Abstract
Frontal cortex - basal ganglia circuitry supports behavioral switching when a change in outcome information is used to adapt response patterns. Less is known about whether specific frontal cortex-basal ganglia circuitry supports behavioral switching when cues signal that a change in response patterns should occur. The present experiments investigated whether the prelimbic cortex and subthalamic nucleus in male Long-Evans rats supports cue-guided switching in a conditional discrimination test. Rats learned in a cross-maze that a start arm cue (black or white) signaled which of two maze arms to enter for a food reward. The cue was switched every 3-6 trials. Baclofen and muscimol infused into the prelimbic cortex significantly impaired performance by increasing switch trial errors, as well as trials immediately following a switch trial (perseveration) and after initially making a correct switch (maintenance error). NMDA receptor blockade in the subthalamic nucleus significantly impaired performance by increasing switch errors and perseveration. Contralateral disconnection of these areas significantly reduced conditional discrimination performance by increasing switch and perseverative errors. These findings suggest that the prelimbic area and subthalamic nucleus support the use of cue information to facilitate an initial switch away from a previously relevant response pattern.
Keywords: Prelimbic Cortex, Subthalamic Nucleus, Reversal Learning, NMDA, Muscimol, Executive Functioning
1. Introduction
Fundamental to daily living and survival is the ability to learn associations among stimuli, actions and outcomes, as well as switching between learned associations as environmental contingences change. These switches in behavior can be guided by changes in outcomes or by cues which predict the appropriate response pattern to be selected (Hikosaka and Isoda, 2010). The findings from several studies indicate that the rodent prefrontal cortex is involved in behavioral switching under certain conditions in which a learned response pattern is followed by a change in outcomes such that a new response pattern must be learned. In particular, infusion of local anesthetics, GABA agonists, or NMDA receptor antagonists into the prelimbic cortex impair extra-dimensional shifts when a response pattern based on particular stimulus information e.g. spatial cues, is no longer reinforced and a different pattern based on other stimulus information, e.g. odor cues, is now reinforced (Birrell and Brown, 2000; Floresco, Block, and Tse, 2008; Ng, Noblejas, Rodefer, Smith, and Poremba, 2007; Ragozzino, Kim, Hassert, Minniti, and Kiang, 2003; Ragozzino, Wilcox, Raso, and Kesner, 1999b; Stefani, Groth, and Moghaddam, 2003). Several of these studies also revealed that manipulations of the prelimbic cortex impair extra-dimensional shifts, by increasing perseveration of the previously learned response pattern in the initial trials of a shift, but not after a rat chooses the new correct response (Dias and Aggleton, 2000; Ragozzino, 2002; Ragozzino, Detrick, and Kesner, 1999a; Ragozzino et al., 2003; Ragozzino et al., 1999b). Taken together, the findings from past studies indicate that when information about outcomes must be used to enable a behavioral switch, the prelimbic cortex selectively supports extra-dimensional shifts by initially inhibiting preservation of the previously learned response pattern.
There has been significantly less examination of whether the prelimbic cortex supports behavioral switching when cues can be used to shift response patterns for an upcoming choice. Past studies found that prelimbic lesions alone or prelimbic and infralimbic lesions do not impair acquisition of a conditional discrimination task (Chudasama, Bussey, and Muir, 2001; Delatour and Gisquet-Verrier, 1999). Importantly, past conditional discriminations have pseudorandomly switched between conditions with at most 3 consecutive trials of the same contingency which may limit an establishment of a response set and any switch costs. A more recent study trained rats on a conditional discrimination task in which two different cues indicated making a distinct response to obtain a reward (if white noise press the left lever – if lights press the right lever). A cue was presented for 5-10 consecutive trials before a switch to presentations of the other cue occurred (Leenaars, Joosten, Zwart, Sandberg, Ruimschotel, Hanegraaf, Dematteis, Feenstra, and van Someren, 2012). This is similar to task-switching tests commonly administered to humans which cause an established choice pattern and induces a switch cost between blocks of trials (Monsell, 2003). After learning this cue-guided switch test, prelimbic inactivation with GABA agonists was found to impair performance for the switch trial, but not the fifth trial in each block (Leenaars et al., 2012). These findings suggest that the prelimbic cortex also supports behavioral switching when cue-information must be used to enable a switch from an established choice pattern in order to obtain a reward. However, while this study examined the first (switch) and fifth trial in each block, unclear is whether prelimbic inactivation also increases perseveration of the previous response pattern and/or impairs maintenance of the currently correct response pattern. Therefore, unknown is whether the prelimbic cortex supports a similar process when a change in outcomes signals a switch, e.g. inhibiting perseveration of a previously relevant response pattern, as when cues can be used to switch a response pattern.
The prefrontal cortex, including the prelimbic area, has extensive projections to basal ganglia structures and together these areas may act in a cooperative manner to facilitate behavioral switching when a change in outcomes or a change in cues guides a behavioral switch (Afsharpour, 1985; Chudasama and Robbins, 2006; Jahfari, Waldorp, van den Wildenberg, Scholte, Ridderinkhof, and Forstmann, 2011; Kehagia, Murray, and Robbins, 2010; Mailly, Aliane, Groenewegen, Haber, and Deniau, 2013). The subthalamic nucleus is one basal ganglia area that receives direct excitatory input from the prelimbic cortex that is mediated, at least in part, by NMDA receptors (Magill, Sharott, Bolam, and Brown, 2006; Maurice, Deniau, Glowinski, and Thierry, 1998a; Nambu, Tokuno, Hamada, Kita, Imanishi, Akazawa, Ikeuchi, and Hasegawa, 2000). Individual neurons in the non-human primate subthalamic nucleus show increased activity in response to a cue that signals when a switch from one response pattern to another will be rewarded (Isoda and Hikosaka, 2008). One possibility is that the rodent subthalamic nucleus also enables rapid and repeated behavioral switches when cue information can be used to proactively switch. Moreover, because the prelimbic cortex and subthalamic nucleus are interconnected and these two areas are involved in behavioral switching, both brain regions may need to be intact in order to facilitate cue-guided behavioral switching.
To determine whether the prelimbic cortex and subthalamic nucleus together are necessary to enable cue-guided behavior switching, the present experiments used a contralateral disconnection approach as in a past study (Chudasama, Baunez, and Robbins, 2003). This involved infusions of the GABA agonists, baclofen and muscimol into the prelimbic cortex (Leenaars et al., 2012) and the NMDA receptor antagonist, AP-5 into the subthalamic nucleus (Baunez and Robbins, 1999). The experiments further determined whether these pharmacological manipulations affected switch trial performance, initial perseveration of a previously relevant response pattern and/or maintenance of the currently relevant response pattern once selected.
2. Materials and Methods
2.1 Subjects
Adult, male Long–Evans rats weighing between 300 and 350 g at the time of testing served as subjects (n = 49). Rats were individually housed in plastic cages (26.5 × 50 × 20 cm) in a temperature (22°C) and humidity (30%) controlled environment and placed on a 12 h light/dark cycle (lights on at 7:00 A.M.). Rats were food restricted to 85–90% of their ad libitum body weight during the experiment, and water was available ad libitum. Animal care and use was in accordance with the National Institutes for Health Guide for the Care and Use of Laboratory Animals and approved by the University of Illinois at Chicago Institutional Laboratory Animal Care and Use Committee.
2.2 Apparatus
Training and testing occurred in a four arm cross maze made of black acrylic. Maze arms contained a base that was 10 cm wide × 55 cm long, two side walls that were 15 cm high by 55 cm long and a back wall that was 8 cm wide and 15 cm high. A 10 × 10 cm square base piece connected all four arms together. A circular food well (3.2 cm diameter and 1.6 cm deep) was located 3 cm away from the end of each arm. The maze was elevated 72 cm above the floor in a room with various extra-maze cues.
2.3 Surgery
Prior to behavioral training, all rats underwent stereotaxic surgery for bilateral implantation of guide cannulae aimed at both the prelimbic cortex and subthalamic nucleus. Thus, each rat had a total of 4 guide cannulae implanted. Although rats in Experiments 1 and 2 (see below) only received infusions into the either the prelimbic cortex or subthalamic nucleus, 4 guide cannulae were implanted in all rats to control for the possibility that effects observed in the contralateral disconnection study were partially due to the number of cannulae implanted. For surgery, rats received 0.2 mL atropine sulfate (250 ug/mL solution) 20 min prior to injection of sodium pentobarbital (50 mg/kg, i.p.). Twenty-two gauge stainless steel guide cannulae (Plastics One, Roanoke, VA) were implanted into the prelimbic cortex at a 15° angle. The stereotaxic coordinates were A-P +3.0; M-L ± 1.8; D-V -3.0 (mm). For the subthalamic nucleus, cannulae were implanted at a 10° angle. The stereotaxic coordinates were A-P -3.6; M-L ± 4.0; D-V -6.7. Cannulae were implanted at an angle because both the prelimbic cortex and subthalamic nucleus are located relatively medial allowing sufficient space for accurate placements. The coordinates were based on the stereotaxic atlas by Paxinos and Watson (1998). Four jeweler screws were positioned in the skull surrounding the cannulae and secured with dental acrylic (Stoetling, Wood Dale, IL). Stylets were placed into the guide cannulae to prevent clogging. During the surgical procedure, meloxicam (1 mg/kg) was administered to manage pain post-operatively. Rats recovered for 7 days after surgery before commencing behavioral training. For 5 days following surgery, rats were fed ad libitum and subsequently food restricted as described above. Following this period, subjects were handled approximately 10 minutes per day.
2.4 Training
One week after surgery, behavioral training commenced in the cross maze. Each arm of the maze was designated either “East”, “West”, “North” or “South”. Each rat received a training procedure in multiple phases. In the first phase, a rat was allowed to consume a quarter piece of Froot Loops cereal (Kelloggs, Battle Creek, MI) in each food well. A rat was also picked up after consuming cereal pieces to acclimate being handled in the maze as in past studies (Baker, Thompson, Sweeney, and Ragozzino, 2011; Brown, Baker, and Ragozzino, 2010). This stage of training lasted 3-7 sessions.
In the second training phase, the “North” and “South” arms always served as the choice arms. The “East” and “West” arms were pseudorandomly alternated to serve as start arms. In this phase, a plastic block was placed in either the “East” or “West” arm giving the maze a T-shape (Figure 1A). The stem arm served as the start arm and the other two arms served as choice arms. The choice arms always remained the same throughout training and testing. Each rat learned to use a visual cue (white or black) in the stem arm to choose the choice arm that contained a cereal reinforcement. The plastic block was moved pseudorandomly between the “East” and “West” arms such that a start arm was used a maximum of 2 consecutive trials. The visual cues were acrylic inserts that covered the walls and floor of the stem arm. Each visual cue color was always associated with one maze arm containing a cereal reinforcement. For example, if the cue was black, the reinforcement would be in the “North” arm, while a white cue indicated the reinforcement would be in the “South” arm. Once a rat made a choice, it was allowed to travel down to the end of the maze arm and explore the food well. If the choice was correct, it was allowed to consume the cereal piece after which it was picked up and placed on top of its home cage. The home cage was placed on a table adjacent to the maze. If an incorrect choice was made, a rat was allowed to proceed to the food well and examine it after which it was picked up and returned to the top of its home cage. In this second phase of training, a rat was exposed to a single cue in a 28 trial session. One session was given every day thus a rat was exposed to a particular visual cue every other day (session). This continued until a rat achieved at least 80% reinforcement on two consecutive days. This training phase lasted 5-9 sessions.
