Abstract
Centrosomes are composed of two centrioles surrounded by pericentriolar material (PCM). However, the sperm and the oocyte modify or lose their centrosomes. Consequently, how the zygote establishes its first centrosome, and in particular, the origin of the second zygotic centriole, is uncertain. Drosophila melanogaster spermatids contain a single centriole called the Giant Centriole (GC) and a Proximal centriole-like (PCL) structure whose function is unknown. We found that, like the centriole, the PCL loses its protein markers at the end of spermiogenesis. After fertilization, the first two centrioles are observed via the recruitment of the zygotic PCM proteins and are seen in asterless mutant embryos that cannot form centrioles. The zygote’s centriolar proteins label only the daughter centrioles of the first two centrioles. These observations demonstrate that the PCL is the origin for the second centriole in the Drosophila zygote and that a paternal centriole precursor, without centriolar proteins, is transmitted to the egg during fertilization.
Keywords: PCL, centriole, Asterless, spermiogenesis, fertilization
CENTRIOLES, in the cytoplasm and basal bodies at the plasma membrane, are conserved microtubule-based organelles essential for cell division and cilium formation (Nigg and Raff 2009). Centrioles are essential for fertilization, development, and animal physiological functions (Nigg and Raff 2009). In the newly fertilized egg (i.e., zygote), a centriole normally functions by recruiting pericentriolar material (PCM) and becoming the primary centrosome (Delattre and Gonczy 2004). This centrosome, in the zygote, acts as a microtubule-organizing center and nucleates the astral microtubules that mediate the migration of the female and male nuclei toward each other (Callaini and Riparbelli 1996).
A centriole forms by one of two pathways. In the “duplication pathway,” a pre-existing centriole acts as a scaffold to ensure that only a daughter centriole is formed per cell cycle. However, the pre-existing centriole does not appear to impart structural information to the daughter (Rodrigues-Martins et al. 2007). In the “de novo pathway,” a centriole forms without a pre-existing centriole and forms more than two centrioles. This pathway occurs when multiple centrioles are required in a cell or in the unusual situation where pre-existing centrioles are absent (Uetake et al. 2007).
Most resting cells have two centrioles. A cell preparing to divide duplicates its centrioles and consequently has four centrioles; each mother/daughter centriole pair forms a centrosome at opposite poles of the cell. Having precisely two centrioles before commitment to cell division and four centrioles during mitosis is particularly critical for proper cell division and an organism’s development (Fukasawa 2007). Having no centrioles interferes with the proper orientation of the spindle axis (Basto et al. 2006), while too many centrioles results in an increase in aneuploidy and defects in cilium formation (Basto et al. 2008; Mahjoub and Stearns 2012). Because a cell requires two centrioles to function, the zygote is expected to require two centrioles. In animals, during oogenesis centrioles are lost, and therefore, oocytes lack centrioles and do not contribute any centrioles to the zygote (Sun and Schatten 2007). Instead, it has been reported that in many animals centrioles are inherited by the zygote from the sperm (Sun and Schatten 2007). However, in many other animals, a single functional centriole is observed. Humans and most mammalian sperm have only a single centriole because during the last phase of spermatogenesis the spermatid centrioles are modified and degraded in a process known as centrosome reduction (Manandhar et al. 2005). In sea urchins, frogs, and Caenorhabditis elegans, the sperm provides two centrioles (Longo and Anderson 1968; Felix et al. 1994; Leidel 2005). The origin of this second zygotic centriole is uncertain. To help explain these uncertainties, four hypotheses have been put forth:
The “de novo/maternal-precursor hypothesis” attempts to explain the observation that, due to a dramatic centrosome reduction, rodent sperm lack a recognizable centriole, yet centrioles are observed when the embryo reaches the 64-cell stage (Schatten et al. 1986; Gueth-Hallonet et al. 1993). It has been proposed that the embryonic centriole forms de novo (Howe and Fitzharris 2013). Alternatively, it has been claimed that the oocyte contains centriolar precursors that give rise to the embryo’s centrioles (Calarco 2000). This hypothesis may also apply to some insects that reproduce by parthenogenesis (Ferree et al. 2006).
The “regeneration hypothesis” proposes that nonrodent mammalian sperm cells have an intact centriole (the proximal centriole) and, due to centrosome reduction, a degenerated centriole (the distal centriole). After fertilization, the degenerated centriole regenerates to form a second centriolar structure (Manandhar et al. 2005; Schatten and Sun 2009). It is claimed that both the intact and the “regenerated” centriole duplicate to form two pairs of centrioles, together resulting in three centrioles and one regenerated centriole.
