Abstract
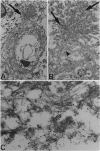
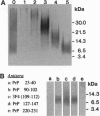
Deposition of PrP amyloid in cerebral vessels in conjunction with neurofibrillary lesions is the neuropathologic hallmark of the dementia associated with a stop mutation at codon 145 of PRNP, the gene encoding the prion protein (PrP). In this disorder, the vascular amyloid in tissue sections and the approximately 7.5-kDa fragment extracted from amyloid are labeled by antibodies to epitopes located in the PrP sequence including amino acids 90-147. Amyloid-laden vessels are also labeled by antibodies against the C terminus, suggesting that PrP from the normal allele is involved in the pathologic process. Abundant neurofibrillary lesions are present in the cerebral gray matter. They are composed of paired helical filaments, are labeled with antibodies that recognize multiple phosphorylation sites in tau protein, and are similar to those observed in Alzheimer disease. A PrP cerebral amyloid angiopathy has not been reported in diseases caused by PRNP mutations or in human transmissible spongiform encephalopathies; we propose to name this phenotype PrP cerebral amyloid angiopathy (PrP-CAA).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsop D., Ikeda S., Bruce M., Glenner G. G. Cerebrovascular amyloid in scrapie-affected sheep reacts with antibodies to prion protein. Neurosci Lett. 1988 Oct 5;92(2):234–239. doi: 10.1016/0304-3940(88)90067-5. [DOI] [PubMed] [Google Scholar]
- Brown H. R., Goller N. L., Rudelli R. D., Merz G. S., Wolfe G. C., Wisniewski H. M., Robakis N. K. The mRNA encoding the scrapie agent protein is present in a variety of non-neuronal cells. Acta Neuropathol. 1990;80(1):1–6. doi: 10.1007/BF00294214. [DOI] [PubMed] [Google Scholar]
- Castaño E. M., Frangione B. Alzheimer's disease from the perspective of the systemic and localized forms of amyloidosis. Brain Pathol. 1991 Jul;1(4):263–271. doi: 10.1111/j.1750-3639.1991.tb00669.x. [DOI] [PubMed] [Google Scholar]
- Dlouhy S. R., Hsiao K., Farlow M. R., Foroud T., Conneally P. M., Johnson P., Prusiner S. B., Hodes M. E., Ghetti B. Linkage of the Indiana kindred of Gerstmann-Sträussler-Scheinker disease to the prion protein gene. Nat Genet. 1992 Apr;1(1):64–67. doi: 10.1038/ng0492-64. [DOI] [PubMed] [Google Scholar]
- Ghetti B., Dlouhy S. R., Giaccone G., Bugiani O., Frangione B., Farlow M. R., Tagliavini F. Gerstmann-Sträussler-Scheinker disease and the Indiana kindred. Brain Pathol. 1995 Jan;5(1):61–75. doi: 10.1111/j.1750-3639.1995.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Ghetti B., Tagliavini F., Giaccone G., Bugiani O., Frangione B., Farlow M. R., Dlouhy S. R. Familial Gerstmann-Sträussler-Scheinker disease with neurofibrillary tangles. Mol Neurobiol. 1994 Feb;8(1):41–48. doi: 10.1007/BF02778006. [DOI] [PubMed] [Google Scholar]
- Ghiso J., Plant G. T., Révész T., Wisniewski T., Frangione B. Familial cerebral amyloid angiopathy (British type) with nonneuritic amyloid plaque formation may be due to a novel amyloid protein. J Neurol Sci. 1995 Mar;129(1):74–75. doi: 10.1016/0022-510x(94)00274-r. [DOI] [PubMed] [Google Scholar]
- Giaccone G., Verga L., Bugiani O., Frangione B., Serban D., Prusiner S. B., Farlow M. R., Ghetti B., Tagliavini F. Prion protein preamyloid and amyloid deposits in Gerstmann-Sträussler-Scheinker disease, Indiana kindred. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9349–9353. doi: 10.1073/pnas.89.19.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Jakes R., Crowther R. A., Cohen P., Vanmechelen E., Vandermeeren M., Cras P. Epitope mapping of monoclonal antibodies to the paired helical filaments of Alzheimer's disease: identification of phosphorylation sites in tau protein. Biochem J. 1994 Aug 1;301(Pt 3):871–877. doi: 10.1042/bj3010871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Jakes R., Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett. 1995 Apr 21;189(3):167–169. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R. Localization of the Alz-50 epitope in recombinant human microtubule-associated protein tau. Neurosci Lett. 1991 May 27;126(2):149–154. doi: 10.1016/0304-3940(91)90541-z. [DOI] [PubMed] [Google Scholar]
