1. Introduction
Protein kinases have emerged as the largest family of signaling proteins in eukaryotic cells and are involved in every aspect of cellular regulation. There are over 500 protein kinases in the human genome.1,2 The vast majority are Ser/Thr protein kinases. The Ser/Thr protein kinases interact with diverse substrates ranging from enzymes, including other kinases, to transcription factors, receptors, and other regulatory proteins. Thus, mechanisms to assure specificity must be present. However, from emerging structural data it is becoming apparent that the ways in which protein kinases interact with their substrates local to the active site are relatively few. Instead, docking interactions, in pockets or grooves outside the active site of the kinase, are used to recognize substrates and other interacting proteins. Docking motifs in substrates bind in docking grooves within the kinase domain or adaptor protein. Docking interactions have been defined for CDKs (cyclin dependent kinases), MAPKs (mitogen activated protein kinases), and members of the AGC group (cAMP-dependent (PKA), cGMP-dependent, PKC), as well as several other kinases. Further, structural data is revealing that docking interactions regulate kinase activity by unanticipated allosteric mechanisms that probably promote pathway specificity.
In this review, we outline the current structural data available on distinct Ser/Thr protein kinases. How kinases bind substrates at the active site is described, focusing on the P+1 pocket, which is remodeled in inactive forms of several protein kinases. Substrate docking interactions, outside the active site, observed in MAP kinases, CDKs and AGC kinases will be described. How specificity among these different families of kinases is achieved from the organization of the binding site and other factors will be discussed. Further, available data suggesting that docking interactions control kinase activity allosterically will be reviewed. Recent reviews of topics under discussion,3–10 and related topics11–17 are available.
2. Architecture and Available Structural Data
Among eukaryotic protein kinases,1 the Ser/Thr kinases have been classified into six large groups. These are named the AGC group, the CaMK group (for calcium-calmodulin dependent), the CMGC group (for CDK, MAP kinase, glycogen synthase kinase, and CDK-like), the STE group (homologs of STE11 and STE20), and the CK1 group (for casein kinase-1), and TKL (tyrosine kinase like). Structural data is now available for representatives of each of the well-populated groups, as well as smaller groups, such as WNKs (with no lysine),18 revealing that the protein kinases have a common architecture.
2.1. Architecture of Protein Kinases
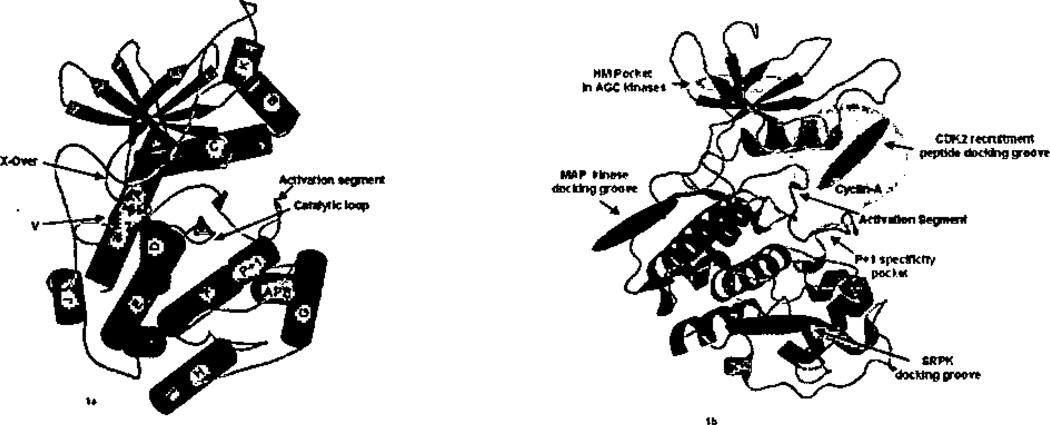
Protein kinases possess a two-lobe architecture that has been reviewed several times (Figure 1a).6,7,19,20,21 Briefly, the N-terminal lobe is composed of a five-stranded β-sheet and a single well-conserved helix, labeled helix C based on the structure of PKA.19 The C-terminal lobe possesses 6 large helices (D, E, F, G, H, and I) and two β-ribbons, β7–β8 and β6–β9. The β7–β8 ribbon is present in both active and inactive protein kinases. Further, an additional β-strand interacts with β7–β8 forming a 3-stranded β-sheet in most protein kinases, but not in PKA. The β-strand is labeled β5D for its placement in the structure between β-strand 5 and helix D (Figure 1a). The β6–β9 ribbon is present only in active kinases (Figure 1a);21 β9 is part of the activation segment. Two smaller helices, labeled P+1 and APE (also called helix αEF)21 in Figure 1a, are conserved in active protein kinases. The activation segment and the catalytic loop are also in the C-terminal lobe. The catalytic loop refers to a 7-residue segment (Asp166-Asn172 in PKA) that houses the catalytic aspartate (Asp 166) and lysine residue (Lys168). The activation segment refers to the sequence flanked by the conserved motifs DFG (following β8; subdomain VII in the nomenclature of Hanks and Hunter22) and APE (subdomain VIII) (also referred to as “activation loop” or Lip). This segment is variable in size and in many kinases possesses one or more phosphorylation sites that tend to be activating.21 The primary substrate recognition pocket, the P+1 binding site, is adjacent to, and contiguous in sequence with, the activation segment (Figure 1b). Further, relatively short (~50 residue) N- and C-terminal extensions from the kinase core may pack on the core, and are present for all of the Ser/Thr kinases studied crystallographically, including the smallest, CDK2.23 Longer N- and C-terminal extensions are known to fold into a variety of separate domains (as reviewed in ref 1). Structural data for Ser/Thr kinases possessing separately folded domains (either a separate subunit or folding unit) is available for twitchin24, p21-activated protein kinase (PAK1),25 CK2 (casein kinase-2),26 G-protein-coupled receptor kinase-2 (GRK2),27 and PKA.28
Figure 1.
a) Secondary structure of protein kinases based on PKA. Helices are cyan, β-strands magenta, and loops deep salmon, b) The MAP kinase docking groove, the AGC hydrophobic motif (HM) pocket, and CDK2 recruitment peptide docking groove and SR docking groove are in violet. The cyclin-A binding site on CDK2 is in gray. Figures generated using PyMOL (Delano Scientific, San Carlos. CA).
Protein kinases have grooves on the surface of the kinase core (Figure 1b). The grooves are a consequence of the architecture, and tend to be conserved. For example, in the structure of PKA, a groove is present between helix C and the N-terminal domain β-sheet, which is conserved in AGC kinases. The grooves serve different functions in homologous kinases, as discussed below.
2.2. Available Structural Data
Structural information is available for both active and inactive forms of at least one member of each of the major subgroups of Ser/Thr kinases6 (Table 1). Active conformations are closely similar among all groups, such that structural signatures for active kinases have been defined.7,23,29 The categorization of “active” versus “inactive” in Table 1 is based upon the position of a conserved threonine, discussed below, as well as other signatures. In the AGC group, PKA,30 PDK1 (phosphoinositide dependent protein kinase),31 PKB32 and GRK233 have known structures. Most AGC structures are in their active conformations; however, an inactive form of PKB has been determined34. In the CaMK group, the structures of twitchin,24,35 titin,36 CaMK-I,37 PHK (phosphorylase kinase)38 are available. Most of these structures are inactive conformations (Table 1), although the structure of PHK has been solved in an active, substrate bound conformation.39 In the CMGC group, the structures of CDK223 and many other CDKs (Table 1) in both inactive and active forms have also been determined.40,42 The MAP kinases ERK2,29,43 p38α,44,45 JNK346 and others have been solved. Both inactive and active conformations are available for rat ERK2.29,43 In addition, glycogen synthase kinase GSK313 and CK2,26,47,48 and more49,51 of the CMGC group have been studied. In the casein kinase 1 group (CK1), CK1 from S. pombe52 and rat CK1δ,53 both in the active conformations are available. Further, data are at hand for the CMGC group SR protein kinases (SRPKs) Sky1p49 and SRPK1 (Table 1). In the STE family, structures are available for inactive p21-activated protein kinase (PAK1)25 and active forms conformation of the MAP3K TAO254 and PAK1.55 The data available in the STE group has recently doubled through the efforts of the Structural Genomics Consortium, Oxford (SGC, Table 1). In the tyrosine kinase-like group (TKL), structures are available for the kinase domains of B-RAF (inactive)56 and the TGFβ receptor.57,58 Also, structures are available for small groups, including WNK1 and PknB.59,60 Current comprehensive listing of protein kinase Protein Data Bank entries can be found at http://cellsignaling.lanl.gov/structure/kinase and http://www.kinasenet.org, and sequence information is available at http://www.kinase.com/.
Table 1.
Crystal structures of Ser/Thr Protein kinases
| Group | Kinase | PDB | Organism | Ligation State | Phos. State |
Activation State | Origin of Inactivity |
Reference |
|---|---|---|---|---|---|---|---|---|
| AGC | PKA | 1ATP | Mouse | MnATP and peptide | P | Active | - | a1 |
| 1BKX | Mouse | Adenosine | P | Active | - | a2 | ||
| 1CDK | Pig | PKI peptide | P | Inhibited | - | 62 | ||
| 1CTP | Pig | Inhibitor peptide | P | Inhibited | - | 29 | ||
| 1FMO | Mouse | PKI inhibitor and adenosine | P | Inhibited | - | a3 | ||
| 1U7E | Mouse | AMPPNP, R-subunit (bovine) | P | Inhibited | 27 | |||
| 1J3H | Mouse | apo | P | Open | - | a4 | ||
| 1JLU | Mouse | Substrate peptide | P | Active | - | 133 | ||
| 1L3R | Mouse | Transition state | P | Active | - | a5 | ||
| PKB | 1MRV | Human | apo | U | Inactive | No helix C | 33 | |
| 1MRY | Human | apo | U | Inactive | No helix C | 33 | ||
| 1O6K | Human | GSK3 peptide, AMPPNP | P | Active | - | 31 | ||
| 1O6L | Human | AMP-PNP, GSK3 peptide | P | Active | - | 31 | ||
| 1GZK | Human | apo | U | Inactive | DFG Out, Thr | 75 | ||
| 1GZN | Human | apo | U | Inactive | DFG Out, Thr | 75 | ||
| 1GZO | Human | apo | U | Inactive | DFG Out, Thr | a6 | ||
| PKC-I | 1ZRZ | Human | BIN-1 inhibitor | P | Active | - | a7 | |
| PDK1 | 1H1W | Human | ATP | P | Active | - | 28 | |
| GRK2 | 1YM7 | Bovine | Apo | U | Active | - | 29 | |
| 2BCJ | Bovine | Gα, Gβ subunits, GDP, AIF4− | U | Active | - | 80 | ||
| GRK6 | 2ACX | Human | AMPPNP, Mg | U | Active | - | 79 | |
| Aurora | 2C6D | Human | ADPNP | U | Active | - | a8 | |
| 1MUO | Human | Apo | U | Inactive | DFG Out | a9 | ||
| 1OL5 | Human | TPX-2 peptide | P | Active | - | 77 | ||
| 2BFX | Frog | Incenp peptide | P | Active | - | 78 | ||
| ROCK | 2F2U | Bovine | Apo | U | Active | - | a10 | |
| CAMK | CamK1 | 1A06 | Rat | Apo | U | Autoinhibited | DFG Out, Thr | 37 |
| CamK1δ | 2JC6 | Human | Inhibitor | U | Inactive | Act loop, autoinhibited | To be published | |
| CamK1γ | 2JAM | Human | Inhibitor | U | Inactive | dimer | To be published | |
| ChKI | 1IA8 | Human | Apo | U | Active | - | a11 | |
| 1ZYS | Human | Inhibitor | U | Active | - | To be published | ||
| DAPK | 1IG1 | Human | AMPPNP, Mn | U | Active | - | a12 | |
| 1JKS | Human | Apo | U | Active | - | a12 | ||
| DAPK2 | 1WMK | Human | Apo | U | Inactive | Dimerized | To be published | |
| DAPK3 | 1YRP | Human | Apo | U | Inactive | Dimerized | To be published | |
| 2J90 | Human | JAK-Inhibitor | P | Active | - | To be published | ||
| Twitchin | 1KOA | C.elegans | Apo | U | Autoinhibited | - | 35 | |
| 1KOB | Aplysia | Apo | U | Autoinhibited | - | a13 | ||
| MAPKAP-K2 | 1KWP | Human | Apo | U | Autoinhibited | - | a14 | |
| 1NY3 | Human | ADP | U | Active | - | a15 | ||
| PHK | 1PHK | Rabbit | MnATP | U | Active | - | 38 | |
| 2PHK | Rabbit | Phosphorylase peptide, MnATP | U | Active | - | 39 | ||
| Titin | 1TKI | Human | Apo | U | Autoinhibited | - | 36 | |
