Abstract
Nanoparticles (NP) have emerged as a novel class of therapeutic agents that overcome many of the limitations of current cancer chemotherapeutics. However, a major challenge to many current NP platforms is unfavorable biodistribution, and limited tumor uptake, upon systemic delivery. Delivery, therefore, remains a critical barrier to widespread clinical adoption of NP therapeutics. To overcome these limitations, we have adapted the techniques of image-guided local drug delivery to develop nano-ablation and nano-embolization. Nano-ablation is a tumor ablative strategy that employs image-guided placement of electrodes into tumor tissue to electroporate tumor cells, resulting in rapid influx of NPs that is not dependent on cellular uptake machinery or stage of the cell cycle. Nano-embolization involves the image-guided delivery of NPs and embolic agents directly into the blood supply of tumors. We describe the design and testing of our innovative local delivery strategies using doxorubicin functionalized superparamagnetic iron oxide nanoparticles (DOX-SPIOs) in cell culture, and the N1S1 hepatoma and VX2 tumor models, imaged by high resolution 7T MRI. We demonstrate that local delivery techniques result in significantly increased intra-tumoral DOX-SPIO uptake, with limited off-target delivery in tumor bearing animal models. The techniques described are versatile enough to be extended to any NP platform, targeting any solid organ malignancy that can be accessed via imaging guidance.
Keywords: Drug Delivery, Imaging Guidance, Magnetic Nanoparticles, Tumor Targeting, Cancer Therapy, Magnetic Resonance Imaging
Current cancer treatments rely on systemically administered therapies that indiscriminately affect tumor and healthy tissue alike, and therefore are toxic to both tissue types. As such, current therapies are limited by a narrow therapeutic index (ratio of therapeutic to toxic effects) and severe systemic side effects. Nanoparticle (NP) based therapeutics offer an innovative method to overcome the limitations of current agents for several reasons. First, NPs possess unique properties enabling them to serve as simultaneous imaging probes and therapeutic agents, unlike current drugs. Second, they can be tailored to deliver specific drugs1-3or target specific molecular mechanisms of cancer.4-6 The impact of these new agents can only be realized if they are able to reach tumor tissues in sufficient concentrations to exert therapeutic effect.
Conventional intravenous (IV) delivery of most NP platforms results in their sequestration by organs of the reticuloendothelial system (RES), limiting uptake within target tumors, leading to subtherapeutic dosing. Moreover, while all NPs exploit an “enhanced permeability and retention effect” (EPR) for tumor uptake, EPR is still inefficient with relatively low concentrations of NPs reaching tumors.7 Heterogeneity across tumor types also leads to low and unpredictable rates of NP extravasation.8 Large tumors, especially metastases, are marked by vascular heterogeneity, reducing perfusion and overall uptake.9-11 Strategies employing tumor targeting ligands have been met with limited success secondary to tumor heterogeneity (no single target) and limited total tumor uptake.12-16 Long-term efficacy of targeting ligands may also be limited by down-regulation of cell-surface targets over time.17, 18 Several groups have demonstrated that the rate-limiting step for tumor localization is vascular extravasation, which is primarily driven by circulation time.10, 13, 19 These barriers have therefore limited the utility and clinical translation of many potential therapeutic agents.
To address issues stemming from unfavorable biodistribution, we have the adapted the therapeutic techniques of interventional radiology (IR) to nanoparticle delivery. Interventional radiologists use a wide range of image-guided procedures to treat cancer patients. Real-time imaging guidance and sophisticated navigation techniques are employed for a variety of procedures including tumor ablation, microparticle embolization of vascular tumors, and intra-arterial delivery of chemotherapeutic agents for tumor chemoembolization.20 These minimally invasive image-guided procedures result in fewer complications, faster recoveries, and reduced costs versus traditional therapies.20 As such, IR may be used to help overcome the limitation of current generation NP platforms with its suite of image-guided techniques. We hypothesized that local, targeted delivery strategies could be exploited for NP-based drug delivery to overcome current unfavorable biodistribution issues. In these proof-of-principle studies, we describe the design, development, and testing of our innovative local delivery strategies.
An essential requirement of this therapeutic approach is a nanoplatform than can be imaged in vivo using current generation imaging techniques. To this end, we utilized doxorubicin-functionalized superparamagnetic iron oxide nanoparticles (DOX-SPIO) permitting 7T high-resolution magnetic resonance imaging (MRI). Clinically, SPIO-platforms have been used as MRI contrast agents to stage tumors, plan treatment, and assess response.21, 22 SPIO-based NPs have a long blood retention time, are biodegradable, and have low inherent toxicity.23, 24 They can be functionalized to carry drugs, and their imaging properties can be exploited to monitor drug distribution in target tissue,24 unlike current drug delivery methods. SPIOs generate tissue contrast via local magnetic field disturbance, causing spin dephasing and signal voids. These susceptibility effects reduce T2* or equivalently increase R2* (relaxation rates).25 These agents can be localized using R2* parametric maps, with local concentration quantified by measuring proportional changes in R2*.26 Previous studies have used this linear relationship between concentration and ΔR2* to quantify tumor vascularity.27 R2* relaxation measurements can estimate local magnetic dose of SPIOs, and used as proxy to quantify attached moieties.28 Use of SPIOs permitted imaging and quantification of therapeutic delivery in our studies.
Results and Discussion
15-nm doxorubicin functionalized SPIOs were fabricated as previously reported.29 Briefly, DOX-SPIOs at a concentration of 4 mg/ml were loaded with 0.2 mg doxorubicin per mg iron oxide (IO) (IO MW=3.7×106 g/mol) with 73% loading efficiency (Ocean Nanotech, Springdale, AR). The DOX was coupled to the iron core via a pH-labile bond, that permitted selective release within the tumor microenvironment29.
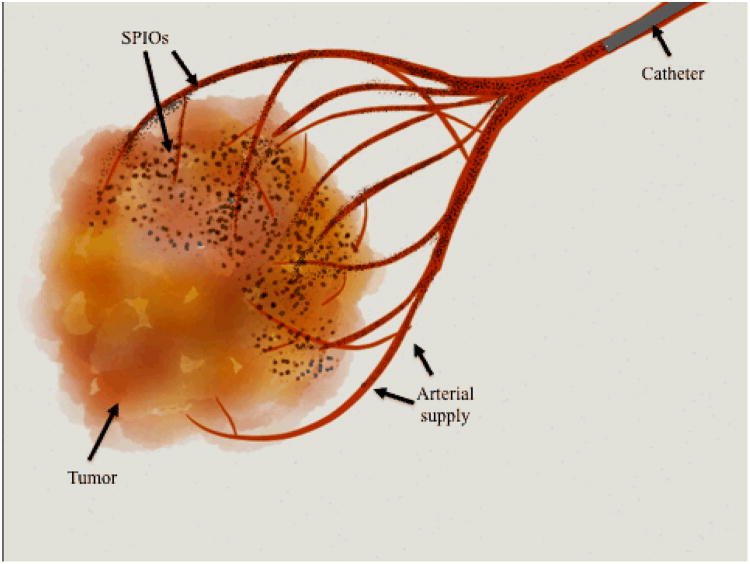
Two different delivery strategies were developed, targeting the cellular microenvironment and tumor vasculature at different levels. First we devised nano-ablation (Figure 1), a tumor ablative strategy that employs image-guided placement of electrodes into tumor tissue to electroporate tumor cells. Electroporation induces nano-scale defects in cells' plasma membranes. This permits rapid influx of extracellular material that is not dependent on cellular uptake machinery or stage of the cell cycle.30, 31 Additionally, electroporation induces transient vascular hypoperfusion within the treated zone,32 reducing drug washout. Therapeutic effect is further localized to tumors because normal tissue recovers more rapidly33-35.
