Abstract
Metastasis, the leading cause of cancer deaths, is an intricate process involving many important tumor and stromal proteins that have yet to be fully defined. This review discusses critical components necessary for the metastatic cascade, including hypoxia, inflammation, and the tumor microenvironment. More specifically, this review focuses on tumor cell and stroma interactions, which allow cell detachment from a primary tumor, intravasation to the blood stream, and extravasation at a distant site where cells can seed and tumor metastases can form. Central players involved in this process and discussed in this review include integrins, matrix metalloproteinases, and soluble growth factors/matrix proteins, including the connective tissue growth factor and lysyl oxidase.
Keywords: Connective tissue growth factor, Lysyl oxidase, Metastasis, Hypoxia
1 Introduction
Cancer accounts for one in four deaths in the USA, corresponding to over 500,000 deaths in 2009. With more than 1,400,000 new cases diagnosed over the same time period in the USA alone, the gravity of the diseases we collectively call cancer is brought to light [1]. In order to better understand cancer and translate basic research into clinical treatments with improved patient outcomes, it becomes necessary to examine the component of cancer which causes the most deaths, metastatic spread of the disease, or metastasis. This review focuses on the metastatic process and the role of both tumor cell and extracellular matrix components that contribute to the progression of metastasis.
2 Cancer metastasis
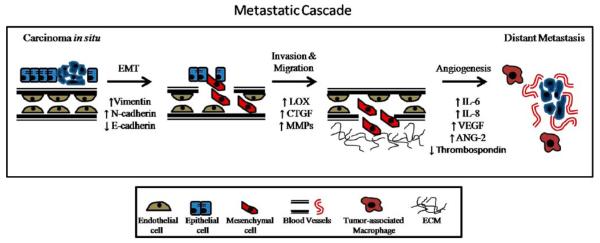
The main cause of cancer death is metastasis of the primary tumor, which involves detachment of cells from the original tumor, invasion through the basement membrane, intravasation into the blood stream, and eventual extravasation from the blood stream at a distant site where implantation and tumor cell proliferation give rise to cancer metastases. In order for this process to successfully transpire, there has to be remodeling of the extracellular matrix, changes in expression, and localization of key tumor-related and cytoskeletal proteins, proteases, and growth factors. Individual tumor cell and stromal components that are necessary for the metastatic process will be discussed within this section.
2.1 Epithelial to mesenchymal transition
Epithelial to mesenchymal transition, or EMT, was first coined by Krug et al. in 1987 but not characterized until 1995 [2, 3]. EMT is defined as the switch from non-motile, polarized epithelial cells to motile, non-polarized mesenchymal cells, with the potential to migrate from a primary tumor site to distant organs, at which they can seed and grow. While there is still debate as to whether EMT occurs in human patients, there is a breadth of literature describing this morphological alteration of cells in vitro and in mice in vivo, as well as changes in expression and localization of EMT markers, including E-cadherin, N-cadherin, Snail 1, Slug, and Twist [4–6]. A hallmark of EMT is a process termed the “cadherin switch,” defined by the loss of E-cadherin, the major component of adherens junctions, and a simultaneous gain of mesenchymal N-cadherin. This switch is characterized by dissolution of the adherens junctions, allowing cells to lose adhesive affinity for other epithelial cells and become more migratory and invasive [7, 8]. N-cadherin, which attaches to the cytoskeleton through interactions with both α- and β-catenin, signals through the GTPases Rac1 and Cdc42, and interacts with the platelet-derived growth factor (PDGF) receptor, to initiate actin remodeling, thereby causing alterations in both cell adhesive properties and migratory status [9]. Partially responsible for this “cadherin switch” are transcriptional repressors of E-cadherin: Snail 1, Snail 2 (Slug), and Twist, which perform pro-invasive functions, such as inducing αvβ3-integrin expression, fibronectin, and matrix metalloproteinase-9 (MMP-9) [10]. In addition to transcriptional repressors, one of the most potent inducers of EMT is the transforming growth factor-β (TGF-β), a known pluripotent growth factor able to induce EMT in mammary, lung, pancreatic, colon, and many other cell types [11–13]. While there are many studies examining individual components of EMT, the process as a whole still needs further defining both in an in vitro setting as well as in a clinical setting.
3 Tumor microenvironment
Under normal conditions, the cellular microenvironment is able to suppress malignant cell growth while tumor–stroma interactions modulate the microenvironment to be more permissive of malignant cell proliferation, motility, and adhesion. The tumor microenvironment is thought to be composed of endothelial cells, fibroblasts, perivascular cells, and inflammatory cells which regulate critical components of the tumorigenic process: angiogenesis, desmoplasia, lymphanogenesis, and inflammation, respectively. A specialized extracellular matrix, termed the basement membrane, separates epithelial and endothelial cells from stromal support components. It is composed of collagen IV, entactin, laminin, and heparin-sulfate proteoglycans, which confer tissue specificity, epithelial polarity, and functionality [14]. The basement membrane composition and structure is commonly altered in cancer, along with changes in growth factor expression, recruitment of inflammatory cytokines, and increased fibroblast proliferation, all of which aid in the metastatic spread of tumor cells [15]. In addition to the tumor microenvironment being capable of influencing malignant cell growth by releasing ECM proteins, growth factors, and cytokines, tumors themselves secrete growth factors and proteases that are able to modify their local microenvironment making it more permissive for cell motility and adhesion.
