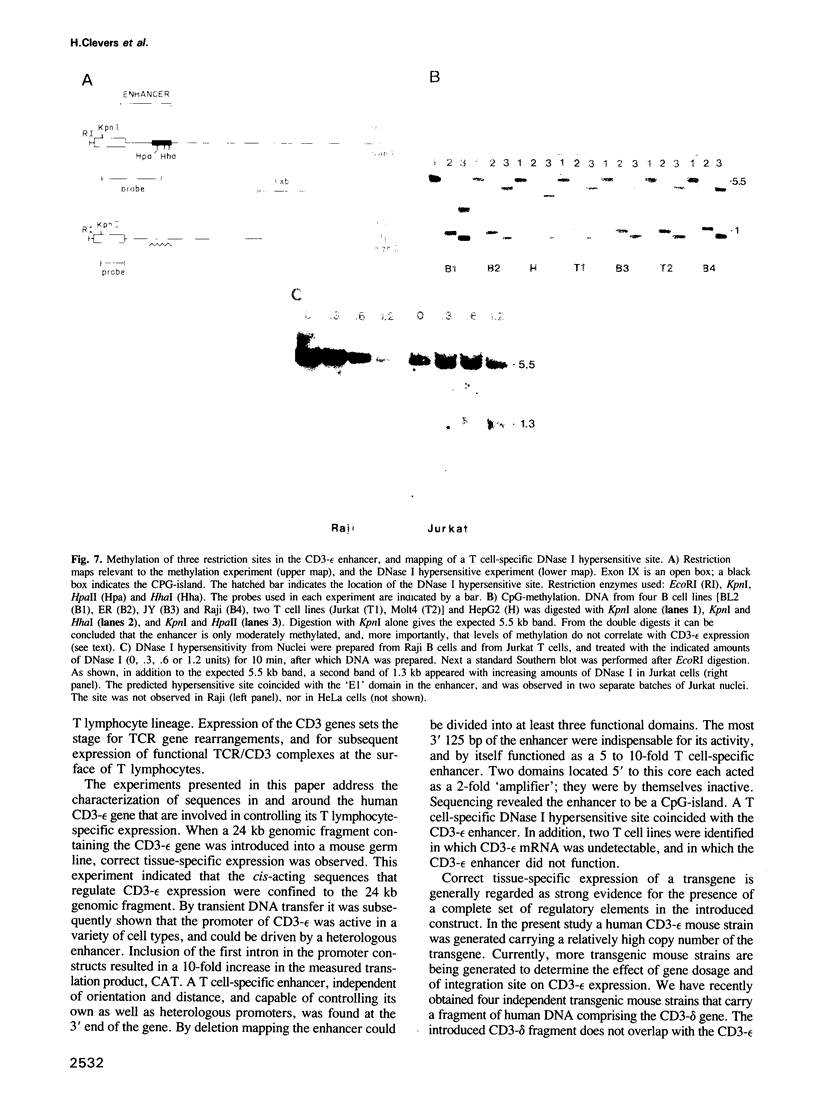
Abstract
The gene encoding the CD3-epsilon chain of the T cell receptor (TCR/CD3) complex is uniquely transcribed in all T lymphocyte lineage cells. The human CD3-epsilon gene, when introduced into the mouse germ line, was expressed in correct tissue-specific fashion. The gene was then screened for T lymphocyte-specific cis-acting elements in transient chloramphenicol transferase assays. The promoter (-228 to +100) functioned irrespective of cell type. A 1225 bp enhancer with strict T cell-specificity was found in a DNase I hypersensitive site downstream of the last exon, 12 kb from the promoter. This site was present in T cells only. The CD3-epsilon enhancer did not display sequence similarity with the T cell-specific enhancer of CD3-delta, a related gene co-regulated with CD3-epsilon during intrathymic differentiation. The CD3-epsilon enhancer was unusual in that it constituted a CpG island, and was hypomethylated independent of tissue type. Two HTLV I-transformed T cell lines were identified in which the CD3-epsilon gene was not expressed, and in which the enhancer was inactive.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atchison M. L. Enhancers: mechanisms of action and cell specificity. Annu Rev Cell Biol. 1988;4:127–153. doi: 10.1146/annurev.cb.04.110188.001015. [DOI] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Bergman Y., Rice D., Grosschedl R., Baltimore D. Two regulatory elements for immunoglobulin kappa light chain gene expression. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7041–7045. doi: 10.1073/pnas.81.22.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Bornstein P., McKay J., Liska D. J., Apone S., Devarayalu S. Interactions between the promoter and first intron are involved in transcriptional control of alpha 1(I) collagen gene expression. Mol Cell Biol. 1988 Nov;8(11):4851–4857. doi: 10.1128/mcb.8.11.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. R., Berg P. Comparison of intron-dependent and intron-independent gene expression. Mol Cell Biol. 1988 Oct;8(10):4395–4405. doi: 10.1128/mcb.8.10.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. C., Dunlap S., Wileman T. E., Terhorst C. Human CD3-epsilon gene contains three miniexons and is transcribed from a non-TATA promoter. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8156–8160. doi: 10.1073/pnas.85.21.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Clevers H., Dunlap S., Saito H., Georgopoulos K., Wileman T., Terhorst C. Characterization and expression of the murine CD3-epsilon gene. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8623–8627. doi: 10.1073/pnas.85.22.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Furley A. J., Mizutani S., Weilbaecher K., Dhaliwal H. S., Ford A. M., Chan L. C., Molgaard H. V., Toyonaga B., Mak T., van den Elsen P. Developmentally regulated rearrangement and expression of genes encoding the T cell receptor-T3 complex. Cell. 1986 Jul 4;46(1):75–87. doi: 10.1016/0092-8674(86)90861-5. [DOI] [PubMed] [Google Scholar]
