Abstract
Ultrasound induced intracellular drug delivery, sonoporation, is an appealing and promising technique for next generation drug delivery system. Many types of molecules, such as plasmid DNAs, siRNAs and peptides, have been demonstrated to be delivered into the cell by ultrasound with the aid of microbubbles both in vitro and in vivo. Although there are many reports on in vitro sonoporation, the efficiency of successful sonoporation and the viabilities of cells after the procedure documented in each report vary in a wide range, and the reasons for these differences are not fully understood. In this study, we have investigated how different experimental settings would affect sonoporation efficiency and cell viabilities after the procedure. Our results show that the fashion of cell culture (e.g. in suspension or in monolayer culture) and the presence of standing wave have a great impact on the overall results. These results indicate that in vitro sonoporation settings should be carefully evaluated in each experiment. The fact that standing wave is necessary to achieve high sonoporation efficiency may be a problematic issue for clinical application of sonoporation, as it may be difficult (although not impossible) to create standing wave in a human body.
Keywords: sonoporation, standing wave, siRNA, gene therapy
Introduction
Today the advancement of acoustic technology has made it possible to produce various kinds of bio-effects both in vitro and in vivo by ultrasound. These bio-effects include hyperthermia, such as coagulation of tissues [1-12], mechnical tissue destruction [13, 14], disruption of tight junctions of the vasculature for enhanced local drug delivery [15-20], and sonoporation, a transient enhanced permeability of the cell membrane caused by the combination of ultrasound and microbubbles [21-38]. As all of these bio-effects can be created non-invasively in vivo, ultrasound is gathering a great attention as a next generation surgical or drug delivery system. Among them, sonoporation has been vigorously investigated, as it allows plasmid DNAs, siRNAs and peptides to be delivered intracellularly without the use of carriers or vectors both in vitro and in vivo. This concept has a great significance especially in the medical field, since it will widen the use of cell membrane impermeable agents. However, when we look into the published reports, sonoporation efficiency and the magnitude of cell toxicity, one of the most important factors for making sonoporation as a practical tool, varies in a very wide range. Some reports, including our previous reports, show only a small amount of successfully sonoporated cells accompanying a large number of non-viable cells [25, 26, 30, 31, 35]. Others report otherwise [34, 38]. Although some reports have pursued the possibility of optimizing ultrasound parameters [26, 35, 39, 40], there has been no report on investigating how the technical differences of experimental settings can impact the results. Solving this question is significantly important for refining sonoporation technology and for bringing it to practical application.
In this study, we have investigated how different experimental settings will affect sonoporation efficiencies and cell viabilities after the procedure. As reports on in vitro sonoporation experiment are mixed with cells sonicated in a suspension and cells sonicated in a monolayer culture, first we have investigated whether or not this difference will produce a difference in sonoporation efficiency. We have also tested if siRNA can be delivered by sonoporation in a monolayer cell culture. Finally, we investigated the role of standing wave in sonoporation. Standing wave is a condition where the reflected ultrasound beam from any sort of interface and the progressive ultrasound beam merge together, boosting the acoustic pressure in the field and altering the 3D structure of the ultrasound field from that created only by progressive ultrasound beam. Although producing standing wave in a human body is difficult, except for structures around the bone, in most of the reported sonoporation experiments, the presence or absence of standing wave is not clearly described. In addition, when standing wave is present, it is difficult to presume the true acoustic power applied to the sonicated samples. In this study, we have carefully created several ultrasound fields where standing wave is considered to be almost completely eliminated and evaluated its impact on sonoporation efficiency.
Materials and Methods
Cell lines and culture
Rat C166 cells and C166-GFP cells, which are the wild type and genetically manipulated cells stably expressing the enhanced green fluorescent protein (EGFP), were obtained from the American Type Culture Collection (ATCC). C166 cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) at 37C° with 5% CO2. Both media were supplemented with 10% fetal calf serum (FCS; Invitrogen). For C166-GFP cells, 0.2mg/ml G418 (Geneticine, Invitrogen) was added in the medium.