| MSKI | 1VZO | Human | Apo | U | Autoinhibited | - | a16 | |
| Pim-1 | IXR1 | Human | AMP-PNP | U | Active | - | a17 | |
| 1XWS | Human | Inhibitor | U | Active | - | to be published | ||
| 1YWV | Human | Apo | U | Active | - | a18 | ||
| IYXT | Human | AMPPNP | U | Active | - | a18 | ||
| 2BIL | Human | Pimtide peptide | U | Active | - | To be published | ||
| 2BIK | Human | BIM-1 inhibitor | P | Active | - | To be published | ||
| MARK | 1ZMU | Rat | Apo | U | Inactive | DFG Out, Thr | a19 | |
| MNK1 | 2HW6 | Human | Apo | U | Inactive | DFG Out, Thr | a20 | |
| MNK2 | 2AC3 | Human | Apo | U | Inactive | DFG Out, Thr | 137 | |
| CamKII | 2BDW | C.elegans | apo | U | Autoinhibited | - | a21 | |
| SNF1 | 2FH9 | Yeast | Apo | U | Inactive | Thr | a22 | |
| Amp-Activated | 2H6D | Human | apo | U | Inactive | DFG Out, Thr | To be published | |
| CMGC | CDK2 | IB38 | Human | ATP | U | Inactive | Thr | 40 |
| 1B39 | Human | ATP | U | Inactive | Thr | 40 | ||
| 1BUH | Human | CKSHS1 | U | Inactive | No helix C | a23 | ||
| 1CKP | Human | Inhibitor | U | Inactive | Thr | a24 | ||
| 1FIN | Human | ATP | U | Inactive | Act loop | a25 | ||
| 1FQ1 | Human | KAP, ATP | P | Inactive | Thr, KAP | a26 | ||
| 1HCK | Human | Mg ATP | U | Inactive | Thr | a27 | ||
| 1HCL | Human | Apo | U | Inactive | Thr | a28 | ||
| 1JST | Human | Cyclin-A and ATP | P | Active | - | 42 | ||
| 1PW2 | Human | Apo | U | Inactive | Thr | a29 | ||
| 1H27 | Human | P27 peptide | P | Active | - | 63 | ||
| 1H28 | Human | P107 peptide | P | Active | - | 63 | ||
| 2CCI | Human | CDC6 peptide | P | Active | - | 67 | ||
| CDK5 | 1H4L | Human | fragment of p35 activator | U | Active | - | a30 | |
| CDK6 | IB17 | Human | P161NK4A | U | Inactive | No helix C | a31 | |
| 1B18 | Human | P19JNK4D | U | Inactive | No helix C | a31 | ||
| 1JOW | Human | Viral cyclin | U | Active | - | a32 | ||
| CDK7 | 1UA2 | Human | ATP | U | Inactive | Thr | a33 | |
| p38 | 1CM8 | Human | AMPPNP | P | Active | - | 45 | |
| 1P38 | Mouse | apo | U | Inactive | Thr | 44 | ||
| 1LEW | Mouse | MEF2 peptide | U | Inactive | Thr | 68 | ||
| 1LEZ | Mouse | MKK3B peptide | U | Inactive | Thr | 68 | ||
| 1WFC | Human | Apo | U | Inactive | Thr | a34 | ||
| 1R39 | Human | Apo | U | Inactive | Thr | a35 | ||
| ERK2 | 1ERK | Rat | Apo | U | Inactive | Act loop | 43 | |
| 2ERK | Rat | Apo | P | Active | - | 28 | ||
| 2GPH | Rat | HePTP peptide | U | Inactive | Thr | 70 | ||
| 2FYS | Rat | MKP3 peptide | U | Inactive | Thr | 71 | ||
| ERK3 | 216L | Human | Apo | U | Inactive | Thr | To be published | |
| JNK1 | 1UKH | Human | JIP peptide | U | Inactive | Thr | 69 | |
| JNK3 | 1JNK | Human | ANP | U | Inactive | Thr | 46 | |
| GSK3 | 1H8F | Human | Inhibitor | U | Active | - | 13 | |
| 1O9U | Human | Axin peptide, Inhibitor | P | Active | - | 114 | ||
| 1GNG | Human | FRATtide | P | Active | - | 19 | ||
| CK2 | 1JWH | Human | Apo | U | Active | - | 23 | |
| 1LP4 | Maize | Mg AMPPNP | U | Active | - | a36 | ||
| 1NA7 | Human | APO | U | Active | - | a37 | ||
| 1PJK | Human | AMPPNP | U | Active | - | a38 | ||
| 1DAW | Maize | MgAMPPNP | U | Inactive | Thr | 47 | ||
| 1DAY | Maize | MgGMPPNP | U | Inctive | Thr | 47 | ||
| 1DS5 | Maize | AMP | U | Inactive | Dimerized | a39 | ||
| Sky1P | 1HOW | Yeast | Apo | U | Active | - | 49 | |
| 1Q8Y | Yeast | ADP | U | Active | - | a40 | ||
| 1Q97 | Yeast | ATP | U | Active | - | a40 | ||
| PKR | 2AIA | Yeast | EIF2α, | P | Inactive | Thr | 50 | |
| 2A19 | Yeast | EIF2α, AMPMPNP | P | Inactive | Thr | 50 | ||
| FUS3 | 2B9F | Yeast | MgADP | U | Inactive | Thr | 51 | |
| 2B9J | Yeast | FARI peptide, MgADP | U | Inactive | Thr | 51 | ||
| 2F49 | Yeast | Ste5 peptide | U | Inactive | Thr | 72 | ||
| CLK1 | 1Z57 | Human | Hymenialdsine | U | Active | - | To be published | |
| CLK3 | 2EU9 | Human | Apo | U | Active | - | To be published | |
| 2EXE | Human | Apo | U | Fragment | - | To be published | ||
| SRPK1 | 1WBP | Human | ASF/SF2 peptide, ADP | U | Active | - | 73 | |
| STE | PAK1 | 1F3M | Human | Apo | U | Autoinhibited | - | 24 |
| 1YHW | Human | Apo K299R | U | Active | - | 55 | ||
| PAK4 | 2BVA | Human | Apo | P | Active | - | To be published | |
| 2J01 | Human | Apo | P | Active | - | To be published | ||
| PAK5 | 2F57 | Human | Apo | P | Active | - | To be published | |
| PAK6 | 2C30 | Human | Apo | P | Active | - | To be published | |
| MEK1 | 1S9J | Human | MgATP, Inhibitor | U | Inactive | Act loop, Thr | a41 | |
| MEK2 | 1S9I | Human | MgATP, Inhibitor | U | Inactive | Act loop Thr | a41 | |
| MEKK5 | 2CLQ | Human | inhibitor | U | Active | - | To be published | |
| TAO2 | IU5Q | Rat | Apo | P | Active | - | 54 | |
| 1U5R | Rat | Mg ATP | P | Active | - | 54 | ||
| GCN2 | 1ZYC | Yeast | Apo | U | Inactive | Thr | a42 | |
| 1ZYD | Yeast | ATP | U | Inactive | Thr | a42 | ||
| TAK1 | 2EVA | Human | TAB1 | U | Inactive | Thr, DFG out | 81 | |
| SLK | 2J51 | Human | Inhibitor | U | Inactive | Thr, dimer | To be published | |
| SLK | 2JFL | Human | K0056 inhibitor | P | Inactive | dimer | To be published | |
| STK10 | 2J7T | Human | SU11274 inhibitor | U | Inactive | dimcr | To be published | |
| CK1 | CK1 | 2CSN | Yeast | CKI7 inhibitor | U | ? | Thr ? | a43 |
| CK1 | 1CSN | Yeast | MgATP | U | ? | Thr? | 52 | |
| CKlγ1 | 2CMW | Human | Purine class Inhibitor | U | ? | ? | To be published | |
| CKlγ2 | 2C47 | Human | Inhibitor | U | Inactive | Act loop, dimer | To be published | |
| CK1γ3 | 2CHL | Human | Inhibitor | U | ? | Thr? | To be published | |
| CK1γ3 | 2IZR | Human | inhibitor | U | Active ? | Thr ?- | To be published | |
| CK1δ | 1CK1 | Rat | Apo | U | Active | - | a44 | |
| TKL | TGFβ TGFβ |
1B6C | Human | FKBP12 | U | Inactive | Act loop | 57 |
| 1PY5 | Human | Inhibitor | U | Inactive | Act loop | a45 | ||
| B-Raf | 1UWH | Human | Inhibitor | U | Inactive | Act loop | 56 | |
| IRAK-4 | 2NRY | Human | Staurosporine | P | Active | - | a46 | |
| OTHER | PKnB | IMRU | Bacteria | ATP-γs | U | Inactive | Thr | 60 |
| 1O6Y | Bacteria | MgAMPPCP | U | Inactive | Thr | a47 | ||
| PknE | 2H34 | Bacteria | apo | U | Inactive | Thr, DFG-out | a48 | |
| WNKI | IT4H | Rat | Apo | U | Inactive | Thr, Act loop | 59 | |
| WEEIA | 1X8B | Human | Inhibitor | U | Active | - | a49 | |
| STK16 | 2BUJ | Human | Staurosporine | U | ? | ? | To be published | |
| NEK2 | 2CL1 | Human | Pyrrole Indolinone | U | Inactive | Thr, DFG-out | To be published |
The designations for origin of inactivity are DFF-Out, if the concerved DFG is displaced from the active conformation, Thr refers to displacement of Thr201 or homologous residue, and Act loop refers to other displacements of the activation loop, but not Thr201. Dimer is used when dimerization has contributed to inactivity, and “?” is used where some ambiguity is present. The table is available at http://www.hhmi.swmed.edu/Labs/bg/Kinase
Zheng, J.; Trafny, E. A.; Knighton, D. R.; Xuong, N.-H.; Taylor, S. S.; Ten Eyck, L. F.; Sowadski, J. M. Acta Cryst. 1993, D49, 362.
Narayana, N.; Cox, S.; Nguyen-huu, X.; Ten Eyck, L. F.; Taylor, S. S. Struct 1997, 5, 921.
Narayana, N.; Cox, S.; Shaltiel, S.; Taylor, S. S.; Xuong, N. Biochem 1997, 36, 4438.
Akamine, P.; Madhusudan; Wu, J.; Xuong, N. H.; Ten Eyck, L. F.; Taylor, S. S. J Mol Biol 2003, 327, 159.
Madhusudan; Akamine, P.; Xuong, N. H.; Taylor, S. S. Nat Struct Biol 2002, 9, 273.
Yang, J.; Cron, P.; Thompson, V.; Good, V. M.; Hess, D.; Hemmings, B. A.; Barford, D. Mol Cell 2002, 9, 1227.
Messerschmidt, A.; Macieira, S.; Velarde, M.; Badeker. M.; Benda, C.; Jestel, A.; Brandstetter, H.; Neuefeind, T.; Blaesse, M. J Mol Biol 2005, 352, 918.
Heron, N. M.; Anderson, M.; Blowers, D. P.; Breed, J.; Eden, J. M.; Green, S.; Hill, G. B,; Johnson, T.; Jung, F. H.; McMiken, H. H.; Mortlock, A. A.; Pannifer, A. D.; Pauptit, R. A.; Pink, J.; Roberts, N. J.; Rowsell, S. Bioorg Med Chem Lett 2006, 16, 1320.
Cheetham, G. M.; Knegtel, R. M.; Coll, J. T.; Renwick, S. B.; Swenson, L.; Weber, P.; Lippke, J. A.; Austen, D. A. J Biol Chem 2002, 277, 42419.
Yamaguchi, H.; Kasa, M.; Amano, M.; Kaibuchi, K.; Hakoshima, T. Struct 2006, 14, 589.
Chen, P.; Luo, C.; Deng, Y.; Ryan, K.; Register, J.; Margosiak, S.; Tempczyk-Russell, A.; Nguyen, B.; Myers, P.; Lundgren, K.; Kan, C. C.; O'Connor, P. M. Cell 2000, 100, 681.
Tereshko, V.; Teplova, M.; Brunzelle, J.; Watterson, D. M.; Egli, M. Nat Struct Biol 2001, 8, 899.
Kobe, B.; Heierhorst, J,; Feil, S. C.; Parker, M. W.; Benian, G. M.; Weiss, K. R.; Kemp, B. E. Embo J l996, 15, 6810.
Meng, W.; Swenson, L. L.; Fitzgibbon, M. J.; Hayakawa, K.; Ter Haar, E.; Behrens, A. E.; Fulghum, J. R.; Lippke, J. A. J Biol Chem 2002, 277, 37401.
Underwood, K. W.; Parris, K. D.; Federico, E.; Mosyak, L.; Czerwinski, R. M.; Shane, T.; Taylor, M.; Svenson, K.; Liu, Y.; Hsiao, C. L.; Wolfrom, S.; Maguire, M.; Malakian, K.; Telliez, J. B.; Lin, L. L.; Kriz, R. W.; Seehra, J.; Somers, W, S.; Stahl, M. L. Struct 2003, 11, 627.
Smith, K. J.; Carter, P. S.; Bridges, A.; Horrocks, P.; Lewis, C.; Pettman, G.; Clarke, A.; Brown, M.; Hughes, J.; Wilkinson, M.; Bax, B.; Reith, A. Struct 2004, 12, 1067.
Qian, K. C.; Wang, L.; Hickcy, E. R.; Studts, J.; Barringer, K.; Peng, C.; Kronkaitis, A.; Li, J.; White, A.; Mische, S.; Farmer, B. J Biol Chem 2005, 280,6130.
Kumar, A.; Mandiyan, V.; Suzuki, Y.; Zhang, C.; Rice, J.; Tsai, J.; Artis, D. R.; Ibrahim, P.; Bremer, R. J Mol Biol 2005, 348, 183.
Panneerselvam, S.; Marx, A.; Mandelkow, E. M.; Mandelkow, E. Struct 2006, 14, 173.
Jauch, R.; Cho, M. K.; Jakel, S.; Netter, C.; Schreiter, K.; Aicher, B.; Zweckstettcr, M.; Jackie, H.; Wahl, M. C. Embo J, 2006, 25, 4020.
Rosenberg, O. S.; Deindl, S.; Sung. R. J.; Naim, A. C.; Kuriyan. J. Cell 2005, 123, 849.
Nayak, V.; Zhao, K.; Wyce, A.; Schwartz, M, F.; Lo, W. S.; Berger, S. L.; Marmorstein, R. Struct 2006, 14, 477.
Bourne, Y.; Watson, M. H.; Hickey, M. J.; Holmes, W.; Rocque, W.; Reed, S. I,; Tainer, J. A. Cell 1996, 84, 863.
Gray, N. S.; Wodicka, L.; Thunnissen, A. M.; Norman, T. C.; Kwon, S.; Espinoza, F. H.; Morgan, D. O.; Barnes, G.; LeClerc, S.; Meijer, L.; Kim, S. H.; Lockhart, D. J.; Schultz, P. G. Science 1998, 281, 533.
Jeffrey, P. D.; Russo, A. A.; Polyak, K.; Gibbs, E.; Hurwitz, J.; Massagué, J.; Pavletich, N. P. Nature 1995, 376, 313.
Song, H.; Hanlon, N.; Brown, N. R.; Noble. M. E.; Johnson, L. N.; Barford, D. Mol Cell 2001, 7, 615.
Schulze-Gahmen, U.; De Bondt, H. L.; Kim, S. H. J Med Chem 1996, 39, 4540.
Schulze-Gahmen, U.; Brandsen, J.; Jones, H. D.; Morgan, D. O.; Meijer, L.; Vesely, J.; Kim, S.-H. PROTEINS:Structure, Function, and Genetics 1995, 22, 378.
Wu, S. Y.; McNae, I.; Kontopidis, G.; McClue, S. J.; Mclnnes, C.; Stewart, K. J.; Wang, S.; Zheleva, D. I.; Marriage, H.; Lane, D. P.; Taylor, P.; Fischer, P. M.; Walkinshaw, M. D. Struct 2003, 11, 399.
Tarricone, C.; Dhavan, R.; Peng, J.; Areces, L. B.; Tsai. L. K.; Musacchio, A. Mol Cell 2001, 8, 657.
Russo, A. A.; Tong. L.; Lee, J. O.; Jeffrey, P. D.; Pavletich, N. P. Nature 1998, 395, 237.
Schulze-Gahmen, U.; Kim, S. H. Nat Struct Biol 2002, 9, 177.
Lolli, G.; Lowe, E. D.; Brown, N. R.; Johnson, L. N. Struct 2004, 12, 2067.