Figure 1.
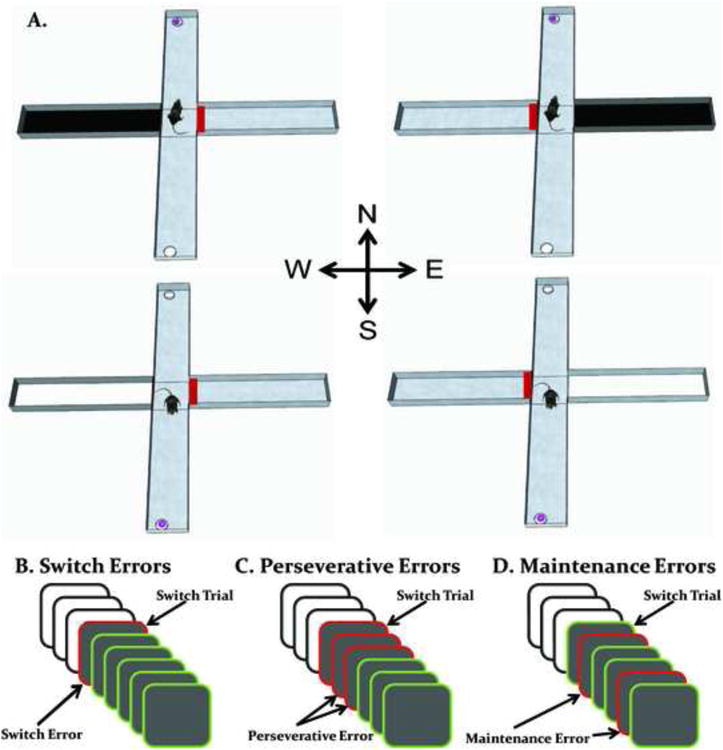
Representation of a modified cross maze used for cue-guided behavioral switching and the different types of errors within a trial block. A. In each trial, a black or white cue was placed in one of two possible start arms (west or east). The opposite arm contained a block (red rectangle) preventing entry into this arm. Rats learned based on the visual cue to enter the same arm to receive a cereal reward (pink ring in a foodwell). In this example, a black cue indicated a rat had to enter the north arm to receive a reward and a white cue indicated a rat had to enter the south arm to receive a reward. Cues were changed every 3-6 trials in a 57 trial test session. N = north, S = south, E = east and W = west. B. Switch errors occur when the first trial of a block is missed. C. Perseverative errors occur when, following a switch trial error, subsequent errors are committed until a correct response is made. D. Once a rat makes a correct response on a given block, any errors following that correct response are considered maintenance errors.
In the third phase of training, rats received both cues on a single daily session. Rats were trained for 40 trials with each cue presented for 10 consecutive trials in alternating blocks. Across sessions, the cue that was presented first in a session was randomized. After a rat achieved at least 80% correct for both black and white cue trials in a session, each visual cue block was reduced to 5 consecutive trials over a total of 40 trials (or eight blocks of alternating cues). A rat had to achieve a minimum of 80% correct for each cue type to advance to the final training phase. Rats required 7-16 sessions to reach criterion in this phase.
In the fourth and final training phase, a rat was tested for 57 trials in which a cue was switched every 3 to 6 trials. This involved a total of 12 switches in a session and each rat received three blocks each of 3, 4, 5, or 6 consecutive trials with an extra 3 trials at the end for the 12th switch. A 57 trial session contained approximately an equal number of presentations for each visual cue (28 or 29). A rat achieved criterion when it accurately discriminated 80% or greater for each visual cue trial type across a 57 trial session. This phase required 1-3 sessions for rats to reach criterion. After achieving criterion, the test phase began.
The test session procedure was the same as the final training phase described above. Specifically, the visual cue was changed every 3-6 trials indicating that a behavioral switch should occur for the upcoming response choice. The relatively short block length was chosen in order to emphasize the need to monitor task cues on every trial while also having a rat establish a response pattern prior to a switch (Leenaars et al., 2012). This is common in a proactive switch task in order to incur a switch cost such that performance is more difficult on a switch trial compared to that of non-switch trials (Hikosaka and Isoda, 2010; Hyafil, Summerfield, and Koechlin, 2009; Konishi, Chikazoe, Jimura, Asari, and Miyashita, 2005).
2.5 Microinfusion Procedure
Five minutes prior to a test session, a rat received an intracranial infusion. Infusions were delivered via 28 gauge injection cannulae which extended 1 mm below the guide cannulae. The injection cannulae were connected by polyethylene tubing to a 10 uL syringe (Hamilton Company, Reno, NV). An infusion into the prelimbic cortex consisted of either saline or GABA agonists baclofen and muscimol (Sigma Aldrich, St. Louis, MO). An infusion into the subthalamic nucleus consisted of either saline or the NMDA receptor antagonist, D-AP 5 (Tocris, Ellisville, MO). An infusion into the prelimbic cortex or subthalamic nucleus alone occurred bilaterally with a total volume of 0.25 uL per hemisphere at a rate of 0.15 uL/min by a microinfusion pump (74900 Series Cole Palmer, Vernon Hills, IL). Injection cannulae were left in place for an additional minute following the injection to allow for diffusion. A similar procedure was used for the contralateral and ipsilateral injection procedure except that a unilateral infusion was made in each brain region. Prior to testing, rats remained in their home cages for five minutes after completion of the injection procedure. As in past studies (Brown et al., 2010; McCool, Patel, Talati, and Ragozzino, 2008), the day prior to the first test procedure, an injection cannula was lowered into each guide cannula and left in place for two minutes. This ensured that any effects observed on the first test day of testing were not due to the initial acute damage caused by the injection cannulae extending 1mm beyond the guide cannulae.
2.6 Switch Costs in Visual Cue – Place Conditional Discrimination
The conditional discrimination test required rats to establish a response based on learned visual cue – place associations and use cue information proactively to switch a response choice. If the procedure led rats to establish a response pattern within a block, then this should lead to a greater switch cost as displayed by a larger percentage of switch errors compared to non-switch errors as observed previously (Leenaars et al., 2012). To determine this, performance in the visual cue-place discrimination test was examined in the vehicle treatment for the percentage of switch trial errors committed vs. the percentage of non-switch trial errors across experiments. In a test session, the switch error percentage was based on a total of 12 switch trials and the non-switch error percentage was based on the remaining 45 trials.
2.7 Experiment 1: The effect of prelimbic cortex inactivation on performance of a visual cue-place conditional discrimination
The visual cue-place conditional discrimination test lasted 57 trials with a visual cue switch occurring every 3-6 trials for a total of 12 switches per test. Five minutes prior to a test session, a rat received a bilateral infusion of either saline (Veh), baclofen 0.005uM-muscimol 0.018uM (Low dose) or baclofen 0.05uM-muscimol 0.18uM (High dose). Doses were selected based on previous studies in which baclofen and muscimol were administered intracranially to affect retroactive switching in a dose-dependent manner (Brown et al., 2010; Floresco, Ghods-Sharifi, Vexelman, and Magyar, 2006a). The order of treatments administered was counterbalanced across rats. There were a total of 8 rats included in the final analysis based on accurate cannula placements out of a 12 total tested. Each rat received each treatment with a minimum of two days between test sessions. The day after testing, a rat received no testing. The following day, each rat received a test session, but did not receive an intracranial infusion prior to the test. This procedure was carried out to ensure that there were no lasting effects of a given treatment on the rat's ability to discriminate between the cues. If a rat was unable to perform the discrimination with at least 80% accuracy on each cue, additional sessions were given until criterion was achieved (This occurred only twice with one rat. One time a rat required a single additional session and the other time required two additional sessions to reach criterion). Once a rat had demonstrated the ability to discriminate accurately, the following day another test was performed. This procedure continued until a rat received all three treatments.
In each test session the percent correct was calculated. Similar to past behavioral switching studies (Baker et al., 2011; Dias and Aggleton, 2000; Floresco, Magyar, Ghods-Sharifi, Vexelman, and Tse, 2006b), an analysis of the errors committed during each block of trials was calculated to determine whether a treatment affected the initial switch, perseveration of the previously correct response after the switch and/or the inability to maintain the currently correct response. Errors were separated into switch, perseverative, and maintenance errors similar to that as in past studies (Baker et al., 2011; Mohler, Baker, Gannon, Jones, Shacham, Sweeney, and Ragozzino, 2011). A switch error was defined as a rat failing to initially switch to the currently relevant response when the visual cue changed (Figure 1B). Perseverative errors were only committed in a block in which an initial switch error occurred. Specifically, perseverative errors were committed when any subsequent errors were made after a switch error and prior to making a correct response in that block (Figure 1C). Once a rat successfully switched from the previous response to the currently relevant one, it was no longer possible to commit a perseverative error. However, if a rat made a correct response in a block and reverted back to the other response choice in that same block, then this constituted a maintenance error (Figure 1D).
Because the task has several different blocks that vary in length, the total number of errors does not provide information about the degree to which certain errors occurred. For example, there may be a significant increase in the total number of perseverative errors following a treatment that does not result because such an error was committed across more trial blocks, but because more perseverative errors were committed in a single or small number of trial blocks. To understand the degree to which certain errors occurred, an analysis was carried out to determine the percentage of blocks in which a particular error was committed based on the total possible blocks such an error was possible. Specifically, the percent score was based on the total number of blocks in which a particular error occurred divided by the total number of possible blocks in which a particular could occur. For perseverative errors, a perseverative error could only occur in blocks in which a switch error was committed. For maintenance errors, an error could only occur when a correct choice was made prior to the last trial in a block. For example, if a rat only committed three switch errors and only made a perseverative error in one of these blocks then the percent of perseverative error blocks would be 33.3%.
The total number of errors also does not provide information about the consistency of errors across a test session. For example, a certain error type may preferentially occur early in a test session, but not late in a test session. To more fully understand the consistency in which errors were committed, the number of errors committed in the first half were compared to those in the second half.