- Greenberg S. G., Davies P., Schein J. D., Binder L. I. Hydrofluoric acid-treated tau PHF proteins display the same biochemical properties as normal tau. J Biol Chem. 1992 Jan 5;267(1):564–569. [PubMed] [Google Scholar]
- Hyman B. T., Tanzi R. E., Marzloff K., Barbour R., Schenk D. Kunitz protease inhibitor-containing amyloid beta protein precursor immunoreactivity in Alzheimer's disease. J Neuropathol Exp Neurol. 1992 Jan;51(1):76–83. doi: 10.1097/00005072-199201000-00009. [DOI] [PubMed] [Google Scholar]
- Kascsak R. J., Rubenstein R., Merz P. A., Tonna-DeMasi M., Fersko R., Carp R. I., Wisniewski H. M., Diringer H. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987 Dec;61(12):3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T., Iizuka R., Tateishi J. An amber mutation of prion protein in Gerstmann-Sträussler syndrome with mutant PrP plaques. Biochem Biophys Res Commun. 1993 Apr 30;192(2):525–531. doi: 10.1006/bbrc.1993.1447. [DOI] [PubMed] [Google Scholar]
- Matsuo E. S., Shin R. W., Billingsley M. L., Van deVoorde A., O'Connor M., Trojanowski J. Q., Lee V. M. Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer's disease paired helical filament tau. Neuron. 1994 Oct;13(4):989–1002. doi: 10.1016/0896-6273(94)90264-x. [DOI] [PubMed] [Google Scholar]
- Otvos L., Jr, Feiner L., Lang E., Szendrei G. I., Goedert M., Lee V. M. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994 Dec 15;39(6):669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- Piccardo P., Ghetti B., Dickson D. W., Vinters H. V., Giaccone G., Bugiani O., Tagliavini F., Young K., Dlouhy S. R., Seiler C. Gerstmann-Sträussler-Scheinker disease (PRNP P102L): amyloid deposits are best recognized by antibodies directed to epitopes in PrP region 90-165. J Neuropathol Exp Neurol. 1995 Nov;54(6):790–801. doi: 10.1097/00005072-199511000-00006. [DOI] [PubMed] [Google Scholar]
- Rogers M., Serban D., Gyuris T., Scott M., Torchia T., Prusiner S. B. Epitope mapping of the Syrian hamster prion protein utilizing chimeric and mutant genes in a vaccinia virus expression system. J Immunol. 1991 Nov 15;147(10):3568–3574. [PubMed] [Google Scholar]
- Shaw C. M. Primary idiopathic cerebrovascular amyloidosis in a child. Brain. 1979 Mar;102(1):177–192. doi: 10.1093/brain/102.1.177. [DOI] [PubMed] [Google Scholar]
- Tagliavini F., Prelli F., Porro M., Rossi G., Giaccone G., Farlow M. R., Dlouhy S. R., Ghetti B., Bugiani O., Frangione B. Amyloid fibrils in Gerstmann-Sträussler-Scheinker disease (Indiana and Swedish kindreds) express only PrP peptides encoded by the mutant allele. Cell. 1994 Nov 18;79(4):695–703. doi: 10.1016/0092-8674(94)90554-1. [DOI] [PubMed] [Google Scholar]
- Tagliavini F., Prelli F., Porro M., Salmona M., Bugiani O., Frangione B. A soluble form of prion protein in human cerebrospinal fluid: implications for prion-related encephalopathies. Biochem Biophys Res Commun. 1992 May 15;184(3):1398–1404. doi: 10.1016/s0006-291x(05)80038-5. [DOI] [PubMed] [Google Scholar]
- Tagliavini F., Prelli F., Verga L., Giaccone G., Sarma R., Gorevic P., Ghetti B., Passerini F., Ghibaudi E., Forloni G. Synthetic peptides homologous to prion protein residues 106-147 form amyloid-like fibrils in vitro. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9678–9682. doi: 10.1073/pnas.90.20.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiyama M., Ikeda S., Yanagisawa N. Transthyretin-type cerebral amyloid angiopathy in type I familial amyloid polyneuropathy. Acta Neuropathol. 1991;81(5):524–528. doi: 10.1007/BF00310133. [DOI] [PubMed] [Google Scholar]
- Wattendorff A. R., Frangione B., Luyendijk W., Bots G. T. Hereditary cerebral haemorrhage with amyloidosis, Dutch type (HCHWA-D): clinicopathological studies. J Neurol Neurosurg Psychiatry. 1995 Jun;58(6):699–705. doi: 10.1136/jnnp.58.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B. L., Pruchnicki A., Dickson D. W., Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986 May 2;232(4750):648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]