Wilson, K. P.; Fitzgibbon, M. J.; Caron, P. R.; Griffith, J. P.; Chen, W. Y.; McCaffrey, P. G.; Chambers, S. P.; Su, M. S.-S. The J of Biol Chem 1996, 271, 27696.
Patel, S. B.; Cameron, P. M.; Frantz-Wattley, B.; O'Neill, E.; Becker. J, W.; Scapin, G. Biochim Biophys Acta 2004, 1696, 67.
Yde, C. W.; Ermakova, I.; Issinger, O. G.; Niefind, K. J Mol Biol 2005, 347, 399.
Pechkova, E.; Zanotti, G.; Nicolini. C. Acta Crystallogr D Biol Crystallogr 2003, 59, 2133.
Ermakova, I.; Boldyreff, B.; Issinger, O. G.; Niefind, K. J Mol Biol 2003, 330, 925.
Battistutta, R.; Samo, S.; De Moliner, E.; Marin, O.; Issinger, O. G.; Zanotti, G.; Pinna, L. A. Eur J Biochem 2000, 267, 5184.
Nolen, B.; Ngo, J.; Chakrabarti, S.; Vu, D.; Adams, J. A.; Ghosh, G. Biochem 2003, 42, 9575.
Ohren, J. F.; Chen, H.; Pavlovsky, A.; Whitehead, C.; Zhang, E.; Kuffa, P.; Yan, C.; McConnell, P.; Spessard, C.; Banotai, C.; Mueller, W. T.; Delaney, A.; Omer, C.; Sebolt-Leopold, J.; Dudley, D. T.; Leung, I. K.; Flamme. C.; Warmus, J.; Kaufman, M.; Barrett, S.; Tecle, H.; Hasemann, C. A. Nat Struct Mol Biol 2004, 11, 1192.
Padyana, A. K.; Qiu, H.; Roll-Mecak, A.; Hinnebusch, A. G.; Burley, S. K. J Biol Chem 2005, 280, 29289.
Xu, R.-M.; Carmel, G.; Kuret, J.; Cheng, X. Proc. Natl. Acad. Sci. 1996, 93, 6308.
Longenecker, K. L.; Roach, P. J.; Hurley, T. D. The J Mol Biol 1996, 257, 618.
Sawyer, J. S.; Beight, D. W.; Britt, K. S.; Anderson, B. D.; Campbell, R. M.; Goodson. T., Jr.; Herron, D. K.; Li, H. Y.; McMillen, W. T.; Mort, N.; Parsons, S.; Smith, E. C.; Wagner, J. R.; Yan, L.; Zhang, F.; Yingling, J. M. Bioorg Med Chem Lett 2004, 14, 3581.
Wang, Z.; Liu, J.; Sudom, A.; Ayres, M.; Li, S.; Wesche, H.; Powers, J.P.; Walker, N. P. Struct. 2006, 14, 1835
Ortiz-Lombardia, M.; Pompeo, F.; Boitel, B.; Alzari, P.M. J Biol Chem 2003, 278, 13094.
Gay, L. M.; Ng, H. L; Alber, T. J Mol Biol 2006, 360, 409.
Squire, C. J.; Dickson, J. M.; Ivanovic, I.; Baker, E. N. Struct 2005, 13, 541.
Ser/Thr kinases have been studied structurally in complex with peptides derived from substrates and other interacting proteins (Table 2). Peptides encompassing the site of phosphorylation in substrate, which bind to the active site, have been defined structurally in relatively few kinases. These are PKA30,51,62 and PKB32 in AGC group, phosphorylase kinase γ (PHK)39 and Pirn-112 in the CaMK group, and CDK263,64 in the CMGC group. The paucity of structural data may be due to poor Km’s (~0.5 mM range) of active-site directed peptides.39,65–67 On the other hand, at this point it is usually apparent when a structure is that of an active kinase. For example, the structures of two CK1s, and the STE20s TAO2 and PAK1 (Table 1) have been defined in uncomplexed but active conformations, thus providing some information about their substrate binding modes. Below we discuss interactions in the P+1 specificity pocket.
Table 2.
Crystal structures of protein kinases in complex with substrate and other peptides
| Group | Kinase | PDB | Complex | Reference |
|---|---|---|---|---|
| AGC | PKA | 1ATP 1CDK |
PKI peptide PKI peptide |
27,61 62 |
| PKB | 1O6K | GSK3 peptide | 31 | |
| Aurora | 1OL5 | TPX-2 peptide | 77 | |
| Aurora | 2BFX | lncenp peptide | 78 | |
| CAMK | PHK | 2PHK | Phospohorylase peptide | 39 |
| Pim-1 | 2BIL | Pimtide peptide | To be published | |
| CMGC | CDK2 | 1JST | Cyclin-A | 42 |
| 1H28 | P107 peptide | 63 | ||
| 1H27 | P27 peptide | 63 | ||
| 2CCI | CDC6 Peptide | 67 | ||
| P38 | 1LEW 1LEZ |
MEF2A peptide MKK3b peptide |
68 68 |
|
| ERK2 | 2FYS 2GPH |
MKP-3 peptide HePTP peptide |
71 70 |
|
| JNK1 | 1UKH | JIP peptide | 69 | |
| GSK3 | 1GNG 1O9U |
FRATtide Axin peptide |
17 114 |
|
| FUS3 | 2B9J 2F49 |
FAR1 peptide Ste5 peptide |
51 72 |
|
| Other | PKR | 2A1A | eIF2 | 50 |
Rather than binding substrates tightly at the active site, many kinases utilize docking interactions in grooves outside the active site to engage “docking motifs” in substrates and other molecules. Docking interactions have been defined structurally in the CMGC group and in the AGC group (Table 2, Figure 1b). The first to be published was CDK2/cyclin A in complex with a peptide derived from p27Kip,42 but several other cyclin A/peptide interactions have been studied.63,67 In MAP kinases, part of the CMGC group, a docking groove is present in the C-terminal lobe, and structural data are available for p38α68 and JNK1,69 as well as ERK2,70,71 and the S. cerevisiae MAP kinase Fus3 in complexes with substrates51 and other interacting proteins.72 Further, SR protein kinases (SRPKs) are CMGC family members that phosphorylate serine arginine rich (SR) protein splicing factors, and a complex of SRPK1 in complex with a substrate derived peptide has been studied73. In the AGC kinases, a pocket is present in the N-terminal lobe (the hydrophobic motif (HM) pocket) that is utilized by phosphoinositide-dependent protein kinase (PDK1) to bind substrates, and by other AGC-group kinases for diverse purposes. Structural data are available for PDK1 lacking HM peptide31,74 and for PKB both with32 and without75 HM peptide. Some of these structures have been reviewed.4,76 In addition, data are available for Aurora kinase77–78 and G-protein-coupled receptor kinase GRK2,27,79,80 which also utilize the HM pocket for subunit interactions. A docking interaction has also been defined recently for the STE group kinase TAK1, in complex with its activator TAB1.81
3. Recognition in the P+1 Specificity Pocket
With data available on all of the major groups of Ser/Thr protein kinases, much of the spectrum of active site substrate recognition modes can be defined. This is especially tractable for the P+1 specificity pocket. It is the only pocket constructed to accept a single amino acid from substrate, and is a frequent specificity determinant.14,82,83 Further, the P+1 pocket is a regulatory site and is often refolded in inactive forms of protein kinases.21 Here we review the three major P+1 pocket recognition modes in Ser/Thr protein kinases. This site is the locus of specificity determination in tyrosine kinases as well50,84 and binds the P0 tyrosine.
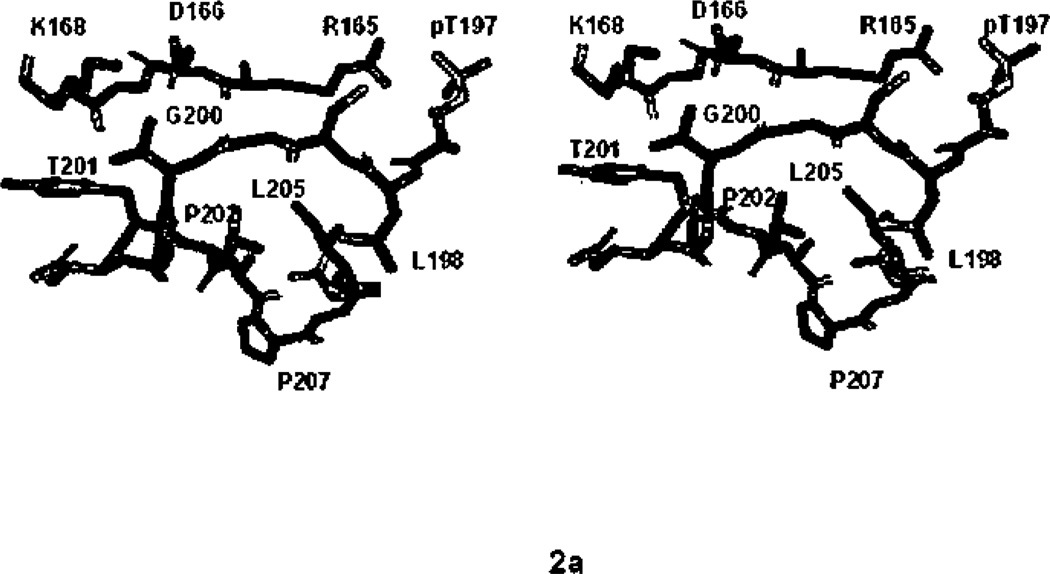
The P+1 binding pocket of all Ser/Thr protein kinases adopts a similar architecture with respect to the backbone of the polypeptide, as typified by PKA (Figure 2a).7,85 In PKA, Leu198–Leu205, residues directly following the activation segment phosphorylation site form the pocket. The salient features are a helical turn (from Thr201 to Leu205) that forms one wall of the pocket, the residues preceding the helix, which form the upper rim of the pocket, and a hydrophobic residue, Leu205 at the bottom. Thr201, a catalytic residue, faces the active site (discussed below). Residues on the outside of the helix, Glu203 and Tyr204, face the interior of the protein. Following this structure is the APE helix (Table 3, Figures 1a, 2b–2e).
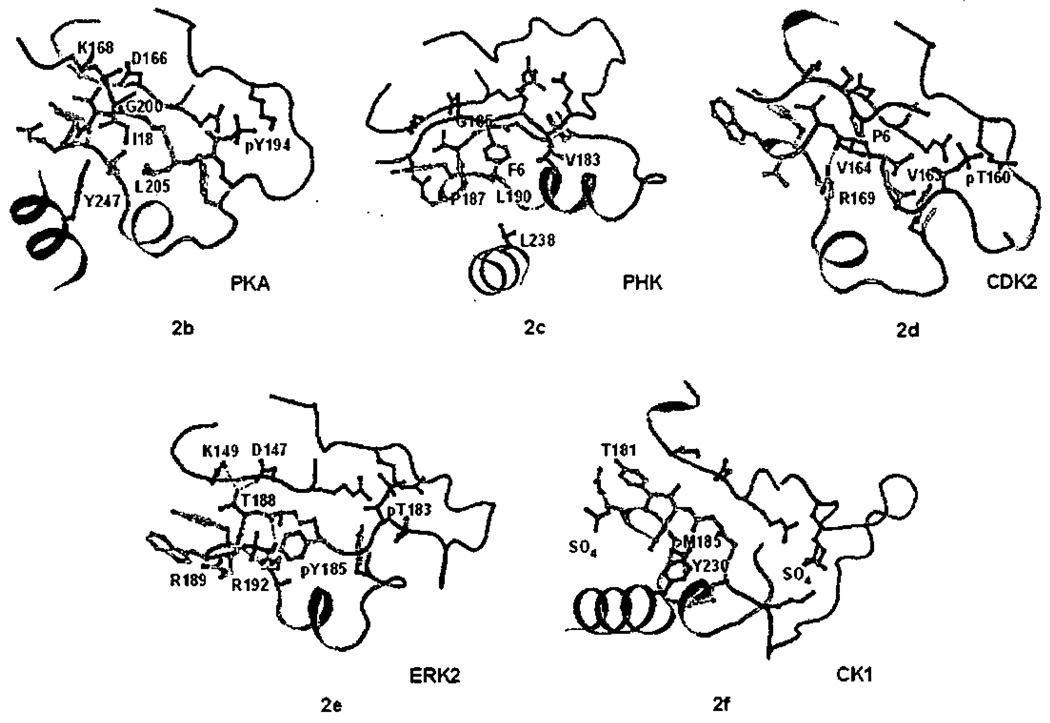
Figure 2.
The P+1 specificity pocket of Ser/Thr protein kinases a). All atom stereoview of the P+1 specificity pocket of PKA (PDB file 1ATP), carbon in green, oxygen in red, nitrogen in blue, phosphorus in orange and sulfur in yellow, b) P+1 specificity pocket of PKA (PDB file 1ATP), c) PHK (2PHK), d) CDK2 (1QMZ), e) ERK2 (2ERK), and f) CK1 (1CSN). In Figures 2b–2f, carbon atoms of the bound peptide are light blue, the P+1 pocket and APE helix are yellow, the activation segment is green, the catalytic loop is magenta, and helix G is cyan. In Figures 2b–2f only the P+1 pocket, close by catalytic residues, and the activation segment phosphorylation site are shown rendered in ball-and-stick representation. Hydrogen bonds are shown in red dashed lines.
Table 3.
Activation loop and P+1 sites of Ser/Thr protein kinases

|
Sequences between the underlined residues are remodeled in inactive structures. Residues in the P+1 pocket and APE helix are green, the conserved threonine is red, the conserved glycine is cyan, and its replacement in CMGC kinases dark blue, the conserved hydrophobic residue at the bottom of the P+1 pocket is lavender, and its replacement in CMGC kinases magenta.