The “duplication hypothesis” postulates that a single functional centriole is inherited from the sperm, which is duplicated soon after fertilization. In Drosophila, only one functional centriole is known (Fuller 1993); the second zygotic centriole is presumed to be the product of the sperm centriole duplicating in the zygote (Callaini and Riparbelli 1996). In this hypothesis, a round of centriole duplication happens prior to the zygote’s first cell division.
The “paternal precursor hypothesis” theorizes that a sperm provides both a centriole and a centriolar precursor that originated during spermiogenesis, but did not mature to a centriole; in the zygote, the precursor becomes the second centriole (Crozet et al. 2000).
We have recently discovered that, in addition to the giant centriole (GC), which is attached to the plasma membrane and is equivalent to a basal body, Drosophila sperm contain an unidentified centriolar structure that lacks the distinctive structural characteristic of a centriole; we named it the proximal centriole-like (PCL) (Blachon et al. 2009). The PCL is a centriole precursor. Initially, the PCL forms in a similar way to a centriole, as they both use the same molecular pathways. However, the PCL diverges from the centriolar formation pathway prior to when a centriole acquires centriolar microtubules, a defining characteristic of a centriole. The PCL may be the predicted precursor of the precursor hypothesis.
Here we report that, like the GC, the PCL also undergoes centrosome reduction. As a result, currently, there is no way to stably label the PCL of the Drosophila sperm to directly test the PCL hypothesis. However, the PCL hypothesis can be differentiated from the duplication hypothesis and the maternal-precursor/de novo hypothesis using three criteria. First, the PCL hypothesis predicts that the first two zygotic centriolar structures appear in the zygote simultaneously, immediately after fertilization. Second, in the zygote that is generated from an oocyte expressing GFP-tagged early centriolar proteins, only the daughter centrioles of the first two zygotic centrioles should be labeled by GFP proteins that initiate centriole formation. Third, in a zygote that is generated from a mutant oocyte that cannot support centriole duplication, two centrioles should be observed after fertilization. Here, we test these predictions and provide evidence for the PCL/paternal precursor hypothesis.
Materials and Methods
Fluorescence microscopy
For embryo imaging, 50 male and 50 virgin female flies, <5 days old, were placed in an egg collection chamber with a grape agar plate with yeast paste. Chambers were used for 3 days and embryos were collected every 4 min. Immediately after collection, the embryos were placed in a mac-tech dish and washed with 100 μl of distilled water and then a wash buffer (0.7% NaCl + 0.05% Triton 100×). Afterward, 50% bleach solution was added onto the embryos until the appendages of the embryos disassociated. The embryos were rinsed twice with wash buffer, fixed in a 1:1 solution of heptane and methanol, and shaken vigorously by a vortex until the embryos settled down in the methanol layer. This was followed by removal of the fixative and suspending the embryos in acetone. At this stage, the embryos were usually stored at −20°. Then the embryos were rehydrated sequentially in 70, 50, 30, and 10% methanol in PBS and then in PBS alone. The embryos were then incubated in PBT (PBS+1% Triton) for 30 min and blocked in PBST (PBT+3% BSA) for 1 hr. Then the embryos were incubated with primary antibodies in PBST for 1 hr at room temperature. After three 5-min washes in PBT, the embryos were incubated with secondary antibodies and 1 μg/ml of DAPI for 1 hr at room temperature. The embryos were washed three times with PBT for 5 min each and then washed in PBS for 5 min. The embryos were mounted on a slide using a mounting medium (PBS, 50% glycerol, 0.5% N-propyl-gallate) and imaged. Testis imaging was performed as described in Basiri et al. (2013). Images were taken by a Leica SP5 or SP8 scanning confocal microscope as Z stacks. Maximal projection images were then modified using Adobe Photoshop and annotated using Adobe Illustrator.
Embryo development
To analyze the embryo development, 50 male and 50 female flies were allowed to mate overnight in a chamber. Then the agar plate with the laid embryos was incubated at 25° for 24 hr, and the number of larvae were counted.
Statistical methods
Experiments were repeated at least three times, and statistical analyses (±SEM) were done with GraphPad Prism 5. A two-tailed, unpaired Student’s t-test was used.