One possibility is that prelimbic inactivation produces a conditional discrimination deficit principally unrelated to switch, perseveration or maintenance errors, but alternatively biases a rat to preferentially use an egocentric response strategy (e.g. always turn right) or an allocentric place strategy that was largely independent of the relevant cue-place response. To determine this, turn bias and place bias scores were measured for each treatment. Turn bias scores were calculated by determining a percentage of the number of errors committed to the more common egocentric response divided by the total number of errors. For example, if a rat made a total of 12 errors and 9 resulted because a rat turned left when it should have turned right into the correct location, then it would have a percent bias score of 75%. Likewise, a place bias score was calculated by determining a percentage of errors for the more common place location divided by the total number of errors. For example, if a rat made a total of 10 errors and 7 resulted because a rat entered the south arm when it should have entered the north arm, then it would have a percent bias score of 70%.
2.8 Experiment 2: The effect of NMDA receptor blockade into the subthalamic nucleus on performance in a visual cue-place conditional discrimination
The conditional discrimination was the same as described in Experiment 1. A separate group of rats (n = 14) were tested in this experiment with 7 rats included in the final analysis based on accurate cannula placements. Five minutes prior to a test session, a rat received a bilateral infusion of either saline (Veh), D-AP 5 0.2uM (Low dose), and D-AP 5 10uM (High dose) into the subthalamic nucleus. Doses were based on previous studies in which NMDA receptor blockade in the subthalamic nucleus or striatum were shown to disrupt behavior (Baunez and Robbins, 1999; Palencia and Ragozzino, 2004). All other aspects of this experimen were as described in Experiment 1.
2.9 Experiment 3: The effect of contralateral disconnection and ipsilateral disconnection of the prelimbic cortex and subthalamic areas on performance of a visual cue-place conditional discrimination
To determine whether a bilaterally intact prelimbic cortex and subthalamic nucleus are necessary for performance of the visual cue-place conditional discrimination, a contralateral disconnection of the two brain areas was carried out. As a control, the effect of an ipsilateral disconnection of the prelimbic cortex and subthalamic thalamic nucleus was also investigated. The test procedure was the same as described in Experiment 1. A separate group of 13 rats were tested in this experiment with a total of 7 rats included in the final analysis based on accurate cannula placements. For this study there were six test sessions that involved intracranial infusions. The injections were counterbalanced for hemisphere injected as well as treatment received across rats. This design led to a maximum of four injections through any one cannula for a rat.This total number of injections into a given cannula is comparable with past studies utilizing a microinjection procedure with these drugs (Barker and Warburton, 2008; Churchwell and Kesner, 2011; Uekita and Okaichi, 2009; Winters, Bartko, Saksida, and Bussey, 2010). The contralateral disconnection manipulation involved a unilateral infusion into the prelimbic cortex and a unilateral infusion into the opposite hemisphere of the subthalamic nucleus. Doses for each brain area remained the same as in Experiments 1 and 2 (e.g. prelimbic low dose was the same as the low dose of baclofen and muscimol injected during ipsilateral and contralateral treatments). The three contralateral disconnection treatments were as follows: 1) Contralateral injection of saline (Contra Veh); 2) Contralateral low doses of baclofen/muscimol into the prelimbic cortex and D-AP5 into the subthalamic nucleus (Contra Low) and 3) prelimbic cortex baclofen/muscimol and subthalamic nucleus high doses (Contra High). The ipsilateral disconnection manipulation involved a unilateral infusion into the prelimbic cortex and a unilateral infusion into the same hemisphere of the subthalamic nucleus. The three ipsilateral disconnection treatments were as follows: 1) prelimbic cortex-subthalamic nucleus injection of saline (Ipsi Veh); 2) Ipislateral injection of the prelimbic and subthalamic area low doses (Ipsi Low) and 3) high doses of drug into the prelimbic cortex and subthalamic nucleus (Ipsi High). All aspects of the testing procedure were the same as in Experiments 1 and 2.
2.10 Experiment 4: The effect of prelimbic cortex inactivation, NMDA receptor blockade of the subthalamic nucleus, or contralateral disconnection of the prelimbic cortex-subthalamic nucleus in a non-switch cue-association test
If pharmacological manipulation of the prelimbic cortex, subthalamic nucleus or contralateral disconnection of these structures impairs conditional discrimination performance, this may result because of a basic impairment in discrimination performance. To determine this, another group of rats were tested in a discrimination task in which only one of the cues was presented throughout a given session. The training procedure was similar as described above except that training was limited to the procedure in which rats receive a single visual cue per session. Thus, rats were trained to discriminate between the different visual cues but this occurred across sessions and not within a session. Once rats completed two consecutive days of training at 80% or higher accuracy, they were advanced to the test phase. The test was identical to the training phase in that rats were tested on a single visual cue discrimination for 28 trials. Rats (n = 10) received a total of six intracranial injections in this experiment with a total of 7 rats included in the final analysis based on accurate cannula placements. Each visual cue was used for three test sessions. The order of treatments was pseudorandomly administered across rats. Each rat received the following treatments: 1) bilateral saline infusion into the prelimbic cortex (PL Veh); 2) bilateral baclofen/muscimol high dose infusion into the prelimbic cortex (PL High); 3) bilateral saline infusion into the subthalamic nucleus (STN Veh); 4) bilateral D-AP5 high dose infusion into the subthalamic nucleus(STN High); 5) contralateral saline infusion into the prelimbic cortex and subthalamic nucleus (Contra Veh), and 6) contralateral baclofen/muscimol high dose infusion into the prelimbic cortex and D-AP5 high dose infusion into the subthalamic nucleus (Contra High). The same procedure was employed for the interval between test sessions as described previously.
2.11 Histology
After completion of behavioral testing, rats were given an overdose of sodium pentobarbital. Rats were intracardially perfused with 0.9% phosphate buffered saline followed by 4% formaldehyde solution. The brain was removed and stored in formaldehyde until sectioning. Brains were frozen and cut into 50-μm coronal sections on a cryostat. Sections were immediately mounted on slides, dried, and then stained with cresyl violet. Placements were then verified with reference to the stereotaxic atlas of Paxinos and Watson (1998).
2.12 Statistical Analysis
In experiments 1-4, a repeated measures design (treatment-by-subjects) was used as each subject received all treatments in an experiment. Specifically, separate repeated measures ANOVA was used to test the effects of drug treatments on performance accuracy, switch errors, perseverative errors, and maintenance errors. Turn bias and place bias scores were analyzed with repeated measures ANOVAs as well. A significant treatment effect was followed by Tukey's post hoc tests to determine significant differences between treatments. Switch cost analysis was carried out by using paired student's t-test comparing percent error rates on switch vs. non switch trials.
3. Results
3.1 Histology
Rats included in the behavioral analysis were restricted to those who had cannulae placements in the prelimbic cortex and subthalamic nucleus. Figure 2 shows placements of cannula tip locations for the prelimbic area (Figure 2A) and subthalamic nucleus (Figure 2B) that were included across the different experiments. Prelimbic cannula placements were primarily located 2.7-3.8mm anterior to bregma. Subthalamic nucleus cannulae were principally located in the portion of the nucleus located 3.6-4.2mm posterior to bregma.
Figure 2.
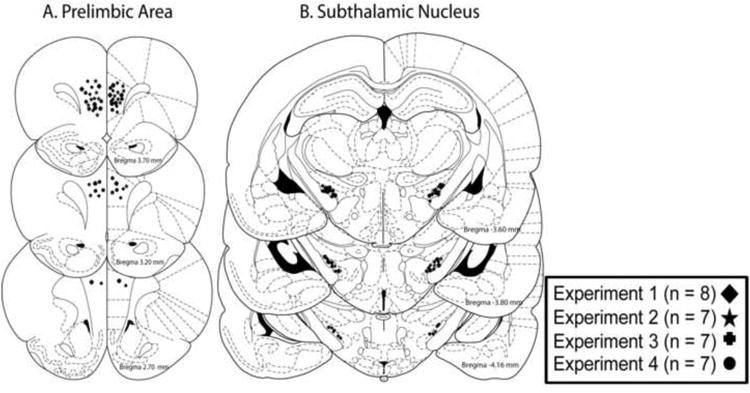
Cannula tip placements in the prelimbic cortex and the subthalamic nucleus in the various experiments. Only placements within the respective areas were included in the final analyses. Final numbers for each experiment are shown. A. Representation of cannula placements in the prelimbic cortex. B. Representation of cannula placements targeting the subthalamic nucleus. Adapted from The Rat Brain in Stereotaxic Coordinates (Paxinos and Watson, 1998).
Twenty rats were excluded from the analyses because of misplacements. In Experiments 1-4, four rats were excluded due to placements outside the prelimbic area. Three misplacements were anterior to the prelimbic cortex located in the medial orbital subregion and one rat had ventral cannula placements located in the infralimbic cortex. One rat who had an anterior cannula placement exhibited motor deficits when infused with the high dose of baclofen/muscimol and could not complete testing. This was the only rat with a cannula misplacement for the prelimbic cortex that exhibited any motor problems. There was a total of 16 rats excluded from analyses in Experiments 1-4 because of cannula placements outside the subthalamic nucleus. Five rats had unilateral (n = 2) or bilateral placements (n = 3) anterior to the subthalamic nucleus in the internal capsule/subincertal nucleus. Six rats had unilateral (n = 2) or bilateral (n = 4) placements dorsal to the subthalamic nucleus in the zona incerta. One rat had a unilateral placement in the substantia nigra pars reticulata that exhibited motor problems under the highest dose of D-AP 5 and could not complete testing. This was the only rat who had a subthalamic nucleus cannula misplacement that exhibited motor impairments. Four rats had bilateral placements ventral to the subthalamic nucleus located in the ventromedial internal capsule.
3.2 Switch Costs in Visual Cue – Place Conditional Discrimination
Figure 3 shows the results from vehicle treatment collapsed across experiments for switch trials errors vs. non-switch trial errors. Following vehicle treatment, the percent error rate for switch trials was approximately double (25.86 ± 2.35% error rate) compared to that of non-switch trials (14.38 ± 1.09%). A paired t-test revealed that the difference in switch trials errors vs. non-switch trial errors was significant, t(28) = 5.01, p < 0.01.
Figure 3.
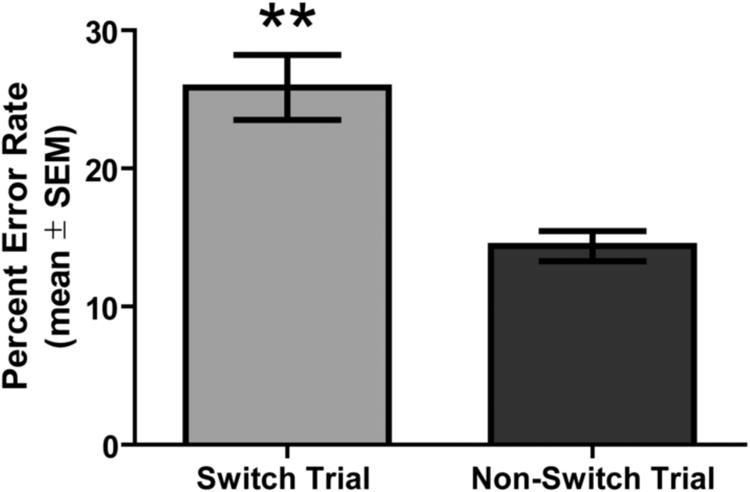
Switch costs incurred during performance of the visual cue-place conditional discrimination task in vehicle-treated rats. All saline treatments across experiments 1-3 were collapsed into one group to examine performance (mean ± SEM) on switch vs. non-switch trials. The percent error rate for switch and non-switch trials was calculated based on the number of errors divided by the total number of trials of that type. Vehicle-treated rats were more likely to commit an error on switch vs. non-switch trials. **p < 0.01.