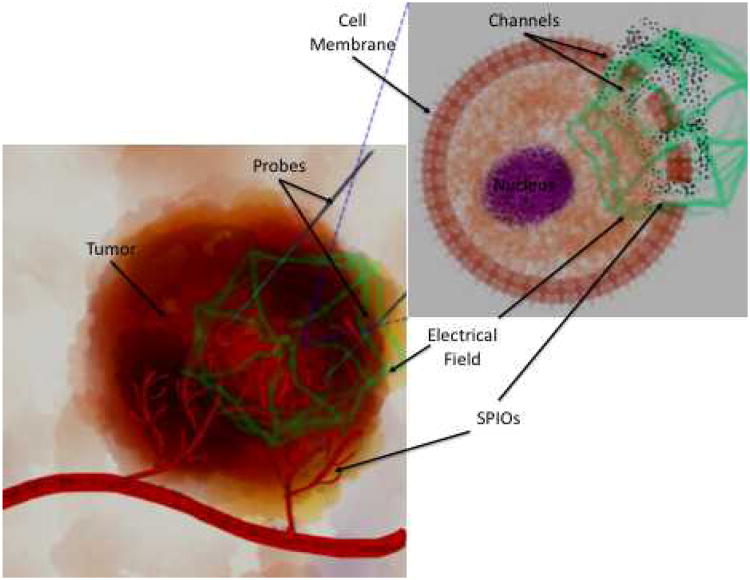
Figure 1.
Schematic of Nano-ablation. Tumor tissue on left, tumor cell on right. Ablation probes are inserted in tumor tissue, generating an electrical field (green). This induces formation of temporary channels in the tumor's cells' plasma membranes, permitting rapid SPIO entry.
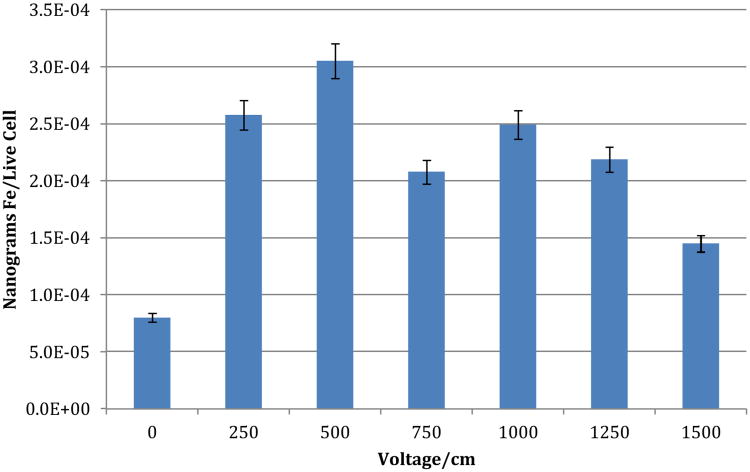
To determine optimal parameters for nano-ablation, in vitro testing with the N1S1 hepatoma cell line was performed. The goal was to maximize cellular uptake with minimal cellular death. 2 million cells/well were plated in 48 well plates. DOX-SPIOs were dissolved in DMEM culture medium, and added to individual wells for a final concentration of 50 nM of DOX-SPIOs. Cells were subsequently electroporated using an ECM830 function generator (BTX, Hollinston, MA). The following parameters, based on prior in vivo studies,36, 37 were utilized: 8 pulses, 99 us pulse duration, 1 Hz frequency, 100 msec pulse interval. Voltage was varied from 50 V/cm to 1500 V/cm to determine optimal uptake parameters. All studies were performed in triplicate. Cell viability was determined by flow cytometry, and SPIO uptake was quantified by inductively coupled plasma mass spectrometry (ICP-MS). A dose response curve was subsequently generated demonstrating maximum SPIO uptake with minimal cell death at 500 V/cm (Figure 2).
Figure 2.
In vitro Nano-ablation testing in N1S1 cell line. Optimal nano-ablation parameters were determined in vitro, by varying voltage strength (V/cm), and examining cellular iron (Fe) content via ICP-MS, and viability via flow cytometry. Optimal uptake was observed at field strength of 500 V/cm.
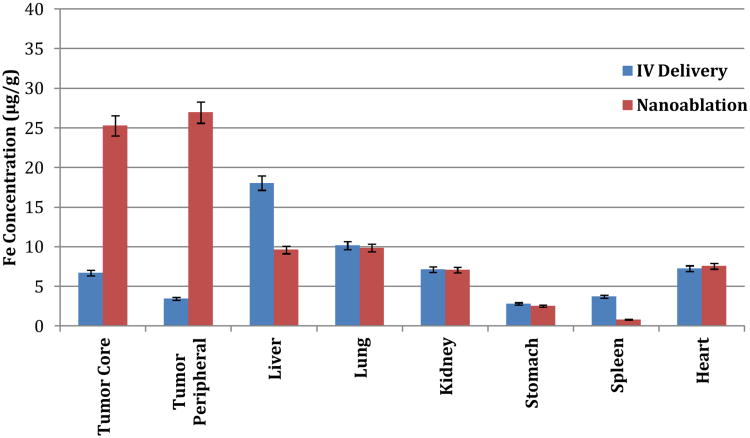
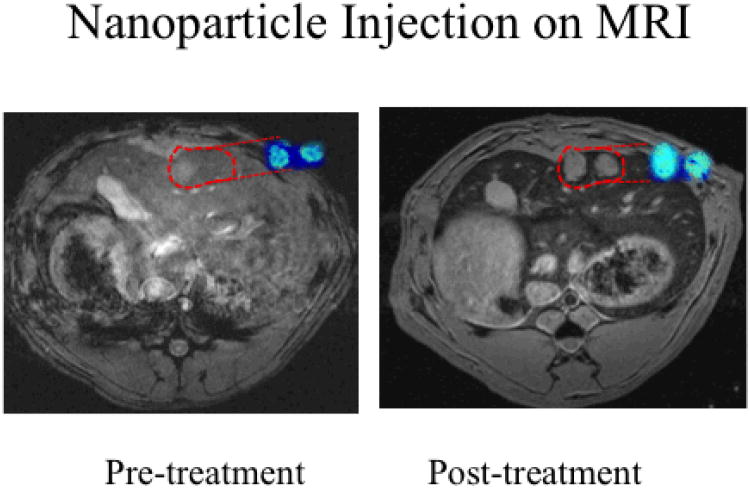
To determine if this enhanced tumor uptake could be translated in vivo, we employed the N1S1 rat model of hepatoma. We hypothesized that localized nano-ablation would enhance intratumoral SPIO uptake over surrounding tissues, compared to standard IV delivery. Twenty animals were implanted with N1S1 tumors according to previously published protocols,36, 37 and divided into nano-ablation and control groups. Dynamic T2* weighted 7T MRI images were obtained before and 24 hours after SPIO administration in both groups. DOX-SPIOs (4mg/ml) suspended in PBS, were injected systemically via tail vein at a dose of 0.5 mg/kg in both groups. Nano-ablation group animals immediately underwent electroporation using the previously determined settings at 500 V/cm field strength. MRI ΔR2* measurements were applied to quantify intratumoral SPIO uptake non-invasively with R2* maps produced for tumor tissues across all image slices. Dynamic T2* weighted images demonstrated dramatically decreased signal intensity within treated tumors, indicative of SPIO uptake (Figures 3-4). Minimal signal change was noted in control tumors. Nano-ablation resulted in significant R2 signal change in treated animals versus controls (103.4 ms vs. 33.5 ms, p<0.05). To confirm this tumor specific uptake and biodistribution, tumors and organs known to accumulate SPIOs were harvested. SPIO content was quantified with ICP-MS. Nano-ablation resulted in significantly higher SPIO uptake within the tumor periphery (27.0 ug/g vs. 3.5 ug/g, p<0.05) and tumor core (25.3 ug/g vs. 6.7 ug/g, p<0.05) compared to IV delivery (Figure 5). Furthermore, nano-ablation resulted in significantly less off-target delivery to the healthy liver and spleen (p<0.05).