Results
PCL and GC undergo centrosome reduction during late spermiogenesis
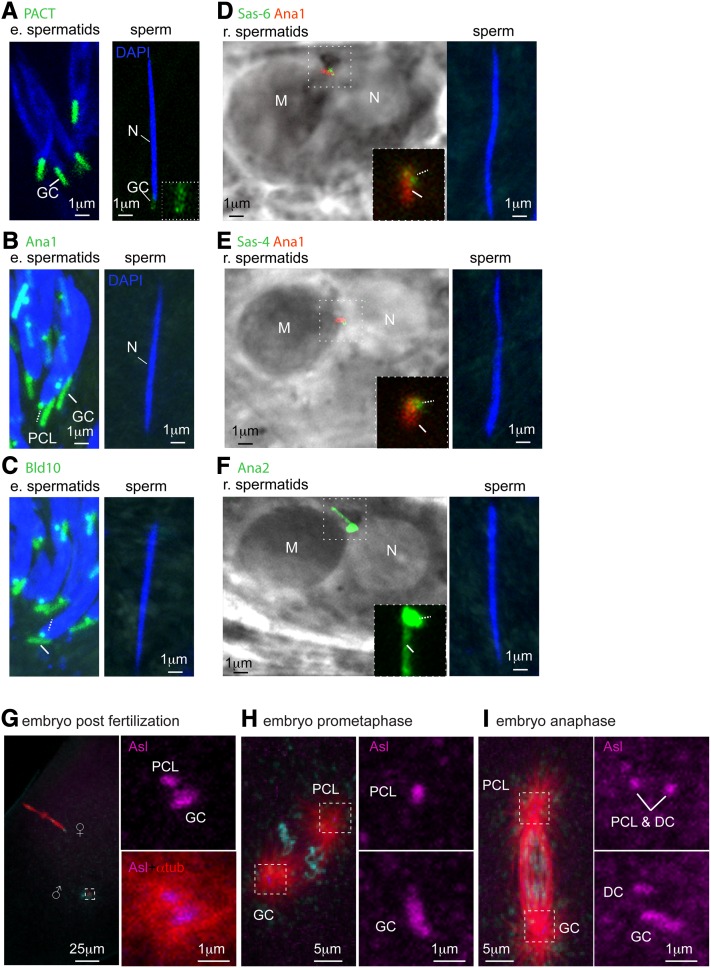
At the end of spermiogenesis, mammalian centrosomes undergo a process by which they lose their PCM and centriole structure is degraded, resulting in sperm with modified centrioles (Schatten 1994; Manandhar and Schatten 2000; Manandhar et al. 2000, 2005). Similarly, during Drosophila spermiogenesis, the PCM proteins γ-tubulin (Wilson et al. 1997) and Cnn (Li et al. 1998) are eliminated from the GC, indicating that centrosome reduction also happens in Drosophila. The GC marker PACT-GFP, which can be intensely observed in the spermatid GC, is hardly observed in the sperm GC (Figure 1A) (Martinez-Campos et al. 2004). To test if the PCL also undergoes centrosome reduction, and whether during centrosome reduction centriolar proteins are also eliminated from the GC, we studied the localization of GFP-tagged centriolar proteins during spermiogenesis and in sperm. We observed PACT-GFP, Ana1-GFP, BLD10-GFP, Ana2-GFP, Sas-6-GFP, and Sas-4-GFP in intermediate or round spermatids, in the giant centriole, and in the PCL (Figure 1, A-F). We found that all of these centriolar proteins are missing from mature sperm, indicating centrosome reduction takes place in both the GC and the PCL. This indicates that the PCL, which was formed during early spermiogenesis and without microtubules (Blachon et al. 2009), loses many of the proteins that formed it during centrosome reduction.
Figure 1.
The PCL and GC undergo centrosome reduction and recruit PCM after fertilization. (A) The GC (solid line) is intensely labeled by PACT-GFP (which is overexpressed by the strong ubiquitin promoter in intermediate spermatids), but can be barely observed at the base of the sperm nucleus (see inset for magnification of this GC). (B–F) The PCL (dashed line) and GC (solid line) are observed in intermediate spermatids by Ana1-GFP (B) and BLD10-GFP (C), as are round spermatids by Sas-6 GFP (D), Sas-4-GFP (E), and Ana2-GFP (F). The GC is also marked by Ana1-td tomato (D–F). However, none of these proteins are observed in sperm found in the seminal vesicle. Note that Ana2-GFP (Stevens et al. 2010) is strongly expressed using the ubiquitin promoter, yet it is completely eliminated during centrosome reduction. N, nuclei. (G–I) Drosophila zygotes contain two centrioles immediately after fertilization and four during mitosis (G). After fertilization, and during maternal meiosis II (twin meiosis II spindles are labeled red, ♀), the GC and second centriolar structure, which is likely to be the PCL, are labeled by Asl; these structures form microtubule asters (red) that associate with the male pronucleus (♂). (H). During prometaphase, the GC and the PCL form distinct microtubule asters (I). During anaphase, the GC and the PCL are each accompanied by an Asl-labeled daughter centriole, indicating that they each gave rise to a DC.