3.1 Extracellular matrix
The extracellular matrix is a dynamic 3D structure composed of specialized proteins and proteoglycans whose interactions regulate many cellular processes, including cell survival, proliferation, differentiation, cell migration, and invasion. In order to better understand the extracellular matrix and its role in tumorigenesis, a few key extracellular matrix components, including integrins, matrix metalloproteinases, and soluble growth factors, such as lysyl oxidase (LOX) and the connective tissue growth factor (CTGF), will be discussed in the next few sections.
3.1.1 Integrins
Integrins are heterodimeric, type I transmembrane adhesion and signaling proteins, consisting of a combination of an α and β-chain. There are 18 known α-chains and 8 β-chains, allowing for at least 24 unique heterodimers. When an integrin interacts with intracellular proteins, this causes a switch from an inactive, low affinity to active, high affinity state. Multiple heterodimer combinations allow for specific crosstalk with oncogenes and growth factor receptors on both tumor and tumor-associated cells, interactions with the extracellular matrix to provide traction necessary for cell motility and invasion, as well as assisting in matrix remodeling by directing localization of proteases [16]. Integrin expression varies between normal epithelial cells and tumor cells. Most notably, αvβ3 expression is strongly correlated with tumor growth and metastasis of breast, prostate, pancreatic, glioblastoma, cervical, and ovarian cancers [17–23]. Integrins are effectors of signaling cascades including MAP kinase, Jun, NFκB, and β-catenin as well as direct downstream targets of Src-family kinases, focal adhesion kinase (FAK) and protein kinase B [9]. Interactions with these signaling cascades allow integrins to modulate cell survival, proliferation, cell migration, and invasion. Since certain integrins, including αvβ3, are expressed on both tumor and angiogenic endothelial cells, they would make good therapeutic targets. Indeed, many clinical studies are underway, including one with etaracizumab, an early clinical integrin agonist. Additionally, an integrin-specific monoclonal antibody, CNTO 95, that targets both αvβ3 and αvβ5 integrins, has demonstrated anti-tumor activity in initial phase I trials [24]. Another set of therapies specifically target β1 integrins, an example of this is volociximab, a function-blocking antibody against αvβ1. In phase I trials, it was well tolerated and demonstrated possible clinical efficacy in patients with solid malignancies [16, 25]. Additional integrin agonists are currently in phase II and phase III clinical trials [16].
3.1.2 Matrix metalloproteinases
Matrix metalloproteinases are members of a family of endopeptidases critical to the tumor microenvironment and process of EMT. The first member of this family was discovered in 1962 through its ability to degrade collagen during tadpole tail metamorphogenesis [26]. Since then, 24 human family members have been characterized. They comprise a collection of zinc-dependent endopeptidases that function at physiological pH and, at low levels, regulate normal remodeling of human connective tissue as well as being involved in ovulation. These metalloproteinases are secreted as inactive proteins, through an association between a conserved cysteine within the pro-domain, “Pro-Arg-Cys-Gly-X-Pro-Asp,” and a zinc within the catalytic site [27]. Proteolytic cleavage is necessary for full enzymatic activity. The family is divided into subclasses based on their substrates: collagenases, stromelysins, matrilysins, and gelatinases [28]. Once activated, the majority of MMPs function to degrade collagen (types I, II, and III), the most abundant protein in the human body accounting for nearly 30% of the total, as well as liberating growth factors and peptides anchored in the extracellular matrix. More specifically, MMP-13 is unique to collagen type II degradation in cartilage, while MMP-2 and MMP-9 degrade type IV collagen, and MMP-1 is robustly expressed and degrades all three types of collagen [29]. Under normal disease-free conditions, MMPs tend to be expressed at low levels and are kept in check by tissue inhibitors of metalloproteinases (TIMPs). This family consists of four protease inhibitors, which function to block MMP activity at a 1:1 molar ratio by forming a complex with the activated catalytic zinc in the MMPs [27]. During disease pathogenesis, MMP levels often rise more quickly than TIMPs, causing MMP activation and remodeling of the tumor microenvironment as well as activation of cell surface receptors and their downstream signaling cascades. MMP-2 and MMP-9 are commonly linked to the process of EMT because they are known to degrade type IV collagen, the main component of the extracellular matrix on which the epithelium resides. Numerous studies have examined the expression of MMPs in disease progression, and consistently, MMP-2 and MMP-9, as well as other family members, have been shown to be increased in patients with advanced stage breast, lung, pancreatic, prostate, ovarian, and colorectal cancers and are often correlated with decreased survival [28, 30–32]. In addition to being valuable biomarkers, MMP inhibitors (MMPIs) are being examined as potential tools to be utilized in a clinical setting. While initial studies utilized broad spectrum inhibitors causing off-target effects, more recent studies have been taking advantage of structural knowledge to produce targeted MMP inhibitors [33]. For example, one study took this target-based approach and discovered a series of non-zinc chelating compounds with potent MMP-12 affinity in the nanomolar range [34]. While there is a lot of convincing literature supporting the role of MMPs in metastatic progression, more work needs to be done to examine whether MMPIs will be clinically effective.
3.2 Hypoxia
A key environmental stressor associated with tumor progression and poor clinical prognosis is tumor cell oxygen deficiency, termed hypoxia [35]. This condition is known to induce genes involved in the regulation of cell proliferation, extracellular matrix production, cell adhesion, and other hallmarks of tumorigenesis. The mechanism behind these effects is frequently accomplished through induction of the hypoxia-inducible factor (HIF) family of transcription factors. This family consists of three members, HIF-1,-2, and -3, which act to regulate cellular processes including glucose metabolism, angiogenesis, cell proliferation, and tissue remodeling in response to low oxygen levels. Under normal oxygen conditions, a group of prolyl-4-hydroxylases (PHDs) hydroxylate HIF-1α on two conserved residues, proline 402 and proline 564 [36–38]. This allows recognition HIF-1α by the von Hippel-Lindau (VHL) tumor suppressor. VHL functions as an E3 ubiquitin ligase and modifies HIF-1α by adding ubiquitin ladders, targeting it to the proteasome for degradation. Under conditions of acute or chronic hypoxia, HIF-1α is stabilized in an oxygen-dependent manner because the hydroxylation and subsequent degradation of HIF-1α by PHDs requires oxygen and 2-oxyglutarate as substrates, as well as iron and ascorbate as cofactors [39, 40]. Once stabilized, HIF-1α can form a heterodimer with HIF-1β, allowing this transcription factor to bind a core sequence of 5′-RCGTG-3′ and increase transcription of target genes [41].