- Georgopoulos K., van den Elsen P., Bier E., Maxam A., Terhorst C. A T cell-specific enhancer is located in a DNase I-hypersensitive area at the 3' end of the CD3-delta gene. EMBO J. 1988 Aug;7(8):2401–2407. doi: 10.1002/j.1460-2075.1988.tb03085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Gold D. P., Puck J. M., Pettey C. L., Cho M., Coligan J., Woody J. N., Terhorst C. Isolation of cDNA clones encoding the 20K non-glycosylated polypeptide chain of the human T-cell receptor/T3 complex. Nature. 1986 May 22;321(6068):431–434. doi: 10.1038/321431a0. [DOI] [PubMed] [Google Scholar]
- Gold D. P., van Dongen J. J., Morton C. C., Bruns G. A., van den Elsen P., Geurts van Kessel A. H., Terhorst C. The gene encoding the epsilon subunit of the T3/T-cell receptor complex maps to chromosome 11 in humans and to chromosome 9 in mice. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1664–1668. doi: 10.1073/pnas.84.6.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Krimpenfort P., de Jong R., Uematsu Y., Dembic Z., Ryser S., von Boehmer H., Steinmetz M., Berns A. Transcription of T cell receptor beta-chain genes is controlled by a downstream regulatory element. EMBO J. 1988 Mar;7(3):745–750. doi: 10.1002/j.1460-2075.1988.tb02871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M., Pierce J. W., Baltimore D. Protein-binding sites in Ig gene enhancers determine transcriptional activity and inducibility. Science. 1987 Jun 19;236(4808):1573–1577. doi: 10.1126/science.3109035. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S., Gross G., Horowitz M., Givol D. Promoter and enhancer elements in the rearranged alpha chain gene of the human T cell receptor. EMBO J. 1987 Nov;6(11):3307–3312. doi: 10.1002/j.1460-2075.1987.tb02650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- McDougall S., Peterson C. L., Calame K. A transcriptional enhancer 3' of C beta 2 in the T cell receptor beta locus. Science. 1988 Jul 8;241(4862):205–208. doi: 10.1126/science.2968651. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Wood W. I., Dolan M., Engel J. D., Felsenfeld G. A 200 base pair region at the 5' end of the chicken adult beta-globin gene is accessible to nuclease digestion. Cell. 1981 Nov;27(1 Pt 2):45–55. doi: 10.1016/0092-8674(81)90359-7. [DOI] [PubMed] [Google Scholar]
- Parslow T. G., Granner D. K. Chromatin changes accompany immunoglobulin kappa gene activation: a potential control region within the gene. Nature. 1982 Sep 30;299(5882):449–451. doi: 10.1038/299449a0. [DOI] [PubMed] [Google Scholar]