Zygote has two centrioles immediately after fertilization
After fertilization, the centrioles that lost their proteins via centrosome reduction can be detected by labeling the PCM components that they recruit from the oocyte’s cytoplasm (Callaini and Riparbelli 1996). To image the zygote’s centrioles, we performed immunofluorescence using an antibody against the PCM protein Asterless (Asl) and an anti-α-tubulin antibody that labels microtubule asters (Blachon et al. 2008). We found that immediately after fertilization, when the twin meiosis II spindles of the female are observed, the two Asl-labeled centrioles are present in the zygote. One of the centrioles is longer and is likely to be the GC (Riparbelli and Callaini 2010), and the other one is a smaller centriole (Figure 1G). Because the two centrioles are observed immediately post-fertilization, this second smaller centriole is likely to be derived from the PCL (Figure 1G, left; see below). Both centrioles are marked by Asl, which they must have recruited from the cytoplasm, together with the rest of the PCM, to form their own aster microtubules. This suggests that both the GC and PCL, which lose many of their components during centrosome reduction, are capable of recruiting PCM and anchoring astral microtubules. During the zygote prometaphase, the two centrioles are at opposite spindle poles (Figure 1H). During anaphase, centriole duplication has already occurred, and daughter centrioles are present near each of the pre-existing centrioles [daughter centrioles (DC), Figure 1I]. These data suggest that the PCL, like a typical centriole, can serve as a scaffold for daughter centriole formation and forms a second centriole. Altogether, these data indicate that either the sperm transported both the GC and the PCL or that the GC duplicated immediately after fertilization and before the completion of maternal meiosis II.
Unlike their daughter centrioles, the first two zygotic centrioles do not incorporate centriolar proteins
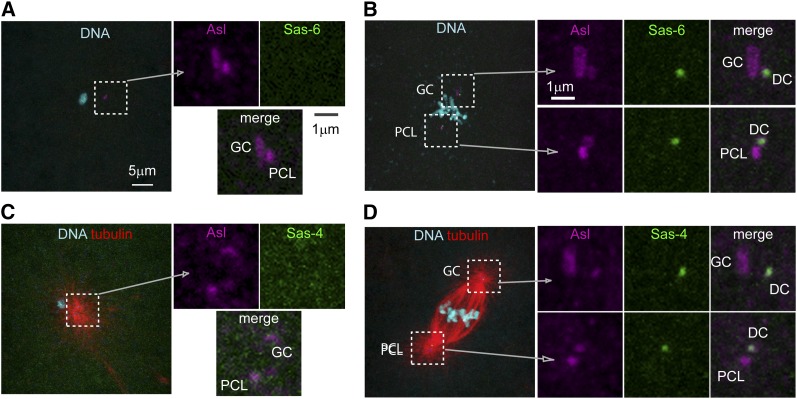
Centrioles, during their formation, incorporate centriolar proteins such as Sas-6 and Sas-4, which are available in the cytoplasm (Kirkham et al. 2003; Leidel and Gonczy 2003). Therefore, to test whether the first two zygotic centrioles are formed in the oocyte, we used oocytes expressing the centriolar proteins Sas-6-GFP and Sas-4-GFP. These oocytes were fertilized with sperm centrioles, which can be observed once they recruit PCM. As expected, immediately after fertilization, there are two zygotic centrioles labeled by the PCM protein Asterless (Figure 2, A and C). However, neither of these centrioles was labeled by Sas-6-GFP or Sas-4-GFP, indicating that they did not form in the zygote and that their origin must be from the sperm. One of these centriolar structures is large, suggesting that it is the GC, while the second is smaller, suggesting that it is the PCL. Later, during mitosis, Asterless staining identifies four centrioles. Of these, two are labeled only by Asterless, and the remaining two are labeled by Asl and Sas-6-GFP or Sas-4-GFP (Figure 2, B and D). The two centrioles labeled by only Asterless are likely to be the GC and the PCL. The two centriolar structures labeled by Sas-6-GFP or Sas-4 -GFP are likely to be the DCs of the GC and the PCL (Figure 2, B and C, left).
Figure 2.