3.1 AGC, CAMK and STE Kinases
The P+1 sites of AGC kinase substrates are known to be hydrophobic.83 The structure of PKA61 revealed hydrophobic residues (Pro202, Leu198, and Leu205) lining the P+1 pocket in the sequence 198LCGTPEYL205 (Table 3), The substrate is recognized also by Gly200, which adopts a left hand configuration (ϕ=147°, ψ/=157°) (Figure 2a) such that the carbonyl of Gly200 points up and can accept a hydrogen bond from the substrate (Figure 2b). Tyr247 at the beginning of helix G also contributes to the pocket. Similar interactions are observed in PKB32. As can be seen in Figure 2b, the P+1 pocket is situated adjacent to active site residues, such as Asp166 and Lys168; and is contiguous with the activation segment and phosphothreonine, pThr197.86
Two other major groups of protein kinases are hydrophobic-directed in the P+1 site, the CaMK group and the STE group. Two structures are available of members of the CaMK group, phosphorylase kinase γ (PHK),7,39 and Pirn-1.12 The structure of PHK, in complex with a heptapeptide possessing a phenylalanine at the P+1 site (Figure 2c), reveals similarities to PKA. PHK has the glycine (Gly185), proline (Pro187), and other hydrophobic residues that are hallmarks of hydrophobic-directed kinases including Leu190 at the bottom of the pocket (Table 3). Pim-1 offers a slight variation, with a specificity for alanine at P+1. The structure of Pim-1 kinase in complex with a substrate derived peptide reveals the glycine (Gly203 in Pim-1) accepting a hydrogen bond from substrate, as in PKA. However, the proline is replaced by alanine, and the P+1 pocket is filled with the side-chain of Phe201 in the activation segment, reducing the space for substrate (not shown). CaMK and AGC kinases have been shown to be especially poor enzymes toward substrates that have proline in the P+1 site.86
STE group kinases are also directed toward substrates with a hydrophobic residue in the P+1 site. This is apparent from the sequences of known substrates and from screening for kinase activity with peptide libraries.82 The STE group is named for several of the proteins that produce sterile phenotypes in yeast,87 STE11, STE7 and STE20.1,88 STE11 and STE7 are STE kinases involved in three-tiered kinase signaling modules, the MAP kinase cascades (reviewed in refs 89–93). Each MAP kinase cascade activates a specific MAP kinase (which are CMGC kinases, discussed below). STE11 s are MAP3Ks (or MEKK (for MAP/ERK kinase kinase), phosphorylating MAP2Ks) and STE7s are MAP2Ks (or MEKs (for MAP/ERK kinase), that phoshorylate MAPKs). STE20 phosphorylates and activates STE11 and is thus referred to as a MAP4K.94 Close STE20 homologs such as PAK1 are putative MAP4Ks.88,90,95,96 MAP3Ks (including STE11) phosphorylate MAP2Ks on two residues in their activation segments.97 The MAP2K MEK2 has the sequence DS*MANS*F,97 and both of the phosphorylation sites are followed by a hydrophobic residue. Similarly, the MAP2Ks MEKs 3 and 6, which are phosphorylated, for example, by the MAP3K TAO2,98 have the phosphorylation motif DS*VAKT*I. MAP4Ks phosphorylate MAP3Ks on a Ser/Thr followed by a hydrophobic residue (in TA02, the sequence is PANS*F). Other MAP3Ks have related sequences. Although structures of STE group kinase substrate complexes are not available, the structures of the STE kinases TAO254 and PAK155 have been solved in active conformations. The P+1 pockets of these proteins resemble that of PKA. In TAO2, for example, the pocket is lined by Phe182, Gly184, Pro 186, Met189, and Leu234, all having homologous counterparts in PKA. On the other hand, the MAP2Ks (and STE7s), which are members of the STE group, exhibit dual specificity99 toward Ser/Thr and tyrosine,100,101 and thus must have unique interactions in the P+1 pocket. The mechanism for this dual specificity has yet to be elucidated.
In addition to the similarities in P+1 site recognition discussed above, AGC, CaMK and STE kinases have been shown recently to have common recognition modes for residues N-terminal to the P0 site of substrates.102
3.2. CMGC Kinases
The specificity of CMGC kinases in the P+1 site is either for proline or the P+1 site is not a strong specificity determinant. CDKs and the MAP kinases are proline directed at the P+1 site of substrates.14 In the CMGC kinases GSK3, CK2, and SR kinases, the P+1 site is not a strong specificity determinant. The structure of CDK2/cyclin A in complex with substrate peptide reveals how the P+1 pocket is arranged to bind proline in CDK2.64 The P+1 pocket is formed by the sequence is 163VVTLWYR169 (Table 3). The backbone of the polypeptide forms a helical turn, as in PKA (Figure 2d). However, the P+1 pocket is filled by the side chain of Arg 171 (Leu205 in PKA), so there is no room for a side chain from substrate. Further, Gly200 of PKA is replaced by Vai163. Vai163 also adopts a left-hand configuration, and the carbonyl accepts a hydrogen bond from Arg171, creating a flat surface with no potential for hydrogen bonding with substrate. Since the proline in substrate has no hydrogen-bonding potential, this feature probably contributes to the proline specificity of CDK2.10,29,103
MAPKs are CMGC kinases that phosphorylate microtubule associated protein-2 (MAP-2) kinase in response to insulin,104 and were cloned105 based on its activity as an S6-kinase, and the regulation of these proteins by MAP kinase has been extensively studied.89,90,106 MAP kinases are also proline directed.65,107,108 The phospho-tyrosme of active ERK2 is in the P+1 site (Figure 2e).100 From the structure of active ERK2,29 and by homology with CDK2, it appears that the proline residue of substrate may interact directly with pTyr185, although no structural data is available for a MAP kinase with substrate bound in the active site. pTyr185 binds to Arg192, which extends out from the bottom of the P+1 pocket, and with Arg189, which is on the edge of the pocket (Figure 2e). Phosphotyrosine 185 is required for ERK2 activity and cannot be replaced by other amino acids.109 This may be partly explained by the remodeling of the activation segment induced by pTyr185.29 The structure of doubly phosphorylated p38γ,45 another MAP kinase, is similar. (The active site of ERK2 is reviewed in ref 10.)
Glycogen synthase kinase-3 (GSK3) is another CMGC group member.110 GSK3 has recently become the object of intense study due to its involvement in pattern formation (reviewed in ref 111), neurodegeneration,112 and insulin signaling, and is a drug target for diabetes, Alzheimer’s disease and cancer.113 Structures of GSK3 are available,13,17,114–116 and have been reviewed.4–10 Its substrate specificity,110,115,117 and its pathway involvement117 have also been reviewed. Many substrates of GSK3 require substrate “priming” (prior phosphorylation) at the P+4 site of substrate,115 and the importance of the P+1 site appears to be diminished. The structures of GSK3 were surprising though, because GSK3 is phosphorylated on Tyr216 in the activation segment, and resembles ERK2, which is proline-directed. Quite recent studies show that GSK3 phosphorylates a bone fide substrate, LDL-related protein-6, at positions containing proline in the P+1 site, and do not require priming.118 This result is consistent with the similarity of GSK3 and ERK2.
SR protein kinases (SRPKs) are CMGC family members that phosphorylate serine arginine rich (SR) protein splicing factors119 processively120. The SRPKs 1 and 2 are specific for sequences in substrates containing arginine in the P+1 position, but can accept proline,121,122 whereas the SR kinase Sky1p123,124 is proline directed. The structures of SRPK173 and Sky1p49 have been determined. Although no active site complex is available, the structures are very similar to ERK2, with two arginines in positions homologous to Arg189 and Arg192 of ERK2 (Figure 2e). The phosphotyrosine of ERK2 is replaced by an aspartic acid in SRPK1, which may contribute to the specificity for arginine. The homologous residue in the proline-directed Sky1p is serine. Thus the SR proteins are similar to GSK3, discussed above, and can accept both proline and other residues in the P+1 site. The SR kinases are interesting also because they build a P+1 pocket very similar to other kinases, but do not rely on activation segment phosphorlation.21,49
Casein kinase 2’s (CK2s, or CKIIs) are a subgroup of CMGC kinases for which the P+1 site of substrates is not a strong specificity determinant, but sometimes has specificity for negatively charged residues. CK2s have hundreds of putative substrates.125 The physiological substrates, and the pathways in which CK2s function are currently being defined.126 Studies based on peptide libraries show that the P+1 site of substrate may be proline, aspartic acid, glutamic acid, or serine.127,128 Although no structural data is available for CK2 in complex with substrates, based on the uncomplexed structure of CK2 CKIIα26,47 it has been proposed10 that a lysine residue (Lys198 in CKIIα), that replaces the Arg191 of ERK2, (Figure 2e) may explain the specificity for acidic residues.
3.3. CK1 Kinases
The CK1s (casein kinase 1) are Ser/Thr protein kinases involved in DNA repair, vesicle trafficking, cell cycle progression and WNT signaling129, and common in C. elegans.130 The P+1 site is not a strong specificity determinant. Instead, as with GSK3, substrate priming at sites C-terminal to P0,131,132 as well as an acidic patch N-terminal to the phosphorylation site,14 dominate interactions. Sequences of CK1s are similar to each other but diverge from other protein kinases both at the beginning and end of the P+1 pocket (Table 3). Although no substrate complex is available for CK1 family members, active conformations of two CK1s have been determined. The structure of CK1 from S. pombe52 is in an active conformation in the P+1 site (discussed below). The P+1 site looks quite similar to PKA, except that a methionine corresponding to Leu205 of PKA completely fills the pocket, leaving a shallow depression (Figure 2f). In CK1 S. pombe52 Thr181 in the activation segment, and Tyr230 in helix G occlude the pocket (Figure 2f). In both of these CK1s, the unusual sequence SINTH following the P+1 binding pocket mediates different interactions with helix I.
3.4. The Active Site Residue Thr201 and Remodeling of the P+1 Pocket
Thr201 (PKA numbering) is on the upper rim of the P+1 pocket. It is highly conserved, although replaced by Ser in CK2s. Thr201 forms hydrogen bonding interactions with the catalytic aspartate in Ser/Thr protein kinases (Asp166) and a catalytic lysine (Lys168) (Figures 2b).7,10,85,133 Mutation of this residue abolishes catalytic activity toward peptide substrates and autophosphorylation.85 Phosphorylation of this residue inhibits the kinase MARK/PAR-1.134
The placement of this conserved threonine residue is affected by activation segment phosphorylation. Activation segment phosphorylation induces conformational changes7,29,135 that can extend from the DFG at the N-terminus7,135,136 through the P+1 specificity pocket at the C-terminus of the loop (the extent of remodeling is indicated in Table 3). In many kinases, the remodeling includes Thr201 (or the homologous residue). Thus the placement of Thr201 is one hallmark of the active conformation.
Several protein kinases are massively remodeled in the P+1 site (Table 3) in inactive versus active structures. The STE20 kinase PAK1, the kinase WNK1, and the CaMK group kinase MNK-1 exhibit the greatest extent of remodeling (Table 3). In the STE20 PAK1, remodeling involves most of the P+1 site helix, extending to Trp430 into the motif 429YWMAPE434.25,54 Tyr429–Trp430 are involved in an elaborate conformational switch between inactive and active PAK1.25,54,55 This motif is conserved in most STE20 homologs,54 and thus similar activation mechanisms may be present in other STE group kinases. A similar degree of remodeling is present in the low activity form of WNK1.59 The CaMK group kinase MAP kinase interacting kinase (MNK-1) is remodeled from the DFG motif at the beginning of the activation segment through the beginning of helix F137. In several cases, inactive forms involve conformational changes in Thr201 (or the homologous residue). Thus, remodeling in this region affects both substrate binding and catalytic activity. It is interesting that remodeling of the P+1 pocket has been observed in at least one member of each of the major groups of kinases (Table 3).
To summarize the discussion of the P+1 pocket recognition and remodeling, first, it is becoming clear that there are only a few binding modes for the P+1 residue in substrates of Ser/Thr protein kinases. Second, the recognition modes tend to be maintained within each group. The AGC, CaMK, and STE groups recognize hydrophobic residues in the P+1 site. Many members of the CMGC group are proline-directed. Otherwise, the P+1 site is not a strong determinant, and the P+1 pocket has been reduced to a surface depression. Third, it is interesting that the P+1 specificity can be overridden. Examples include CK2, and CK1, each of which recognizes “primed” substrates. Nevertheless, the default P+1 specificity is also used for some substrates. Fourth, structural data on uncomplexed kinases support observed P+1 site preferences, even when structures of complexes are not available. Fifth, the P+1 pocket is remodeled in inactive forms of at least one member of each group of protein kinases. Finally, the fact that the catalytic residue Thr201 is remodeled, along with other residues in the P+1 pocket, implies that inactive forms are deficient in both substrate recognition and catalysis.
4. Docking Interactions
One solution to conferring specificity in the very large protein kinase family is the evolution of new binding sites for substrates and other interaction proteins. Binding sites outside the active site are referred to as docking grooves, and occur on the surface of the kinase domain or in adaptor proteins. These grooves interact with contiguous peptide sequences in substrates referred to as docking motifs or docking sites. Here we will focus on five of these interactions, those of the MAP kinase ERK2, PKA and PKB, CDK2/cyclinA/p107, and SR protein kinases. Mechanisms of specificity determination will be compared. The effect of docking interactions on substrate affinity, and the data suggestive that allosteric conformational changes contribute to pathway specificity will be discussed.
4.1. Docking Interactions in MAP Kinases
Twelve years ago it was discovered that the MAP kinase c-Jun kinase (JNK) binds its substrates outside the active site, engaging substrate sequences distal from the site of phosphorylation.l38,l39 Since then, a large body of data has accumulated showing that all MAP kinases studied use docking interactions to bind substrates. Further, the MAP kinases engage their activating kinases, phosphatases, and scaffolding proteins using similar docking interactions (reviewed in refs 4,140–147). The “D-motif” docking site, present in MAP kinase interacting proteins, is best studied. D-motif peptide complexes with target MAP kinases have been the object of six recent crystallographic studies (Table 2). The structures span the spectrum of MAP kinase binding partners. Structures of p38α were determined in complex with substrate and activating enzyme-derived peptides,68 JNK1 in complex with a scaffold-derived peptide,69 S. cerevisiae MAPK Fus3 in complex with substrate,51 MAP2K3,51 and scaffold72 derived peptides, and ERK2 in complex with MAP2K-derived70 and two different phosphatase-derived peptides.70,71 Each peptide binds to homologous loci. In this review, we focus on ERK2 in complex with a peptide derived from hematopoetic protein tyrosine phosphatase (HePTP), the structure of which we determined recently, and which revealed the most extensive interactions seen to date. The structural data show that D-motif docking interactions contribute to specificity not only through unique interactions in the docking groove, but through allosteric effects that remodel the activation segment and active site of the MAP kinase.