While hypoxia has been strongly linked to tumor metastasis and poor clinical outcome for patients, it seems to actually have a dual role: insufficient oxygen limits tumor cell division while at the same time selecting for more malignant cells and inducing cell adaptations allowing for more invasive behavior. Regarding tumor growth, cancer cells, similar to normal cells, need oxygen to generate energy as well as acting as a substrate for many fundamental cellular processes, including generating macromolecules. Yet, hypoxia is also strongly associated with tumor progression and metastatic disease. This is likely because low oxygen tension is able to increase cell invasiveness, cause cells to switch to anaerobic metabolism, increase genetic instability, and promote angiogenesis [42–47].
The ability of tumor cells to invade is a critical step in metastasis. There are several methods through which hypoxia can cause an increase in metastatic potential of tumor cells. One way is through HIF-1α binding to hypoxia-response elements within the c-met promoter activating transcription of this gene. Overexpression of the Met protein on the cell surface of tumor cells leads them to be more susceptible to hepatocyte growth factor stimulation. This causes extracellular matrix degradation, cell dissociation, and escape from hypoxic areas to more oxygen-rich environments at a secondary site [46]. Additionally, a study in renal cell carcinomas demonstrated that hypoxia-induced HIF expression is necessary and sufficient to cause E-cadherin loss, a critical step in EMT [48, 49], while yet another study showed that activation of the Wnt/beta-catenin signaling pathway, through HIF-1α, can induce prostate cancer cells to be more motile and invasive [50]. Moreover, expression of hypoxia-induced factors, such as lysyl oxidase (discussed in further detail below), can cause cancer cells to favor metastatic spread as demonstrated in both in vitro and in vivo experiments (Fig. 1) [51].
Fig. 1.
Role of hypoxia and inflammatory-regulated genes on metastasis. The process of metastasis often occurs in regions of oxygen deprivation, termed hypoxia, in which HIF can induce expression of pro-invasive and pro-angiogenic factors, such as LOX, CTGF, VEGF, Ang-2 and decreases in E-cadherin and thrombospondin. Additionally, the inflammatory response induces tumor-associated macrophages which stimulate pro-angiogenic factors, including IL-6, IL-8, and VEGF. Together, hypoxia and inflammation promote an environment permissive of metastatic disease
Angiogenesis, another critical step in tumorigenesis, is defined by the formation of new blood vessels which provide nutrients to the growing tumor as well as an escape route for cancer cells to travel to distant sites in the body. Due to the growing demands of solid tumors, invariably their vasculature is impaired with a lack of strong interconnections and pericytes, leakiness, and transient changes in oxygenation [52, 53]. Hypoxia, through HIF-1α, induces the secretion of pro-angiogenic factors including vascular endothelial growth factor (VEGF), angiopoietin 2 (Ang-2), PDGF, and fibroblast growth factor [54–57]. In addition to these factors, HIF-1 works to decrease the activity of angiogenic inhibitors, such as thrombospondin, thereby creating a pro-angiogenic environment [58].
Clinically, both HIF-1α and HIF-2α are increased in tumors, including renal, breast, colon, and pancreatic cancers [59]. With oxygen deprivation having such a large effect on many steps of the metastatic cascade, targeting hypoxia for human therapies makes sense. But hypoxia poses a variety of problems in the treatment of cancers by decreasing the effectiveness of chemotherapy and radiotherapy [35, 60]. The majority of cytotoxic chemotherapies require proliferating cells to induce cytotoxicity. However, tumor cells in regions of hypoxia divide slower, and therefore, chemotherapies are less effective in the hypoxic regions of solid tumors. Initial treatments to target hypoxia include hyperbaric oxygen (intermittent administration of 100% oxygen at high pressure), as well as systemic erythropoietin treatment [61, 62]. A recent study which utilized genetic manipulation of the hypoxic tumor environment by ectopically expressing myoglobin (Mb) in human A549 lung cancer cell xenografts proved more successful. Myoglobin is a cytoplasmic protein involved in oxygen transport and free radical scavenging. It is thought that Mb can decrease HIF-1α stabilization while allowing cancer cells to utilize oxygen more effectively in a hypoxic environment resulting in delayed engraftment, tumor growth, and microvascular density in vivo [63].
3.2.1 Lysyl oxidase
Lysyl oxidase, or LOX, is a copper-dependent amine oxidase that cross-links collagen and elastin molecules in the extracellular matrix [64]. This highly conserved protein was discovered in 1968 and is part of a larger family consisting of five members: LOXL, LOXL2, LOXL3, LOXL4, and LOX [65]. It is translated as a 48-kDa preproprotein which is subject to cleavage of its N-terminal signal peptide and is glycosylated prior to secretion. Once secreted, the catalytically inactive pro-enzyme protein is further cleaved by extracellular proteases releasing the 32-kDa mature and catalytically active LOX enzyme [66–68]. In addition to being found extracellularly, mature LOX has also been found to be localized to the nucleus in a NIH3T3 cell culture system, suggesting nuclear activity [69, 70]. Indeed, intranuclear LOX can interact with histone H1 and is therefore implicated in modifying gene expression [71, 72]. The collagen and elastin cross-links formed by this enzyme lead to increases in tensile strength and structural integrity necessary for normal connective tissue function, embryonic development, tissue remodeling, and repair of diseased and aging connective tissues [73, 74]. LOX−/− mice die shortly after birth due to structural and functional defects in the cardiovascular system, lungs, and skin [75, 76]. Localization of LOX occurs in extracellular compartments within many regions of the body, including skin, liver, heart, lungs, skeletal muscle, cartilage, and kidney and pancreas in mammals [70, 77, 78].