Maternally GFP-tagged centriolar proteins are incorporated into the daughter centrioles of the GC and the PCL. (A and C) Immediately post-fertilization, the first two zygotic centrioles are not labeled by maternal centriolar proteins, but are labeled by Asl (magenta). (B and D) During the zygote’s first mitosis, an antibody against Asl labels all centrioles, but Sas-6-GFP (B) or Sas-4-GFP (D) labels only two centrioles. This indicates that three types of centrioles are observed: a GC, the presumed PCL, and two DCs.
Altogether, this experiment strongly argues against both the maternal precursor hypothesis and the duplication hypothesis. Indeed, maternal centrioles and centrioles duplicated in the zygote should have been labeled by Sas-6-GFP or Sas-4-GFP. These data, however, are consistent with the PCL hypothesis.
Homozygote aslmecD zygotes have two centrioles
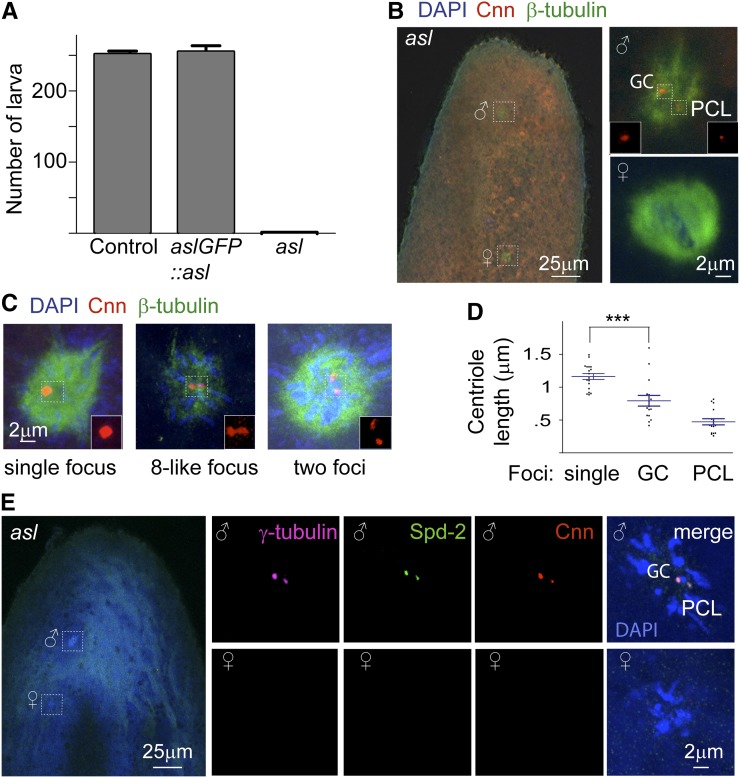
Drosophila Asterless and its vertebrate homolog Cep152 are essential proteins for centriole duplication (Blachon et al. 2008) and embryo development (Varmark et al. 2007). Depletion of Cep152 prevents both centriole duplication and Plk4-induced de novo centriole formation (Cizmecioglu et al. 2010; Hatch et al. 2010), indicating that Asterless/Cep152 is essential for centriole formation by both the duplication and de novo pathways. We recently showed that aslmecD completely blocks centriole duplication in flies (Blachon et al. 2008). Because the aslmecD flies die shortly after emerging from their pupal cases, these flies are not capable of mating. To remedy this, we generated aslmecD oocyte clones that lack the Asterless protein in heterozygous aslmecD females (Supporting Information, Figure S1, File S1). To test for the role of Asl in fertilization, these adult females were mated to normal males, and their zygotes were analyzed. As expected, we found that aslmecD embryos cannot develop into larvae (Figure 3A). Importantly, introduction of Asl-GFP using germline transformation reverted the aslmecD phenotype, allowing embryo development (Figure 3A).
Figure 3.
Two centrioles are observed in an oocyte mutant for centriole duplication. (A) Embryo development is arrested in embryos generated by aslmecD oocytes and is rescued in the presence of Asl-GFP. (B) A zygote generated by an aslmecD oocyte with a paternal pronucleus (♂) labeled by two Cnn-labeled centrioles and a maternal pronucleus (♀) lacking centrioles. (C) Paternal pronuclei in zygotes generated by aslmecD oocytes have two centrioles that are superimposed (single focus), near each other (8-like focus), or apart from each other (two foci). (D) Graph depicting the length of the Cnn foci in embryos with one Cnn focus (single) as well as long and short CNN foci in embryos with two separate Cnn foci, demonstrating that most of the apparent foci are two superimposed foci. ***P < 0.0003. (E) Cnn, γ-tubulin, and Spd-2 are colabeling the two zygotic centrioles.