Sonication device setup
Details of the experimental setup are shown in Fig. 1A. C166 or C166-GFP cells were cultured in a 6-well plate either in a suspension or in a monolayer fashion. Total number of cells in each well was fixed as 0.6 × 106 cells /1.5 ml serum free DMEM. We used a 1.706 MHz planer ultrasound transducer with a surface area of 7 cm2. The transducer's efficiency was equal to approximately 80% and it was used to convert the electrical power measured by the amplifier into acoustical power. The radiofrequency (RF) signals to drive the ultrasound transducers were generated by a pulsed-output ultrasound driving system (model UDS 04PF-CSA, Advanced Surgical Systems, Inc., Tuscon, AZ). The transducer was submerged in degassed water and the samples were placed 6 cm above the surface of the transducer (Fig. 1A). The ultrasound parameters used were, 0 ∼ 4 W/cm2 acoustic power (AP) in continuous wave (CW; 100% duty cycle) mode for 15 ∼ 120 sec. Max temperature rise was less than 4.6 °C with 1.6 W/cm2 AP × CW 120 sec. This was measured in a 1.5 ml degassed water sonicated with standing present and microbubbles absent. The base line temperature was 36.5 °C. We also used OPTISON®, a microbubble ultrasound contrast agent available from Amersham Health Inc. (Princeton, NJ), with a concentration of 5 or 10 % (v/v) to aid cavitation production.
Fig. 1. Experimental setup for cell sonication using planner ultrasound.
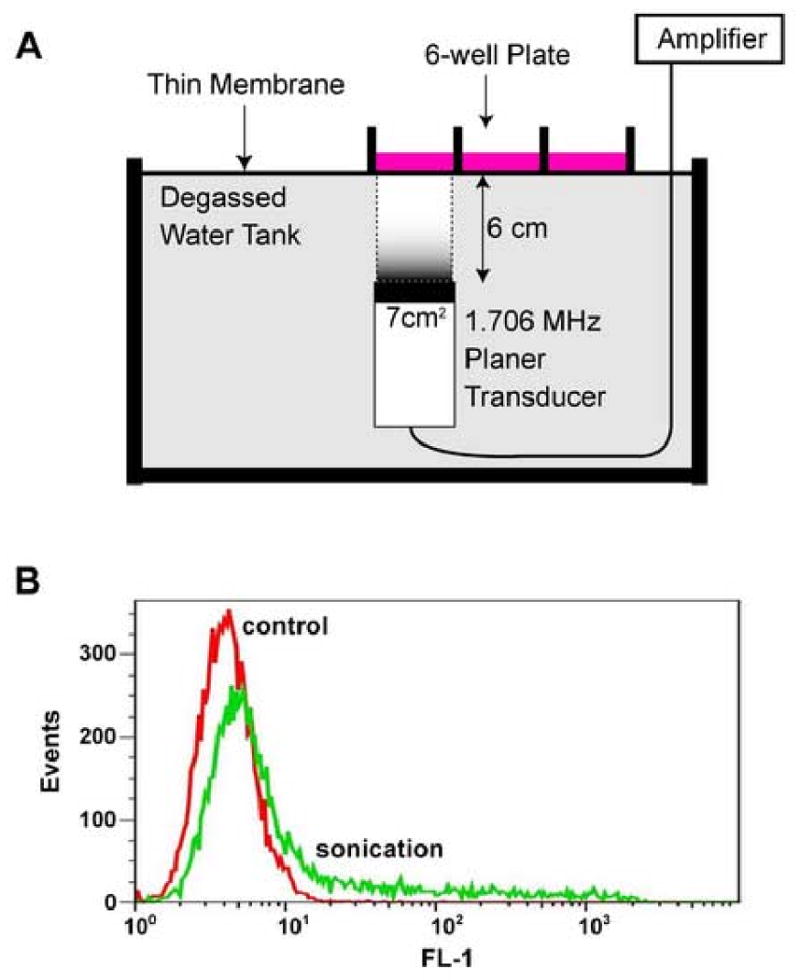
A, A polystyrene 6-well plate was placed on a device designed for planner ultrasound exposure. Planner ultrasound (1.706 MHz center frequency) was delivered vertically to the cells from the bottom through a water tank and each sample was sonicated separately by moving the plate to the target of the focused ultrasound. B, Sonoporated cells were detected using flowcytometric analysis. Positive cells were labeled with calcein and detected on FL-1 channel.