3.3 Experiment 1: The effect of prelimbic cortex inactivation on performance of a visual cue-place conditional discrimination
Rats following all three treatments into the prelimbic cortex required approximately 30 minutes to complete a test session. The difference in time to completion among the treatments was not significant, F(2,14) = 0.02, p > 0.05.
Behavioral performance following prelimbic inactivation is shown in Figure 4. Vehicle-treated rats made the correct choice on 84.25 ± 1.67% of trials (mean ± SEM). The low dose of baclofen/muscimol led to a similar accuracy (mean = 81.38 ± 1.58%) as vehicle controls. However, the high dose, of baclofen/muscimol infused into the prelimbic cortex reduced performance to a mean of 60.50 ± 2.77% correct. A repeated measures ANOVA revealed a significant effect of treatment on performance accuracy, F(2,14) = 61.90, p < 0.01. Tukey HSD post hoc tests indicated that the high dose of baclofen/muscimol led to a significant reduction in performance accuracy compared to that of vehicle or the low dose of baclofen/muscimol (p values < 0.01).
Figure 4.
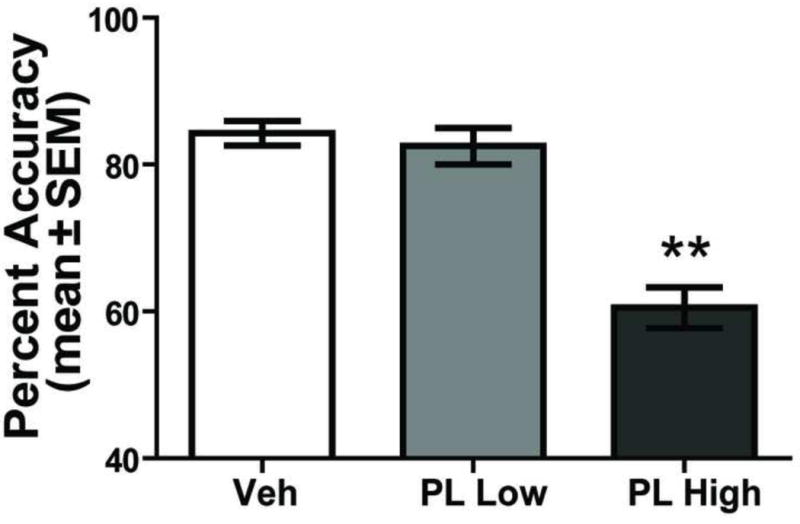
PL inactivation impairs conditional discrimination performance. Each rat received a bilateral injection into the PL area of saline (Veh), baclofen 0.005uM-muscimol 0.018uM (PL Low), and baclofen 0.05uM-muscimol 0.18uM (PL High) in a random order 5 min before testing. Percent accuracy (mean ± SEM) in the conditional discrimination is significantly impaired in the PL High treatment compared with Veh and PL Low dose. **p < 0.01.
An analysis of the errors committed in the conditional discrimination test (Figure 5A-C) revealed that there was a significant difference in switch errors among the treatment conditions, F(2,14) = 18.38, p < 0.01. The high dose of baclofen/muscimol significantly increased switch errors compared to that of the vehicle and the low dose treatments (p values < 0.01). There was also a significant effect of treatment on perseverative errors, F(2,14) = 4.66, p < 0.05. The high dose of baclofen/muscimol increased perseveration compared to the vehicle treatment (p values < 0.05). The low dose was not significantly different from any other treatment. Comparable to switch and perseverative errors, there was also a significant treatment effect for maintenance errors, F(2, 14) = 37.00, p < 0.01. The high dose treatment significantly elevated maintenance errors compared to that of the vehicle and low dose treatments (p values < 0.01). Thus, prelimbic inactivation at the high dose impaired performance by increasing switch, perseverative, and maintenance errors.
Figure 5.
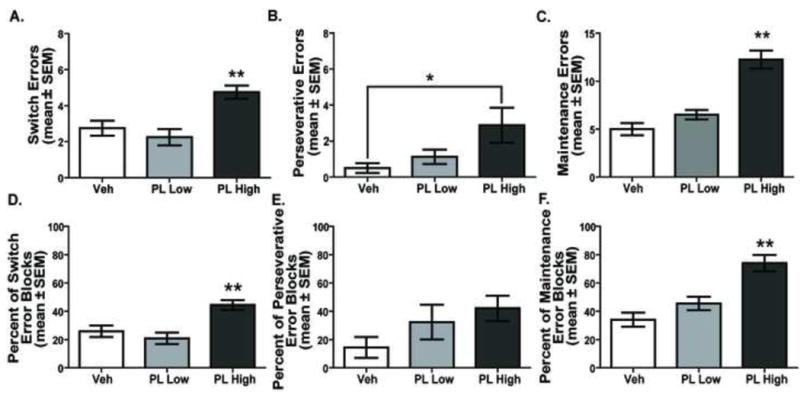
Distribution of errors in the visual cue-place conditional discrimination task following PL inactivation. A. Total switch errors. The number of switch errors increased in the PL High treatment compared to that of all other treatments. **p < 0.01. B. The PL High dose led to significantly more perseverative errors than Veh treatment. *p < 0.05. C. Maintenance errors committed. PL High dose resulted in significantly more maintenance errors than the PL Low and Veh doses. **p < 0.01. D. Percent of switch error blocks committed. PL High dose led to a significantly higher percentage of switch errors blocks compared with that of Veh and PL Low dose. **p < 0.01. E. Percent of perseverative error blocks. Overall there was an effect of treatment on the percentage of error blocks committed (p < 0.05). Post hoc comparisons did not reveal any differences between specific treatments. F. Percent of maintenance error blocks. PL High dose treatment led to a significantly higher percentage of maintenance errors compared to that of PL Low dose and Veh treatment. **p < 0.01.
To further understand the degree to which certain errors occurred across blocks, a percent score was calculated on the total number of blocks in which a particular error occurred divided by the total number of possible blocks in which a particular error could occur. This was analyzed for all error types across all treatments. A comparable pattern of results for percent of error blocks was observed as that of the total error measures (see Figure 5D-F). In particular, there was a significant treatment effect on percent of switch error blocks, F(2, 14) = 17.63, p < 0.01. Post-hoc tests revealed that the high dose of baclofen/muscimol treatment significantly increased the percentage of blocks in which a switch error was committed compared to that of vehicle or the low dose treatment (p's < 0.01). There was not a significant difference in the percentage of switch error blocks between the vehicle and low dose treatments (p > 0.05). There was a trend for the percentage of perseverative error blocks to be increased by the high dose treatment (mean = 42.1%) compared to that of vehicle (14.3%). The low dose treatment (32.2%) also exhibited an increase compared to that of vehicle treatment. However, the difference in percentage of perseverative error blocks across treatments was not significant, F(2,14) = 2.27, p >0.05. For the percent of maintenance error blocks, there was a significant treatment effect, F(2, 14) = 20.06, p < 0.01. Post-hoc tests indicated that the high dose of baclofen/muscimol treatment significantly increased the percentage of blocks in which a maintenance error was committed compared to that of vehicle or the low dose treatment (p's < 0.01). There was not a significant difference in the percentage of maintenance error blocks between the vehicle and low dose treatments (p > 0.05).
To understand the consistency in which errors were committed across a session for the high dose treatment, the number of errors committed in the first half were compared to those in the second half (see supplemental figure 1). The high dose of baclofen/muscimol did not lead to a significant difference in the number of switch, t(7) = 1.1, p > 0.05, perseverative, t(7) = 0.36, p > 0.05 or maintenance errors, t(7) = 0.01, p > 0.05 in the first half of trials compared to the second of half of trials.
An analysis was also carried out to determine whether a treatment biased a rat to preferentially use an egocentric response strategy (e.g. always turn right) or an allocentric place strategy that was largely independent of the relevant cue-guided choice patterns. A repeated measures ANOVA revealed a significant effect of treatment on turn bias, F(2, 14) = 3.77, p < 0.05. Specifically, there was a significantly greater turn bias for the high dose treatment (mean = 0.83 ± 0.06) compared to that of the low dose (mean = 0.67 ± 0.03) [p < 0.05]. The difference in the turn bias score between the high dose and vehicle treatment (mean = 0.71 ± 0.04) approached significance (p = 0.07). In contrast, there was not a treatment effect for the place bias scores, F(2, 14) = 2.70, p > 0.05.
3.4 Experiment 2: The effect of NMDA receptor blockade into the subthalamic nucleus on performance in a visual cue-place conditional discrimination
Rats following all three treatments into the subthalamic nucleus required approximately 30-35 minutes to complete a test session. The difference in time to completion among the treatments was not significant, F(2,12) = 2.45, p > 0.05.
The results on D-AP5 infusions into the subthalamic nucleus are shown in Figure 6. An analysis on percent correct trials revealed that there was a significant effect of drug treatment, F(2, 12) = 21.95, p < 0.01. Post-hoc tests revealed that the high dose of D-AP5 (mean = 66.29% ± 2.77) significantly reduced behavioral performance compared to that of vehicle (mean = 82.86% ± 1.71) or the low dose of D-AP5 (mean = 80.86% ± 1.88) [p values < 0.01].
Figure 6.
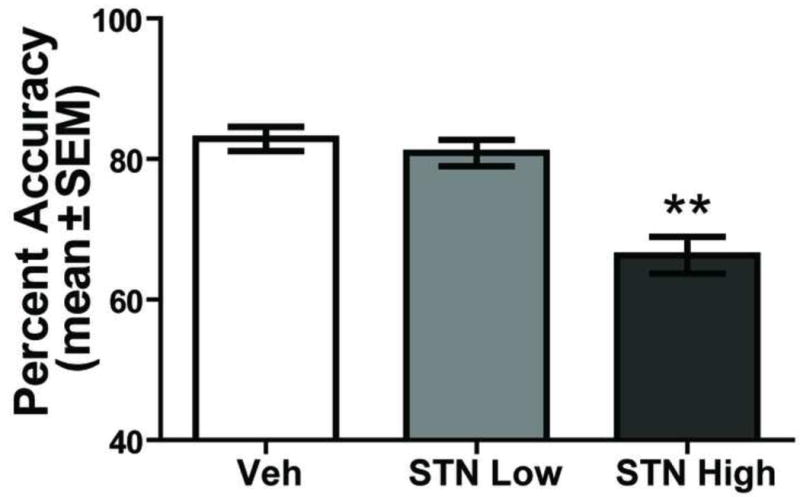
NMDA receptor blockade in the STN impairs visual cue-place conditional discrimination performance. Each rat received a bilateral injection into the STN of saline (Veh), D-AP5 2uM (STN Low), and D-AP5 10uM (STN High) in a random order 5 min prior to testing. Percent accuracy (mean ± SEM) in the STN High condition was significantly impaired compared to that of Veh and STN Low treatments. **p < 0.01.