We then analyzed the phenotype of zygotes generated by aslmecD oocytes. Embryos collected immediately after they were laid were stained with the nuclear marker DAPI, an antibody for microtubules, and an antibody for the centrosomal protein Cnn. Two populations of embryos were found. The first population has two to four nuclei associated with the microtubule spindles, which are lacking centrosomes as judged by the absence of PCM markers (36% or 28/77), indicating that they are unfertilized oocytes. The second population has one paternal pronucleus that possesses Cnn labeling and one maternal pronucleus that lacks Cnn labeling, indicating that the second population are fertilized oocytes (i.e., an embryo) (64% 49/77) (Figure 3B). The maternal and paternal pronuclei were not close to each other, indicating that the embryo’s development arrested before completion of pronuclei migration.
Analysis of the Cnn labeling of the paternal pronuclei found that 32% of embryos (16/49) have two clearly separated Cnn foci; 30% (15/49) have two close, but distinct, Cnn foci that look like the “figure 8”; and 36% (18/49) have a single Cnn focus (Figure 3C). Because the size of the single Cnn focus is larger than a clearly separated Cnn focus (Figure 3D), it is likely that many of the single foci are two centriolar structures that are closely associated with each other. Similar to Cnn, γ-tubulin and DSpd-2 also label both centrioles of the zygote generated by the aslmecD oocytes (Figure 3E). Altogether, this analysis demonstrates that an embryo that cannot duplicate centrioles has two centriolar structures, consistent with the PCL hypothesis.
Discussion
We have shown previously that the Drosophila sperm contain a second centriolar structure, the PCL (Blachon et al. 2009). In this article, we demonstrated that two centrioles are present in the zygote after fertilization; that these two centrioles are not marked by maternally contributed centriolar proteins; and that, in a zygote unable to duplicate centrioles, there are two centrioles present. Therefore, the sperm must have brought two centrioles to the zygote.
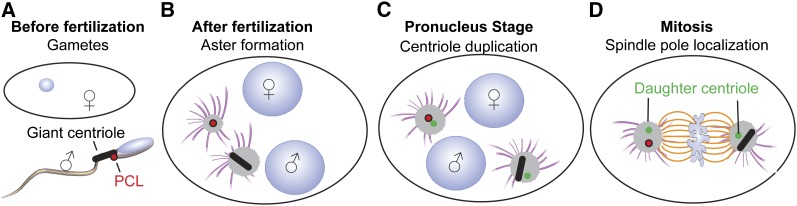
We therefore propose the following model for centriole inheritance in Drosophila: the sperm provides a GC and a PCL, and both lack PCM and many centriolar proteins, while the oocyte is lacking any centrioles (Figure 4A). Immediately after fertilization, both the GC and the PCL recruit the PCM and nucleate astral microtubules, but they do not incorporate centriolar proteins (Figure 4B). Later, the centriole and PCL each template a daughter centriole, which is made from maternally contributed proteins (Figure 4C). Finally, the two centrosomes, one derived from the GC, and the other derived from the PCL, migrate to the spindle poles of the embryo’s first spindle (Figure 4D). Because after fertilization the PCL does not incorporate maternal Sas-6 and Sas-4, it is possible that the PCL does not mature to a typical centriole.
Figure 4.
The PCL in Drosophila fertilization. (A) During fertilization, the oocyte lacks centrioles, whereas the sperm contains a GC and a PCL. (B) After fertilization, the centriole and the PCL form asters. (C) During the pronuclear stage, the centriole and PCL are duplicated, resulting in two DCs. (D) During mitosis, each pole has a centrosome that consists of a centriole with its daughter or of a PCL with its daughter.
Our data extend the similarity between the PCL and centrioles. First, like the GC, the PCL also undergoes centrosome reduction at the end of spermiogenesis. Second, after fertilization, both the GC and the PCL do not recruit centriolar proteins, despite losing them. The GC and the PCL still recruit PCM proteins, form astral microtubules, and give rise to a daughter centriole. How the GC and the PCL can accomplish this without Sas-6 and Sas-4, and possibly other centriolar proteins, remains a mystery.
Importantly, the PCL hypothesis may be applicable to vertebrates; it may explain why only three centrioles have been observed in a mammalian zygote during mitosis (Sathananthan et al. 1996; Crozet et al. 2000), although it is known that four centrioles are required for normal mitosis in a cell. Perhaps the PCL, which was unseen in any organism until our discovery in Drosophila, remains undiscovered in mammalian sperm.