In human disease, there have been numerous studies linking LOX to tumorigenesis. Currently, LOX is the only tumor-secreted factor shown to be directly involved in pre-metastatic niche formation. Increased LOX expression correlating to disease progression, metastasis, and poor overall survival has been shown in breast cancer, head and neck cancers, as well as oral cancers [51, 79–81]. LOX expression is associated with hypoxia and is increased in hypoxic tumors (as determined by CAIX expression) in breast and head and neck cancers [82]. Additionally, LOX expression coincides with hypoxia and correlates to decreased metastasis-free and overall survival in patients. In vitro studies have demonstrated a role for LOX in invasion of human cutaneous melanoma and breast cancer cell lines as well as cell migration through its regulation of FAK activity [51, 83]. LOX can also regulate cell adhesion through increased cyclin D1 and β-catenin expression [84]. Furthermore, the catalytic domain of LOX and LOX family members has been shown to bind Snail, suggesting that these proteins play a role in EMT [85].
Since LOX expression and activity regulate a number of important steps in the metastatic process, there are studies examining its potential as a therapeutic target. Utilizing a specific antibody to LOX in an in vivo orthotopic breast cancer model, lung and liver metastases were dramatically decreased while primary tumor growth was unaffected [51]. This demonstrates the importance of LOX in late-stage metastatic disease. Further studies examining the mechanisms behind LOX’s contribution to the metastatic process and its potential as a therapeutic target still need to be investigated.
3.2.2 Connective tissue growth factor
Another hypoxia-regulated extracellular matrix protein is CTGF, or connective tissue growth factor. CTGF is a transcriptional target of HIF-1, which directly interacts with HREs located in the 5′ region of the protein [86]. It is a member of the CCN family of secreted proteins, which are cysteine-rich matricellular proteins composed of four modular domains with homology to insulin-like growth factor-binding proteins (domain 1), a von Wille-brand factor type C repeat (domain 2), a thrombospondin type 1 repeat (domain 3), and a cysteine-knot domain (domain 4) [87].
While CTGF’s function in normal tissue has been well studied, the knowledge of its role in tumor biology is still being discovered. It is known that CTGF exerts a dual role as both a tumor suppressor and tumor promoter, depending on the cancer context. Specifically, the expression of CTGF has been demonstrated to be increased during tumor growth in many cancers including pancreatic [88], glioblastoma [89], and melanoma [90]. Both CTGF messenger RNA (mRNA) and protein have been shown to be induced in response to hypoxia in a human breast cancer model in vitro [91]. Studies examining human pancreatic specimens also displayed a 40- to 50-fold increase in CTGF mRNA expression in tumor compared to normal samples [88, 92]. Additionally, CTGF co-localized with regions of hypoxia in human tumor xenografts as well as clinical pancreatic adenocarcinoma specimens [93]. CTGF secretion was also increased in hypoxic tumor cells in vitro [93]. In an in vivo Panc 1 model of pancreatic cancer, subcutaneous and orthotopic tumor growth was found to be critically dependent on CTGF being expressed by the tumor cells. In addition, CTGF expression increased anchorage-independent growth of pancreatic tumor cells in soft agar while decreasing apoptosis in response to hypoxic stress [93]. Similar results were found in another model of pancreatic cancer utilizing MiaPaca2 cells, where CTGF was able to promote growth in soft agar as well as in vivo primary tumor growth. A neutralizing CTGF-specific monoclonal antibody, FG-3019, successfully inhibited both anchorage-independent growth in vitro as well as primary and lymph node metastasis in vivo [94]. These studies demonstrate the importance of CTGF as a potential therapeutic target, especially in pancreatic cancer. Additional studies have shown CTGF to be upregulated during inflammation, fibrotic disorders, and angiogenesis, all critical factors for primary tumor growth and metastasis [95–98]. It is important to note that while CTGF expression generally correlates with decreased survival, exceptions have been found in esophageal squamous cell carcinoma and chondrosarcoma [99, 100].
3.3 Inflammation
Another aspect of the tumor microenvironment that has a significant role in neoplastic progression is inflammation. Under normal conditions, such as wound healing, an influx of cytokines and chemokines work in a self-limiting approach to heal the wound. But dysregulation of this process can lead to abnormalities in the inflammatory response and ultimately tumorigenesis. Worldwide, approximately 1.2 million cancer cases are thought to arise from chronic inflammation and infections [101, 102]. One of the best examples of this is colon cancers which often develop following inflammatory bowel disease. Inflammation in carcinomas involves different factors than what is seen with a typical inflammatory response. For example, an influx of pro-inflammatory cytokines, including tumor necrosis factor-α and TGF-β, as well as cytotoxic mediators, proteases, MMPs, interleukins, and interferons, produce potent lymphanogiogenic and angiogenic growth factors allowing tumor growth and metastatic spread to the lymph nodes [103, 104]. More specifically, tumor-associated macrophages produce pro-angiogenic growth factors, such as vascular endothelial growth factors (VEGF-C, -D) and VEGF receptor 3. Tumor cells themselves produce cytokines which attract neutrophils, macrophages, lymphocytes, and dendritic cells all attributing to tumorigenic growth and metastatic potential [105].