Sonoporation efficiency and cell viability assay for C166 cells
In order to determine the sonoporation efficiency of C166 cells under various ultrasound exposure conditions, cell membrane impermeable calcein (Sigma-Aldrich, Saint Louis, MO) was added to the cell suspension at a final concentration of 37.5 μM. Cells were sonicated as mentioned above under various conditions and recovered, washed twice with phosphate buffer saline (PBS) and analyzed by flow cytometry (FACScan; Becton Dickinson, Mountain View, CA). Successfully sonoporated cells would uptake calcein and were detected by FL1 channel (Fig. 1B). To measure the viability of sonicated cells, we used CellTiter-Blue assay (Promega; Madison, WI). Sonicated cells were cultured for 2 hr with the reagent and their viability was estimated by measuring the amount of reduced resorufin by its fluorescent intensity (ex. 560 nm, em. 590 nm) using a fluorescent microplate reader (SPECTRAmax GEMINI XS, Sunnyvale, CA).
siRNA transfection for EGFP suppression using microbubble-enhanced ultrasound
EGFP-specific siRNA; egfp siRNA (sense; 5′- GCA AGC UGA CCC UGA AGU UCA U -3′, antisense; 5′- GAA CUU CAG GGU CAG CUU GCC G -3′) and control siRNA (sense; 5′-UUC UCC GAA CGU GUC ACG UdTdT -3′,antisense; 5′- ACG UGA CAC GUU CGG AGA AdTdT -3′) duplexes were purchased from Qiagen (Valencia, CA). The siRNA duplexes were suspended in the provided buffer solution and prepared according to the instruction supplied by the manufacture. C166-GFP cells cultured in monolayer were suspended in their respective culture medium without serum. OPTISON® was added to the cell suspension at a final concentration of 5%. SiRNAs were added at a concentration of 15μg/ml for gene silencing. After sonication, cells were recovered and 1.5ml 10% serum and 50 units/ml penicillin (Invitrogen) supplemented medium was added and cultured for 48 hours followed by flow cytometric analysis of EGFP expression.
Flow cytometric analysis
After cells were cultured for the indicated time, cells were harvested, recovered and washed twice with PBS. Flow cytometric data collection was done using FACScan (Becton Dickinson) and EGFP or calcein positive cells were detected by FL1 channel. Acquired data was analyzed by FlowJo (Tree Star Inc, Ashland, OR). Two cell populations were identified on the forward scatter (FSC) vs. side scatter (SSC) plot and the population having larger values of both the FSC and SSC was gated as viable cells.
Special settings to avoid standing wave production
For experiments where the production of standing wave had to be avoided, we built a water chamber above the cell culture samples with a thin plastic membrane separating the samples and the water chamber. Air bubbles in the cell culture medium were carefully removed, allowing the plastic membrane to have a direct contact with the medium. 3 different settings were prepared as shown in Fig. 3.
Fig. 3. Experimental setting eliminating standing wave.
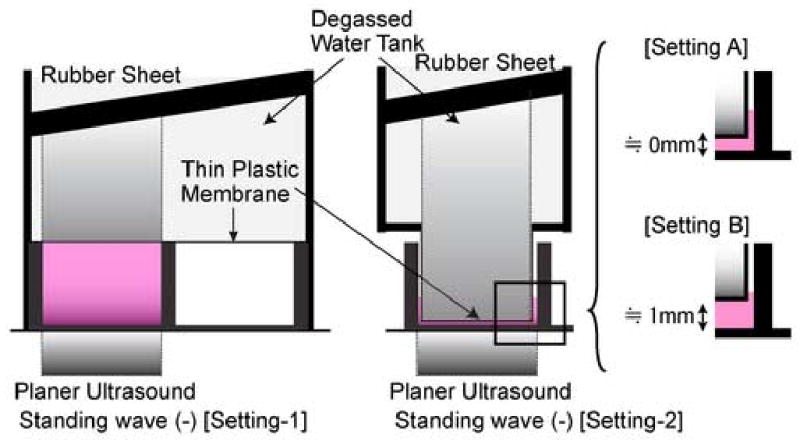
Experimental settings eliminating standing wave. Cells were cultured in a monolayer fashion on the bottom of the dish. A water chamber was constructed above the samples which allow the ultrasound beam to propagate through the sample without causing standing wave. In some cases, the interface of the water chamber and the sonicated cells was settled close to 0 mm [setting A] or 1 mm above [setting B] the bottom of the dish.