The finding that the PCL is the second centriole that the sperm provides argues for an almost universal mechanism of centriole inheritance among animals that involves paternal inheritance of two centrioles. These may be two centrioles (C. elegans, frogs and sea urchin), a regenerated centriole (or potentially a PCL), and one centriole (nonrodent mammals) or one centriole and one PCL (Drosophila). Altogether, we have shown here that the PCL is a centriole precursor that contributes to the first zygote in Drosophila and, as a result, is the origin of half of the fly’s centrioles. Similar mechanisms may exist in other animals.
Supplementary Material
Acknowledgments
We thank Alberto Vizuet-Torre, Suma Kolla, Marli Emad Gabriel, and Vidita Reddy for technical assistance; Sarah E. Hynek for editing this manuscript; and Jarema Malicki for commenting on the manuscript. We also thank Maurizio Gatti for the DSpd-2 antibody, Thomas C. Kaufman for the Cnn antibody, and Jordan Raff for the Ana2-GFP and PACT-GFP flies. This work was supported by National Science Foundation grant 1121176. The authors declare no competing financial interests.
Footnotes
Communicating editor: L. Cooley
Literature Cited
- Basiri M. L., Blachon S., Chim Y. C., Avidor-Reiss T., 2013. Imaging centrosomes in fly testes. J. Vis. Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basto R., Lau J., Vinogradova T., Gardiol A., Woods C. G., et al. , 2006. Flies without centrioles. Cell 125: 1375–1386. [DOI] [PubMed] [Google Scholar]
- Basto R., Brunk K., Vinadogrova T., Peel N., Franz A., et al. , 2008. Centrosome amplification can initiate tumorigenesis in flies. Cell 133: 1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachon S., Gopalakrishnan J., Omori Y., Polyanovsky A., Church A., et al. , 2008. Drosophila asterless and vertebrate Cep152 Are orthologs essential for centriole duplication. Genetics 180: 2081–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachon S., Cai X., Roberts K. A., Yang K., Polyanovsky A., et al. , 2009. A proximal centriole-like structure is present in Drosophila spermatids and can serve as a model to study centriole duplication. Genetics 182: 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarco P. G., 2000. Centrosome precursors in the acentriolar mouse oocyte. Microsc. Res. Tech. 49: 428–434. [DOI] [PubMed] [Google Scholar]
- Callaini G., Riparbelli M. G., 1996. Fertilization in Drosophila melanogaster: centrosome inheritance and organization of the first mitotic spindle. Dev. Biol. 176: 199–208. [DOI] [PubMed] [Google Scholar]
- Cizmecioglu O., Arnold M., Bahtz R., Settele F., Ehret L., et al. , 2010. Cep152 acts as a scaffold for recruitment of Plk4 and CPAP to the centrosome. J. Cell Biol. 191: 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozet N., Dahirel M., Chesne P., 2000. Centrosome inheritance in sheep zygotes: centrioles are contributed by the sperm. Microsc. Res. Tech. 49: 445–450. [DOI] [PubMed] [Google Scholar]
- Delattre M., Gonczy P., 2004. The arithmetic of centrosome biogenesis. J. Cell Sci. 117: 1619–1630. [DOI] [PubMed] [Google Scholar]
- Felix M. A., Antony C., Wright M., Maro B., 1994. Centrosome assembly in vitro: role of gamma-tubulin recruitment in Xenopus sperm aster formation. J Cell Biol 124: 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree P. M., McDonald K., Fasulo B., Sullivan W., 2006. The origin of centrosomes in parthenogenetic hymenopteran insects. Curr. Biol. 16: 801–807. [DOI] [PubMed] [Google Scholar]
- Fukasawa K., 2007. Oncogenes and tumour suppressors take on centrosomes. Nat. Rev. Cancer 7: 911–924. [DOI] [PubMed] [Google Scholar]
- Fuller M. T., 1993. Spermatogenesis, pp. 71–174 in The Development of Drosophila melanogaster, edited by Bate M., Martinez-Arias A. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Gueth-Hallonet C., Antony C., Aghion J., Santa-Maria A., Lajoie-Mazenc I., et al. , 1993. gamma-Tubulin is present in acentriolar MTOCs during early mouse development. J. Cell Sci. 105(Pt 1): 157–166. [DOI] [PubMed] [Google Scholar]
- Hatch E. M., Kulukian A., Holland A. J., Cleveland D. W., Stearns T., 2010. Cep152 interacts with Plk4 and is required for centriole duplication. J. Cell Biol. 191: 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K., Fitzharris G., 2013. A non-canonical mode of microtubule organization operates throughout pre-implantation development in mouse. Cell Cycle 12: 1616–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham M., Muller-Reichert T., Oegema K., Grill S., Hyman A. A., 2003. SAS-4 is a C. elegans centriolar protein that controls centrosome size. Cell 112: 575–587. [DOI] [PubMed] [Google Scholar]
