Abstract
Prostate cancer growth depends on androgen receptor (AR) signaling. Androgen ablation therapy induces expression of constitutively active AR splice variants which drive disease progression. Taxanes are a standard of care therapy in castration-resistant prostate cancer (CRPC), however, mechanisms underlying the clinical activity of taxanes are poorly understood. Recent work suggests that the microtubule network of prostate cells is critical for AR nuclear translocation and activity. In this study, we employed a set of AR deletion mutants to identify the microtubule-binding domain of AR, which encompasses the DNA binding domain plus hinge region. We report that two clinically relevant AR splice variants, ARv567 and ARv7, differentially associate with microtubules and dynein motor protein, thereby resulting in differential taxane sensitivity in vitro and in vivo. ARv7, which lacks the hinge region, did not co-sediment with microtubules or co-precipitate with dynein motor protein, unlike ARv567. Mechanistic investigations revealed that the nuclear accumulation and transcriptional activity of ARv7 was unaffected by taxane treatment. In contrast, the microtubule-interacting splice variant ARv567 was sensitive to taxane-induced microtubule stabilization. In ARv567-expressing LuCap86.2 tumor xenografts, docetaxel treatment was highly efficacious, whereas ARv7-expressing LuCap23.1 tumors xenografts displayed docetaxel resistance. Our results suggest that AR variants which accumulate in CRPC cells utilize distinct pathways of nuclear import that affect the antitumor efficacy of taxanes, suggesting a mechanistic rationale to customize treatments for CRPC patients which might improve outcomes.
Keywords: Androgen receptor, Taxane, Androgen receptor variants, Prostate cancer, resistance to therapy
Introduction
Prostate cancer progression is dependent on continuous androgen receptor (AR) signaling and transcriptional activity. Thus, strategies designed to effectively inhibit AR transcriptional activity and signaling, are at the forefront of current research in prostate cancer. The importance of the AR in prostate cancer disease progression is further highlighted by the fact that many recent therapies are designed to target the androgen axis, such as enzalutamide (formerly MDV3100) (1, 2) an AR antagonist, and abiraterone (3) a CYP17 inhibitor that inhibits androgen synthesis. However, resistance to all forms of androgen deprivation therapy (ADT), including these next-generation compounds, occurs eventually and results in disease progression. In fact, despite androgen ablation, the progression to castration-resistant prostate cancer (CRPC) remains AR driven (4) due to several mechanisms including AR gene amplification, in situ androgen production (5), the presence of ligand-independent AR splice variants which localize to the nucleus and are constitutively active (6, 7) and the appearance of the recently identified ligand-binding domain (LBD) mutant form of ARF876L (8, 9).
Following disease progression on ADT, CRPC patients are commonly treated with taxanes, microtubule-stabilizing drugs, which represent the only class of chemotherapy drugs that prolongs survival in CRPC (10, 11) and are used as standard first- and second-line chemotherapy. We and others have recently shown that AR signaling, is inhibited by taxane treatment, as drug-induced microtubule stabilization abrogates AR's nuclear translocation and transcriptional activity (12, 13). Specifically, we showed that following ligand stimulation, AR traffics on microtubules with the aid of the microtubule-based minus-end directed motor protein dynein, in order to be efficiently trafficked to the nucleus, where it exerts its transcriptional activity (12). Importantly, using CRPC patient-derived circulating tumor cells, we observed a significant correlation between AR cytoplasmic sequestration and clinical response to taxane chemotherapy. These results demonstrate that AR inhibition can occur as a result of microtubule stabilization and is a critical mechanism underlying clinical activity of taxanes in prostate cancer (14).
This newly identified mechanism of taxane activity predicts that the combination of taxanes with next-generation AR targeting drugs would be synergistic since they both inhibit AR signaling, by targeting different components of AR signaling axis (14). For example, abiraterone, inhibits ligand production while taxanes inhibit the nuclear translocation of the receptor-ligand complex. Despite this prediction, a recent clinical study reported that the activity of docetaxel post- abiraterone was lower than expected while no responses to docetaxel were observed in abiraterone-refractory patients (15). These data suggested that perhaps besides the common mechanism of action (inhibition of AR axis) these two classes of drugs, taxanes and AR inhibitors, might also share a common mechanism of resistance.