Figure 7A-C illustrates the different errors committed following the various treatments into the subthalamic nucleus. A further analysis of task performance indicated that there was a significant treatment effect for switch errors, F(2, 12) = 22.20, p < 0.01. The high dose of D-AP5 significantly increased switch errors compared to that of vehicle treatment and the low dose of D-AP5 (p values < 0.01). Likewise, an effect of treatment was observed on perseverative errors, F(2, 12) = 8.29, p < 0.01. The high dose of D-AP5 significantly increased perseveration compared to that of vehicle (p < 0.05) and low dose treatments (p < 0.01). In contrast, there was not a significant treatment effect for maintenance errors, F(2,12) = 1.74, p > 0.05.
Figure 7.
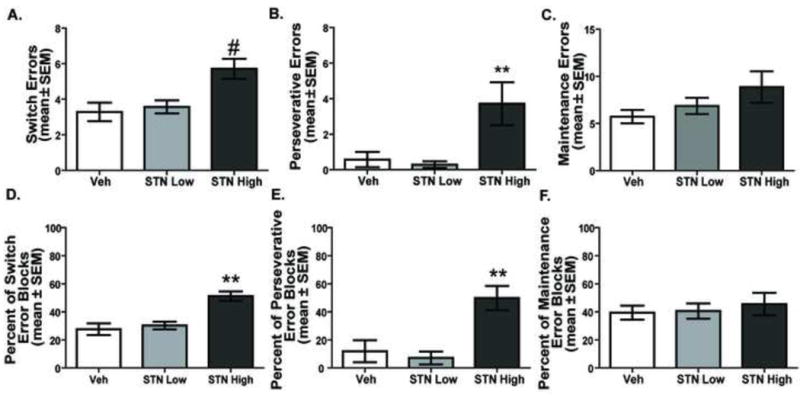
Distribution of errors for STN NMDA receptor blockade in the visual cue-place conditional discrimination task. A. Switch errors. The STN High condition led to more switch errors than the STN Low and Veh treatments. #p < 0.05 vs. Veh, p < 0.01 vs. STN Low. B. Perseverative errors committed. The STN High condition significantly increased perseverative errors compared to that of STN Low or Veh treatments. **p < 0.01. C. Maintenance errors committed. No differences were observed in the number of maintenance errors committed among the treatments. D. Percent of switch error blocks. STN High treatment led to a significantly higher percentage of switch error blocks versus Veh and STN Low treatment. **p < 0.01. E. Percent of perseverative error blocks. STN High treatment led to a significantly higher percentage of perseverative error blocks then either Veh or STN Low dose treatment. **p < 0.01. F. No differences were observed among treatments in the percent of maintenance error blocks performance.
The results from the percentage of error blocks are shown in Figure 7D-F. The pattern of findings matched that for the total errors. An examination of the percentage of error blocks following the different treatments revealed that there was a significant treatment effect for switch error blocks F(2,12) = 29.56, p < 0.01; a significant treatment effect for perseverative error blocks, F(2,12) = 13.00, p < 0.01, but not a significant treatment effect for maintenance error blocks, F(2,12) = 0.28, p > 0.05. Further analyses indicated that the high dose treatment significantly increased the percentage of switch and perseverative error blocks compared to that of vehicle and the low dose treatment (p's < 0.01). However, there was not a significant difference in percent switch error blocks or percent perseverative error blocks between the vehicle and low dose treatment groups (p's > 0.05).
Although the high dose of D-AP5 significantly increased the total switch and perseverative errors, as well as the percent of switch and perseverative error blocks, it did not affect the number of errors committed in the first half compared to those in the second half (see supplemental Figure 2). The high dose of D-AP5 did not lead to a significant difference in the number of switch, t(6) = 0.42, p > 0.05, perseverative, t(6) = 0.18, p > 0.05 or maintenance errors, t(6) = 0.01, p > 0.05 in the first half of trials compared to the second of half of trials. Additionally, there was no effect of treatment on turn bias scores, F(2, 12) = 0.04, p > 0.05, or place bias scores, F(2, 12) = 3.41, p > 0.05.
3.5 Experiment 3: The effect of contralateral disconnection and ipsilateral disconnection of the prelimbic cortex and subthalamic areas on performance of a visual cue-place conditional discrimination
Rats following all six treatments for the ipsilateral and contralateral manipulations required approximately 30-35 minutes to complete a test session. The difference in time to completion among the treatments was not significant, F(5,30) = 0.62, p > 0.05.
The results from contralateral disconnection and ipsilateral disconnection of the prelimbic cortex and subthalamic nucleus are shown in Figure 8. There was a significant treatment effect for percent accuracy, F(5, 30) = 7.75, p < 0.01. The Contra High dose significantly reduced accuracy compared to that of all other contralateral and ipsilateral treatment groups (p values < 0.01).
Figure 8.
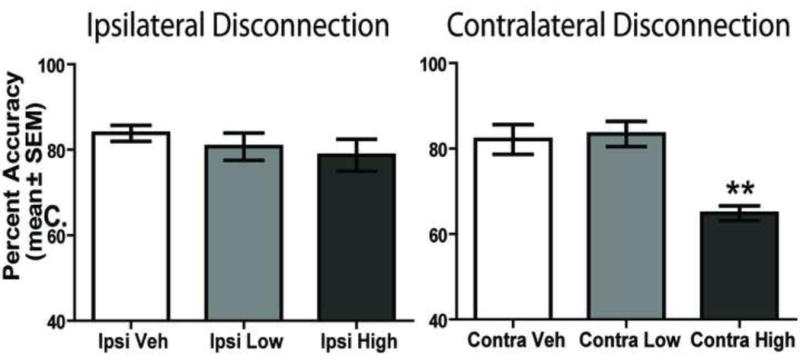
Contralateral disconnection, but not ipsilateral disconnection, of the PL-STN nucleus impairs visual cue-place conditional discrimination performance. Each rat received 3 treatments involving ipsilateral injections of saline (Ipsi Veh), combined low dose of baclofen/muscimol in PL and D-AP5 in the STN (Ipsi Low) and combined high dose of baclofen/muscimol in PL and D-AP5 in the STN (Ipsi High). Each rat also received 3 treatments consisting of contralateral injections of saline (Contra Veh), combined low dose of baclofen/muscimol in PL and D-AP5 in the STN (Contra Low) and combined high dose of baclofen/muscimol in PL and D-AP5 in the STN (Contra High). A. Percent accuracy (mean ± SEM) during the switching test. The Contra High dose significantly impaired performance on the task compared with that of all other treatments. **p < 0.01.
An analysis of the errors revealed that there was a significant effect of drug treatment on switch errors, F(5, 30) = 2.73, p < 0.05 (Figure 9A-C). Contra High treatment led to significantly more switch errors compared to that of the Contra Low and Vehicle treatment conditions (p values < 0.05). There was also a significant effect of drug treatment on perseverative errors, F(5, 30) = 15.62, p < 0.01. Post-hoc tests revealed that the Contra High treatment led to significantly more perseverative errors compared to that of all other treatment conditions (p values < 0.01). However, no effect of drug treatment was found on maintenance errors, F(5, 30) = 1.68, p > 0.05. The contralateral disconnection treatment produced a similar pattern of results for percent of error blocks as observed for the total error measures (see Figure 9D-F). For switch errors, the Contra Veh and Contra Low treatment had a percent error block score of approximately 20% while the Contra High treatment had a percent score of approximately 40%. Although, all the Ipsi treatments led to percent scores of approximately 30%. There was a significant treatment effect on percent of switch error blocks, F(5,30) = 2.72, p < 0.05. Contra High treatment significantly increased the percentage of blocks in which a switch error was committed compared to that of Contra Veh (p < 0.05). No other comparisons were significant. For perseverative errors, the Contra Veh and Contra Low treatment had a percent error block score between 10- 20% while the Contra High treatment had a percent score of approximately 50%. The difference in the percentage of perseverative error blocks among the treatments was significant, F(5,30) = 2.96, p < 0.05. However, the post-hoc analyses revealed that there were no significant differences between any of the comparisons. As observed with total errors, the difference in percent maintenance error blocks among the treatments was not significant, F(5,30) = 2.44, p > 0.05.
Figure 9.
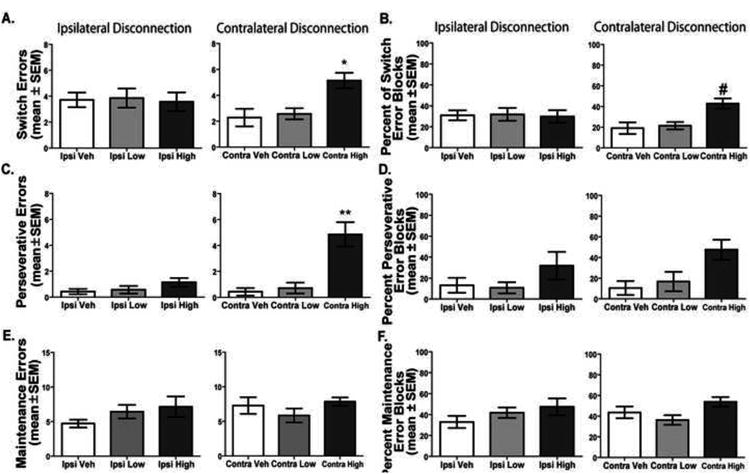
Distribution of errors with ipsilateral and contralateral disconnection in the visual cue-place conditional discrimination task. A. Switch errors (mean ± SEM) during the visual cue-place discrimination task. Contra High dose lead to significantly more switch errors than Veh and Low dose conditions. *p < 0.05. B. Percent of switch block errors (mean ± SEM). Contra High dose led to a significantly higher percentage of switch errors compared to the Contra Veh treatment. # = p < 0.05 Contra High vs. Contra Veh. C. Perseverative errors (mean ± SEM) committed. Treatment with the Contra High dose significantly elevated perseverative errors compared to that of all other treatments. **p < 0.01. D. Percent of perseverative error blocks (mean ± SEM). E. Maintenance errors (mean ± SEM) committed. No differences were observed among treatments on the number of maintenance errors. F. Percent of maintenance error blocks (mean ± SEM). No differences were observed between treatments on the percentage of maintenance error opportunities.