4 Conclusions
Since metastasis is the main cause of death from cancer, exploring the sources of metastatic disease is critical for the development of therapies to be used clinically. A great deal of research has focused on the tumor microenvironment and the role of extracellular matrix proteins in disease progression. This review highlighted the importance of integrins, matrix metalloproteinases, and extracellular matrix proteins, such as LOX and CTGF, and their roles in epithelial to mesenchymal transition. Additionally, this review also covered the importance of oxygen deprivation and inflammation on tumorigenesis. As more components of the metastatic cascade are discovered and interactions between known extracellular matrix proteins and tumor-expressed proteins are brought to light, new classes of therapeutic targets will emerge.
Acknowledgments
Due to the broad scope of this review, we apologize to anyone we failed to cite. This work was supported by the National Institutes of Health (RO1 CA-116685, PO1 CA-67166, and T32 CA121940 to E.C.F and to A.J.G.).
References
- 1.Jemal A, et al. Cancer statistics, 2009. CA: A Cancer Journal for Clinicians. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Krug EL, Mjaatvedt CH, Markwald RR. Extracellular matrix from embryonic myocardium elicits an early morphogenetic event in cardiac endothelial differentiation. Developmental Biology. 1987;120(2):348–355. doi: 10.1016/0012-1606(87)90237-5. [DOI] [PubMed] [Google Scholar]
- 3.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anatomica (Basel) 1995;154(1):8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 4.Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Research. 2005;65(14):5996–6000. doi: 10.1158/0008-5472.CAN-05-0699. discussion 6000-1. [DOI] [PubMed] [Google Scholar]
- 5.Birchmeier W, Behrens J. Cadherin expression in carcinomas: Role in the formation of cell junctions and the prevention of invasiveness. Biochimica et Biophysica Acta. 1994;1198(1):11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 6.Hotz B, et al. Epithelial to mesenchymal transition: Expression of the regulators snail, slug, and twist in pancreatic cancer. Clinical Cancer Research. 2007;13(16):4769–4776. doi: 10.1158/1078-0432.CCR-06-2926. [DOI] [PubMed] [Google Scholar]
- 7.Gravdal K, et al. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clinical Cancer Research. 2007;13(23):7003–7011. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- 8.Margulis A, et al. E-cadherin suppression accelerates squamous cell carcinoma progression in three-dimensional, human tissue constructs. Cancer Research. 2005;65(5):1783–1791. doi: 10.1158/0008-5472.CAN-04-3399. [DOI] [PubMed] [Google Scholar]
- 9.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer and Metastasis Reviews. 2009;28(1–2):15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 10.Haraguchi M, et al. Snail regulates cell-matrix adhesion by regulation of the expression of integrins and basement membrane proteins. Journal of Biological Chemistry. 2008;283(35):23514–23523. doi: 10.1074/jbc.M801125200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon KJ, et al. Loss of type III transforming growth factor beta receptor expression increases motility and invasiveness associated with epithelial to mesenchymal transition during pancreatic cancer progression. Carcinogenesis. 2008;29(2):252–262. doi: 10.1093/carcin/bgm249. [DOI] [PubMed] [Google Scholar]
- 12.Ozdamar B, et al. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307(5715):1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- 13.Dumont N, Bakin AV, Arteaga CL. Autocrine transforming growth factor-beta signaling mediates Smad-independent motility in human cancer cells. Journal of Biological Chemistry. 2003;278(5):3275–3285. doi: 10.1074/jbc.M204623200. [DOI] [PubMed] [Google Scholar]
- 14.Kalluri R. Basement membranes: Structure, assembly and role in tumour angiogenesis. Nature Reviews Cancer. 2003;3(6):422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 15.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(7015):332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desgrosellier JS, Cheresh DA. Integrins in cancer: Biological implications and therapeutic opportunities. Nataure Reviews Cancer. 2010;10(1):9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takayama S, et al. The relationship between bone metastasis from human breast cancer and integrin alpha(v)beta3 expression. Anticancer Research. 2005;25(1A):79–83. [PubMed] [Google Scholar]
- 18.Liapis H, Flath A, Kitazawa S. Integrin alpha V beta 3 expression by bone-residing breast cancer metastases. Diagnostic Molecular Pathology. 1996;5(2):127–135. doi: 10.1097/00019606-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 19.McCabe NP, et al. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26(42):6238–6243. doi: 10.1038/sj.onc.1210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosotani R, et al. Expression of integrin alphaVbeta3 in pancreatic carcinoma: Relation to MMP-2 activation and lymph node metastasis. Pancreas. 2002;25(2):e30–e35. doi: 10.1097/00006676-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Gruber G, et al. Correlation between the tumoral expression of beta3-integrin and outcome in cervical cancer patients who had undergone radiotherapy. British Journal of Cancer. 2005;92(1):41–46. doi: 10.1038/sj.bjc.6602278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landen CN, et al. Tumor-selective response to antibody-mediated targeting of alphavbeta3 integrin in ovarian cancer. Neoplasia. 2008;10(11):1259–1267. doi: 10.1593/neo.08740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bello L, et al. Alpha(v)beta3 and alpha(v)beta5 integrin expression in glioma periphery. Neurosurgery. 2001;49(2):380–9. doi: 10.1097/00006123-200108000-00022. discussion 390. [DOI] [PubMed] [Google Scholar]