Interestingly recent reports have shown that constitutively active AR splice variants are overexpressed in CRPC (16-19) and confer resistance to both enzalutamide and abiraterone (20, 21). AR variants ARv567 and ARv7 appear to be the two most clinically prevalent splice variants, with ARv567 present in 59% of tumor specimens from CRPC patients (19) and arising in response to ADT or abiraterone treatment (20) and ARv7 present in both benign and malignant prostate tissues but mostly enriched in metastatic disease (17, 22). Together these studies show that the presence of AR splice variants is common in CRPC and associates with resistance to current androgen deprivation therapies (20, 21).
The impact of AR variant expression on taxane sensitivity and resistance has not been evaluated. Here, we set out to investigate the mechanisms underlying the variants ARv567 and ARv7 nuclear translocation and constitutive activity, and whether their presence would affect taxane sensitivity. Our results show that ARv567, but not ARv7, utilizes dynamic microtubules and the dynein motor protein for its nuclear accumulation and resultant transcriptional activity and as such only taxane treatment sequesters ARv567 “inactive” in the cytoplasm, while it has no effect on ARv7 subcellular localization and nuclear activity. We have also identified the minimum microtubule-binding domain on the AR, which lies within the DNA-binding domain and the hinge region. Interestingly, ARv7, which does not display microtubule-binding properties lacks the hinge region and thus lacks the main part of the bipartite nuclear localization signal (NLS) (23-26). We show that ARv567, expression confers docetaxel sensitivity in vivo, while expression of the microtubule-independent ARv7 variant confers resistance. Taken together, our data identify distinct mechanisms of cytoplasmic-to nuclear-trafficking for the two AR variants, which underlie their differential sensitivity to taxane treatment suggesting that variant expression could be used as a potential predictive biomarker of taxane clinical activity in CRPC.
Materials and Methods
Cell lines and reagents
PC3 and HEK293T cells were obtained from ATCC (Manassas, VA) and a PC3 stable cell line expressing mCherry-tubulin (PC3:mCherry-tub) was generated and maintained as previously described (12).
M12 cell line is derived by human prostate epithelial cells immortalized with the SV40T antigen to produce the poorly tumorigenic P69SV40T cell line. After injection into athymic nude mice the produced tumor nodules were reimplanted in athymic mice leading to the generation of the M12 cells, which demonstrated a shorter latency period to tumor formation and were locally invasive and metastatic compared to the initial P69SV40T. We chose M12 cells for subsequent transfection experiments with GFP-tagged AR-wt or AR variants as they do not express endogenous AR protein. GFP+ cells from all three AR cell lines were sorted using fluorescence activated cell sorting (FACS) on a BD FACSAria II cell sorter (BD Biosciences, Franklin Lakes, NJ, USA) and subsequently expanded in media containing G418 (400 ng/mL).
M12 stable cell lines expressing untagged wild-type AR (AR-wt), ARv567es and ARv7 or expressing Cumate-inducible FLAG-tagged AR-wt or variants were generated in Dr. Plymate's laboratory (University of Washington School of Medicine, Seattle, WA) and maintained in RPMI 1640 supplemented with 5% FBS, 0.01 μM dexamethasone (Sigma Aldrich, St Louis, MO), 10 ng/ml epidermal growth factor (Invitrogen), 10 ml/L insulin-transferrin-selenium (Cellgro, Manassas, VA), 100 I.U./ml penicillin plus 100 μg/μl streptomycin at 37°C with 5% CO2.
Unless otherwise stated, all reagents used were from Sigma Aldrich (St. Louis, MO). For immunofluorescence we used the following ptimary and secondary antibodies: rat monoclonal anti α-tubulin (Novus Biologicals, Littleton, CO), rabbit polyclonal anti AR-N21 developed in our lab using as an antigen the first 21 amino acids of AR and species-matching Alexa Fluor 488 and Alexa Fluor 568 conjugated antibodies from Invitrogen (Carlsbad, CA). For immunoprecipitation we used rat monoclonal anti α-tubulin, rabbit polyclonal anti-GFP (Novus Biologicals) and mouse anti-dynein (Covance, Emeryville, CA) antibodies. For the immunoblot assays we used mouse monoclonal anti-AR 441 (Novus Biologicals); rabbit monoclonal anti-AR (EP670Y from Abcam, Cambridge, MA) specific for the C-terminus; mouse monoclonal anti-ARv7 (Precision Antibody, Columbia, MD) specific for ARv7 variant; mouse monoclonal anti-FLAG M2 (Sigma Aldrich); rat monoclonal anti α-tubulin; rabbit polyclonal anti-GFP (Abcam) and rabbit polyclonal anti-actin (Sigma Aldrich) antibodies. Alexa Fluor 680 (Invitrogen) and IRDye 800 (Rockland) conjugated antibodies were as secondary antibodies. Methyltrienolone (R1881) and Paclitaxel were purchased from Sigma Aldrich and docetaxel from Sanofi Aventis (Kansas City, MO).