The contralateral disconnection did not affect the number of errors committed in the first half compared to those in the second half (see supplemental Figure 3). The Contra High did not lead to a significant difference in the number of switch, t(6) = 0.36, p > 0.05, perseverative, t(6) = 1.18, p > 0.05 or maintenance errors, t(6) = 0.67, p > 0.05 in the first half of trials compared to the second of half of trials. Additionally, there was not a significant effect for either turn bias, F(5, 30) = 0.67, p > 0.05 or place bias, F(5, 30) = 0.92, p > 0.05.
3.6 Initial Block Performance In the Visual Cue - Place Conditional Discrimination
Experiments 1-3 found that prelimbic inactivation, NMDA receptor blockade in the subthalamic nucleus, and contralateral disconnection of the prelimbic and subthalamic areas impaired performance in the conditional discrimination task. One possibility is that the deficits arose because the drug manipulations impaired expression of the learned visual cue-place associations and/or general discrimination performance as opposed to behavioral switching. If this was the case, then a deficit should emerge within the first block before a rat has to switch. To assess this, performance on the first block of trials during experiments 1-3 were compared among treatments to examine whether performance is affected before the initial switch in a test session. Repeated measures ANOVAs revealed that no significant effect of drug treatment was observed in the first block of trials for Experiment 1 (F(2, 14) = 0.64, p > 0.05), Experiment 2 (F(2, 12) = 0.12, p > 0.05), or Experiment 3, F(5, 30) = 0.12, p > 0.05. Thus, initial discrimination performance was not significantly affected by treatments that impaired behavioral switching.
3.7 Experiment 4: The effect of prelimbic cortex inactivation, NMDA receptor blockade of the subthalamic nucleus, or contralateral disconnection of the prelimbic cortex- subthalamic nucleus in a non-switch cued-association test
To further examine whether treatment effects in the conditional discrimination test resulted from a more fundamental deficit in discrimination performance, non-switch discrimination performance was tested under all effective treatments (Figure 10). A repeated measures ANOVA revealed there was no significant effect of treatment on percent accuracy during non-switch discrimination performance, F(5, 30) = 2.15, p > 0.05. Thus, prelimbic inactivation, NMDA receptor blockade in the subthalamic nucleus or contralateral disconnection had no effect on performance of a learned cue-place association.
Figure 10.
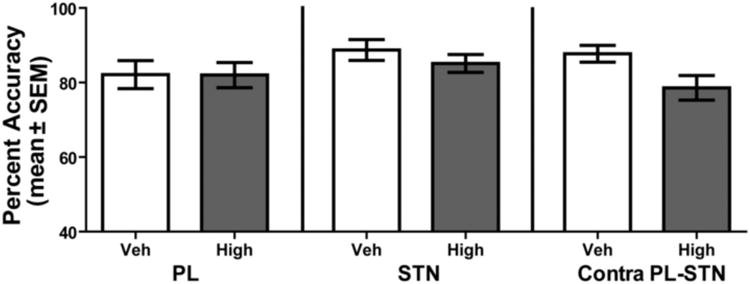
Prelimbic cortex (PL), subthalamic nucleus (STN), and contralateral disconnection of the prelimbic-subthalamic nucleus (Contra PL-STN) does not affect performance during a non-switch discrimination. Each rat received 6 treatments during 6 separate non-switch discriminations. These included the following: Two PL infusions involving a saline injection (PL Veh) or baclofen/muscimol (PL High); Two STN infusions involving a saline control (STN Veh) or the NMDA receptor antagonist D-AP5 (STN High); Two contralateral infusions of the PL-STN occurred involving a saline injection (PL-STN Veh), and baclofen/muscimol high dose in the PL and D-AP5 high dose in the STN (PL-STN High). No differences in performance were observed between treatments in a non-switch discrimination.
3.8 The effect of drug infusions in rats with cannula misplacement on visual cue-place conditional discrimination performance
In rats that had cannula placements outside of the prelimbic cortex in Experiment 1, the high dose treatment led to a percent accuracy of 79.00 ± 3.61% comparable to that of vehicle treatment which resulted in 80.67 ± 3.53% accuracy. Because seven rats had cannula misplacements outside the subthalamic nucleus in Experiment 2 (described above), an ANOVA was carried out to determine whether there was a treatment effect. As a group, the misplaced subthalamic nucleus placements did not show an effect of drug treatment on performance (F(2, 12) = 3.34, p > 0.05) with the high dose treatment leading to performance of 74.00 ± 3.89% compared with vehicle treatment and the low dose treatments (80.71 ± 3.21% and 80.71 ± 1.81% accuracy respectively). Analysis of the six rats which had misplacements in Experiment 3 revealed that as a group, no treatments affected performance of the task (F(5, 25) = 0.93, p > 0.05) with performance ranging from 77.17 ± 4.19% in the contralateral high dose treatment to 84.14 ± 2.85% with the ipsilateral vehicle treatment. Thus, rats with cannula misplacements in Experiments 1-3 did not exhibit a conditional discrimination deficit when the high doses of the drugs were infused.
4. Discussion
The present studies employed a conditional discrimination test that required both an establishment of a response pattern and the use of cue information to produce a behavioral switch. Consistent with the task having switch costs, vehicle treated rats committed a significantly greater percentage of errors on switch trials compared to that of non-switch trials. The studies also found that bilateral injections of GABA agonists into the prelimbic cortex or the NMDA receptor antagonist D-AP5 into the subthalamic nucleus impaired conditional discrimination performance. This was, in part, due to an increase in switch errors. In a similar manner, Experiment 3 demonstrated that contralateral disconnection of the prelimbic cortex and subthalamic nucleus also increased switch errors in the conditional discrimination test. However, the contralateral disconnection also increased perseverative errors leading a rat to repeatedly choose the previously relevant response pattern after the initial switch trial. Taken together, the findings suggest that both an intact prelimbic cortex and subthalamic nucleus is necessary for using cue information to proactively switch and initially inhibit perseveration of the previously relevant response pattern. In monkeys, neurons in the presupplementary area and subthalamic nucleus exhibit switch-selective activity in a saccade switching test (Hikosaka and Isoda, 2008; Isoda and Hikosaka, 2008). Because the actions of subthalamic neurons appear to mainly suppress an on-going response pattern using a saccade overriding procedure, Isoda and Hikosaka (2008; 2010) have proposed that the subthalamic nucleus mediates a signal from the medial frontal cortex that allows inhibition of a response pattern that is no longer correct. The rodent prelimbic cortex may be comparable to the paralimbic cortex and/or anterior cingulate region, as opposed to the presupplementary area in primates (Uylings and van Eden, 1990). However, similar to the presupplementary area, the prelimbic cortex directly projects to the subthalamic nucleus (Maurice et al., 1998a). These anatomical findings combined with the contralateral disconnection results, raise the possibility that the prelimbic cortex sends a signal to the subthalamic nucleus, which is in part mediated by NMDA receptors, to inhibit an ongoing response pattern and enable a proactive switch to a different response pattern.
In monkeys, neurons in the presupplementary area and subthalamic nucleus exhibit switch-selective activity in a saccade switching test (Hikosaka and Isoda, 2008; Isoda and Hikosaka, 2008). Because the actions of subthalamic neurons appear to mainly suppress an on-going response pattern using a saccade overriding procedure, Isoda and Hikosaka (2008; 2010) have proposed that the subthalamic nucleus mediates a signal from the medial frontal cortex that allows inhibition of a response pattern that is no longer correct. The rodent prelimbic cortex may be comparable to the paralimbic cortex and/or anterior cingulate region, as opposed to the presupplementary area in primates (Uylings and van Eden, 1990). However, similar to the presupplementary area, the prelimbic cortex directly projects to the subthalamic nucleus (Maurice et al., 1998a). These anatomical findings combined with the contralateral disconnection results, raise the possibility that the prelimbic cortex sends a signal to the subthalamic nucleus, which is in part mediated by NMDA receptors, to inhibit an ongoing response pattern and enable a proactive switch to a different response pattern.
Previous contralateral disconnection studies have utilized either lesions (Christakou, Robbins, and Everitt, 2001; Chudasama et al., 2003) or injections of GABA agonists (Gilmartin, Kwapis, and Helmstetter, 2012; Jo and Lee, 2010) into both target regions. Because the prelimbic area has a direct, ipsilateral excitatory projection to the subthalamic nucleus that may be mediated in part by NMDA receptors (Maurice et al., 1998a; Maurice, Deniau, Menetrey, Glowinski, and Thierry, 1998b), the goal of the present study was to determine whether excitatory input from the prelimbic area to the subthalamic nucleus affects cue-guided behavioral switching. To this end, we employed a contralateral disconnection of the prelimbic cortex and subthalamic nucleus by infusing GABA agonists into the prelimbic cortex and a NMDA receptor antagonist into the subthalamic nucleus. Investigating the effects of NMDA receptor blockade in the subthalamic nucleus was carried out because in non-human primates an NMDA receptor antagonist blocks an excitatory response in the subthalamic nucleus from the motor and sensory cortex (Nambu et al., 2000). While this does not demonstrate that prelimbic cortex input to the subthalamic nucleus is mediated by NMDA receptors, it does raise the possibility. Furthermore, NMDA receptor blockade in the subthalamic nucleus does impair sustained attention and a contralateral disconnection of prelimbic cortex and subthalamic nucleus also impairs sustained attention (Baunez and Robbins, 1999; Chudasama et al., 2003).
Similar to sustained attention tests, i.e., the 5-choice serial reaction time test, rats in the conditional discrimination must maintain attention to the cues presented across the entire test session. This is because the cues change repeatedly in a session requiring rats to maintain attention to these cues in order to execute the appropriate response pattern. Comparable to that observed in the present study, contralateral lesions of the prelimbic cortex and subthalamic nucleus decreases task performance and increase perseverative responding in a sustained attention test (Chudasama et al., 2003). However, in Experiment 4 where rats had to attend to a single cue that did not change across a session, manipulations of the prelimbic cortex and subthalamic nucleus did not affect performance suggesting that these manipulations do not produce general attentional deficits. In the present conditions, when a rat must monitor cue information that periodically changes in order to select a recently used response pattern or an alternative response pattern, the prelimbic cortex and subthalamic nucleus are two brain areas that are critical for allowing a fluid, cue-guided behavioral switch beyond facilitating general attentional processes.