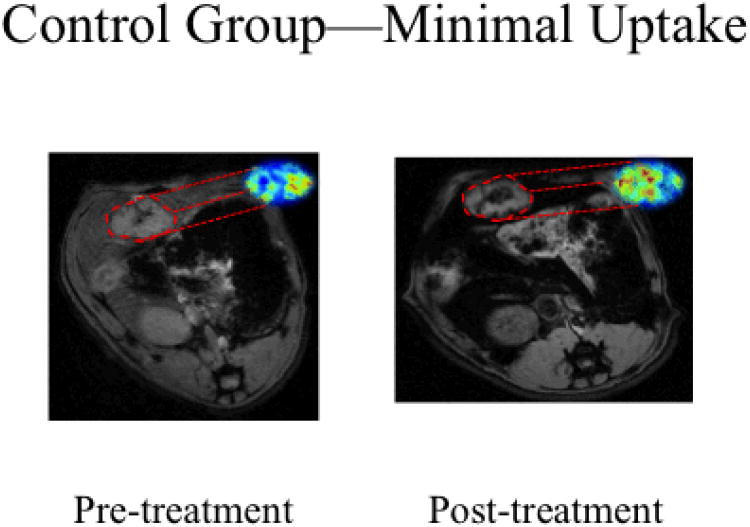
Figure 3.
SPIO IV delivery in control N1S1 rats on 7T MRI. Representative axial T2*W GRE images (TE: 11.9 ms) images with corresponding with R2* parametric maps from the same animal. Signal intensities were measured in the same plane to determine tumor SPIO uptake. Following SPIO delivery in the control group we saw minimal signal change in the tumor tissue, highlighted here in red, confirming limited uptake with conventional intravenous delivery
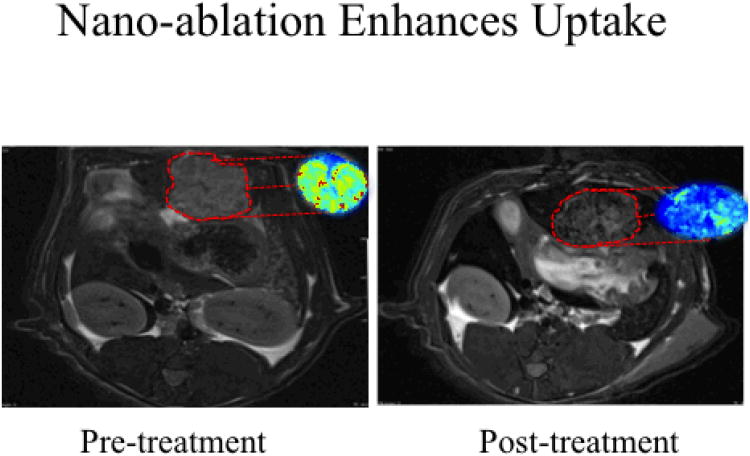
Figure 4.
SPIO delivery in nano-ablation treated N1S1 rats on 7T MRI. Representative axial T2*W GRE images (TE: 11.9 ms) images with corresponding with R2* parametric maps from the same animal. Signal intensities were measured in the same plane to determine tumor SPIO uptake. Following nano-ablation, animals in the treatment group demonstrated significant R2* signal changes within tumor tissues, highlighted in red.
Figure 5.
Biodistribution of DOX-SPIOs following administration in N1S1 model. Animals in the nano-ablation group demonstrated significantly higher DOX-SPIO uptake within tumor tissue, as well as decreased uptake within healthy liver and spleen tissue, compared to controls (p<0.05).
To further maximize local tumor SPIO uptake and enhance local (tumor-specific) circulation time, we developed nano-embolization. Nano-embolization involves the direct delivery of SPIOs combined with embolic agents, directly into a tumor's blood supply (Figure 6). The goal of embolization is to reduce antegrade blood flow locally to the tumor, thereby increasing the dwell time of the therapeutic agent within the target tumor. Embolization techniques utilizing standard chemotherapeutics or radiopharmaceuticals have been proven to be beneficial in the treatment of primary hepatic and metastatic liver tumors.38, 39 The improved customizability and imaging characteristics of SPIOs may make these agents the ideal next-generation embolic therapy. Furthermore, direct arterial delivery may overcome issues with unfavorable biodistribution and inefficient tumor uptake that limited current therapies prior to arterial-directed delivery.
Figure 6.
Schematic of Nano-embolization: SPIOs along with embolic agents are delivered directly to a tumor's arterial supply via an intra-arterial microcatheter. This maximizes intra-tumoral uptake, and minimizes off-target delivery.
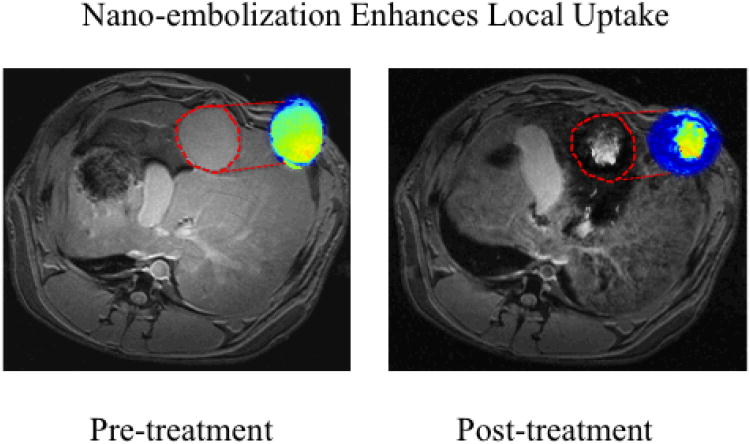
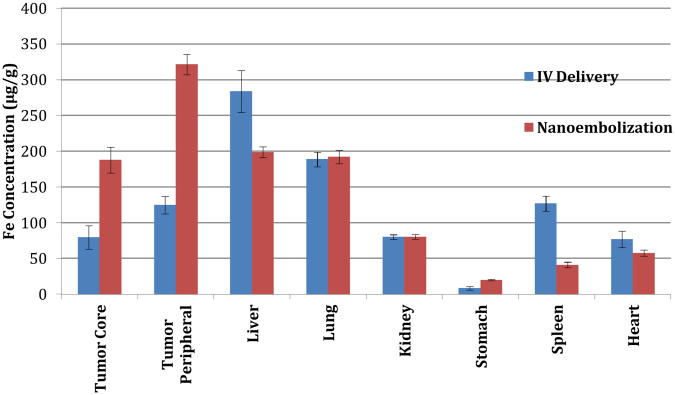
To test this hypothesis in vivo, we utilized the VX2 rabbit model of liver cancer (supporting info).40-49 This model permitted catheterization of the tumor's arterial supply, and represented an ideal model in which to test local delivery techniques by placing the tumor directly within competing RES tissue. 30 animals were divided into treatment and control groups. Again, animals in both groups were imaged prior to and after SPIO delivery with 7T T2* weighted MRI to quantify SPIO uptake. Control animals received DOX-SPIOs suspended in PBS, via ear vein injection (0.5 mg/kg). In the nano-embolization group, a microcatheter was selectively advanced into the hepatic artery feeding the tumor under image-guidance. DOX-SPIOs emulsified in lipiodol, the standard chemoembolic agent,50-52 were delivered intra-arterially (0.5 mg/kg). ΔR2* measurements and ICP-MS were used to quantify intratumoral SPIO uptake. Significant increases in R2* (corresponding to signal reduction in T2*-weighted images) were demonstrated within treated tumors (47.4 ms vs. 18.9 ms, p<0.05) compared to controls (Figures 7-8). This was confirmed pathologically with ICP-MS, which demonstrated a 240% increase in SPIO nanoparticle delivery to the tumor core (188 ug/g vs. 80 ug/g, p<0.05), and a 260% increase in delivery to the tumor periphery (322 ug/g vs. 125 ug/g, p<0.05) (Figure 9). Furthermore, nano-embolization resulted in 32% less off-target delivery to healthy liver tissue, and 68% less off-target delivery to the spleen (p<0.05).