- 24.Mullamitha SA, et al. Phase I evaluation of a fully human anti-alphav integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clinincal Cancer Reseach. 2007;13(7):2128–2135. doi: 10.1158/1078-0432.CCR-06-2779. [DOI] [PubMed] [Google Scholar]
- 25.Ricart AD, et al. Volociximab, a chimeric monoclonal antibody that specifically binds alpha5beta1 integrin: A phase I, pharmacokinetic, and biological correlative study. Clinical Cancer Research. 2008;14(23):7924–7929. doi: 10.1158/1078-0432.CCR-08-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proceedings of the National Academy of Science of the United States America. 1962;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: A tail of a frog that became a prince. Nature Reviews Molecular Cell Biology. 2002;3(3):207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 28.Zucker S, et al. Measurement of matrix metalloproteinases and tissue inhibitors of metalloproteinases in blood and tissues. Clinical and experimental applications. Annals of the New York Academy of Sciences. 1999;878:212–227. doi: 10.1111/j.1749-6632.1999.tb07687.x. [DOI] [PubMed] [Google Scholar]
- 29.Burrage P, et al. Matrix metalloproteinases: Role in arthritis. Frontiers in Bioscience. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 30.Koc M, et al. Matrix metalloproteinase-9 (MMP-9) elevated in serum but not in bronchial lavage fluid in patients with lung cancer. Tumori. 2006;92(2):149–154. doi: 10.1177/030089160609200211. [DOI] [PubMed] [Google Scholar]
- 31.Hilska M, et al. Prognostic significance of matrix metalloproteinases-1, -2, -7 and -13 and tissue inhibitors of metalloproteinases-1, -2, -3 and -4 in colorectal cancer. International Journal of Cancer. 2007;121(4):714–723. doi: 10.1002/ijc.22747. [DOI] [PubMed] [Google Scholar]
- 32.Lengyel E, et al. Expression of latent matrix metalloproteinase 9 (MMP-9) predicts survival in advanced ovarian cancer. Gynecologic Oncology. 2001;82(2):291–298. doi: 10.1006/gyno.2001.6243. [DOI] [PubMed] [Google Scholar]
- 33.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. Journal of Clinical Oncology. 2009;27(31):5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dublanchet AC, et al. Structure-based design and synthesis of novel non-zinc chelating MMP-12 inhibitors. Bioorganic & Medicinal Chemistry Letters. 2005;15(16):3787–3790. doi: 10.1016/j.bmcl.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 35.Chan DA, Giaccia AJ. Hypoxia, gene expression, and metastasis. Cancer and Metastasis Reviews. 2007;26(2):333–339. doi: 10.1007/s10555-007-9063-1. [DOI] [PubMed] [Google Scholar]
- 36.Masson N, et al. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO Journal. 2001;20(18):5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan DA, et al. Role of prolyl hydroxylation in oncogenically stabilized hypoxia-inducible factor-1alpha. Journal Biological Chemistry. 2002;277(42):40112–40117. doi: 10.1074/jbc.M206922200. [DOI] [PubMed] [Google Scholar]
- 38.Bedogni B, Powell MB. Hypoxia, melanocytes and melanoma—Survival and tumor development in the permissive microenvironment of the skin. Pigment Cell Melanoma Research. 2009;22(2):166–174. doi: 10.1111/j.1755-148X.2009.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epstein AC, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107(1):43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 40.Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 41.Mole DR, et al. Genome-wide association of hypoxiainducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. Journal of Biological Chemistry. 2009;284(25):16767–16775. doi: 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michieli P. Hypoxia, angiogenesis and cancer therapy: To breathe or not to breathe? Cell Cycle. 2009;8(20):3291–3296. doi: 10.4161/cc.8.20.9741. [DOI] [PubMed] [Google Scholar]
- 43.Kim JW, et al. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism. 2006;3(3):177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Bindra RS, et al. Alterations in DNA repair gene expression under hypoxia: Elucidating the mechanisms of hypoxia-induced genetic instability. Annals of the New York Academy of Sciences. 2005;1059:184–195. doi: 10.1196/annals.1339.049. [DOI] [PubMed] [Google Scholar]
- 45.Tang N, et al. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6(5):485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 46.Pennacchietti S, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3(4):347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 47.Canning MT, et al. Oxygen-mediated regulation of gelatinase and tissue inhibitor of metalloproteinases-1 expression by invasive cells. Experimental Cell Research. 2001;267(1):88–94. doi: 10.1006/excr.2001.5243. [DOI] [PubMed] [Google Scholar]
- 48.Esteban MA, et al. Regulation of E-cadherin expression by VHL and hypoxia-inducible factor. Cancer Research. 2006;66(7):3567–3575. doi: 10.1158/0008-5472.CAN-05-2670. [DOI] [PubMed] [Google Scholar]
- 49.Imai T, et al. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. American Journal of Pathology. 2003;163(4):1437–1447. doi: 10.1016/S0002-9440(10)63501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang YG, et al. Role of Wnt/beta-catenin signaling pathway in epithelial–mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha. International Journal of Urology. 2007;14(11):1034–1039. doi: 10.1111/j.1442-2042.2007.01866.x. [DOI] [PubMed] [Google Scholar]