Similar to the contralateral disconnection results, bilateral prelimbic inactivation also impaired cue-guided behavioral switching. However, prelimbic cortex inactivation significantly increased switch, perseverative, and maintenance errors. This contrasts with past studies in which prelimbic cortex inactivation selectively increased perseveration of the previously relevant response in an extra-dimensional shift task (Block, Dhanji, Thompson-Tardif, and Floresco, 2007; Dias and Aggleton, 2000; Ragozzino, 2007; Ragozzino et al., 1999b). Committing errors beyond the switch trial in a block is not likely due to an overall decrease in discrimination performance because prelimbic inactivation did not affect performance in the non-switch discrimination test. The increase in multiple types of errors following prelimbic inactivation may more likely reflect the inability to flexibly apply learned visual cue-place associations that leads to a more rigid and fixed response pattern. More specifically, bilateral prelimbic inactivation in the conditional discrimination test increased a turn bias that was independent of current cue information. Rats, even under saline treatment, exhibited a turn bias in the test, but this was significantly enhanced under the high dose of baclofen/muscimol. However, the exaggerated turn bias is not a necessary consequence of prelimbic inactivation as this did not occur in the non-switch discrimination test. Overall, the results suggest that the prelimbic cortex supports the use of cue information to allow the proactive selection of an alternative response pattern and maintenance of that response pattern when conditions require a behavioral switch.
The conditional discrimination test required a rat to reverse which place it entered every few trials. Past studies found that prelimbic inactivation does not impair place reversal learning in which the prior outcome information is used to initiate a behavioral switch (Birrell and Brown, 2000; Boulougouris, Dalley, and Robbins, 2007; Ragozzino et al., 1999a). This is the case even when the level of difficulty is enhanced by increasing the number of maze locations (Ragozzino et al., 2003). The present results suggest that the prelimbic cortex supports a place reversal, but only under conditions in which cue information is used to guide a behavioral switch. Alternatively, prelimbic cortex inactivation in the present study may have impaired performance not based on requiring the use of cue information, but because multiple switches within the same session were required, as opposed to a single switch as required in past reversal learning studies (Chudasama et al., 2001; Ragozzino et al., 2003; Young and Shapiro, 2009). However, this is unlikely the case as medial prefrontal cortex lesions that include the prelimbic cortex do not impair multiple reversals within a session that require switching based on outcome information (Birrell and Brown, 2000; Boulougouris et al., 2007; Rich and Shapiro, 2007).
Some studies have failed to observe deficits in conditional discrimination performance with medial prefrontal cortex lesions that include the prelimbic area or prelimbic cortex specific lesions (Bussey, Muir, Everitt, and Robbins, 1997; Chudasama et al., 2001; Delatour and Gisquet-Verrier, 1999). This is even the case when rats with prelimbic cortex lesions are required to reverse task contingencies (Chudasama et al., 2001). These studies have utilized discriminations in which the contingencies are switched pseudo-randomly with at most 3 consecutive trials. More recently Leenaars and colleagues (2012) used a conditional discrimination test similar to that in the present study with block length varying between 5-10 consecutive trials. Although this conditional discrimination test was carried out in an operant chamber, they reported that medial prefrontal cortex inactivation decreased accuracy on switch trials comparable with the present results. This study along with the present studies suggest that tests which use longer trial blocks, e.g. 3 trials and greater, may form a response set and require greater inhibitory demands to supplant the current response pattern with a different and more optimal response pattern. The prelimbic cortex may be important for producing inhibition of an ongoing response pattern under these conditions and therefore when inactivated leads to a performance deficit. The idea that a response set forms with longer trial blocks that then leads to greater inhibitory demands during a behavioral switch is consistent with the present findings showing significant switch costs. The present results also suggest that not only the prelimbic cortex, but the subthalamic nucleus is important for cue-guided behavioral switching under conditions in which a response set is formed.
The findings from Experiment 2 reveal that, similar to prelimbic cortex inactivation, NMDA receptor blockade in the subthalamic nucleus impaired performance in the visual cue-place conditional discrimination. However, in contrast to the effects of prelimbic cortex inactivation, NMDA receptor blockade in the subthalamic nucleus selectively increased switch and perseverative errors, but did not affect maintenance errors. The findings are comparable to those in which subthalamic nucleus lesions impair inhibition of an initiated response in the stop-signal test (Eagle, Baunez, Hutcheson, Lehmann, Shah, and Robbins, 2008) and further suggest that the subthalamic nucleus is critical not only for inhibiting an initiated response, but also for inhibiting an ongoing response pattern when cues indicate an alternate response pattern should occur. Stop-signal findings in rats have implicated the orbitofrontal cortex to subthalamic nucleus connection (Eagle et al., 2008). Future studies can determine whether the orbitofrontal cortex input to the subthalamic nucleus may also support cue-guided behavioral switching.
One common set of findings across Experiments 1-3 is that the different pharmacological manipulations, with the exception of ipsilateral disconnection, impaired conditional discrimination performance and increased the likelihood of committing a perseverative error within a trial block. Perseverative errors, as operationally defined, can only occur after a switch error. Therefore, following a switch trial error a rat can use both cue information and outcome information to select the appropriate response pattern. The present results indicate that neither cue and outcome information is sufficient to accurately select a response pattern when contralateral disconnection of the prelimbic cortex and subthalamic nucleus occurs.
Another common set of findings across Experiments1-3 is that the performance impairing treatments led to a similar number of errors in the first half of testing as in the second half of testing. Thus, a treatment that impaired performance produced a similar effect across the entire test session. All treatments that led to a conditional discrimination deficit increased the number of switch errors. In addition, D-AP5 infusion into the subthalamic nucleus and contralateral disconnection of the prelimbic cortex and subthalamic nucleus also increased perseverative errors. Moreover, prelimbic cortex inactivation alone significantly increased perseverative and maintenance errors. One possibility is that an increase in perseverative and/or maintenance errors results from a large number of these errors occurring in only one or two trial blocks. Alternatively, an increase in perseverative or maintenance errors may occur because one of these errors occurred in a greater number of trials blocks across a test session. Measuring the percentage of trial blocks an error was committed revealed that a behavioral impairing treatment increased the number of blocks an error was committed. Therefore, the findings based on errors in the first half vs. second half of testing, as well as percent of error blocks indicated that conditional discrimination deficits resulted from errors consistently occurring across an entire test session.
A conditional discrimination impairment following contralateral disconnection of the prelimbic cortex and subthalamic nucleus indicates that this disconnection is sufficient to disrupt cue-guided behavioral switching. One possibility is that a deficit occurs because a prelimbic cortex-subthalamic nucleus “hyperdirect” pathway supports this type of cue-guided behavioral switching. Another possibility is that while a contralateral disconnection of the prelimbic cortex and subthalamic nucleus is sufficient to impair conditional discrimination performance, a greater complexity of frontal cortex and basal ganglia circuitry supports cue-guided behavioral switching. Besides a “hyperdirect” connection from the prelimbic cortex to the subthalamic nucleus, there is an indirect pathway in which the prelimbic cortex projects to the dorsomedial striatum which projects to the entopeduncular nucleus that in turn projects to the subthalamic nucleus (Albin, Young, and Penney, 1989; Mathai and Smith, 2011). The indirect pathway may also play a role in cue-guided behavioral switching as well. In support of this, a recent fMRI study using a behavioral switching task suggested that both the hyperdirect and indirect pathways support response inhibition when a behavioral switch is required (Jahfari et al., 2011). These findings would support the idea that multiple frontal cortex-basal ganglia circuits contribute to behavioral switching and combined with the present results raise the possibility that disrupting one of these circuits is sufficient to induce a behavioral deficit.
Because contralateral disconnection of the prelimbic cortex and subthalamic nucleus selectively increased switch and perseverative errors, but prelimbic cortex inactivation increased switch, perseverative, and maintenance errors, the prelimbic cortex may be involved in a top-down monitoring of task demands to enable a switch and maintenance of the currently correct response pattern. Narayanan and Laubach (2006; 2009) have proposed that the dorsomedial frontal cortex encodes both prepotent responses and proactive inhibition such that when neurons encoding proactive inhibition predominate, a rat will be less likely to make a premature response in tests that have a delay component. We suggest a somewhat similar top-down model in which the prelimbic cortex encodes inhibition of an ongoing strategy and maintenance of a relevant strategy. When excitatory input from the prelimbic area to the subthalamic area predominates, this allows an inhibition of the ongoing response and selection of a different response pattern. However, when excitatory input from the prelimbic cortex to other basal ganglia structures such as the dorsomedial striatum (Braun and Hauber, 2011; Castane, Theobald, and Robbins, 2010; O'Neill and Brown, 2007; Palencia and Ragozzino, 2004; Pastuzyn, Chapman, Wilcox, and Keefe, 2012; Pisa and Cyr, 1990; Ragozzino and Choi, 2004) or the nucleus accumbens (Floresco et al., 2006a) predominates, this supports the execution of the ongoing response pattern. This proposal is consistent with recent findings indicating that the dorsomedial striatum is part of a neural system that supports cue-guided behavioral switching (Bradfield and Balleine, 2013).
Alternative to a top-down model, another possibility is that the subthalamic nucleus through a “bottom-up” mechanism influences prelimbic cortex to inhibit a recently selected response pattern and select an alternative response pattern. This may indirectly occur through the thalamus or through direct input into the prelimbic cortex as the subthalamic nucleus projects to layers I-III of the prelimbic cortex (Degos, Deniau, Le Cam, Mailly, and Maurice, 2008). Still another possibility is that there are both top-down and bottom up influences that produce dynamic interactions between these structures to allow rapid and repeated cue-guide behavioral switches.
4.1 Conclusion
In conclusion, the present findings suggest that the prelimbic cortex is able to monitor visual cues in order to select appropriate responses under conditions that demand repeated and rapid behavioral switching. This may occur by dynamically interacting with multiple basal ganglia structures to allow a fluid and flexible use of various response patterns at appropriate times. The contralateral disconnection findings indicate that the prelimbic cortex and subthalamic nucleus together support cue-guided behavioral switching. More specifically, our findings support the idea that the prelimbic cortex and subthalamic nucleus are critical for a proactive switch from one response pattern to an alternative pattern. Overall, the present experiments reveal some of the specific frontal cortex-basal ganglia circuitry that enables behavioral flexibility under conditions that require cue-guided behavioral switching.
Supplementary Material
Highlights for Review.