Generation of AR truncated mutants
The full-length GFP-AR plasmid (pEGFP-C1-AR-Q22) was generously provided by Dr. Michael Mancini (Baylor College of Medicine, Houston, TX) and used as the template to generate all AR-truncated mutant constructs. All PCR-generated AR-truncated mutant constructs were sub-cloned into the expression vector pEGFP-C1. All cloning was performed using AccuPrime Taq DNA Polymerase High Fidelity (Invitrogen), with 10 μM forward and reverse cloning primers (IDT, San Diego, CA). All primers were designed using the human AR mRNA reference sequence (GenBank NM_000044) and are shown in Table 1.
Table 1.
Primers used to generate AR-truncated mutant constructs
| Segment | Forward | Reverse |
|---|---|---|
| AR N-terminal domain | 5’-cgcgtcgacatggaagtgcagttagggctg-3’ | 5’ cgggatcctcacgcatgtccccgtaaggt-3’ |
| AR C-terminal domain | 5’-cgcgtcgacatgcgtttggagactgccag-3’ | 5’- cgggatcctcactgggtgtggaaatagatggg-3’ |
| AR 540-558 | 5’-cgcacgaattctatgttggagactgccagggacc-3’ | 5’-cgcacggatcctcacttctggggtggaaagtaatag-3’ |
| AR 559-624 (DBD) | 5’-cgcacgaattctatgacctgcctgatctgtggag-3’ | 5’- cgcacggatcctcaccctgcttcataacatttccg-3’ |
| AR 559-663 (DBD-Hinge) | 5’- cgcacgaattctatgacctgcctgatctgtggag-3’ | 5’- cgcacggatcctcatgacactgtcagcttctggg-3’ |
| AR 624-663 (Hinge) | 5’- cgcacgaattctatgactctgggagcccgg-3’ | 5’- cgcacggatcctcatgacactgtcagcttctggg-3’ |
| AR 664-724 | 5’-cgcacgaattctatgcacattgaaggctatgaatgtc-3’ | 5’-cgcacggatcctcaaggcaaggccttggcccac-3’ |
AR 540-724 and AR 725-919 were subcloned into the p3xFLAG-CMV-14 expression vector (Sigma) with the following specific primers: AR 540-724 forward: 5’-cgcacgatatcgccaccatgttggagactgccagggacc-3’; reverse: 5’-cgcacggatccaggcaaggccttggcccac-3’ and AR 725-919 forward 5’-cgcacgatatcgccaccatgggcttccgcaacttacacgtg-3’; reverse: 5’-cgcacggatccctgggtgtggaaatagatggg-3’. Amplification products were analyzed by Sanger sequencing to confirm the integrity of all constructs.
Immunoprecipitation and Western Blotting
HEK293T cells transiently transfected with GFP-tagged AR-wt AR, ARv567 or ARv7 were lysed in TNES buffer and subjected to immunoprecipitation as previously described (12).