Results and Discussions
Although there are many reports on sonoporation [21-38], each experimental setting differs from one another and the reported sonoporation efficiency, which is one of the most significant information in sonoporation, varies. In order to understand the effect of the experimental settings themselves on sonoporation experiments, we first investigated the impact of the conditions of the cultured cells on sonoporation efficiency. When cells were sonicated in a suspension, although sonoporation was achieved among variable cells (Fig. 2B), majority of the cells were non-viable (Fig. 2A). On the other hand, when cells were cultured on the dish in a monolayer fashion, although the ratio of successfully sonoporated cells among viable cells did not differ from that in a condition with cells in suspension, the total viability was significantly higher than in suspensions (Fig. 2A and B). Increasing the concentration of OPTISON from 5 to 10% did not contribute in improving the overall outcome (Fig. 2C), and extending the sonication time only decreased the total cell viability while sonoporation efficiency saturated at around 15 to 30 sec CW exposure (Fig. 2C and D). This is a similar observation compared to what we have previously seen in cells sonicated in a suspension [30, 31]. Delivery of siRNA against EGFP into cells with cell attached on the culture dish was also demonstrated possible (Fig. 4C) and approximately half of the cell sonoporated showed decreased EGFP production (Fig. 4C). These findings suggest that the conditions where cells are cultured have a great influence on the total outcome. As cells are structured in a matrix in vivo, it is assumed that sonoporation efficacy in vivo might be better compared to those performed in vitro with cell cultured in a suspension.
Fig. 2. Impact of the condition of cell culture on sonoporation.
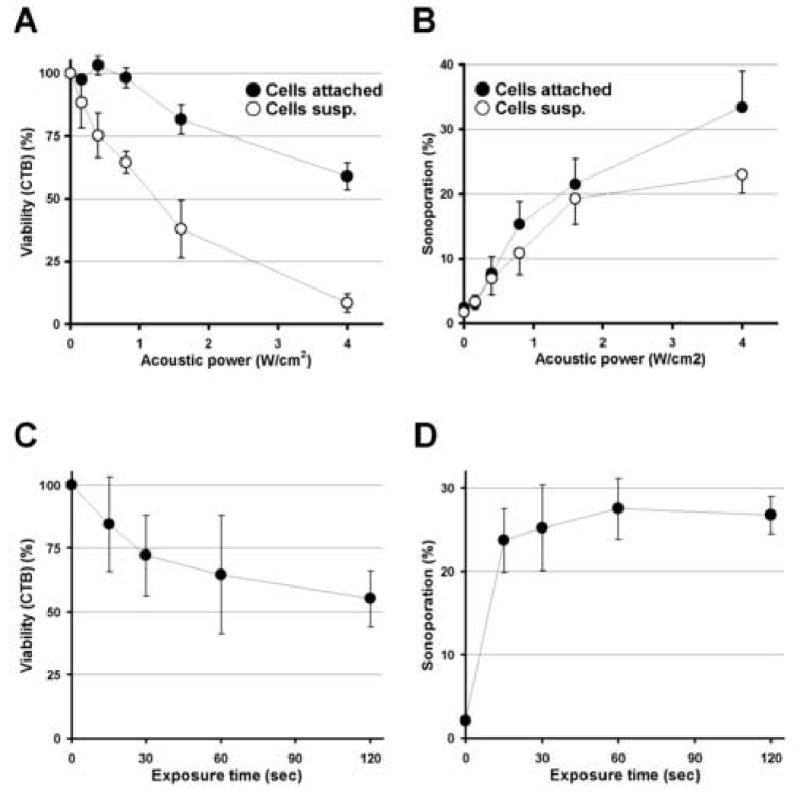
A and B, Sonoporation with cells in suspension v.s. attached to the dish. The concentration of OPTISON was 5% and the exposure time was fixed to 15 sec. Cell viabilities (A) and sonoporation efficiencies among viable cells (B) are plotted as a function of the applied acoustic power. Data obtained from two conditions; cells in suspension (○) and cells cultured in monolayer (●) are presented. C and D, Sonoporation efficiency as a function of exposure time. The concentration of OPTISON was increased up to 10% and the power was fixed to 1.6 W/cm2 AP and the cells cultured in monolayer were sonoporated. Cell viabilities (C) and sonoporation efficiencies among viable cells (D) are plotted as a function of exposure time. All data are presented as mean±SD of 3 independent experiments.