- Leidel S., Gonczy P., 2003. SAS-4 is essential for centrosome duplication in C elegans and is recruited to daughter centrioles once per cell cycle. Dev. Cell 4: 431–439. [DOI] [PubMed] [Google Scholar]
- Leidel S., Gonczy P., 2005. Centrosome duplication and nematodes: recent insights from an old relationship. Dev. Cell 9: 317–325. [DOI] [PubMed] [Google Scholar]
- Li K., Xu E. Y., Cecil J. K., Turner F. R., Megraw T. L., et al. , 1998. Drosophila centrosomin protein is required for male meiosis and assembly of the flagellar axoneme. J. Cell Biol. 141: 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo F. J., Anderson E., 1968. The fine structure of pronuclear development and fusion in the sea urchin, Arbacia punctulata. J. Cell Biol. 39: 339–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahjoub M. R., Stearns T., 2012. Supernumerary centrosomes nucleate extra cilia and compromise primary cilium signaling. Curr. Biol. 22: 1628–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manandhar G., Schatten G., 2000. Centrosome reduction during Rhesus spermiogenesis: gamma-tubulin, centrin, and centriole degeneration. Mol. Reprod. Dev. 56: 502–511. [DOI] [PubMed] [Google Scholar]
- Manandhar G., Simerly C., Schatten G., 2000. Centrosome reduction during mammalian spermiogenesis. Curr. Top. Dev. Biol. 49: 343–363. [DOI] [PubMed] [Google Scholar]
- Manandhar G., Schatten H., Sutovsky P., 2005. Centrosome reduction during gametogenesis and its significance. Biol. Reprod. 72: 2–13. [DOI] [PubMed] [Google Scholar]
- Martinez-Campos M., Basto R., Baker J., Kernan M., Raff J. W., 2004. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensable for mitosis. J. Cell Biol. 165: 673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Raff J. W., 2009. Centrioles, centrosomes, and cilia in health and disease. Cell 139: 663–678. [DOI] [PubMed] [Google Scholar]
- Riparbelli M. G., Callaini G., 2010. Detachment of the basal body from the sperm tail is not required to organize functional centrosomes during Drosophila embryogenesis. Cytoskeleton (Hoboken) 67: 251–258. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Martins A., Riparbelli M., Callaini G., Glover D. M., Bettencourt-Dias M., 2007. Revisiting the role of the mother centriole in centriole biogenesis. Science 316: 1046–1050. [DOI] [PubMed] [Google Scholar]
- Sathananthan A. H., Ratnam S. S., Ng S. C., Tarin J. J., Gianaroli L., et al. , 1996. The sperm centriole: its inheritance, replication and perpetuation in early human embryos. Hum. Reprod. 11: 345–356. [DOI] [PubMed] [Google Scholar]
- Schatten G., 1994. The centrosome and its mode of inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev. Biol. 165: 299–335. [DOI] [PubMed] [Google Scholar]
- Schatten H., Sun Q. Y., 2009. The role of centrosomes in mammalian fertilization and its significance for ICSI. Mol. Hum. Reprod. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten H., Schatten G., Mazia D., Balczon R., Simerly C., 1986. Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc. Natl. Acad. Sci. USA 83: 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens N. R., Dobbelaere J., Brunk K., Franz A., Raff J. W., 2010. Drosophila Ana2 is a conserved centriole duplication factor. J. Cell Biol. 188: 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q. Y., Schatten H., 2007. Centrosome inheritance after fertilization and nuclear transfer in mammals. Adv. Exp. Med. Biol. 591: 58–71. [DOI] [PubMed] [Google Scholar]
- Uetake Y., Loncarek J., Nordberg J. J., English C. N., La Terra S., et al. , 2007. Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J. Cell Biol. 176: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmark H., Llamazares S., Rebollo E., Lange B., Reina J., et al. , 2007. Asterless is a centriolar protein required for centrosome function and embryo development in Drosophila. Curr. Biol. 17: 1735–1745. [DOI] [PubMed] [Google Scholar]
- Wilson P. G., Zheng Y., Oakley C. E., Oakley B. R., Borisy G. G., et al. , 1997. Differential expression of two gamma-tubulin isoforms during gametogenesis and development in Drosophila. Dev. Biol. 184: 207–221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.