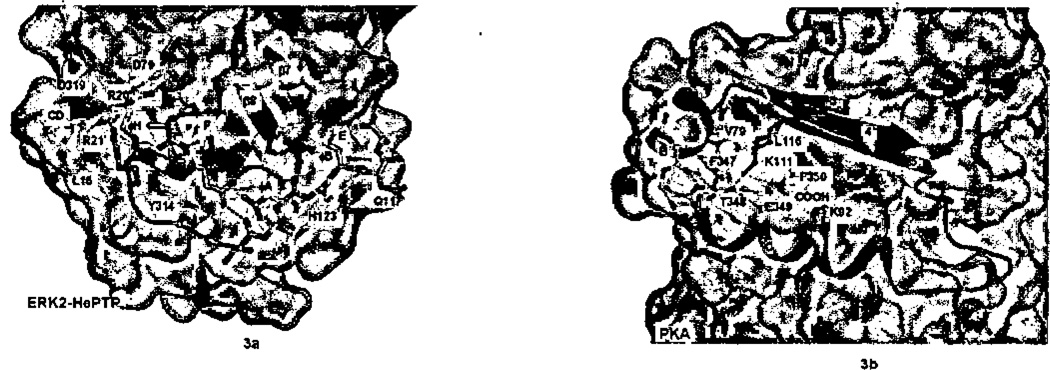
D-motifs directed toward MAP kinases encompass ~13–16 residues, X ∅H XX(R/K)2-X2–4-∅A-X-∅B, featuring a separation of charged (R/K) and hydrophobic elements (∅). The docking groove for D-motifs in MAP kinases was defined by mutational analyses.143,148–150 The docking groove was found to be in the C-terminal lobe of the kinase, near the β7–β8 reverse turn (Figure 3a), and an acidic patch in L16 (the linker at the C-terminus of the kinase lobe43) labeled the “CD”, for common docking domain.143 In the ERK2/pepHePTP complex, the 16-mer peptide from HePTP phosphatase forms an alpha helix over the first six residues and then is extended.70 The helix binds in a shallow depression formed where the CD domain in L16 binds atop helix F. The complex reveals the importance of the ∅H: it gives the helix a hydrophobic face. The helix orients the basic residues (R20’and R21’) in the peptide toward the CD domain forming an elaborate network of ionic hydrogen bonds. The extended segment of pepHePTP, including the ∅A-X-∅B motif, is laced perpendicularly over two helices, D and E and under the β7–β8 reverse turn. The backbone of the peptide is recognized through hydrogen bonds to Gln117 and His 123 (in the loop between helices D and E of ERK2). The ∅A-X-∅B motif contacts residues in the two helices ∅A to helix E and ∅B to helix D. There is considerable heterogeneity among the MAP kinases in their interactions with D-motifs, both with the CD domain and the hydrophobic docking groove,51,70 which doubtless contributes to specificity among MAP kinases. The binding site that forms the MAP kinase docking groove is present in many protein kinases, and is often utilized to bind the kinase C-terminal tail.4,54
Figure 3.
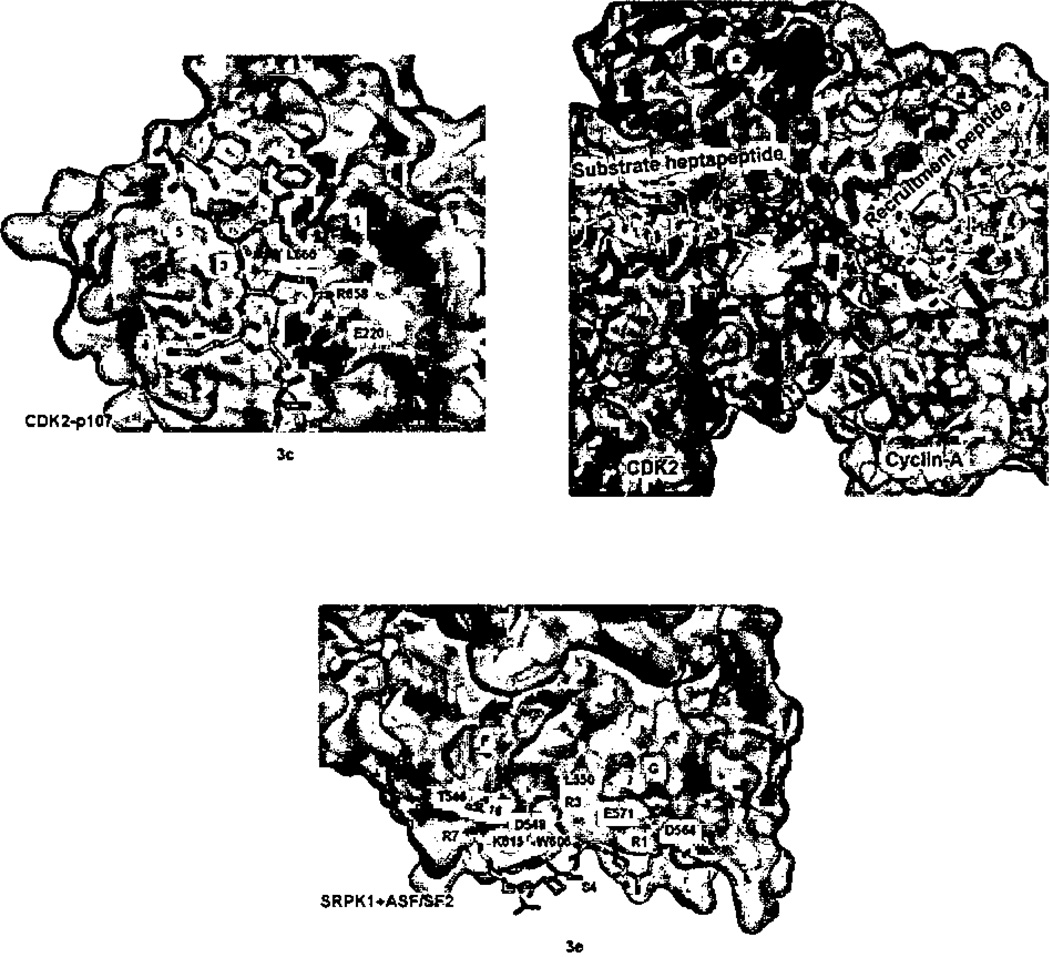
Docking interactions between kinase and corresponding peptides with the kinase surface rendered in gray, otherwise the coloring scheme is the same as in Figure 1. a) ERK2 and docking peptide HePTP (2GPH). Carbon atoms of the peptide are blue. Note how the peptide helix orients the Arg20’ and Arg21’ toward the ERK2 acidic patch. b) PKA (1ATP). C-terminus of PKA in blue binds in the kinase HM pocket. Note how the reverse turn (at Thr348-Glu349) brings Phe347 and Phe35O close together. The side-chain of Thr348, which caps the helical turn, has been eliminated for clarity, c) CDK2 with recruitment peptide pi07 (1H28). Peptide, shown in blue, is bound in the hydrophobic crevice formed by kinase helices 1 and 3. d) Surface representation of pCDK2/cyclin-A with CDC6 bis-substrate inhibitor spanning active site and recruitment site. Visible residues shown in stick representation; disordered residues indicated by green balls. e) SRPK1 with docking peptide ASF/SF2 in blue (1WBP) showing arginine-mediated interactions.
4.2. HM Motifs in AGC Kinases and AGC Kinase Substrates
In the AGC group, a group-specific pocket called the HM pocket for (hydrophobic motif binding pocket) in the N-terminal lobe is formed by helix B, which prevents helix C from packing tightly on the core β-sheet (Figures 1b and 3b). This pocket serves different purposes in different AGC kinases.3,4 In PKA, the C-terminal sequence FTEF-COOH binds in the pocket (Figure 3b). In other AGC kinases,3,4 such as PKB, the kinase C-terminal tail possesses a hydrophobic motif (HM) FXXF-S/T-F/Y, and must be phosphorylated (or replaced with a phosphorylation site mimetic) to bind to the pocket.151 The AGC kinase phosphoinositide-dependent protein kinase (PDK1) lacks the hydrophobic motif altogether. Instead, the HM is present in PDK1 substrates. The binding of substrates both activates152,153 and stabilizes74,154 PDK1. This mechanism is potentially very powerful at conferring specificity, since PDK1 is not active in the absence of its substrate.
Structural data illuminating how HM-peptides may interact with AGC kinases came first from the structure of PKA (Figure 3b).155 The four residues Phe347–Phe350 form a hairpin turn that brings the two phenylalanine residues in close proximity. Phe347 packs against a valine in helix B, and Phe350 contacts the aliphatic part of the side-chains of two lysine residues, one in helix C (Lys92) and one extending β4 (Lys111). The terminal carboxylate of PKA is also bound by the same two lysine residues. The backbone of the hairpin turn is contacted by Lys111. Thus, there is an exquisite interaction, requiring two phenylalanine residues separated by two intervening residues, accompanied by a negative charge adjacent to the second phenylalanine. Structural data on PKB HM interactions were obtained by mutating the C-terminal phosphorylation site to aspartic acid (S474D) and by making a chimera with another HM.75 The interactions are similar but more extensive (not shown). Other AGC kinase-HM interactions have been studied structurally. The G-protein coupled receptor kinase GRK2 uses this site intramolecularly to bind the RGS-homology domain,27,79,80 whereas Aurora kinase uses the same groove binds interacting proteins.77,78 Finally, the TKL family enzyme TGFβ receptor (transforming growth factor receptor-beta) also uses this groove to bind the interacting protein FKBP12.58
4.3. CDK2/Cyclin A Recruitment Peptide Interactions
CDKs bind substrates and inhibitors through cyclin mediated interactions (reviewed in refs 155,156). The cyclin inhibitor p21 binds cyclin A via a recruitment peptide sequence, ACRRLFGP, and similar sequences are present in related inhibitors.158 Substrates of CDK2 such as the tumor suppressor Rb and p107, and the transcription factors E2F also possess related recruitment sequences.159 Only a short motif, RXLΦ, where the hydrophobic amino acid Φ is either adjacent to RXL, or displaced by one residue, is conserved (Table 4). Several complexes of CDK2/cyclin A with inhibitor or substrate derived recruitment peptides have been studied structurally (Table 2), and also well-illustrated.41,63 We focus on the structure the of CDK2/cyclin A/p 107 peptide (Figure 3c) as an example.63 This has two domains, the first of which binds recruitment peptides. The recruitment peptide binding domain is comprised of five helices, the first three of which resemble the first three helices of an antiparallel four-helix bundle.160
Table 4.
Docking Peptide Motifs
| a) Docking site peptides for MAPK | |
| HePTP | RLQERRGSNVALMLDV |
| ELK1 | PQKGRKPRDLELPLSP |
| MEK2 | MKARRKPVLPALTINP |
| b) Recruitment Peptide Motifs for CDKs | |
| p27 | KPSACRNLFGP |
| p53 | STSRHKKLMFK |
| p107 | AGSAKRRLFGE |
| pRB | PPKPLKKLRFD |
| c) Hydrophobic Motifs for AGC kinases | |
| PKA | EKCGKE–FTEF |
| PKB | DSERRPHFPQFSYSASTA |
| PRK2 | EEEQEM–FRDFDYIADWC |
Two short helices pack orthogonally on one of the major helices, helix 3, such that the loop connecting them falls near the intersection of helices 1 and 3. The peptide binding groove is a shallow V-shaped hydrophobic crevice between helices 1 and 3 and the helix 4–helix 5 loop. The recruitment peptide adopts a primarily extended conformation. The conserved leucine in the motif RXLΦ is defined as P0 by Lowe et al.63 The leucine, as well as the Φ residue in the P1 position, binds in the crevice between helices 1 and 3. The conserved arginine (P−2) lies across the top of helix 1, and forms an ion pair with Glu220 in helix 1. Interactions with the peptide backbone are made by a glutamine in helix 3 (Gln254), and the backbone of a residue in the loop between helix 4 the helix 5 (Ile281). This simple binding groove, then, confers recognition of a positive charge separated by one residue from two hydrophobic amino acids.
The recruitment site in cyclin A is about 35 Å away from the catalytic site, and the mechanism for enhancing substrate phosphorylation was originally proposed to raise the local concentration of the protein substrate.161 A recent elegant study67 utilizing a ATP-mimetic bisubstrate inhibitor162 has allowed a 30 residue peptide spanning from the active site to the recruitment site to be visualized. Indeed, several residues between the recruitment site and active site are disordered, validating the concept that recruitment interactions increase the local concentration of substrate. The authors also show that improvement in catalytic efficiency is primarily a Km effect, which goes down 18-fold for substrate with RXL containing substrates (see also 163). No conformational changes are induced in CDK2/Cyclin A by the interaction with recruitment peptide63 or bisubstrate peptide67.
4.4. SRPK Docking Interactions
SRPKs phosphorylate subsets of available serine phosphorylation sites within the target “RS”-domains of substrates, and the exact position of phosphorylation is thought to direct the outcome of alternative splicing mediated by the SR proteins.122,73 Ngo et al73 have determined the structure of a truncation of SRPK 1 in the presence of substrate derived peptide (RERSPTR), and found that the peptide binds in a docking groove, rather than at the active site. They further demonstrate the presence of this docking site in one of its substrates, the splicing factor ASF. The docking interaction appears to sequester phosphorylation sites as well as limit the phosphorylation at other sites73. The docking groove is formed where an insert in the connection between helices F and G unique to the SR kinases causes helix G and a two turn helix in the MAP kinase insertion (labeled α3L1443 (Figures 1b, and 3e) to be farther separated than in MAP kinases. Arg1 of the peptide (Figure 3e) is recognized by two Asp564 and Glu571 in helix G, residues separated by two turns of helix. Arg3 contacts two carbonyls in the helix F/helix G connection, and Arg7 contacts the backbone of Tyr181 at the terminus of helix D and Thr546 in the helix F/G connection. The peptide backbone makes only a single contact Lys615 at the end of helix G. The only hydrophobic interactions are with the aliphatic portion of the Arg3, representing a major departure from the themes observed in other kinases. Peptide interactions do not induce any conformational changes in SRPK1 (see 1 WAK.pdb). The recent structure of STE group kinase TAK1, in complex with its activator TABl reveals a similar locus of interactions to ASF/SF2, but the binding site is more extensive.81
4.5. Similarities and Differences in Docking Interactions
The docking interactions described above have some interesting parallels. First, each peptide primarily adopts an extended conformation, but may have some internal hydrogen bonding. This hydrogen bonding can be significant, such as the small helix as in HePTP bound to ERK2, to just a few hydrogen bonds, as in p107 bound to CDK2/cyclin A. Second, the backbone of the peptide is recognized in each case. In ERK2 and other MAP kinases, a glutamine, or a glutamine and histidine make hydrogen bonds with the peptide backbone. In cyclin/p107 a histidine residue as well as the backbone of the helix 4 – helix 5 loop contact the peptide. In PKA, a lysine residue (Lys111) contacts the peptide backbone. Similarly, in SRPK1, a lysine contacts the peptide backbone. A third similarity, among ERK2, PKA, and CDK2/cylcin A, is the presence of binding sites for two or more hydrophobic residues. Finally, the binding grooves invariably involve two or more helices, as well as a loop or additional secondary structure.