Fig. 4. The impact of standing wave on sonoporation and inhibition of EGFP stable expression in C166-GFP cells by microbubble-enhanced ultrasound delivered siRNA.
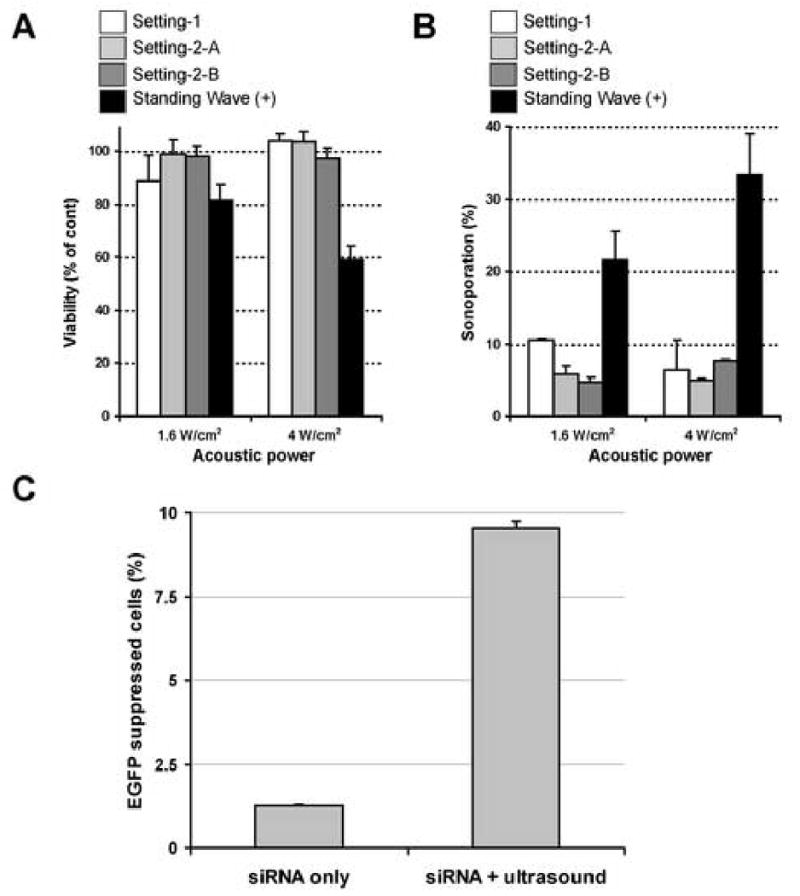
A and B, Sonoporation without the presence of standing wave. The concentration of OPTISON was 5% and the exposure time was fixed to 15 sec. Note that both loss of cell viability (A) and sonoporation efficacy (B) have dramatically dropped when standing wave is absent. All data are presented as mean±SD of 3 independent experiments.
C, EGFP gene suppression with intracellular siRNA delivery. The conditions were 5% OPTISON with 1.6 W/cm2 AP × 15 sec exposure and the cells were cultured in a monolyaer fashion. The concentration of siRNA was the same as previously reported where cells were sonicated in suspesion [30]. Approximately half of the sonoporated cells underwent gene supression (mean±SD of 3 independent experiments).