Microtubule co-sedimentation assay
PC3-mCherry-tubulin and HEK293T cells were transiently transfected with GFP-tagged AR-wt or GFP-ARv7 or HA-tagged ARv567 and subjected to microtubule co-sedimentation assay as previously described (27). Cells were grown in RPMI 10% FBS+1%P/S till transfection in which the media were replaced with CSS media. No R1881 was added. For the cosedimentation assay, briefly, 1 mg of total cell lysate was first precleared by high-speed centrifugation. The pellet (HSP) containing mostly insoluble cell debris was discarded after loading a small amount on the gel to identify whether there was significant loss of AR in the cell debris, while the supernatant (HSS) was supplemented with exogenous purified bovine brain tubulin (Cytoskeleton, Denver, CO) reconstituted at a final concentration of 10 μM in the presence of 1 mM GTP, and 20 μM paclitaxel (PTX) and subjected to a cycle of polymerization for 30 min at 37°C. Samples were centrifuged at 100,000 × g for 30 min at room temperature and the warm supernatant (WS) was separated from the warm pellet (WP), which was resuspended in an equal volume of PEM buffer. Equal volumes from each respective fraction were loaded onto a SDS-PAGE and transferred and immunoblotted with antibodies against GFP, AR, α-tubulin and actin.
Densitometry for each respective protein was performed using ImageJ (National Institutes of Health) software and the percentage of the protein present in the pellet fraction was calculated using the following formula: % P = 100*WP/(WP+WS).
Quantitative real time PCR
M12-cumate inducible AR-wt or variant cells were treated with cumate (Cu) for 48h and then starved for 24h in CSS media. Cells were treated with 1 μM docetaxel (TXT) for 4 h either alone or followed by 10 nM R1881 overnight. QPCR for TMPRSS2, FKBP51 and GAPDH was performed as previously described (28).
Dynamitin overexpression, immunofluorescence and confocal microscopy
M12 cells stably expressing the untagged AR constructs were transiently transfected with c-myc-tagged pCMVH50m plasmid containing dynamitin (gifted by R. Vallee, Columbia University, New York, NY) using FuGENE 6, according to the manufacturer's instructions. Twenty four hours post-transfection, the cells were treated with the indicated drugs. Images were acquired by confocal microscopy and image analysis was performed as previously described (12).
Live cell imaging
PC3-mCherry-tubulin cells were plated on MatTek (Ashland, MA) 5 mm, Poly-d-lysine coated glass bottom dishes and allowed to adhere overnight. Cells were maintained in charcoal-stripped RPMI 1640 at 37°C and 5% CO2 for 1h, pressure-microinjected intranuclearly with plasmids encoding the different GFP-AR cDNAs as previously described (12) and then treated post-microinjection for 2hrs with TXT or Noc to affect MTs. After protein expression time-lapse images were taken using a Spinning Disk microscopy system consisting of a Zeiss Axiovert 200 system fitted with a Yokogawa CSU-X1 spinning disk head (Tokio, Japan). Time lapse microscopy and image analyses were performed as previously described (12).
Xenograft tumors
LuCaP human prostate cancer xenografts were grown as previously described (20, 29) in non-castrate SCID mice. Briefly, when the tumor volume reached an estimated size of 200mm3 (lxw2/2) mice was treated with either vehicle or docetaxel 5 or 20-mgm/kg i.p. weekly until tumors in the control group reached a tumor volume of 1000 mm3 . At this time all animals in a group were sacrificed. The LuCaP 23.1 and LuCaP 86.2 prostate cancer xenograft lines were developed by co-author RLV at the University of Washington. The pre-clinical studies presented here were performed in his laboratory. All animal studies were approved by the University of Washington Institutional Animal Care and Use Committee.
Immunohistochemistry
AR immunostaining of explanted LuCaP tumors was conducted as previously described (30)
Statistical analysis
The p-values for AR nuclear localization in control, taxane and nocodazole treated cells were calculated using one-way analysis of variance followed by multiple comparisons with Bonferroni adjustments using Stata Statistical Software: release 10. The p-value for AR nuclear versus cytoplasmic localization in M12 cells were calculated using two-tailed unpaired T-Test
Image Analysis
For all images segmentation of the nuclei and the cellular area was done using MATLAB, MathWorks. Using the DAPI images, we applied a unimodal thresholding algorithm (31) to identify the nuclear areas for each cell. We then computed the total intensity for the nuclear areas in the AR images and obtained the overall AR nuclear intensity for each cell. We used the coordinates of the centroids of the nuclei to build a Voronoi diagram (32) and identify the approximate area of the cytoplasm for each cell. We then computed the total intensity in the AR images for each cellular area, including the cytoplasmic and nuclear areas. The percentage of AR nuclear accumulation was calculated as the ratio of the nuclear to cellular AR for each individual cell.