There are also interesting differences which surely confer specificity among these groups of kinases. Among the groups that bind hydrophobic residues, the docking grooves are organized to bind residues separated by a different spacing: Cyclins bind adjacent hydrophobic residues or residues separated by one amino acid in a depression formed by two parallel helices in contact that form the docking groove. MAP kinases recognize hydrophobic residues separated by one amino acid, through well-separated pockets, on two different helices. A glutamine residue contacts the peptide backbone between the two hydrophobic residues. AGC kinases bind HM peptides that possess hydrophobes separated by two amino acids. The binding pockets are deep and close together, and an intricate network of hydrogen bonds recognizes a hairpin turn in the HM peptide. A second obvious difference between the binding grooves in MAP kinases, AGC kinases and cyclins is the relative orientation of the hydrophobic versus charged and hydrogen bonding functionalities, which is necessary to bind the distinctive docking motifs (Table 4).
As more kinase-substrate complexes are studied, it is becoming clear that larger surfaces of substrates can be involved in recognition. For example, the complex of PKR, a CMGC kinase, with its native substrate eukaryotic translation initiation factor 2α (eIF2α)50 involves helix G of the kinase and an entire surface of the eIF2α β-barrel (not shown). Interestingly, the interaction of PKR with eIF2 α works allosterically on the eIF2α inducing disorder in the loop containing the phosphorylation site. This may be important for active site recognition.
4.6. Allosteric Properties of Docking Interactions
The recent structures of MAP kinase docking peptide complexes and the AGC kinase PKB have revealed allosteric conformational changes that serve functions other than enhancing substrate recognition. In PKB, the allostery serves to activate the kinase. In MAP kinases, the allostery does effect active site binding of substrates, but also influences binding of processing kinases and phosphatases that act on the activation loop. These conformational changes appear to be potent methods for enhancing pathway specificity.
4.6.1 Allostery in MAP Kinase Docking Interactions
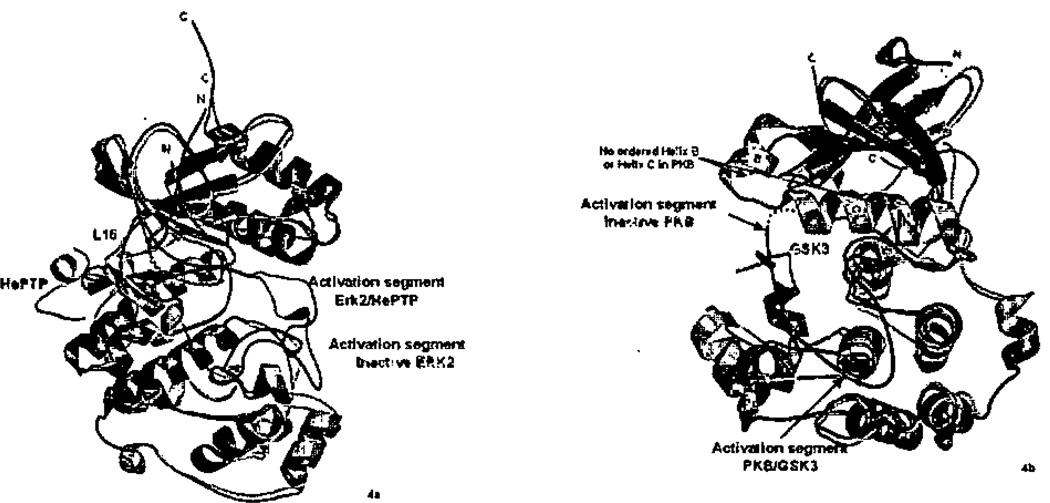
In both ERK270 and p38α,68 D-motif docking peptides induce conformational changes in the activation segment (Figure 4a). In ERK2, a new conformation of the activation segment is adopted (Figure 4a). In p38α, the activation segment becomes disordered and more susceptible to proteolysis (unpublished results). Conformational changes in ERK2 occur in solution also.164 What is the function of these conformational changes? Structural studies are available exclusively for unphosphorylated enzymes, and thus the most physiologically relevant structures involve MAP2K-derived peptides. Consider the action of MEKs, which phosphorylate MAP kinases. The phosphorylation sites of MAP kinases are sequestered or well-tethered in the low activity conformers. In unphosphorylated ERK2,43 Tyr 185, the residue phosphorylated first by MEK1/2,101 is buried under the backbone of the activation segment. In p38α,44,45 although the Tyr182 phosphorylation site is on the surface, it forms numerous intramolecular contacts. These contacts probably prevent other kinases from accessing MAP kinase phosphorylation sites. Thus, this allosteric mechanism may contribute to pathway specificity. A prediction is that docking peptides added in trans to docking-motif truncated kinases (or phosphatases) might facilitate activity, but this has not been reported so far. It may be such studies must be conducted at a very high protein concentrations to account for the local concentration effect of the docking interaction, as observed for CDK2/cyclin A67.
Figure 4.
Peptide induced allostery in MAP kinases and AGC kinases. a) ERK2/pepHePTP (2GPH) superimposed with unphosphorylated unliganded ERK2 (1ERK). Note the very large changes in the activation segment, and changes in the N-terminus. b) PKB/GSK3 peptide (1O6K) superimposed with unphosphorylated PKB (1MRY). Helices B and C, as well as the activation segment become ordered in peptide-bound PKB. Unliganded conformations are rendered in cyan, and activation segment green. Peptide-bound structures are pink, activation segment red, and peptide yellow.
The docking study in p38α showed that substrate derived peptides also induce conformational changes. But function of the conformational change induced by substrate is presumably different. The study of peptide-bound ERK2 provides the best insight, since structural data are available for both inactive and active ERK2. The peptide induced structure is closer to the active form than the inactive form (rmsd 1.8 Å versus 2.4 Å). Thus, it may be that substrate peptides are complimentary to a structure closer to the active form.
The conformational changes induced by docking motif peptides are shown for ERK2 in Figure 4a. The changes occur in the activation segment, and along L16, a linker that contacts both the peptide HePTP, and the activation segment. In p38α, peptides also if induce changes, but the changes are different in detail. In p38α, the largest change is in the activation segment, but near the peptide binding site the changes are in helix D, and the loop between helix D and helix E (not shown). The linkage between peptide binding and activation segment disorder is unclear, but may involve a relaxation process within the C-terminal lobe of p38α. Apparently, functionally similar conformational changes are induced by different mechanisms. This difference in allosteric mechanism may contribute further to specificity determination among MAP kinases70.
4.6.2. Allosteric Properties HM Motif Interactions in AGC Kinases
As discussed above, AGC kinases use the HM-pocket for different purposes in different kinases: part of the active structure as in PKA, as a binding site for a regulatory segment of the same protein as in PKB or GRK2, or other subunit as in Aurora kinase, or as a substrate binding site as in PDK1. PDK1 is the most intriguing, because it has been shown that the substrate HM both binds and activates PDK1,74,152–154 The mechanism for how this might be accomplished comes from the structure of a truncated form of PKB,75 lacking the HM (as well as a PH domain at the N-terminus of the kinase) (Figure 4b). This structure reveals massive disorder in helices B and C. Thus, apparently the HM is required to build the active structure of the PKB. The structure of PDK1 has also been solved in the absence of an HM.74,151 No similar disorder was observed, but a lattice contact may have stabilized the HM-pocket.151 Biochemical data (reviewed in ref 4) shows that HM interactions are required for PDK1 to have any activity. Thus, there very probably is an allosteric mechanism activating PDK1 through docking interactions, if not as dramatic as those observed in PKB. The allosteric activation in AGC kinases is very reminiscent of the allosterically induced activation loop changes in MAPKs.
No similar peptide-induced conformational changes have been observed in the CDK2/cyclin A/peptide complexes.42,67 On the other hand, docking interaction greatly improves the Km, as discussed above. Activation segment phosphorylated CDK2 (on Thr160), in the absence of a cyclin, adopts an inactive structure,40 and cyclin binding appears to be required to form the active enzyme.41
4.6.4. Conformational Change Energy
Conformational changes require energy. The conformational change energies (CCE)165 cannot be measured for these docking interactions. However, CCE’s have been measured for several proteins, including hemoglobin, aspartate transcarbamylase, phosphofructokinase166 and serpins.167 The observed CCEs range from 3 to 6 kcals (or 3 to 6 orders of magnitude of affinity), with 11 kcals as an upper limit (serpins). Affinities of docking peptides have been measured for the MAP kinase ERK2,150 and for the AGC kinase PDK1152,168. The affinities were found to be in the micromolar range for both. If similar CCE’s apply to MAP kinase and AGC kinase docking interactions, the intrinsic binding energy169 should be better by at least 3 kcals, or 3 orders of magnitude in affinity. This effect could reduce a nanomolar intrinsic affinity into the observed micromolar range. Thus, it seems probable that allostery may serve to gain high specificity at modest affinities. We think that allostery will prove to be very prevalent in signal-transducing protein-protein interactions for this reason, perhaps as important as compartmentalization170 and scaffolding171 in specificity determination. A further prediction can be drawn that the docking groove should bind yet-to-be-identified compounds or peptides more tightly than the native substrates, and thus could be a target for drug discovery.
5. Conclusions
With structural data now available on active and inactive forms of each of the major groups of Ser/Thr kinases defined by Hunter and colleagues, several conclusions can now be drawn. First, the P+1 specificity pocket distinguishes the kinase groups from one another. The AGC, CaMK, and Ste groups are hydrophobic directed for P+1 sites, the CMGC group is proline directed (or P +1 is not a strong determinant) and in the CK1 group, the P+1 site is not a strong determinant. The P+1 pocket is also important because it houses a catalytic threonine, Thr201 of PKA. The P+1 pocket and the threonine are remodeled in inactive forms of at least one member of every group of protein kinases. Second, biochemical and structural data have revealed that at least two groups of protein kinases utilize docking in grooves outside the active site to bind substrates and other molecules. Docking strategies are group specific. Third, docking interactions often involve allosteric conformational changes that affect the active site or activation segment. Allostery appears to be a powerful mechanism to confer pathway specificity by preventing reactions between inappropriate partners. In MAP kinases. one function may be to make the MAP kinase phosphorylation sites available for processing, while inappropriate kinases and phosphatases cannot access these sites. The docking interactions also promote the active structure. In PKB, the HM interaction is used to make the PKB activity dependent on phosphorylation, while in PDK1, the substrate provides the HM peptide. Further, conformational changes absorb intrinsic binding energy, reducing the affinity of substrates, but perhaps not affecting the substrate specificity. For these reasons, allosteric effects may prove to be prevalent in signal-transducing machinery, as mechanisms for conferring specificity among similar proteins. We look forward to further biochemical studies to better understand how kinases are regulated, and to the elucidation of larger protein-protein interactions that serve to define the specificity of protein kinases in the cellular context.
Acknowledgements
We apologize for sins of omission in this review and thank Louise Johnson, Natalie Ahn, Bostjan Kobe, and Zhulun Wang for reading and suggestions. This research was supported by National Institutes of Health grant (DK46993) and from Welch Foundation (I1128).