Next, we evaluated the impact of the presence or absence of standing wave on sonoporation efficiency. In this experiment, sonoporation was performed with cell attached on the bottom of the dish and standing wave was eliminated by building a water chamber above the sonication samples, allowing the progressive ultrasound wave to propagate through the sonication samples without causing reflected ultrasound wave (Fig. 3 left). When standing wave was nearly completely eliminated from the experimental settings, only a negligible amount of cells achieved sonoporation, even under condition where maximum sonoporation was achieved in the presence of standing wave (Fig. 4B). Cell viability after sonoporation was nearly 100% even after 5 W/cm2 exposures (Fig. 4A). As there is a possibility that all the OPTISON® microbubbles were pushed away from the cultured cells during sonication, we prepared 2 different settings in addition. One with the water chamber directly contacting the cells attached on the bottom of the wells (Fig. 3 right setting-2A), and the other lifting the water chamber 1 mm above the cells attached on the bottom (Fig. 3 right setting-2B). Again, when standing wave was absent, both of the settings failed to achieve a satisfactory amount of sonoporation, even up to 5 W/cm2 exposures (Fig. 4A and B). These results suggest that creation of standing wave is one of the key elements in achieving successful sonoporation in in vitro experiments. This finding could be crucial in applying sonoporation to real clinical use, as the production of standing wave may be difficult in the human body and special arrangements may be needed to establish the desired acoustic fields. When we look into the reported sonoporation experiments in vivo, the animals often used for demonstration are rats or rabbits [27, 29, 33, 34, 37]. Again, the animals are sonicated under a condition where it is difficult to assess the true ultrasound field and the presence or absence of standing wave is not clear. However, based on the data available, it is likely that standing waves were generated in many of these experiments. As the information on the relationship between the applied ultrasound field characteristics and the achieved sonoporation efficiency is crucial to render this technique practical in the future clinical application, we suggest that researchers should be aware of the above mentioned matters in performing and reporting sonoporation experiments.
Acknowledgments
This investigation was supported by the Shinya International Exchange Fund, the Osaka Medical Research Foundation for Incurable Diseases, the Osaka Neurological Institute and NIH grant (EB003268).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chapelon JY, Margonari J, Vernier F, Gorry F, Ecochard R, Gelet A. In vivo effects of high-intensity ultrasound on prostatic adenocarcinoma Dunning R3327. Cancer Res. 1992;52:6353–6357. [PubMed] [Google Scholar]
- 2.Chopra R, Burtnyk M, Haider MA, Bronskill MJ. Method for MRI-guided conformal thermal therapy of prostate with planar transurethral ultrasound heating applicators. Phys Med Biol. 2005;50:4957–4975. doi: 10.1088/0031-9155/50/21/001. [DOI] [PubMed] [Google Scholar]
- 3.Diederich CJ. Thermal ablation and high-temperature thermal therapy: overview of technology and clinical implementation. Int J Hyperthermia. 2005;21:745–753. doi: 10.1080/02656730500271692. [DOI] [PubMed] [Google Scholar]
- 4.Fry WJ, Barnard JW, Fry FJ, Brennan JF. Ultrasonically produced localized selective lesions in the central nervous system. Am J Phys Med. 1955;34:413–423. [PubMed] [Google Scholar]
- 5.Hazle JD, Diederich CJ, Kangasniemi M, Price RE, Olsson LE, Stafford RJ. MRI-guided thermal therapy of transplanted tumors in the canine prostate using a directional transurethral ultrasound applicator. J Magn Reson Imaging. 2002;15:409–417. doi: 10.1002/jmri.10076. [DOI] [PubMed] [Google Scholar]
- 6.Sanghvi NT, Foster RS, Bihrle R, Casey R, Uchida T, Phillips MH, Syrus J, Zaitsev AV, Marich KW, Fry FJ. Noninvasive surgery of prostate tissue by high intensity focused ultrasound: an updated report. Eur J Ultrasound. 1999;9:19–29. doi: 10.1016/s0929-8266(99)00010-5. [DOI] [PubMed] [Google Scholar]