Contralateral disconnection of the prelimbic cortex and subthalamic nucleus impairs cue-guided behavioral switching
Contralateral disconnection of the prelimbic cortex and subthalamic nucleus increases switch and perserverative errors in a conditional discrimination test
Prelimbic cortex inactivation impairs cue-guided behavioral switching by biasing rats to use an ineffective response strategy
NMDA receptor blockade in the subthalamic nucleus impairs cue-guided behavioral switching by increasing switch and perserverative errors in a conditional discrimination test
Acknowledgments
This research was supported by grant NIH grant P50 HD055751 to MER. PMB was supported by the University of Illinois at Chicago Center for Clinical and Translational Science (CCTS), Award Number TL1RR029877 from the National Center For Research Resources. We would like to thank Gena M. Grospe for her help with behavioral testing and Jamie D. Roitman for her input into an earlier version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afsharpour S. Topographical projections of the cerebral cortex to the subthalamic nucleus. J Comp Neurol. 1985;236:14–28. doi: 10.1002/cne.902360103. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Baker PM, Thompson JL, Sweeney JA, Ragozzino ME. Differential effects of 5-HT(2A) and 5-HT(2C) receptor blockade on strategy-switching. Behav Brain Res. 2011;219:123–131. doi: 10.1016/j.bbr.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GRI, Warburton EC. NMDA receptor plasticity in the perirhinal and prefrontal cortices is crucial for the acquisition of long-term object-in-place associative memory. The Journal of neuroscience. 2008;28:2837–2844. doi: 10.1523/JNEUROSCI.4447-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunez C, Robbins TW. Effects of transient inactivation of the subthalamic nucleus by local muscimol and APV infusions on performance on the five-choice serial reaction time task in rats. Psychopharmacology (Berl) 1999;141:57–65. doi: 10.1007/s002130050806. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb Cortex. 2007;17:1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Bradfield LA, Balleine BW. Hierarchical and binary associations compete for behavioral control during instrumental biconditional discrimination. J Exp Psychol Anim Behav Process. 2013;39:2–13. doi: 10.1037/a0030941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S, Hauber W. The dorsomedial striatum mediates flexible choice behavior in spatial tasks. Behav Brain Res. 2011;220:288–293. doi: 10.1016/j.bbr.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Brown HD, Baker PM, Ragozzino ME. The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats. J Neurosci. 2010;30:14390–14398. doi: 10.1523/JNEUROSCI.2167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci. 1997;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- Castane A, Theobald DE, Robbins TW. Selective lesions of the dorsomedial striatum impair serial spatial reversal learning in rats. Behav Brain Res. 2010;210:74–83. doi: 10.1016/j.bbr.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Robbins TW, Everitt BJ. Functional disconnection of a prefrontal cortical-dorsal striatal system disrupts choice reaction time performance: implications for attentional function. Behav Neurosci. 2001;115:812–825. doi: 10.1037//0735-7044.115.4.812. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Baunez C, Robbins TW. Functional disconnection of the medial prefrontal cortex and subthalamic nucleus in attentional performance: evidence for corticosubthalamic interaction. J Neurosci. 2003;23:5477–5485. doi: 10.1523/JNEUROSCI.23-13-05477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Bussey TJ, Muir JL. Effects of selective thalamic and prelimbic cortex lesions on two types of visual discrimination and reversal learning. Eur J Neurosci. 2001;14:1009–1020. doi: 10.1046/j.0953-816x.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Churchwell JC, Kesner RP. Hippocampal-prefrontal dynamics in spatial working memory: interactions and independent parallel processing. Behav Brain Res. 2011;225:389–395. doi: 10.1016/j.bbr.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degos B, Deniau JM, Le Cam J, Mailly P, Maurice N. Evidence for a direct subthalamo-cortical loop circuit in the rat. Eur J Neurosci. 2008;27:2599–2610. doi: 10.1111/j.1460-9568.2008.06229.x. [DOI] [PubMed] [Google Scholar]
- Delatour B, Gisquet-Verrier P. Lesions of the prelimbic-infralimbic cortices in rats do not disrupt response selection processes but induce delay-dependent deficits: evidence for a role in working memory? Behav Neurosci. 1999;113:941–955. doi: 10.1037//0735-7044.113.5.941. [DOI] [PubMed] [Google Scholar]
- Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioural flexibility. Eur J Neurosci. 2000;12:4457–4466. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW. Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb Cortex. 2008;18:178–188. doi: 10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S, Vexelman C, Magyar O. Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. J Neurosci. 2006a;26:2449–2457. doi: 10.1523/JNEUROSCI.4431-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006b;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, Helmstetter FJ. Trace and contextual fear conditioning are impaired following unilateral microinjection of muscimol in the ventral hippocampus or amygdala, but not the medial prefrontal cortex. Neurobiology of learning and memory. 2012;97:452–464. doi: 10.1016/j.nlm.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Isoda M. Brain mechanisms for switching from automatic to controlled eye movements. Prog Brain Res. 2008;171:375–382. doi: 10.1016/S0079-6123(08)00655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Isoda M. Switching from automatic to controlled behavior: cortico-basal ganglia mechanisms. Trends Cogn Sci. 2010;14:154–161. doi: 10.1016/j.tics.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyafil A, Summerfield C, Koechlin E. Two mechanisms for task switching in the prefrontal cortex. J Neurosci. 2009;29:5135–5142. doi: 10.1523/JNEUROSCI.2828-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci. 2008;28:7209–7218. doi: 10.1523/JNEUROSCI.0487-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Waldorp L, van den Wildenberg WPM, Scholte HS, Ridderinkhof KR, Forstmann BU. Effective Connectivity Reveals Important Roles for Both the Hyperdirect (Fronto-Subthalamic) and the Indirect (Fronto-Striatal-Pallidal) Fronto-Basal Ganglia Pathways during Response Inhibition. Journal of Neuroscience. 2011;31:6891–6899. doi: 10.1523/JNEUROSCI.5253-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YS, Lee I. Disconnection of the hippocampal-perirhinal cortical circuits severely disrupts object-place paired associative memory. The Journal of neuroscience. 2010;30:9850–9858. doi: 10.1523/JNEUROSCI.1580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. 2010;20:199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Konishi S, Chikazoe J, Jimura K, Asari T, Miyashita Y. Neural mechanism in anterior prefrontal cortex for inhibition of prolonged set interference. Proc Natl Acad Sci U S A. 2005;102:12584–12588. doi: 10.1073/pnas.0500585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenaars CH, Joosten RN, Zwart A, Sandberg H, Ruimschotel E, Hanegraaf MA, Dematteis M, Feenstra MG, van Someren EJ. Switch-task performance in rats is disturbed by 12 h of sleep deprivation but not by 12 h of sleep fragmentation. Sleep. 2012;35:211–221. doi: 10.5665/sleep.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill PJ, Sharott A, Bolam JP, Brown P. Delayed synchronization of activity in cortex and subthalamic nucleus following cortical stimulation in the rat. J Physiol. 2006;574:929–946. doi: 10.1113/jphysiol.2006.110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailly P, Aliane V, Groenewegen HJ, Haber SN, Deniau JM. The rat prefrontostriatal system analyzed in 3D: evidence for multiple interacting functional units. J Neurosci. 2013;33:5718–5727. doi: 10.1523/JNEUROSCI.5248-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai A, Smith Y. The corticostriatal and corticosubthalamic pathways: two entries, one target. So what? Frontiers in Systems Neuroscience. 2011;5 doi: 10.3389/fnsys.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Deniau JM, Glowinski J, Thierry AM. Relationships between the prefrontal cortex and the basal ganglia in the rat: physiology of the corticosubthalamic circuits. J Neurosci. 1998a;18:9539–9546. doi: 10.1523/JNEUROSCI.18-22-09539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Deniau JM, Menetrey A, Glowinski J, Thierry AM. Prefrontal cortex-basal ganglia circuits in the rat: involvement of ventral pallidum and subthalamic nucleus. Synapse. 1998b;29:363–370. doi: 10.1002/(SICI)1098-2396(199808)29:4<363::AID-SYN8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- McCool MF, Patel S, Talati R, Ragozzino ME. Differential involvement of M1-type and M4-type muscarinic cholinergic receptors in the dorsomedial striatum in task switching. Neurobiol Learn Mem. 2008;89:114–124. doi: 10.1016/j.nlm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler EG, Baker PM, Gannon KS, Jones SS, Shacham S, Sweeney JA, Ragozzino ME. The effects of PRX-07034, a novel 5-HT(6) antagonist, on cognitive flexibility and working memory in rats. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends Cogn Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, Ikeuchi Y, Hasegawa N. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol. 2000;84:289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Delay activity in rodent frontal cortex during a simple reaction time task. J Neurophysiol. 2009;101:2859–2871. doi: 10.1152/jn.90615.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CW, Noblejas MI, Rodefer JS, Smith CB, Poremba A. Double dissociation of attentional resources: prefrontal versus cingulate cortices. J Neurosci. 2007;27:12123–12131. doi: 10.1523/JNEUROSCI.2745-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill M, Brown VJ. The effect of striatal dopamine depletion and the adenosine A2A antagonist KW-6002 on reversal learning in rats. Neurobiol Learn Mem. 2007;88:75–81. doi: 10.1016/j.nlm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The influence of NMDA receptors in the dorsomedial striatum on response reversal learning. Neurobiol Learn Mem. 2004;82:81–89. doi: 10.1016/j.nlm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Pastuzyn ED, Chapman DE, Wilcox KS, Keefe KA. Altered learning and Arc-regulated consolidation of learning in striatum by methamphetamine- induced neurotoxicity. Neuropsychopharmacology. 2012;37:885–895. doi: 10.1038/npp.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3rd. New York: 1998. [DOI] [PubMed] [Google Scholar]
- Pisa M, Cyr J. Regionally selective roles of the rat's striatum in modality-specific discrimination learning and forelimb reaching. Behav Brain Res. 1990;37:281–292. doi: 10.1016/0166-4328(90)90140-a. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The effects of dopamine D(1) receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learn Mem. 2002;9:18–28. doi: 10.1101/lm.45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Choi D. Dynamic changes in acetylcholine output in the medial striatum during place reversal learning. Learn Mem. 2004;11:70–77. doi: 10.1101/lm.65404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic- infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999a;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Kim J, Hassert D, Minniti N, Kiang C. The contribution of the rat prelimbic-infralimbic areas to different forms of task switching. Behav Neurosci. 2003;117:1054–1065. doi: 10.1037/0735-7044.117.5.1054. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Wilcox C, Raso M, Kesner RP. Involvement of rodent prefrontal cortex subregions in strategy switching. Behav Neurosci. 1999b;113:32–41. doi: 10.1037//0735-7044.113.1.32. [DOI] [PubMed] [Google Scholar]
- Rich EL, Shapiro ML. Prelimbic/infralimbic inactivation impairs memory for multiple task switches, but not flexible selection of familiar tasks. J Neurosci. 2007;27:4747–4755. doi: 10.1523/JNEUROSCI.0369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- Uekita T, Okaichi H. Pretraining does not ameliorate spatial learning deficits induced by intrahippocampal infusion of AP5. Behavioral neuroscience. 2009;123:520. doi: 10.1037/a0015672. [DOI] [PubMed] [Google Scholar]
- Uylings HB, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- Winters BD, Bartko SJ, Saksida LM, Bussey TJ. Muscimol, AP5, or scopolamine infused into perirhinal cortex impairs two-choice visual discrimination learning in rats. Neurobiology of Learning and Memory; Neurobiology of Learning and Memory. 2010 doi: 10.1016/j.nlm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Young JJ, Shapiro ML. Double dissociation and hierarchical organization of strategy switches and reversals in the rat PFC. Behav Neurosci. 2009;123:1028–1035. doi: 10.1037/a0016822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