- 51.Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440(7088):1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 52.Dewhirst MW, et al. Morphologic and hemodynamic comparison of tumor and healing normal tissue microvasculature. International Journal of Radiation Oncology, Biology, Physics. 1989;17(1):91–99. doi: 10.1016/0360-3016(89)90375-1. [DOI] [PubMed] [Google Scholar]
- 53.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 54.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 55.Shweiki D, et al. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 56.Holash J, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284(5422):1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 57.Ruan K, Song G, Ouyang G. Role of hypoxia in the hallmarks of human cancer. Journal Cell Biochemistry. 2009;107(6):1053–1062. doi: 10.1002/jcb.22214. [DOI] [PubMed] [Google Scholar]
- 58.Laderoute KR, et al. Opposing effects of hypoxia on expression of the angiogenic inhibitor thrombospondin 1 and the angiogenic inducer vascular endothelial growth factor. Clinincal Cancer Research. 2000;6(7):2941–2950. [PubMed] [Google Scholar]
- 59.Talks KL, et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. American Journal of Pathology. 2000;157(2):411–421. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kallman RF, Dorie MJ. Tumor oxygenation and reoxygenation during radiation therapy: Their importance in predicting tumor response. International Journal of Radiation Oncology, Biology, Physics. 1986;12(4):681–685. doi: 10.1016/0360-3016(86)90080-5. [DOI] [PubMed] [Google Scholar]
- 61.Daruwalla J, Christophi C. Hyperbaric oxygen therapy for malignancy: A review. World Journal of Surgery. 2006;30(12):2112–2131. doi: 10.1007/s00268-006-0190-6. [DOI] [PubMed] [Google Scholar]
- 62.Engert A. Recombinant human erythropoietin in oncology: Current status and further developments. Annals of Oncology. 2005;16(10):1584–1595. doi: 10.1093/annonc/mdi307. [DOI] [PubMed] [Google Scholar]
- 63.Galluzzo M, et al. Prevention of hypoxia by myoglobin expression in human tumor cells promotes differentiation and inhibits metastasis. Journal of Clinical Investigative. 2009;119(4):865–875. doi: 10.1172/JCI36579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Csiszar K. Lysyl oxidases: A novel multifunctional amine oxidase family. Progress in Nucleic Acid Research and Molecular Biology. 2001;70:1–32. doi: 10.1016/s0079-6603(01)70012-8. [DOI] [PubMed] [Google Scholar]
- 65.Pinnell SR, Martin GR. The cross-linking of collagen and elastin: Enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proceedings of the National Academy of Science of the United States of America. 1968;61(2):708–716. doi: 10.1073/pnas.61.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trackman PC, et al. Post-translational glycosylation and proteolytic processing of a lysyl oxidase precursor. Journal of Biological Chemistry. 1992;267(12):8666–8671. [PubMed] [Google Scholar]
- 67.Cronshaw AD, Fothergill-Gilmore LA, Hulmes DJ. The proteolytic processing site of the precursor of lysyl oxidase. Biochemical Journal. 1995;306(Pt 1):279–284. doi: 10.1042/bj3060279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panchenko MV, et al. Metalloproteinase activity secreted by fibrogenic cells in the processing of prolysyl oxidase. Potential role of procollagen C-proteinase. Journal of Biological Chemistry. 1996;271(12):7113–7119. doi: 10.1074/jbc.271.12.7113. [DOI] [PubMed] [Google Scholar]
- 69.Nellaiappan K, et al. Fully processed lysyl oxidase catalyst translocates from the extracellular space into nuclei of aortic smooth-muscle cells. Journal of Cell Biochemistry. 2000;79(4):576–582. doi: 10.1002/1097-4644(20001215)79:4<576::aid-jcb60>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 70.Kagan HM, Li W. Lysyl oxidase: Properties, specificity, and biological roles inside and outside of the cell. Journal of Cell Biochemistry. 2003;88(4):660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 71.Kagan HM, et al. Histone H1 is a substrate for lysyl oxidase and contains endogenous sodium borotritide-reducible residues. Biochemical and Biophysical Research Communications. 1983;115(1):186–192. doi: 10.1016/0006-291x(83)90987-7. [DOI] [PubMed] [Google Scholar]
- 72.Giampuzzi M, Oleggini R, Di Donato A. Demonstration of in vitro interaction between tumor suppressor lysyl oxidase and histones H1 and H2: Definition of the regions involved. Biochimica et Biophysica Acta. 2003;1647(1–2):245–251. doi: 10.1016/s1570-9639(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 73.Warburton D, Shi W. Lo, and the niche is knit: Lysyl oxidase activity and maintenance of lung, aorta, and skin integrity. American Journal of Pathology. 2005;167(4):921–922. doi: 10.1016/S0002-9440(10)61181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maki JM, et al. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. American Journal of Pathology. 2005;167(4):927–936. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maki JM, et al. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106(19):2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 76.Hornstra IK, et al. Lysyl oxidase is required for vascular and diaphragmatic development in mice. Journal of Biology Chemistry. 2003;278(16):14387–14393. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- 77.Hayashi K, et al. Comparative immunocytochemical localization of lysyl oxidase (LOX) and the lysyl oxidase-like (LOXL) proteins: Changes in the expression of LOXL during development and growth of mouse tissues. Journal of Molecular Histology. 2004;35(8–9):845–855. doi: 10.1007/s10735-004-2340-1. [DOI] [PubMed] [Google Scholar]