- 7.ter Haar G, Rivens I, Chen L, Riddler S. High intensity focused ultrasound for the treatment of rat tumours. Phys Med Biol. 1991;36:1495–1501. doi: 10.1088/0031-9155/36/11/009. [DOI] [PubMed] [Google Scholar]
- 8.Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai J, Li KQ, Jin CB, Xie FL, Su HB. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology. 2005;236:1034–1040. doi: 10.1148/radiol.2362041105. [DOI] [PubMed] [Google Scholar]
- 9.McDannold N, Moss M, Killiany R, Rosene DL, King RL, Jolesz FA, Hynynen K. MRI-guided focused ultrasound surgery in the brain: tests in a primate model. Magn Reson Med. 2003;49:1188–1191. doi: 10.1002/mrm.10453. [DOI] [PubMed] [Google Scholar]
- 10.Tempany CM, Stewart EA, McDannold N, Quade BJ, Jolesz FA, Hynynen K. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology. 2003;226:897–905. doi: 10.1148/radiol.2271020395. [DOI] [PubMed] [Google Scholar]
- 11.Hynynen K, Pomeroy O, Smith DN, Huber PE, McDannold NJ, Kettenbach J, Baum J, Singer S, Jolesz FA. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology. 2001;219:176–185. doi: 10.1148/radiology.219.1.r01ap02176. [DOI] [PubMed] [Google Scholar]
- 12.Hynynen K, Clement GT, McDannold N, Vykhodtseva N, King R, White PJ, Vitek S, Jolesz FA. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: a preliminary rabbit study with ex vivo human skulls. Magn Reson Med. 2004;52:100–107. doi: 10.1002/mrm.20118. [DOI] [PubMed] [Google Scholar]
- 13.Smith NB, Hynynen K. The feasibility of using focused ultrasound for transmyocardial revascularization. Ultrasound Med Biol. 1998;24:1045–1054. doi: 10.1016/s0301-5629(98)00086-6. [DOI] [PubMed] [Google Scholar]
- 14.Tran BC, Seo J, Hall TL, Fowlkes JB, Cain CA. Microbubble-enhanced cavitation for noninvasive ultrasound surgery. IEEE Trans Ultrason Ferroelectr Freq Control. 2003;50:1296–1304. doi: 10.1109/tuffc.2003.1244746. [DOI] [PubMed] [Google Scholar]
- 15.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 16.Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24:12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 17.Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA, Sheikov N. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg. 2006;105:445–454. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- 18.McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbits. Ultrasound Med Biol. 2005;31:1527–1537. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun. 2006;340:1085–1090. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- 20.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A. 2006;103:11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anwer K, Kao G, Proctor B, Anscombe I, Florack V, Earls R, Wilson E, McCreery T, Unger E, Rolland A, Sullivan SM. Ultrasound enhancement of cationic lipid-mediated gene transfer to primary tumors following systemic administration. Gene Ther. 2000;7:1833–1839. doi: 10.1038/sj.gt.3301302. [DOI] [PubMed] [Google Scholar]
- 22.Miller DL, Pislaru SV, Greenleaf JE. Sonoporation: mechanical DNA delivery by ultrasonic cavitation. Somat Cell Mol Genet. 2002;27:115–134. doi: 10.1023/a:1022983907223. [DOI] [PubMed] [Google Scholar]
- 23.Azuma H, Tomita N, Kaneda Y, Koike H, Ogihara T, Katsuoka Y, Morishita R. Transfection of NFkappaB-decoy oligodeoxynucleotides using efficient ultrasound-mediated gene transfer into donor kidneys prolonged survival of rat renal allografts. Gene Ther. 2003;10:415–425. doi: 10.1038/sj.gt.3301882. [DOI] [PubMed] [Google Scholar]
- 24.Deng CX, Sieling F, Pan H, Cui J. Ultrasound-induced cell membrane porosity. Ultrasound Med Biol. 2004;30:519–526. doi: 10.1016/j.ultrasmedbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Guzman HR, Nguyen DX, McNamara AJ, Prausnitz MR. Equilibrium loading of cells with macromolecules by ultrasound: effects of molecular size and acoustic energy. J Pharm Sci. 2002;91:1693–1701. doi: 10.1002/jps.10156. [DOI] [PubMed] [Google Scholar]
- 26.Guzman HR, McNamara AJ, Nguyen DX, Prausnitz MR. Bioeffects caused by changes in acoustic cavitation bubble density and cell concentration: a unified explanation based on cell-to-bubble ratio and blast radius. Ultrasound Med Biol. 2003;29:1211–1222. doi: 10.1016/s0301-5629(03)00899-8. [DOI] [PubMed] [Google Scholar]