Results
Microtubule binding is mediated by the C-terminal domain of the androgen receptor
We have previously shown that wild type AR (AR-wt) associates with the microtubule cytoskeleton and that this association is important for AR cytoplasmic to nuclear translocation and transcriptional activity in CRPC (12, 14). However, the AR protein domain required for microtubule association remains unknown. To determine the microtubule binding domain on the AR protein we performed a microtubule co-sedimentation assay using cells expressing overlapping truncated AR mutants, which we generated by a serial mutagenesis approach. The microtubule co-sedimentation assay is an assay routinely used to analyze proteins that specifically bind to microtubule polymers. The basic principles of the assay involve addition of exogenous purified microtubule protein to a crude cell extract which is then subjected to a cycle of polymerisation in the presence of the microtubule stabilizing drug paclitaxel. Any cellular proteins that have affinity for the microtubule polymer will co-sediment with the tubulin pellet following ultracentrifugation at 100,000 x g. Following centrifugation the pellet (microtubules and associated cell proteins) and supernatant (soluble tubulin dimers and all other cell proteins) fractions are loaded on adjacent wells of an SDS-PAGE, transferred and immunoblotted for the proteins of interest.
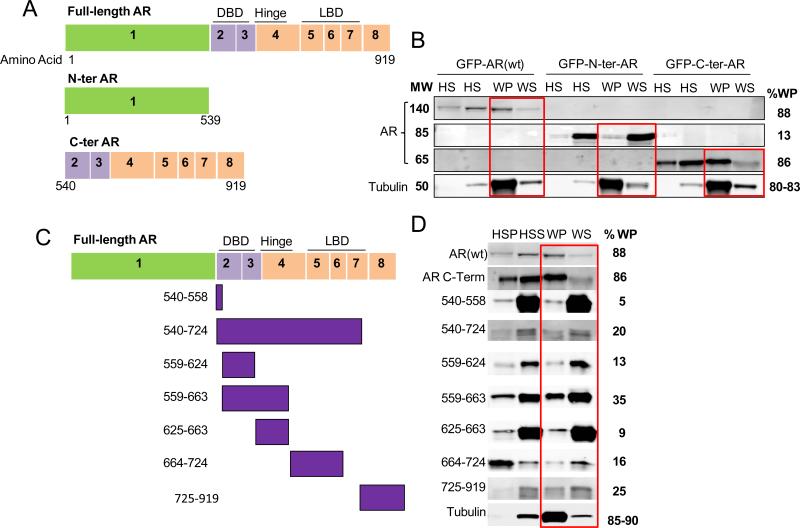
Initially, two large AR truncations were generated and sub-cloned into a pEGFP-C1 vector: the N-terminal domain (N-ter, aa 1-539) encompassing exon 1 of the AR and the C-terminal domain (C-ter, aa 540-919) encompassing the DNA binding domain, the hinge region and the ligand binding domain of AR (Figure 1A). Each deletion mutant was individually expressed in either PC3:mCherry-tub or HEK293T cells by transient transfection and cells were subjected to microtubule co-sedimentation in order to assess each proteins’ association with microtubule polymers. In this assay cell lysates from each condition (HSS) were supplemented with exogenous purified tubulin and subjected to a cycle of microtubule polymerization at 37 °C and in the presence of GTP and Taxol. Under these conditions purified tubulin along with endogenous cellular tubulin is robustly polymerized enabling microtubule interactions with cellular proteins. Following the polymerization reaction high-speed centrifugation separates microtubule polymers along with any cellular proteins with affinity for them into the warm pellet (WP) fraction, while soluble tubulin and other proteins that do not have affinity for microtubules segregate with the warm supernatant (WS). The distribution of AR proteins between the WP and WS fractions indicates its ability to associate with the microtubule polymers. Efficient tubulin polymerization was observed in all conditions.
Figure 1. Microtubule-binding is mediated by the C-terminal domain of AR.