- 78.Kagan HM, et al. Ultrastructural immunolocalization of lysyl oxidase in vascular connective tissue. Journal of Cell Biology. 1986;103(3):1121–1128. doi: 10.1083/jcb.103.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sakai M, et al. Expression of lysyl oxidase is correlated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma. Annals of Surgical Oncology. 2009;16(9):2494–2501. doi: 10.1245/s10434-009-0559-5. [DOI] [PubMed] [Google Scholar]
- 80.Albinger-Hegyi A, et al. Lysyl oxidase expression is an independent marker of prognosis and a predictor of lymph node metastasis in oral and oropharyngeal squamous cell carcinoma (OSCC) International Journal of Cancer. 2009;126(11):2653–2662. doi: 10.1002/ijc.24948. [DOI] [PubMed] [Google Scholar]
- 81.Le QT, et al. Validation of lysyl oxidase as a prognostic marker for metastasis and survival in head and neck squamous cell carcinoma: Radiation Therapy Oncology Group trial 90-03. Journal of Clinical Oncology. 2009;27(26):4281–4286. doi: 10.1200/JCO.2008.20.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erler JT, Giaccia AJ. Lysyl oxidase mediates hypoxic control of metastasis. Cancer Research. 2006;66(21):10238–10241. doi: 10.1158/0008-5472.CAN-06-3197. [DOI] [PubMed] [Google Scholar]
- 83.Kirschmann DA, et al. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Research. 2002;62(15):4478–4483. [PubMed] [Google Scholar]
- 84.Giampuzzi M, et al. Beta-catenin signaling and regulation of cyclin D1 promoter in NRK-49F cells transformed by down-regulation of the tumor suppressor lysyl oxidase. Biochimica et Biophysica Acta. 2005;1745(3):370–381. doi: 10.1016/j.bbamcr.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 85.Peinado H, et al. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO Journal. 2005;24(19):3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Higgins DF, et al. Hypoxic induction of Ctgf is directly mediated by Hif-1. American Journal of Physiology. Renal Physiology. 2004;287(6):F1223–F1232. doi: 10.1152/ajprenal.00245.2004. [DOI] [PubMed] [Google Scholar]
- 87.Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Letters. 1993;327(2):125–130. doi: 10.1016/0014-5793(93)80155-n. [DOI] [PubMed] [Google Scholar]
- 88.Wenger C, et al. Expression and differential regulation of connective tissue growth factor in pancreatic cancer cells. Oncogene. 1999;18(4):1073–1080. doi: 10.1038/sj.onc.1202395. [DOI] [PubMed] [Google Scholar]
- 89.Xie D, et al. Levels of expression of CYR61 and CTGF are prognostic for tumor progression and survival of individuals with gliomas. Clinical Cancer Research. 2004;10(6):2072–2081. doi: 10.1158/1078-0432.ccr-0659-03. [DOI] [PubMed] [Google Scholar]
- 90.Kubo M, et al. Expression of fibrogenic cytokines in desmoplastic malignant melanoma. British Journal of Dermatology. 1998;139(2):192–197. doi: 10.1046/j.1365-2133.1998.02354.x. [DOI] [PubMed] [Google Scholar]
- 91.Shimo T, et al. Connective tissue growth factor as a major angiogenic agent that is induced by hypoxia in a human breast cancer cell line. Cancer Letters. 2001;174(1):57–64. doi: 10.1016/s0304-3835(01)00683-8. [DOI] [PubMed] [Google Scholar]
- 92.Hartel M, et al. Desmoplastic reaction influences pancreatic cancer growth behavior. World Journal of Surgery. 2004;28(8):818–825. doi: 10.1007/s00268-004-7147-4. [DOI] [PubMed] [Google Scholar]
- 93.Bennewith KL, et al. The role of tumor cell-derived connective tissue growth factor (CTGF/CCN2) in pancreatic tumor growth. Cancer Research. 2009;69(3):775–784. doi: 10.1158/0008-5472.CAN-08-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dornhofer N, et al. Connective tissue growth factor-specific monoclonal antibody therapy inhibits pancreatic tumor growth and metastasis. Cancer Research. 2006;66(11):5816–5827. doi: 10.1158/0008-5472.CAN-06-0081. [DOI] [PubMed] [Google Scholar]
- 95.Dammeier J, et al. Connective tissue growth factor: A novel regulator of mucosal repair and fibrosis in inflammatory bowel disease? International Journal of Biochemistry and Cell Biology. 1998;30(8):909–922. doi: 10.1016/s1357-2725(98)00046-6. [DOI] [PubMed] [Google Scholar]
- 96.Igarashi A, et al. Connective tissue growth factor gene expression in tissue sections from localized scleroderma, keloid, and other fibrotic skin disorders. Journal of Investigative Dermatology. 1996;106(4):729–733. doi: 10.1111/1523-1747.ep12345771. [DOI] [PubMed] [Google Scholar]
- 97.Ito Y, et al. Expression of connective tissue growth factor in human renal fibrosis. Kidney International. 1998;53(4):853–861. doi: 10.1111/j.1523-1755.1998.00820.x. [DOI] [PubMed] [Google Scholar]
- 98.Kondo S, et al. Connective tissue growth factor increased by hypoxia may initiate angiogenesis in collaboration with matrix metalloproteinases. Carcinogenesis. 2002;23(5):769–776. doi: 10.1093/carcin/23.5.769. [DOI] [PubMed] [Google Scholar]
- 99.Koliopanos A, et al. Connective tissue growth factor gene expression alters tumor progression in esophageal cancer. World Journal of Surgery. 2002;26(4):420–427. doi: 10.1007/s00268-001-0242-x. [DOI] [PubMed] [Google Scholar]
- 100.Shakunaga T, et al. Expression of connective tissue growth factor in cartilaginous tumors. Cancer. 2000;89(7):1466–1473. [PubMed] [Google Scholar]
- 101.Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. Journal of Internal Medicine. 2000;248(3):171–183. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 102.Pisani P, et al. Cancer and infection: Estimates of the attributable fraction in 1990. Cancer Epidemiology, Biomarkers and Prevention. 1997;6(6):387–400. [PubMed] [Google Scholar]
- 103.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. British Journal of Cancer. 2010;102(4):639–644. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schoppmann SF, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. American Journal of Pathology. 2002;161(3):947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]