Abbreviations
- AGC
cAMP-dependent protein kinase/protein kinase G/protein kinase C extended family
- ASF/SF2
SR human splicing factor
- CaMK
Calcium-calmodulin dependent kinase
- CCE
Coformational change energies
- CDKs
cyclin dependent kinases
- CK1
Casein kinase-1
- CK2
casein kinase-2
- CMGC
CDK, MAP kinase, glycogen synthase kinase, and CDK-like
- ERK
Extracellular responsive kinase
- GRK2
G-protein-coupled receptor kinase-2
- GSK
Glycogen synthase kinase
- HePTP
Hematopoietic protein tyrosine phosphatase
- HM
Hydrophobic motif
- JNK
Jun-N-terminal kinase
- MAPKs
Mitogen activated protein kinases
- MAP2K
Mitogen activated protein kinase kinase
- MAP3K
Mitogen activated protein kinase kinase kinase
- MARK
Microtubule-affinity regulating kinase
- MNK-1
MAP kinase interacting kinase
- Np13p
SR-like RNA binding protein
- PAK1
p21-activated protein kinase
- PDK1
Phosphoinositide dependent protein kinase
- PepHePTP
Peptide from hematopoietic protein tyrosine phosphatase
- PHK
Phosphorylase kinase
- Pim-1
Phosphatidylinositol mannoside-1
- PKA
Protein kinase A
- PKB
Protein kinase B
- PKC
Protein kinase C
- SRPK1
Serine/Arginine rich protein kinases
- STE
homologs of STE11 and STE20
- TAB1
TAK1 binding protein 1
- TAK1
Transforming growth factor-beta activated kinase 1
- TAO2
Thousand and one kinase2
- TGFβ-receptor
transforming growth factor receptor-beta
- TKL
Tyrosine kinase like
- WNKs
With no lysine
References
- 1.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. Science. 2002;298:1912. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 2.Kostich M, English J, Madison V, Gheyas F, Wang L, Qiu P, Greene J, Laz TM. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-9-research0043. Research43.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biondi RM. Trends Biochem. Sci. 2004;29:136. doi: 10.1016/j.tibs.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Biondi RM, Nebreda AR. Biochem. J. 2003;372:1. doi: 10.1042/BJ20021641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu G, Liu Y, Shaw S. Cell Cycle. 2005;4:52. doi: 10.4161/cc.4.1.1353. [DOI] [PubMed] [Google Scholar]
- 6.Taylor SS, Kim C, Vigil D, Haste NM, Yang J, Wu J, Anand GS. Biochim. Biophys. Acta. 2005;1754:25. doi: 10.1016/j.bbapap.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Johnson LN, Lowe ED, Noble ME, Owen DJ. FEBS Lett. 1998;430:1. doi: 10.1016/s0014-5793(98)00606-1. [DOI] [PubMed] [Google Scholar]
- 8.Kobe B, Kemp BE. Nature. 1999;402:373. doi: 10.1038/46478. [DOI] [PubMed] [Google Scholar]
- 9.Kobe B, Kampmann T, Forwood JK, Listwan P, Brinkworth RI. Biochim. Biophys. Acta. 2005;1754:200. doi: 10.1016/j.bbapap.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 10.Kannan N, Neuwald AF. Protein Sci. 2004;13:2059. doi: 10.1110/ps.04637904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubbard SR. Prog. Biophys. Mol Biol. 1999;71:343. doi: 10.1016/s0079-6107(98)00047-9. [DOI] [PubMed] [Google Scholar]
- 12.Bullock AN, Debreczeni J, Amos AL, Knapp S, Turk BE. J. Biol. Chem. 2005;280:41675. doi: 10.1074/jbc.M510711200. [DOI] [PubMed] [Google Scholar]
- 13.Dajani R, Fraser E, Roe SM, Young N, Good V, Dale TC, Pearl LH. Cell. 2001;105:721. doi: 10.1016/s0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 14.Songyang Z, Lu KP, Kwon YT, Tsai L-H, Filhol O, Cochet C, Brickey DA, Soderling TR, Bartleson C, Graves DJ, DeMaggio AJ, Hoekstra MF, Blenis J, Hunter T, Cantley LC. Mol. Cell. Biol. 1996;16:6486. doi: 10.1128/mcb.16.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brinkworth RI, Breinl RA, Kobe BP. Natl. Acad. Sci. U S A. 2003;100:74. doi: 10.1073/pnas.0134224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinna LA, Ruzzene M. Biochim. Biophys. Acta. 1996;1314:191. doi: 10.1016/s0167-4889(96)00083-3. [DOI] [PubMed] [Google Scholar]
- 17.Bax B, Carter PS, Lewis C, Guy AR, Bridges A, Tanner R, Pettman G, Mannix C, Culbert AA, Brown MJ, Smith DG, Reith AD. Structure (Camb) 2001;9:1143. doi: 10.1016/s0969-2126(01)00679-7. [DOI] [PubMed] [Google Scholar]
- 18.Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. J. Biol Chem. 2000;275:16795. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- 19.Knighton DR, Zheng J, Ten Eyck LF, Ashford VA, Xuong N-H, Taylor SS, Sowadski JM. Science. 1991;253:407. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- 20.Taylor SS, Knighton DR, Zheng J, Sowadski JM, Gibs CS, Zoller MJ. Trends Biochem. Sci. 1993;18:84. doi: 10.1016/0968-0004(93)80001-r. [DOI] [PubMed] [Google Scholar]
- 21.Nolen B, Taylor S, Ghosh G. Mol. Cell. 2004;15:661. doi: 10.1016/j.molcel.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Hanks SK, Hunter T. FASEB J. 1995;9:576. [PubMed] [Google Scholar]
- 23.De Bondt HL, Rosenblatt J, Jancarik J, Jones HD, Morgan DO, Kim S-H. Nature. 1993;363:595. doi: 10.1038/363595a0. [DOI] [PubMed] [Google Scholar]
- 24.Kobe B, Heierhorst J, Feil SC, Parker MW, Benian GM, Weiss KR, Kemp BE. Embo J. 1996;15:6810. [PMC free article] [PubMed] [Google Scholar]
- 25.Lei M, Lu W, Meng W, Parrini MC, Eck MJ, Mayer BJ, Harrison SC. Cell. 2000;702:387. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- 26.Niefind K, Guerra B, Ermakowa I, Issinger OG. EMBO J. 2001;20:5320. doi: 10.1093/emboj/20.19.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. Science. 2003;300:1256. doi: 10.1126/science.1082348. [DOI] [PubMed] [Google Scholar]
- 28.Kim C, Xuong NH, Taylor SS. Science. 2005;307:690. doi: 10.1126/science.1104607. [DOI] [PubMed] [Google Scholar]
- 29.Canagarajah BJ, Khokhlatchev A, Cobb MH, Goldsmith EJ. Cell. 1997;90:859. doi: 10.1016/s0092-8674(00)80351-7. [DOI] [PubMed] [Google Scholar]
- 30.Zheng J, Knighton DR, Ten Eyck LF, Karlsson R, Xuong N-H, Taylor SS, Sowadski JM. Biochemistry. 1993;32:2154. doi: 10.1021/bi00060a005. [DOI] [PubMed] [Google Scholar]
- 31.Biondi RM, Komander D, Thomas CC, Lizcano JM, Deak M, Alessi DR, van Aalten DM. EMBO J. 2002;21:4219. doi: 10.1093/emboj/cdf437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Cron P, Good VM, Thompson V, Hemmings BA, Barford D. Nat. Struct. Biol. 2002;9:940. doi: 10.1038/nsb870. [DOI] [PubMed] [Google Scholar]
- 33.Lodowski DT, Barnhill JF, Pyskadlo RM, Ghirlando R, Steme-Marr R, Tesmer JJ. Biochemistry. 2005;44:6958. doi: 10.1021/bi050119q. [DOI] [PubMed] [Google Scholar]
- 34.Huang X, Begley M, Morgenstern KA, Gu Y, Rose P, Zhao H, Zhu X. Structure. 2003;11:21. doi: 10.1016/s0969-2126(02)00937-1. [DOI] [PubMed] [Google Scholar]
- 35.Hu S-H, Parker MW, Lei JY, Wilce MCJ, Benian GM, Kemp BE. Nature. 1994;369:581. doi: 10.1038/369581a0. [DOI] [PubMed] [Google Scholar]
- 36.Mayans O, van der Ven PF, Wilm M, Mues A, Young P, Furst DO, Wilmanns M, Gautel M. Nature. 1998;395:863. doi: 10.1038/27603. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg J, Nairn AC, Kuriyan J. Cell. 1996;84:875. doi: 10.1016/s0092-8674(00)81066-1. [DOI] [PubMed] [Google Scholar]
- 38.Owen DJ, Noble MEM, Garman EF, Papagerorgiou AC, Johnson LN. Structure. 1995;3:467. doi: 10.1016/s0969-2126(01)00180-0. [DOI] [PubMed] [Google Scholar]
- 39.Lowe ED, Noble MEM, Skamnaki VT, Oikonomakos NG, Owen DJ, Johnson LN. EMBO J. 1997;16:6646. doi: 10.1093/emboj/16.22.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown NR, Noble ME, Lawrie AM, Morris MC, Tunnah P, Divita G, Johnson LN, Endicott JA. J. Biol. Chem. 1999;274:8746. doi: 10.1074/jbc.274.13.8746. [DOI] [PubMed] [Google Scholar]
- 41.Russo AA, Jeffrey PD, Patten AK, Massague J, Pavletich NP. Nature. 1996;382:325. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- 42.Russo AA, Jeffrey PD, Pavletich NP. Nat. Struct. Biol. 1996;3:696. doi: 10.1038/nsb0896-696. [DOI] [PubMed] [Google Scholar]
- 43.Zhang F, Strand A, Robbins D, Cobb MH, Goldsmith EJ. Nature. 1994;367:704. doi: 10.1038/367704a0. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Harkins PC, Ulevitch RJ, Han J, Cobb MH, Goldsmith EJ. P. Natl. Acad. Sci. USA. 1997;94:2327. doi: 10.1073/pnas.94.6.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bellon S, Fitzgibbon MJ, Fox T, Hsiao H-M, Wilson KP. Structure. 1999;7:1057. doi: 10.1016/s0969-2126(99)80173-7. [DOI] [PubMed] [Google Scholar]
- 46.Xie X, Gu Y, Fox T, Coll JT, Fleming MA, Markland W, Caron PR, Wilson KP, Su MS. Structure. 1998;6:983. doi: 10.1016/s0969-2126(98)00100-2. [DOI] [PubMed] [Google Scholar]
- 47.Niefind K, Putter M, Guerra B, Issinger OG, Schomburg D. Nat. Struct. Biol. 1999;6:1100. doi: 10.1038/70033. [DOI] [PubMed] [Google Scholar]
- 48.Niefind K, Issinger OG. Mol. Cell. Biochem. 2005;274:3. doi: 10.1007/s11010-005-3114-0. [DOI] [PubMed] [Google Scholar]
- 49.Nolen B, Yun CY, Wong CF, McCammon JA, Fu XD, Ghosh G. Nat. Struct. Biol. 2001;5:176. doi: 10.1038/84178. [DOI] [PubMed] [Google Scholar]
- 50.Dar AC, Dever TE, Sicheri F. Cell. 2005;122:887. doi: 10.1016/j.cell.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 51.Remenyi A, Good MC, Bhattacharyya RP, Lim WA. Mol. Cell. 2005;20:951. doi: 10.1016/j.molcel.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 52.Xu R-M, Carmel G, Sweet RM, Kuret J, Cheng X. EMBO J. 1995;14:1015. doi: 10.1002/j.1460-2075.1995.tb07082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Longenecker KL, Roach PJ, Hurley TD. J. Mol. Biol. 1996;257:618. doi: 10.1006/jmbi.1996.0189. [DOI] [PubMed] [Google Scholar]
- 54.Zhou T, Raman M, Gao Y, Earnest S, Chen Z, Machius M, Cobb MH, Goldsmith EJ. Structure. 2004;12:1891. doi: 10.1016/j.str.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 55.Lei M, Robinson MA, Harrison SC. Structure. 2005;513:769. doi: 10.1016/j.str.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, Marais R. Cell. 2004;116:855. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 57.Huse M, Chen YG, Massague J, Kuriyan J. Cell. 1999;96:425. doi: 10.1016/s0092-8674(00)80555-3. [DOI] [PubMed] [Google Scholar]
- 58.Huse M, Muir TW, Xu L, Chen YG, Kuriyan J, Massague J. Mol Cell. 2001;8:671. doi: 10.1016/s1097-2765(01)00332-x. [DOI] [PubMed] [Google Scholar]
- 59.Min X, Lee BH, Cobb MH, Goldsmith EJ. Structure. 2004;12:1303. doi: 10.1016/j.str.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 60.Young TA, Delagoutte B, Endrizzi JA, Falick AM, Alber T. Nat. Struct. Biol. 2003;10:168. doi: 10.1038/nsb897. [DOI] [PubMed] [Google Scholar]
- 61.Knighton DR, Zheng J, Ten Eyck LF, Xuong N-H, Taylor SS, Sowadski JM. Science. 1991;253:414. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- 62.Bossemeyer D, Engh RA, Kinzel V, Ponstingl H, Huber R. EMBO J. 1993;12:849. doi: 10.1002/j.1460-2075.1993.tb05725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lowe ED, Tews I, Cheng KY, Brown NR, Gul S, Noble ME, Gamblin SJ, Johnson LN. Biochemistry. 2002;41:15625. doi: 10.1021/bi0268910. [DOI] [PubMed] [Google Scholar]
- 64.Brown NR, Noble ME, Endicott JA, Johnson LN. Nat. Cell Biol. 1999;7:438. doi: 10.1038/15674. [DOI] [PubMed] [Google Scholar]
- 65.Haycock JW. J Neurol. sci. Methods. 2002;116:29. [Google Scholar]
- 66.Cox S, Taylor SS. Biochemistry. 1995;34:16203. doi: 10.1021/bi00049a036. [DOI] [PubMed] [Google Scholar]
- 67.Cheng KY, Noble ME, Skamnaki V, Brown NR, Lowe ED, Kontogiannis L, Shen K, Cole PA, Siligardi G, Johnson LN. J. Biol. Chem. 2006 doi: 10.1074/jbc.M600480200. [DOI] [PubMed] [Google Scholar]
- 68.Chang CI, Xu BE, Akella R, Cobb MH, Goldsmith EJ. Mol. Cell. 2002;9:1241. doi: 10.1016/s1097-2765(02)00525-7. [DOI] [PubMed] [Google Scholar]
- 69.Heo YS, Kim SK, Seo CI, Kim YK, Sung BJ, Lee HS, Lee JI, Park SY, Kim JH, Hwang KY, Hyun YL, Jeon YH, Ro S, Cho JM, Lee TG, Yang CH. EMBO J. 2004;23:2185. doi: 10.1038/sj.emboj.7600212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou T, Sun L, Humphreys J, Goldsmith EJ. Structure. 2006;14:1011. doi: 10.1016/j.str.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 71.Liu S, Sun JP, Zhou B, Zhang ZY. P. Natl Acad Sci. U S A. 2006;103:5326. doi: 10.1073/pnas.0510506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhattacharyya RP, Remenyi A, Good MC, Bashor CJ, Falick AM, Lim WA. Science. 2006;311:822. doi: 10.1126/science.1120941. [DOI] [PubMed] [Google Scholar]
- 73.Ngo JC, Chakrabarti S, Ding JH, Velazquez-Dones A, Nolen B, Aubol BE, Adams JA, Fu XD, Ghosh G. Mol. Cell. 2005;20:11. doi: 10.1016/j.molcel.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 74.Komander D, Kular G, Deak M, Alessi DR, van Aalten DM. J. Biol. Chem. 2005;280:18797. doi: 10.1074/jbc.M500977200. [DOI] [PubMed] [Google Scholar]
- 75.Yang J, Cron P, Thompson V, Good VM, Hess D, Hemmings BA, Barford D. Mol. Cell. 2002;9:1227. doi: 10.1016/s1097-2765(02)00550-6. [DOI] [PubMed] [Google Scholar]
- 76.Goldsmith EJ, Chang CI. Structure. 2002;10:888. doi: 10.1016/s0969-2126(02)00798-0. [DOI] [PubMed] [Google Scholar]
- 77.Bayliss R, Sardon T, Vernos I, Conti E. Mol. Cell. 2003;12:851. doi: 10.1016/s1097-2765(03)00392-7. [DOI] [PubMed] [Google Scholar]