Figure 7.
IV SPIO delivery in VX2 rabbit liver cancer model imaged on 7T MRI. Representative axial T2*W GRE images (TE: 11.9 ms) images with corresponding with R2* parametric maps from the same animal. Signal intensities were measured in the same plane to determine tumor SPIO uptake. IV delivery did not result in significant R2* signal changes within tumor tissue, highlighted in red.
Figure 8.
Nano-embolization in VX2 rabbit liver cancer model imaged on 7T MRI. Representative axial T2*W GRE images (TE: 11.9 ms) images with corresponding with R2* parametric maps from the same animal. Signal intensities were measured in the same plane to determine tumor SPIO uptake. Nano-embolization resulted in significant R2* signal changes within tumor tissue, highlighted in red.
Figure 9.
Biodistribution of DOX-SPIOs following administration in VX2 rabbit model. Animals in the nano-embolization group demonstrated significantly higher DOX-SPIO uptake within tumor tissue, as well as decreased uptake within healthy liver and spleen tissue, compared to controls (p<0.05).
Several studies have examined the relative contribution of cellular and vascular uptake mechanisms in tumor therapy.10-12, 53 As nano-ablation and nano-embolization enhance intra-tumoral SPIO uptake via different mechanisms (cellular vs. vascular), we hypothesized that their effects could be additive. A combination approach may promote synergy between these distinct physiologic pathways.
To determine the optimal temporal window to permit maximal SPIO uptake, delivery-time dependent uptake profiles were determined in vivo. Using a VX2 tumor rabbit hindlimb model (supporting info), 8 tumors were implanted. Again, a microcatheter was selectively advanced into the artery feeding the tumor, and SPIOs (0.5 mg/kg), emulsified in lipiodol, were delivered. Electroporation was performed at progressive time points ranging from 3 minutes before to 3 minutes after intra-arterial delivery. ICP-MS and T2*-weighted MR imaging both demonstrated that intra-tumoral SPIO concentrations were significantly increased (2.9 fold, p<0.05) when electroporation was performed 1.5 to 2 minutes following intra-arterial delivery.
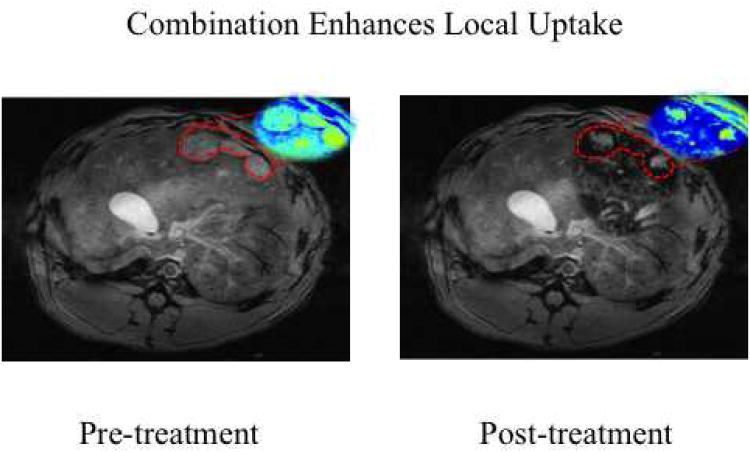
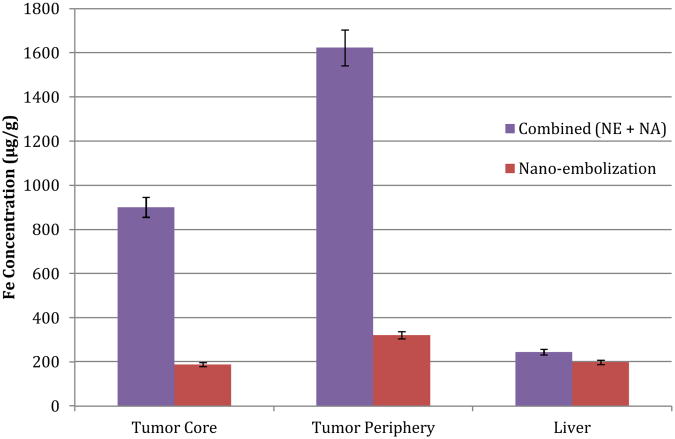
Next, using the VX2 liver cancer model, the synergistic effects of nano-ablation and nano-embolization were studied. Ten animals were implanted with hepatic VX2 tumors, as previously described.40-49 Again, a microcatheter was selectively advanced into the hepatic artery feeding the tumor under image-guidance. DOX-SPIOs emulsified in lipiodol, were delivered, followed by nano-ablation 1.5 minutes later. Animals were imaged via 7T T2* weighted MRI pre- and post-treatment to quantify ΔR2*. Tumor and healthy liver tissue were harvested for SPIO quantification via ICP-MS. Combination therapy (Figures 10-11) resulted in significantly increased SPIO uptake over nano-embolization alone in both the tumor core (901 μg/g vs. 188 μg/g, p<0.05), and tumor periphery (1623 μg/g vs. 321 μg/g, p<0.05).Furthermore, no significant difference in delivery to healthy liver tissue was observed (p=0.67). These results demonstrated the significant impact of our proposed combination approach (nano-ablation + nano-embolization) for tumor targeted SPIO nanoparticle delivery.
Figure 10.
Combination Nano-ablation and Nano-embolization in VX2 rabbit model on 7T MRI. Representative axial T2*W GRE images (TE: 11.9 ms) images with corresponding with R2* parametric maps from the same animal. Signal intensities were measured in the same plane to determine tumor SPIO uptake. Combination therapy resulted in significant signal change within tumor tissue, as depicted here in red.
Figure 11.
Biodistribution of DOX-SPIOs in tumor and liver tissue following combination therapy (nano-embolization followed by nano-ablation) in VX2 rabbit model. Combination therapy resulted in significantly increased delivery to both the tumor core and periphery, compared to nano-embolization alone (p<0.05). Furthermore, a significant difference in off-target delivery, to healthy liver tissue, was not observed (p=ns).
Conclusion
Optimization of drug delivery is a central issue in oncology. There is great potential for image-guided strategies to enhance NP delivery, overcoming the barriers related to non-target uptake by the RES, tumor perfusion, and tissue heterogeneity. To maximize the therapeutic index of novel nanotherapies, it is critical to preferentially deliver these agents to target tissues rather than healthy normal tissue. We have demonstrated this control can be achieved by adopting image-guided ablative and intra-arterial techniques for the delivery of NPs. By applying these local delivery techniques, we have demonstrated enhanced intratumoral SPIO uptake in contrast to standard IV delivery strategies. Furthermore, these strategies limit nonspecific delivery to the RES. The effectiveness of these techniques does not rely on sophisticated targeting mechanisms or cellular uptake machinery. Critically, these benefits do not depend on the composition or specific characteristics of the NP delivered. As such, these techniques are versatile enough to be extended to any NP platform, targeting any solid organ malignancy that can be accessed via image-guidance. Future longitudinal studies will focus on further validating the therapeutic efficacy of these techniques.