- 27.Hashiya N, Aoki M, Tachibana K, Taniyama Y, Yamasaki K, Hiraoka K, Makino H, Yasufumi K, Ogihara T, Morishita R. Local delivery of E2F decoy oligodeoxynucleotides using ultrasound with microbubble agent (Optison) inhibits intimal hyperplasia after balloon injury in rat carotid artery model. Biochem Biophys Res Commun. 2004;317:508–514. doi: 10.1016/j.bbrc.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 28.Inagaki H, Suzuki J, Ogawa M, Taniyama Y, Morishita R, Isobe M. Ultrasound-microbubble-mediated NF-kappaB decoy transfection attenuates neointimal formation after arterial injury in mice. J Vasc Res. 2006;43:12–18. doi: 10.1159/000089103. [DOI] [PubMed] [Google Scholar]
- 29.Huber PE, Mann MJ, Melo LG, Ehsan A, Kong D, Zhang L, Rezvani M, Peschke P, Jolesz F, Dzau VJ, Hynynen K. Focused ultrasound (HIFU) induces localized enhancement of reporter gene expression in rabbit carotid artery. Gene Ther. 2003;10:1600–1607. doi: 10.1038/sj.gt.3302045. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita M, Hynynen K. A novel method for the intracellular delivery of siRNA using microbubble-enhanced focused ultrasound. Biochem Biophys Res Commun. 2005;335:393–399. doi: 10.1016/j.bbrc.2005.07.101. [DOI] [PubMed] [Google Scholar]
- 31.Kinoshita M, Hynynen K. Intracellular delivery of Bak BH3 peptide by microbubble-enhanced ultrasound. Pharm Res. 2005;22:716–720. doi: 10.1007/s11095-005-2586-7. [DOI] [PubMed] [Google Scholar]
- 32.Shimamura M, Sato N, Taniyama Y, Yamamoto S, Endoh M, Kurinami H, Aoki M, Ogihara T, Kaneda Y, Morishita R. Development of efficient plasmid DNA transfer into adult rat central nervous system using microbubble-enhanced ultrasound. Gene Ther. 2004;11:1532–1539. doi: 10.1038/sj.gt.3302323. [DOI] [PubMed] [Google Scholar]
- 33.Shimamura M, Sato N, Taniyama Y, Kurinami H, Tanaka H, Takami T, Ogihara T, Tohyama M, Kaneda Y, Morishita R. Gene transfer into adult rat spinal cord using naked plasmid DNA and ultrasound microbubbles. J Gene Med. 2005;7:1468–1474. doi: 10.1002/jgm.793. [DOI] [PubMed] [Google Scholar]
- 34.Sonoda S, Tachibana K, Uchino E, Okubo A, Yamamoto M, Sakoda K, Hisatomi T, Sonoda KH, Negishi Y, Izumi Y, Takao S, Sakamoto T. Gene transfer to corneal epithelium and keratocytes mediated by ultrasound with microbubbles. Invest Ophthalmol Vis Sci. 2006;47:558–564. doi: 10.1167/iovs.05-0889. [DOI] [PubMed] [Google Scholar]
- 35.Zarnitsyn VG, Prausnitz MR. Physical parameters influencing optimization of ultrasound-mediated DNA transfection. Ultrasound Med Biol. 2004;30:527–538. doi: 10.1016/j.ultrasmedbio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Tsunoda S, Mazda O, Oda Y, Iida Y, Akabame S, Kishida T, Shin-Ya M, Asada H, Gojo S, Imanishi J, Matsubara H, Yoshikawa T. Sonoporation using microbubble BR14 promotes pDNA/siRNA transduction to murine heart. Biochem Biophys Res Commun. 2005;336:118–127. doi: 10.1016/j.bbrc.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 37.Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, Aoki M, Ogihara T, Yasufumi K, Morishita R. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 2002;105:1233–1239. doi: 10.1161/hc1002.105228. [DOI] [PubMed] [Google Scholar]
- 38.Duvshani-Eshet M, Baruch L, Kesselman E, Shimoni E, Machluf M. Therapeutic ultrasound-mediated DNA to cell and nucleus: bioeffects revealed by confocal and atomic force microscopy. Gene Ther. 2006;13:163–172. doi: 10.1038/sj.gt.3302642. [DOI] [PubMed] [Google Scholar]
- 39.Rahim AA, Taylor SL, Bush NL, ter Haar GR, Bamber JC, Porter CD. Spatial and acoustic pressure dependence of microbubble-mediated gene delivery targeted using focused ultrasound. J Gene Med. 2006;8:1347–1357. doi: 10.1002/jgm.962. [DOI] [PubMed] [Google Scholar]
- 40.Rahim A, Taylor SL, Bush NL, ter Haar GR, Bamber JC, Porter CD. Physical parameters affecting ultrasound/microbubble-mediated gene delivery efficiency in vitro. Ultrasound Med Biol. 2006;32:1269–1279. doi: 10.1016/j.ultrasmedbio.2006.04.014. [DOI] [PubMed] [Google Scholar]