- 78.Sessa F, Mapelli M, Ciferri C, Tarricone C, Areces LB, Schneider TR, Stukenberg PT, Musacchio A. Mol. Cell. 2005;18:379. doi: 10.1016/j.molcel.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 79.Lodowski DT, Tesmer VM, Benovic JL, Tesmer JJ. J. Biol Chem. 2006;257:16785. doi: 10.1074/jbc.M601327200. [DOI] [PubMed] [Google Scholar]
- 80.Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. Science. 2005;310:1686. doi: 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- 81.Brown K, Vial SC, Dedi N, Long JM, Dunster NJ, Cheetham GM. J. Mol. Biol. 2005;354:1013. doi: 10.1016/j.jmb.2005.09.098. [DOI] [PubMed] [Google Scholar]
- 82.Songyang Z, Blechner S, Hoagland N, Hoekstra MF, Piwnica-Worms H, Cantley LC. Curr. Biol. 1994;4:973. doi: 10.1016/s0960-9822(00)00221-9. [DOI] [PubMed] [Google Scholar]
- 83.Denis CL, Kemp BE, Zoller MJ. J. Biol. Chem. 1991;266:17932. [PubMed] [Google Scholar]
- 84.Hubbard SR. EMBO J. 1997;16:5572. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moore MJ, Kanter JR, Jones KC, Taylor SS. J Biol. Chem. 2002;277:47878. doi: 10.1074/jbc.M204970200. [DOI] [PubMed] [Google Scholar]
- 86.Zhu G, Fujii K, Belkina N, Liu Y, James M, Herrero J, Shaw S. J Biol Chem. 2005;280:10743. doi: 10.1074/jbc.M413159200. [DOI] [PubMed] [Google Scholar]
- 87.Herskowitz I. Cell. 1995;80:187. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- 88.Dan I, Watanabe NM, Kusumi A. Trends Cell. Biol. 2001;11:220. doi: 10.1016/s0962-8924(01)01980-8. [DOI] [PubMed] [Google Scholar]
- 89.Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH. Chem. Rev. 2001;101:2449. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- 90.Widmann C, Gibson S, Jarpe MB, Johnson GL. Physiol. Rev. 1999;79:143. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 91.Ahn NG. Mol. Cell. Biochem. 1993;127–128:201. doi: 10.1007/BF01076771. [DOI] [PubMed] [Google Scholar]
- 92.Cobb MH, Xu S, Hepler JE, Hutchison M, Frost J, Robbins DJ. Cell. Mol. Biol Res. 1994;40:253. [PubMed] [Google Scholar]
- 93.Nishida E, Gotoh Y. Trends Biochem. Sci. 1993;18:128. doi: 10.1016/0968-0004(93)90019-j. [DOI] [PubMed] [Google Scholar]
- 94.Drogen F, O'Rourke SM, Stucke VM, Jaquenoud M, Neiman AM, Peter M. Curr. Biol. 2000;10:630. doi: 10.1016/s0960-9822(00)00511-x. [DOI] [PubMed] [Google Scholar]
- 95.Chen W, Yazicioglu M, Cobb MH. J Biol. Chem. 2004;279:11129. doi: 10.1074/jbc.M313562200. [DOI] [PubMed] [Google Scholar]
- 96.Kyriakis JM. J Biol. Chem. 1999;274:5259. doi: 10.1074/jbc.274.9.5259. [DOI] [PubMed] [Google Scholar]
- 97.Mansour SJ, Resing KA, Candi JM, Hermann AS, Gloor JW, Herskind KR, Wartmann M, Davis RJ, Ahn NG. J. Biochem. 1994;116:304. doi: 10.1093/oxfordjournals.jbchem.a124524. [DOI] [PubMed] [Google Scholar]
- 98.Chen Z, Raman M, Chen L, Lee SF, Gilman AG, Cobb MH. J. Biol Chem. 2003;278:22278. doi: 10.1074/jbc.M301173200. [DOI] [PubMed] [Google Scholar]
- 99.Ben-David Y, Letwin K, Tannock L, Bernstein A, Pawson T. EMBO J. 1991;70:317. doi: 10.1002/j.1460-2075.1991.tb07952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahn NG, Seger R, Bratlien RL, Diltz CD, Tonks NK, Krebs EG. J. Biol. Chem. 1991;266:4220. [PubMed] [Google Scholar]
- 101.Haystead TA, Dent P, Wu J, Haystead CMM, Sturgill TW. FEBS Lett. 1992;306:17. doi: 10.1016/0014-5793(92)80828-5. [DOI] [PubMed] [Google Scholar]
- 102.Zhu G, Fujii K, Liu Y, Codrea V, Herrero J, Shaw S. J Biol Chem. 2005;280:36372. doi: 10.1074/jbc.M505031200. [DOI] [PubMed] [Google Scholar]
- 103.Khokhlatchev AV, Canagarajah B, Atkinson M, Goldsmith E, Cobb MH. Cell. 1997 doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- 104.Ray LB, Sturgill TW. P. Natl. Acad. Sci. USA. 1987;84:1502. doi: 10.1073/pnas.84.6.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boulton TG, Yancopoulos GD, Gregory JS, Slaughter C, Moomaw C, Hsu J, Cobb MH. Science. 1990;249:64. doi: 10.1126/science.2164259. [DOI] [PubMed] [Google Scholar]
- 106.Lewis TS, Shapiro PS, Ahn NG. Adv. Cancer Res. 1998;74:49. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 107.Clark-Lewis I, Sanghera JS, Pelech SL. J. Biol. Chem. 1991;266:15180. [PubMed] [Google Scholar]
- 108.Haycock JW, Ahn NG, Cobb MH, Krebs EG. Proc. Natl Acad. Sci. USA. 1992;89:2365. doi: 10.1073/pnas.89.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang J, Zhang F, Ebert D, Cobb MH, Goldsmith EJ. Structure. 1995;3:299. doi: 10.1016/s0969-2126(01)00160-5. [DOI] [PubMed] [Google Scholar]
- 110.Cohen P, Frame S. Nat. Rev. Mol Cell Bio. 2001;2:769. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 111.Frame S, Cohen P. Biochem. J. 2001;359:1. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jope RS, Johnson GV. Trends Biochem. Sci. 2004;29:95. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 113.Cohen P, Goedert M. Nat. Rev. Drug Discov. 2004;3:479. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- 114.Dajani R, Fraser E, Roe SM, Yeo M, Good VM, Thompson V, Dale TC, Pearl LH. EMBO J. 2003;22:494. doi: 10.1093/emboj/cdg068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Doble BW, Woodgett JR. J. Cell Sci. 2003;116:1175. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.ter Haar E, Coll JT, Austen DA, Hsiao HM, Swenson L, Jain J. Nat. Struct. Biol. 2001;8:593. doi: 10.1038/89624. [DOI] [PubMed] [Google Scholar]
- 117.Ali A, Hoeflich KP, Woodgett JR. Chem. Rev. 2001;101:2527. doi: 10.1021/cr000110o. [DOI] [PubMed] [Google Scholar]
- 118.Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. Nature. 2005;438:873. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gui JF, Lane WS, Fu XD. Nature. 1994;369:678. doi: 10.1038/369678a0. [DOI] [PubMed] [Google Scholar]
- 120.Aubol BE, Chakrabarti S, Ngo J, Shaffer J, Nolen B, Fu XD, Ghosh G, Adams JA. P. Natl. Acad. Sci. USA. 2003;100:12601. doi: 10.1073/pnas.1635129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Colwill K, Feng LL, Yeakley JM, Gish GD, Caceres JF, Pawson T, Fu XD. J. Biol. Chem. 1996;271:24569. doi: 10.1074/jbc.271.40.24569. [DOI] [PubMed] [Google Scholar]
- 122.Wang HY, Lin W, Dyck JA, Yeakley JM, Songyang Z, Cantley LC, Fu XD. J. Cell Biol. 1998;140:737. doi: 10.1083/jcb.140.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yun CY, Fu XD. J. Cell Biol. 2000;150:707. doi: 10.1083/jcb.150.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aubol BE, Nolen B, Vu D, Ghosh G, Adams JA. Biochemistry. 2002;41:10002. doi: 10.1021/bi020233y. [DOI] [PubMed] [Google Scholar]
- 125.Meggio F, Pinna LA. FASEB J. 2003;17:349. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 126.Litchfield DW. Biochem. J. 2003;369:1. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kemp BE, Pearson RB. Method Enzymol. 1991;200:121. doi: 10.1016/0076-6879(91)00134-i. [DOI] [PubMed] [Google Scholar]
- 128.Sarno S, Boldyreff B, Marin O, Guerra B, Meggio F, Issinger OG, Pinna LA. Biochem. Biophys. Res. Com. 1995;206:171. doi: 10.1006/bbrc.1995.1024. [DOI] [PubMed] [Google Scholar]
- 129.Price MA. Genes Dev. 2006;20:399. doi: 10.1101/gad.1394306. [DOI] [PubMed] [Google Scholar]
- 130.Hunter T, Plowman GD. Trends Biochem. Sci. 1997;22:14. doi: 10.1016/s0968-0004(96)10068-2. [DOI] [PubMed] [Google Scholar]
- 131.Flotow H, Graves PR, Wang AQ, Fiol CJ, Roeske RW, Roach PJ. J. Biol. Chem. 1990;265:14264. [PubMed] [Google Scholar]
- 132.Marin O, Bustos VH, Cesaro L, Meggio F, Pagano MA, Antonelli M, Allende CC, Pinna LA, Allende JE. P. Natl. Acad. Sci. U S A. 2003;100:10193. doi: 10.1073/pnas.1733909100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Madhusudan, Trafny EA, Xuong NH, Adams JA, Ten Eyck LF, Taylor SS, Sowadski JM. Protein Sci. 1994;3:176. doi: 10.1002/pro.5560030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Timm T, Li XY, Biernat J, Jiao J, Mandelkow E, Vandekerckhove J, Mandelkow EM. EMBO J. 2003;22:5090. doi: 10.1093/emboj/cdg447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huse M, Kuriyan J. Cell. 2002;109:275. doi: 10.1016/s0092-8674(02)00741-9. [DOI] [PubMed] [Google Scholar]
- 136.Pargellis C, Tong L, Churchill L, Cirillo PF, Gilmore T, Graham AG, Grob PM, Hickey ER, Moss N, Pav S, Regan J. Nat. Struct. Biol. 2002;9:268. doi: 10.1038/nsb770. [DOI] [PubMed] [Google Scholar]
- 137.Jauch R, Jakel S, Netter C, Schreiter K, Aicher B, Jackie H, Wahl MC. Structure. 2005;13:1559. doi: 10.1016/j.str.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 138.Kallunki T, Su B, Tsigelny I, Sluss HK, Dérijard B, Moore G, Davis R, Karin M. Genes Dev. 1994;8:2996. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- 139.Kallunki T, Deng T, Hibi M, Karin M. Cell. 1996;87:929. doi: 10.1016/s0092-8674(00)81999-6. [DOI] [PubMed] [Google Scholar]
- 140.Bardwell L, Thorner J. Trends Biochem. Sci. 1996;21:373. [PubMed] [Google Scholar]
- 141.Jacobs D, Glossip D, Xing H, Muslin AJ, Kornfeld K. Gene. Dev. 1999;13:163. [PMC free article] [PubMed] [Google Scholar]
- 142.Holland PM, Cooper JA. Curr. Biol. 1999;9:R329. doi: 10.1016/s0960-9822(99)80205-x. [DOI] [PubMed] [Google Scholar]
- 143.Tanoue T, Adachi M, Moriguchi T, Nishida E. Nat. Cell Biol. 2000;2:110. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- 144.Tanoue T, Nishida E. Cell Signal. 2003;15:455. doi: 10.1016/s0898-6568(02)00112-2. [DOI] [PubMed] [Google Scholar]
- 145.Sharrocks AD, Yang SH, Galanis A. Trends Biochem. Sci. 2000;25:448. doi: 10.1016/s0968-0004(00)01627-3. [DOI] [PubMed] [Google Scholar]
- 146.Enslen H, Davis RJ. Biol Cell. 2001;93:5. doi: 10.1016/s0248-4900(01)01156-x. [DOI] [PubMed] [Google Scholar]
- 147.Bogoyevitch MA, Kobe B. Microbiol. Mol. Biol. Rev. 2006;70:1061. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gum RJ, Young PR. Biochem. Biophys. Res. Commun. 1999;266:284. doi: 10.1006/bbrc.1999.1787. [DOI] [PubMed] [Google Scholar]
- 149.Zhou H, Zheng M, Chen J, Xie C, Kolatkar AR, Zarubin T, Ye Z, Akella R, Lin S, Goldsmith EJ, Han J. Mol. Cell. Biol. 2006;26:3824. doi: 10.1128/MCB.26.10.3824-3834.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang J, Zhou B, Zheng CF, Zhang ZY. J. Biol. Chem. 2003;278:29901. doi: 10.1074/jbc.M303909200. [DOI] [PubMed] [Google Scholar]
- 151.Frodin M, Antal TL, Dummler BA, Jensen CJ, Deak M, Gammeltoft S, Biondi RM. EMBO J. 2002;21:5396. doi: 10.1093/emboj/cdf551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Balendran A, Biondi RM, Cheung PC, Casamayor A, Deak M, Alessi DR. J. Biol. Chem. 2000;275:20806. doi: 10.1074/jbc.M000421200. [DOI] [PubMed] [Google Scholar]
- 153.Frodin M, Jensen CJ, Merienne K, Gammeltoft S. Embo J. 2000;19:2924. doi: 10.1093/emboj/19.12.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Biondi RM, Cheung PC, Casamayor A, Deak M, Currie RA, Alessi DR. EMBO J. 2000;19:979. doi: 10.1093/emboj/19.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zheng J, Trafhy EA, Knighton DR, Xuong N-H, Taylor SS, Ten Eyck LF, Sowadski JM. Acta Cryst. 1993;D49:362. doi: 10.1107/S0907444993000423. [DOI] [PubMed] [Google Scholar]
- 156.Harper JW, Adams PD. Chem. Rev. 2001;101:2511. doi: 10.1021/cr0001030. [DOI] [PubMed] [Google Scholar]
- 157.Noble ME, Endicott JA, Brown NR, Johnson LN. Trends Biochem. Sci. 1997;22:482. doi: 10.1016/s0968-0004(97)01144-4. [DOI] [PubMed] [Google Scholar]
- 158.Chen J, Saha P, Kornbluth S, Dynlacht BD, Dutta A. Mol. Cell. Biol. 1996;16:4673. doi: 10.1128/mcb.16.9.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Adams PD, Sellers WR, Sharma SK, Wu AD, Nalin CM, Kaelin WG., Jr Mol. Cell. Biol. 1996;16:6623. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chothia C, Levitt M, Richardson D. J. Mol. Biol. 1981;145:215. doi: 10.1016/0022-2836(81)90341-7. [DOI] [PubMed] [Google Scholar]
- 161.Schulman BA, Lindstrom DL, Harlow E. P. Natl. Acad. Sci. U S A. 1998;95:10453. doi: 10.1073/pnas.95.18.10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Parang K, Till JH, Ablooglu AJ, Kohanski RA, Hubbard SR, Cole PA. Nat. Struct. Biol. 2001;8:37. doi: 10.1038/83028. [DOI] [PubMed] [Google Scholar]
- 163.Stevenson-Lindert LM, Fowler P, Lew J. J Biol Chem. 2003;278:50956. doi: 10.1074/jbc.M306546200. [DOI] [PubMed] [Google Scholar]
- 164.Lee T, Hoofnagle AN, Kabuyama Y, Stroud J, Min X, Goldsmith EJ, Chen L, Resing KA, Ahn NG. Mol. Cell. 2004;14:43. doi: 10.1016/s1097-2765(04)00161-3. [DOI] [PubMed] [Google Scholar]
- 165.Strajbl M, Shurki A, Warshel A. P. Natl. Acad. Sci. USA. 2003;100:14834. doi: 10.1073/pnas.2436328100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Goldsmith EJ. FASEB J. 1996;10:702. doi: 10.1096/fasebj.10.7.8635687. [DOI] [PubMed] [Google Scholar]
- 167.Wang Z, Mottonen J, Goldsmith EJ. Biochemistry. 1996 doi: 10.1021/bi961214p. [DOI] [PubMed] [Google Scholar]
- 168.Gao X, Harris TK. J. Biol. Chem. 2006;281:21670. doi: 10.1074/jbc.M602448200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jencks WP. Methods Enzymol. 1989;171:145. doi: 10.1016/s0076-6879(89)71010-7. [DOI] [PubMed] [Google Scholar]
- 170.Murphy LO, Blenis J. Trends Biochem. Sci. 2006;31:268. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 171.Grewal S, Molina DM, Bardwell L. Cell Signal. 2006;18:123. doi: 10.1016/j.cellsig.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]