Materials and Methods
Doxorubicin Superparamagnetic Iron-oxide Nanoparticles
SPIOs were prepared using iron oxide micropowder as the iron precursor, oleic acid as the ligands, and octadecene as the solvent as described.54 The core size and hydrodynamic size of the SPIOs were measured using transmission electron microscopy (TEM), and light scattering scan, respectively. We used IO nanoparticles with 10 nm core size for this study. The particles were coated with amphiphilic polymers reported previously,55 which stabilizes SPIOs in water and provides reactive carboxyl groups on the particle surface for bioconjugation. To reduce nonspecific binding and uptake by cells, PEG-diamine (Molecular weight of 2000) were conjugated to SPIOs by ethyl-3-dimethyl amino propyl carbodiimide (EDAC) coupling method.
Doxorubicin HCI in 0.15 M NaCl at 0.5 mg/mL was added to 10 nm iron oxide nanoparticles and vortex for 1 hour at room temperature. The free DOX molecules were separated from the encapsulated DOX-SPIOs by magnetic separator (SuperMag Separator, Ocean NanoTech, Springdale AR). The DOX loading amount was about 20% (w/w(Fe)) calculated by free DOX left from the supernatant.
Cell Line and Culture
The N1-S1 rat hepatoma cell line (ATCC, Manassas, VA) was obtained and cultured in Dulbecco's Modified Eagle's Medium (DMEM) (ATCC, Manassas, VA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO) and 1% penicillin streptomycin (Invitrogen, Carlsbad, CA). Cells were maintained in suspension culture flasks at 37°C in a humidified atmosphere containing 5%CO2. Trypan blue staining was performed before each tumor implantation procedure to verify >90% cell viability.
Flow Cytometry and Fluorescence Microscopy
Cells were harvested and suspended in cold PBS with 2% bovine serum albumin at a concentration of 50000 cells/mL, incubated with Propidium Iodide for 30 min at RT, washed three times with cold PBS, and centrifuged at 1250 rpm for 5 min. Afterward, the cells were washed and analyzed using a Beckman Coulter Moflo Cell Sorter, which is equipped with five lasers, with excitation lines ranging from 350nm to 647nm (Beckman Coulter, Brea, CA).
Animal Models
N1S1 Cells and Rat Model
All experiments were approved by an Institutional Animal Care and Use Committee. Twenty male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 250-380 g were used for the experiments, all of which received a standard laboratory diet with free access to water.
Tumor inoculation was performed according to previously published procedure.56 Subjects were anesthetized with a hind limb injection of 100 mg/kg ketamine (Ketaset®, Fort Dodge Animal Health, Fort Dodge, IA) and 4 mg/kg xylazine (Isothesia®; Abbot Laboratories, North Chicago, IL). The left lateral lobe of the liver was exposed via mini-laparotomy and externalized using cotton-tipped applicators. Using a sterile insulin syringe, 5 million cells of N1-S1 suspended in 0.2 mL of DMEM were injected into the left lateral lobe of the liver. The incision was then closed using a double-layer suture technique and a 0.75 × 0.75 cm square of Surgicel hemostat (Ethicon, Somerville, NJ) was placed over the incision. Buprenorphine (2mg/kg) and meloxicam (2mg/kg) were administered twice daily for 48 hours after surgery, and animals were monitored for post-surgical recovery until wound healing was complete.
VX2 Rabbit
Our institutional Animal Care and Use Committee approved all experiments. Forty-five New Zealand white rabbits weighing 4–5 kg were used in these experiments. The VX2 tumor model was used because the VX2 tumor blood supply is almost entirely from the hepatic artery, and rabbit hepatic arteries are sufficiently large to permit catheterization.57 VX2 cells were initially grown in the hind limb of five donor rabbits. Tumor specimens approximately 2 mm in diameter were harvested and implanted in the left lobe of the liver in the 40 remaining rabbits via mini-laparotomy. Tumors were allowed to grow for 3–4 weeks before imaging. During imaging procedures, rabbits were anesthetized with intramuscularly administered ketamine (Ketaset; Fort Dodge Animal Health, Fort Dodge, Iowa), and xylazine (AnaSed Injection; Lloyd Laboratories, Shenandoah, Iowa), followed by inhaled isoflurane (Isothesia; Abbott Laboratories, North Chicago, Ill).
Magnetic Resonance Imaging and Analysis
A Bruker 7T ClinScan MRI horizontal bore scanner (Bruker, Billerica, MA) was employed for all rodent scans. A mixture of 2-5% isofluorane and 2L/min oxygen were supplied via facemask to the subjects throughout imaging. A small animal monitoring system (SA Instruments, Stony Brook, NY) was used to ensure appropriate sedation and monitor physiologic parameters at all times. Localizer and T2-weighted anatomical scans were performed to verify animal positioning and tumor presence. T2* measurements were obtained before and after treatment using the following scan parameters: T2*/R2* measurements were obtained by using a multiple-gradient-echo (mGRE) sequence with TR/TEs = 200/2.6, 5.7, 8.8, 11.9 ms; 30° flip angle, 1-mm section thickness, 65-mm field of view, 192 × 192 matrix, readout bandwidth of 360 Hz/pixel.
Our quantitative T2*/R2* measurements were fit to monoexponential equations voxelwise to extract T2*/R2* maps using Matlab 7.1 (MathWorks, Natick, MA). A specific region of interest (ROI) was drawn in each R2 or R2* map, then averaged over the ROI to generate a mean R2 or R2* value.
Treatment
Rodent Studies
MRI was performed 7-10 days after tumor implantation to confirm tumor growth, and tumor size was quantified by measuring maximum diameter, in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) guidelines.58 After removal from the MRI scanner, nano-ablation (NA) was performed as follows: A mini-laparotomy exposed the liver, and cotton-tipped applicators were employed to externalize the left lateral lobe of the liver and place it on a sheet of gauze. DOX-SPIOs suspended in PBS (0.5 mg/kg) were then injected into the tail vein. Two minutes after DOX-SPIO injection, electroporation was applied via a two-pronged electroporation tool (BTX, Holliston, MA) with two 0.4 mm-diameter needles separated by 1 cm. Eight 500 V pulses of 100 μs each with 100 ms between pulses were delivered with an ECM830 function generator (BTX, Holliston, MA) in accordance with previous electroporation optimization studies.59 The liver was then returned to the abdominal cavity, and the incision was closed via suture. Twenty-four hours following nano-ablation, the subjects were returned to MRI for post-treatment imaging. For IV control animals, DOX-SPIOs suspended in PBS were delivered via tail vein (0.5 mg/kg). Twenty-four hours following delivery animals underwent MR imaging. Subsequently, the subjects in both groups were euthanized using pentobarbital (Euthasol ®, Virbac Animal Health, Fort Worth, TX) for tissue harvest.