- Picard D., Schaffner W. A lymphocyte-specific enhancer in the mouse immunoglobulin kappa gene. Nature. 1984 Jan 5;307(5946):80–82. doi: 10.1038/307080a0. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986 Aug 21;322(6081):697–701. doi: 10.1038/322697a0. [DOI] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Saito H., Koyama T., Georgopoulos K., Clevers H., Haser W. G., LeBien T., Tonegawa S., Terhorst C. Close linkage of the mouse and human CD3 gamma- and delta-chain genes suggests that their transcription is controlled by common regulatory elements. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9131–9134. doi: 10.1073/pnas.84.24.9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Stalder J., Groudine M., Dodgson J. B., Engel J. D., Weintraub H. Hb switching in chickens. Cell. 1980 Apr;19(4):973–980. doi: 10.1016/0092-8674(80)90088-4. [DOI] [PubMed] [Google Scholar]
- Toyonaga B., Mak T. W. Genes of the T-cell antigen receptor in normal and malignant T cells. Annu Rev Immunol. 1987;5:585–620. doi: 10.1146/annurev.iy.05.040187.003101. [DOI] [PubMed] [Google Scholar]
- Tunnacliffe A., Buluwela L., Rabbitts T. H. Physical linkage of three CD3 genes on human chromosome 11. EMBO J. 1987 Oct;6(10):2953–2957. doi: 10.1002/j.1460-2075.1987.tb02600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnacliffe A., Olsson C., Buluwela L., Rabbitts T. H. Organization of the human CD3 locus on chromosome 11. Eur J Immunol. 1988 Oct;18(10):1639–1642. doi: 10.1002/eji.1830181027. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Hou D., Orloff D. G., Modi W. S., Seuanez H., O'Brien S. J., Klausner R. D. Molecular cloning and chromosomal localization of the human T-cell receptor zeta chain: distinction from the molecular CD3 complex. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9709–9713. doi: 10.1073/pnas.85.24.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender E. Compilation of transcription regulating proteins. Nucleic Acids Res. 1988 Mar 25;16(5):1879–1902. doi: 10.1093/nar/16.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]
- Yang J. Q., Remmers E. F., Marcu K. B. The first exon of the c-myc proto-oncogene contains a novel positive control element. EMBO J. 1986 Dec 20;5(13):3553–3562. doi: 10.1002/j.1460-2075.1986.tb04682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen J. J., Krissansen G. W., Wolvers-Tettero I. L., Comans-Bitter W. M., Adriaansen H. J., Hooijkaas H., van Wering E. R., Terhorst C. Cytoplasmic expression of the CD3 antigen as a diagnostic marker for immature T-cell malignancies. Blood. 1988 Mar;71(3):603–612. [PubMed] [Google Scholar]
- van Dongen J. J., Quertermous T., Bartram C. R., Gold D. P., Wolvers-Tettero I. L., Comans-Bitter W. M., Hooijkaas H., Adriaansen H. J., de Klein A., Raghavachar A. T cell receptor-CD3 complex during early T cell differentiation. Analysis of immature T cell acute lymphoblastic leukemias (T-ALL) at DNA, RNA, and cell membrane level. J Immunol. 1987 Feb 15;138(4):1260–1269. [PubMed] [Google Scholar]
- van den Elsen P., Georgopoulos K., Shepley B. A., Orkin S., Terhorst C. Exon/intron organization of the genes coding for the delta chains of the human and murine T-cell receptor/T3 complex. Proc Natl Acad Sci U S A. 1986 May;83(9):2944–2948. doi: 10.1073/pnas.83.9.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]