Rabbit Studies
Each rabbit was catheterized with x-ray fluoroscopic guidance by using a C-arm unit (PowerMobil; Siemens Medical Solutions, Erlangen, Germany). Vascular access was achieved in the femoral artery through surgical cutdown. A 3-F vascular sheath was first placed, and a 2-F catheter was then inserted within this sheath. The 2-F catheter (JB-1; Cook, Bloomington, ID) was advanced over a 0.014-inch-diameter guidewire into the targeted arteries. Conventional digital subtraction angiographic procedures were performed by an attending interventional radiologist (R.A.O., with >15 years of experience). A 4:1 DOX-SPIO:lipiodol embolic emulsion was delivered via arterial catheter. Lipiodol is used clinically in standard chemoembolization protocols as an emulsifier to deliver drugs. It also has an embolic effect, thereby promoting dwell time of therapeutics within the targeted tumor. For animals undergoing combination treatment, electroporation was then performed via our previously described protocol above. The animals were then transferred to an MR imaging unit.
Necropsy and Tissue Analysis
Tissue iron concentration was determined via inductively coupled plasma mass-spectrometry (ICP-MS) and used as proxy for SPIO uptake and Doxorubicin concentration. Necropsy was performed and the tumor was separated from the surrounding hepatic parenchyma. Prior studies in both the VX2 rabbit and N1S1 rat models49, 57, 60-62 have demonstrated a histologic difference between the tumor core and periphery, with highly vascularized tissue found in the tumor periphery, and poorly vascularized tissue in the tumor core. To account for these differences, after necropsy, using surgical microdissection, the central tumor was extracted and the viable margin of each tumor was sectioned into 4 quadrants, according to previously published protocols.60 Specimens were sectioned if necessary such that each sample was less than 0.1g and were placed in an Eppendorf tube (Eppendorf International, Hamburg, Germany). The samples were dissolved in 800 μl of trace metal grade nitric acid (70%). Digested samples were filtered with 0.45 μm PTFE syringe filters, then 20 μl of digested sample were transferred to a 15 ml metal-free tube and diluted with 12 mL laboratory grade water to 2-3% acid. Calibration standards of 0.5, 1, 5, 10, 20, 50, and 100 ppb Fe in 2% nitric acid buffer were prepared, as well as a blank solution. An Yttrium internal standard was added to both standards and samples, and ICP-MS was performed on a Thermo XSeries II ICP-MS (Thermo-Fisher, Waltham, MA). This returned a concentration of iron in ppb, which was converted to μg iron. This returned a concentration of iron in ppb, which was converted to μg iron. From the weight of the tissue, and volume of the digested homogenate, iron levels were normalized to amount per gram of wet tissue. Tissues from animals receiving sham procedures (saline injection) were used as controls for endogenous iron content. Fe content in each organ was subtracted by the corresponding averaged value from sham animals according to previously published protocols.24, 63.
Statistical Analysis
Data are expressed as means ± standard deviation from a minimum of three samples, unless otherwise indicated. Statistical analyses were carried out using a statistics software program (SPSS Statistics, IBM, Armonk, NY). For multiple comparisons, a one-way analysis of variance (ANOVA) with post test (Kruskal-Wallis test with post-test) was used. p< 0.05 was considered to indicate a statistically significant difference.
Acknowledgments
Funding Sources: This work was supported by Robert H. Lurie Comprehensive Cancer Center, and the National Cancer Institute Grants R01CA159178, and R01CA141047
Abbreviations
- NP
nanoparticle
- SPIO
superparamagnetic iron oxide nanoparticle
- RES
reticuloendothelial system
- DOX
doxorubicin
- IR
interventional radiology
- MRI
magnetic resonance imaging
Footnotes
Author Contributions: The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Notes: The authors declare no competing financial interest.
References
- 1.Jain TK, Morales MA, Sahoo SK, Leslie-Pelecky DL, Labhasetwar V. Iron Oxide Nanoparticles for Sustained Delivery of Anticancer Agents. Mol Pharm. 2005 Jan 1;2:194–205. doi: 10.1021/mp0500014. [DOI] [PubMed] [Google Scholar]
- 2.Sun C, Lee JSH, Zhang M. Magnetic Nanoparticles in Mr Imaging and Drug Delivery. Adv Drug Deliv Rev. 2008 Aug 17;60:1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kettering M, Zorn H, Bremer-Streck S, Oehring H, Zeisberger M, Bergemann C, Hergt R, Halbhuber KJ, Kaiser WA, Hilger I. Characterization of Iron Oxide Nanoparticles Adsorbed with Cisplatin for Biomedical Applications. Phys Med Biol. 2009 Sep 7;54:5109–5121. doi: 10.1088/0031-9155/54/17/003. [DOI] [PubMed] [Google Scholar]
- 4.Peng XH, Qian X, Mao H, Wang AY, Chen ZG, Nie S, Shin DM. Targeted Magnetic Iron Oxide Nanoparticles for Tumor Imaging and Therapy. Int J Nanomedicine. 2008 Jan 1;3:311–321. doi: 10.2147/ijn.s2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, Mao H, Cao Z, Wang YA, Peng X, Wang X, Sajja HK, Wang L, Duan H, Ni C, et al. Molecular Imaging of Pancreatic Cancer in an Animal Model Using Targeted Multifunctional Nanoparticles. Gastroenterology. 2009 May;136:1514–1525 e1512. doi: 10.1053/j.gastro.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis ME, Chen ZG, Shin DM. Nanoparticle Therapeutics: An Emerging Treatment Modality for Cancer. Nat Rev Drug Discovery. 2008 Sep 1;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 7.Jain RK, Stylianopoulos T. Delivering Nanomedicine to Solid Tumors. Nat Rev Clin Oncol. 2010 Nov 1;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Zerda A, Bodapati S, Teed R, May SY, Tabakman SM, Liu Z, Khuri-Yakub BT, Chen X, Dai H, Gambhir SS. Family of Enhanced Photoacoustic Imaging Agents for High-Sensitivity and Multiplexing Studies in Living Mice. ACS Nano. 2012 Jun 26;6:4694–4701. doi: 10.1021/nn204352r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang J, Nakamura H, Maeda H. The Epr Effect: Unique Features of Tumor Blood Vessels for Drug Delivery, Factors Involved, and Limitations and Augmentation of the Effect. Adv Drug Deliv Rev. 2010 May 2; doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Minchinton AI, Tannock IF. Drug Penetration in Solid Tumours. Nat Rev Cancer. 2006 Aug 1;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 11.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor Vascular Permeability, Accumulation, and Penetration of Macromolecular Drug Carriers. JNCI Journal of the National Cancer Institute. 2006 Mar 1;98:335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 12.Danquah MK, Zhang XA, Mahato RI. Extravasation of Polymeric Nanomedicines across Tumor Vasculature. Adv Drug Deliv Rev. 2010 Dec 6; doi: 10.1016/j.addr.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Huang X, Peng X, Wang Y, Wang Y, Shin DM, El-Sayed MA, Nie S. A Reexamination of Active and Passive Tumor Targeting by Using Rod-Shaped Gold Nanocrystals and Covalently Conjugated Peptide Ligands. ACS Nano. 2010 Oct 26;4:5887–5896. doi: 10.1021/nn102055s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nie S. Understanding and Overcoming Major Barriers in Cancer Nanomedicine. Nanomedicine (Lond) 2010 Jun 1;5:523–528. doi: 10.2217/nnm.10.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of Tumor-Specific Targeting on the Biodistribution and Efficacy of Sirna Nanoparticles Measured by Multimodality in vivo Imaging. Proc Natl Acad Sci U S A. 2007 Sep 25;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody Targeting of Long-Circulating Lipidic Nanoparticles Does Not Increase Tumor Localization but Does Increase Internalization in Animal Models. Cancer Res. 2006 Jul 1;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 17.Valastyan S, Weinberg RA. Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell. 2011 Oct 14;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-Initiating Cells: Challenges and Opportunities for Anticancer Drug Discovery. Nat Rev Drug Discov. 2009 Oct;8:806–823. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
- 19.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor Vascular Permeability, Accumulation, and Penetration of Macromolecular Drug Carriers. J Natl Cancer Inst. 2006 Mar 1;98:335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 20.Solomon SB, Silverman SG. Imaging in Interventional Oncology. Radiology. 2010 Dec 1;257:624–640. doi: 10.1148/radiol.10081490. [DOI] [PubMed] [Google Scholar]
- 21.Weber MA, Giesel FL, Stieltjes B. Mri for Identification of Progression in Brain Tumors: From Morphology to Function. Expert Rev Neurother. 2008 Oct;8:1507–1525. doi: 10.1586/14737175.8.10.1507. [DOI] [PubMed] [Google Scholar]
- 22.Khoo VS, Joon DL. New Developments in Mri for Target Volume Delineation in Radiotherapy. Br J Radiol. 2006 Sep;79(Spec No 1):S2–15. doi: 10.1259/bjr/41321492. [DOI] [PubMed] [Google Scholar]
- 23.Levy M, Luciani N, Alloyeau D, Elgrabli D, Deveaux V, Pechoux C, Chat S, Wang G, Vats N, Gendron F, et al. Long Term in vivo Biotransformation of Iron Oxide Nanoparticles. Biomaterials. 2011 Jun;32:3988–3999. doi: 10.1016/j.biomaterials.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 24.Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V. Biodistribution, Clearance, and Biocompatibility of Iron Oxide Magnetic Nanoparticles in Rats. Mol Pharm. 2008 Mar-Apr;5:316–327. doi: 10.1021/mp7001285. [DOI] [PubMed] [Google Scholar]
- 25.Winkelmann S, Schaeffter T, Weiss S, Eggers H, Doessel O. Simultaneous Imaging and R2* Mapping Using a Radial Multi-Gradient-Echo (Rmge) Sequence. J Magn Reson Imaging. 2006 Oct;24:939–944. doi: 10.1002/jmri.20712. [DOI] [PubMed] [Google Scholar]
- 26.Yablonskiy DA, Haacke EM. Theory of Nmr Signal Behavior in Magnetically Inhomogeneous Tissues: The Static Dephasing Regime. Magn Reson Med. 1994 Dec;32:749–763. doi: 10.1002/mrm.1910320610. [DOI] [PubMed] [Google Scholar]
- 27.Bremer C, Mustafa M, Bogdanov A, Jr, Ntziachristos V, Petrovsky A, Weissleder R. Steady-State Blood Volume Measurements in Experimental Tumors with Different Angiogenic Burdens a Study in Mice. Radiology. 2003 Jan;226:214–220. doi: 10.1148/radiol.2261012140. [DOI] [PubMed] [Google Scholar]
- 28.Bowen CV, Zhang X, Saab G, Gareau PJ, Rutt BK. Application of the Static Dephasing Regime Theory to Superparamagnetic Iron-Oxide Loaded Cells. Magn Reson Med. 2002 Jul;48:52–61. doi: 10.1002/mrm.10192. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Cao Z, Sajja HK, Mao H, Wang L, Geng H, Xu H, Jiang T, Wood WC, Nie S, et al. Development of Receptor Targeted Magnetic Iron Oxide Nanoparticles for Efficient Drug Delivery and Tumor Imaging. Journal of Biomedical Nanotechnology. 2008;4:439–449. doi: 10.1166/jbn.2008.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hui SW. Overview of Drug Delivery and Alternative Methods to Electroporation. Methods Mol Biol. 2008;423:91–107. doi: 10.1007/978-1-59745-194-9_6. [DOI] [PubMed] [Google Scholar]
- 31.Walczak P, Kedziorek DA, Gilad AA, Lin S, Bulte JW. Instant Mr Labeling of Stem Cells Using Magnetoelectroporation. Magn Reson Med. 2005 Oct;54:769–774. doi: 10.1002/mrm.20701. [DOI] [PubMed] [Google Scholar]
- 32.Mir LM, Orlowski S. The Basis of Electrochemotherapy. Methods Mol Med. 2000;37:99–117. doi: 10.1385/1-59259-080-2:99. [DOI] [PubMed] [Google Scholar]
- 33.Tounekti O, Pron G, Belehradek J, Jr, Mir LM. Bleomycin, an Apoptosis-Mimetic Drug That Induces Two Types of Cell Death Depending on the Number of Molecules Internalized. Cancer Res. 1993 Nov 15;53:5462–5469. [PubMed] [Google Scholar]
- 34.Tounekti O, Belehradek J, Jr, Mir LM. Relationships between DNA Fragmentation, Chromatin Condensation, and Changes in Flow Cytometry Profiles Detected During Apoptosis. Exp Cell Res. 1995 Apr;217:506–516. doi: 10.1006/excr.1995.1116. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell MS. Combining Chemotherapy with Biological Response Modifiers in Treatment of Cancer. J Natl Cancer Inst. 1988 Nov 16;80:1445–1450. doi: 10.1093/jnci/80.18.1445. [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Zhang Y, Klein R, Nijm GM, Sahakian AV, Omary RA, Yang GY, Larson AC. Irreversible Electroporation Therapy in the Liver: Longitudinal Efficacy Studies in a Rat Model of Hepatocellular Carcinoma. Cancer Res. 2010 Feb 15;70:1555–1563. doi: 10.1158/0008-5472.CAN-09-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Guo Y, Ragin AB, Lewandowski RJ, Yang GY, Nijm GM, Sahakian AV, Omary RA, Larson AC. Mr Imaging to Assess Immediate Response to Irreversible Electroporation for Targeted Ablation of Liver Tissues: Preclinical Feasibility Studies in a Rodent Model. Radiology. 2010 Aug 1;256:424–432. doi: 10.1148/radiol.10091955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coldwell D, Sangro B, Salem R, Wasan H, Kennedy A. Radioembolization in the Treatment of Unresectable Liver Tumors: Experience across a Range of Primary Cancers. Am J Clin Oncol. 2010 Nov 30; doi: 10.1097/COC.0b013e3181f47923. [DOI] [PubMed] [Google Scholar]
- 39.Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH, et al. A Comparative Analysis of Transarterial Downstaging for Hepatocellular Carcinoma: Chemoembolization Versus Radioembolization. Am J Transplant. 2009 Aug;9:1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 40.Virmani S, Rhee TK, Ryu RK, Sato KT, Lewandowski RJ, Mulcahy MF, Kulik LM, Szolc-Kowalska B, Woloschak GE, Yang GY, et al. Comparison of Hypoxia-Inducible Factor-1alpha Expression before and after Transcatheter Arterial Embolization in Rabbit Vx2 Liver Tumors. J Vasc Interv Radiol. 2008 Oct;19:1483–1489. doi: 10.1016/j.jvir.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta T, Virmani S, Neidt TM, Szolc-Kowalska B, Sato KT, Ryu RK, Lewandowski RJ, Gates VL, Woloschak GE, Salem R, et al. Mr Tracking of Iron-Labeled Glass Radioembolization Microspheres During Transcatheter Delivery to Rabbit Vx2 Liver Tumors: Feasibility Study. Radiology. 2008 Dec;249:845–854. doi: 10.1148/radiol.2491072027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D, Virmani S, Tang R, Szolc-Kowalska B, Woloschak G, Omary RA, Larson AC. Four-Dimensional Transcatheter Intraarterial Perfusion (Trip)-Mri for Monitoring Liver Tumor Embolization in Vx2 Rabbits. Magn Reson Med. 2008 Oct;60:970–975. doi: 10.1002/mrm.21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Virmani S, Harris KR, Szolc-Kowalska B, Paunesku T, Woloschak GE, Lee FT, Lewandowski RJ, Sato KT, Ryu RK, Salem R, et al. Comparison of Two Different Methods for Inoculating Vx2 Tumors in Rabbit Livers and Hind Limbs. J Vasc Interv Radiol. 2008 Jun;19:931–936. doi: 10.1016/j.jvir.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng J, Virmani S, Young J, Harris K, Yang GY, Rademaker A, Woloschak G, Omary RA, Larson AC. Diffusion-Weighted Propeller Mri for Quantitative Assessment of Liver Tumor Necrotic Fraction and Viable Tumor Volume in Vx2 Rabbits. J Magn Reson Imaging. 2008 May;27:1069–1076. doi: 10.1002/jmri.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang D, Bangash AK, Rhee TK, Woloschak GE, Paunesku T, Salem R, Omary RA, Larson AC. Liver Tumors: Monitoring Embolization in Rabbits with Vx2 Tumors--Transcatheter Intraarterial First-Pass Perfusion Mr Imaging. Radiology. 2007 Oct;245:130–139. doi: 10.1148/radiol.2451061689. [DOI] [PubMed] [Google Scholar]
- 46.Rhee TK, Young JY, Larson AC, Haines GK, 3rd, Sato KT, Salem R, Mulcahy MF, Kulik LM, Paunesku T, Woloschak GE, et al. Effect of Transcatheter Arterial Embolization on Levels of Hypoxia-Inducible Factor-1alpha in Rabbit Vx2 Liver Tumors. J Vasc Interv Radiol. 2007 May;18:639–645. doi: 10.1016/j.jvir.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 47.Rhee TK, Ryu RK, Bangash AK, Wang D, Szolc-Kowalska B, Harris KR, Sato KT, Chrisman HB, Vogelzang RL, Paunesku T, et al. Rabbit Vx2 Tumors as an Animal Model of Uterine Fibroids and for Uterine Artery Embolization. J Vasc Interv Radiol. 2007 Mar;18:411–418. doi: 10.1016/j.jvir.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Larson AC, Rhee TK, Deng J, Wang D, Sato KT, Salem R, Paunesku T, Woloschak G, Mulcahy MF, Li D, et al. Comparison between Intravenous and Intraarterial Contrast Injections for Dynamic 3d Mri of Liver Tumors in the Vx2 Rabbit Model. J Magn Reson Imaging. 2006 Jul;24:242–247. doi: 10.1002/jmri.20623. [DOI] [PubMed] [Google Scholar]
- 49.Deng J, Rhee TK, Sato KT, Salem R, Haines K, Paunesku T, Mulcahy MF, Miller FH, Omary RA, Larson AC. In vivo Diffusion-Weighted Imaging of Liver Tumor Necrosis in the Vx2 Rabbit Model at 1.5 Tesla. Invest Radiol. 2006 Apr;41:410–414. doi: 10.1097/01.rli.0000201232.14903.da. [DOI] [PubMed] [Google Scholar]
- 50.Brown DB, Geschwind JF, Soulen MC, Millward SF, Sacks D. Society of Interventional Radiology Position Statement on Chemoembolization of Hepatic Malignancies. J Vasc Interv Radiol. 2009 Jul;20:S317–323. doi: 10.1016/j.jvir.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 51.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RTP, Fan ST, Wong J. Randomized Controlled Trial of Transarterial Lipiodol Chemoembolization for Unresectable Hepatocellular Carcinoma. Hepatology. 2002 May 1;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 52.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial Embolisation or Chemoembolisation Versus Symptomatic Treatment in Patients with Unresectable Hepatocellular Carcinoma: A Randomised Controlled Trial. Lancet. 2002 May 18;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 53.Torchilin V. Tumor Delivery of Macromolecular Drugs Based on the Epr Effect. Adv Drug Deliv Rev. 2010 Mar 18; doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 54.Yu WW, Falkner JC, Yavuz CT, Colvin VL. Synthesis of Monodisperse Iron Oxide Nanocrystals by Thermal Decomposition of Iron Carboxylate Salts. Chem Commun (Camb) 2004 Oct 21;:2306–2307. doi: 10.1039/b409601k. [DOI] [PubMed] [Google Scholar]
- 55.Duan H, Kuang M, Wang X, Wang Y, Mao H, Nie S. Reexamining the Effects of Particle Size and Surface Chemistry on the Magnetic Properties of Iron Oxide Nanocrystals: New Insights into Spin Disorder and Proton Relaxivity. J Phys Chem C. 2008;112:8127–8131. [Google Scholar]
- 56.Garin E, Denizot B, Roux J, Noiret N, Lepareur N, Moreau M, Mesba H, Laurent JF, Herry JY, Bourguet P, et al. Description and Technical Pitfalls of a Hepatoma Model and of Intra-Arterial Injection of Radiolabelled Lipiodol in the Rat. Lab Anim. 2005 Jul;39:314–320. doi: 10.1258/0023677054307051. [DOI] [PubMed] [Google Scholar]
- 57.Geschwind JF, Artemov D, Abraham S, Omdal D, Huncharek MS, McGee C, Arepally A, Lambert D, Venbrux AC, Lund GB. Chemoembolization of Liver Tumor in a Rabbit Model: Assessment of Tumor Cell Death with Diffusion-Weighted Mr Imaging and Histologic Analysis. J Vasc Interv Radiol. 2000 Nov-Dec;11:1245–1255. doi: 10.1016/s1051-0443(07)61299-8. [DOI] [PubMed] [Google Scholar]
- 58.Therasse P, Eisenhauer EA, Verweij J. Recist Revisited: A Review of Validation Studies on Tumour Assessment. European Journal of Cancer. 2006;42:1031–1039. doi: 10.1016/j.ejca.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 59.Magill D, Mouli S, Sandberg J, Eifler AC, Belkind N, Guo Y, Nicolai J, Klein RA, Lewandowski RJ, Ryu RK, et al. Abstract No. 18: The Timing of Electroporation after Delivery of Therapeutic Nanoparticles Affects Drug Uptake in Vx2 Tumors. J Vasc Interv Radiol. 2011;22:S11–S12. [Google Scholar]
- 60.Gaba RC, Baumgarten S, Omene BO, van Breemen RB, Garcia KD, Larson AC, Omary RA. Ethiodized Oil Uptake Does Not Predict Doxorubicin Drug Delivery after Chemoembolization in Vx2 Liver Tumors. J Vasc Interv Radiol. 2012 Feb;23:265–273. doi: 10.1016/j.jvir.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 61.Thompson SM, Callstrom MR, Knudsen B, Anderson JL, Carter RE, Grande JP, Roberts LR, Woodrum DA. Development and Preliminary Testing of a Translational Model of Hepatocellular Carcinoma for Mr Imaging and Interventional Oncologic Investigations. J Vasc Interv Radiol. 2012 Mar;23:385–395. doi: 10.1016/j.jvir.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo Y, Klein R, Omary RA, Yang GY, Larson AC. Highly Malignant Intra-Hepatic Metastatic Hepatocellular Carcinoma in Rats. Am J Transl Res. 2010;3:114–120. [PMC free article] [PubMed] [Google Scholar]
- 63.Chen H, Wang L, Yeh J, Wu X, Cao Z, Wang YA, Zhang M, Yang L, Mao H. Reducing Non-Specific Binding and Uptake of Nanoparticles and Improving Cell Targeting with an Antifouling Peo-B-Pgammamps Copolymer Coating. Biomaterials. 2010 Jul;31:5397–5407. doi: 10.1016/j.biomaterials.2010.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]