(A, C) Schematic representation of AR deletion mutants as indicated. (B, D) Microtubule co-sedimentation assay of PC3:mCh-tub cells transiently expressing GFP-AR(wt), GFP-N-terminal AR (N-ter AR) or GFP-C-terminal AR (C-ter AR) or each of the smaller deletion mutants as indicated. Cell lysates from each condition (HSS) were supplemented with exogenous tubulin and subjected to microtubule polymerization in the presence of GTP and paclitaxel. The samples were centrifuged at 100,000 g to separate the microtubule polymers (WP) from the soluble tubulin dimers (WS), resolved by SDS-PAGE and immunoblotted for the presence of AR and tubulin as indicated. HSP: High speed pellet containing cellular proteins that aggregated non specifically. HSS: High speed supernatant (cell lysate). WP: warm pellet containing microtubule polymers and associated proteins. WS: warm supernatant containing soluble tubulin dimers and proteins non associated with microtubules. Red boxes highlight the relative protein distribution between the WP and WS fractions from each condition. The percent protein present in the pellet fraction (%WP) was calculated using the following formula: % P = 100*WP/(WP+WS) and is presented to the right of each immunoblot. For tubulin a range of values is shown in the %WP quantitation reflecting each of the three reactions performed. The blots shown in panel B belong to the same gel, which was blotted with anti-AR 441 for AR-wt and N-terminal AR, and anti-AR (EP670Y) specific for the C-terminal AR, anti-tubulin and anti-actin antibodies. In panel D, all AR deletion mutants were detected with an anti-GFP antibody except for the AR 540-724 and AR 725-919 deletion mutants which were detected with an anti-FLAG antibody.
Our results revealed that the C-terminal domain of AR associated preferentially with microtubule polymers in both cell lines (Figures 1B and S1), as 86% of the C-ter AR co-fractionated with microtubules in the WP fraction, similar to the AR-wt. In contrast, N-ter AR showed minimal association (13%) with polymerized tubulin (Figures 1B and S1). Tubulin was efficiently polymerized in all conditions as shown by the majority of tubulin (over 80%) found in respective WP fractions.
In order to further narrow down the minimum AR-microtubule binding domain, we generated seven additional AR truncation mutants within the C-ter region of AR (Figure 1C). The truncations were designed to correspond to AR functional domains as well as to cover the entire C-ter region, as follows: the DNA binding domain (aa 559-624), the hinge region (aa 625-663), the DNA binding domain plus the hinge region (aa 559-663) and fragments aa 540-724, 540-558, 664-724 and 725-919. These deletion mutants were subcloned into a pEGFP-C1 vector, with the exception of aa 540-724 and 725-919 mutants, which were subcloned into a p3XFLAG-CMV vector as the GFP-tag gave rise to a protein of approximately 50 kDa, which is also the size of tubulin, whose excess made the detection of the truncated proteins very challenging. Of the seven truncation mutants, the AR 559-663, corresponding to the DNA BD plus the hinge region, showed the most extensive association with the microtubule polymers with 35% protein seen in the WP (Figure 1D). Surprisingly, none of these AR deletion mutants showed as extensive association with the microtubule polymer fraction as the original C-ter AR (Figure 1B), suggesting that likely there is contribution from different parts of the AR within its C-terminus region for effective tubulin association.
ARv567 associates more extensively with microtubules compared to ARv7
We then sought to investigate whether any of the two most clinically prevalent AR splice variants with truncations in their C-terminus, namely ARv567 (19) and ARv7 (17, 18), would associate with microtubules similar to the AR-wt. Microtubule co-sedimentation revealed that the ARv567 variant co-fractionated almost exclusively (70%) with microtubule polymers in the WP fraction while ARv7, only partially co-fractionated with microtubules at 42% (Figure 2B).
Figure 2. ARv567, but not ARv7, associates with the microtubule polymer.
(A) Schematic representation of full-length AR as well as the splice variants ARv567, encoding exons 1-4 and the first 10 amino acids of exon 8, and ARv7, encoding exons 1-3 and a cryptic exon of 16 amino acids. (B and C) A MT co-sedimentation assay using whole cell lysate (HSS) from PC3:mCh-tub cells transfected with either GFP-AR (wt), HA-ARv567 or GFP-ARv7 was carried out as in Figure 1. AR-wt AR was used as positive controls of MT-binding. Proteins from each of the fractions were immunoblotted using anti-AR specific for the C-terminus (EP670Y) to detect GFP-AR-C-ter; anti-AR 441 to detect GFP-AR (wt), HA-ARv567 and GFP-ARv7; anti-tubulin and anti-actin antibodies. Tubulin was detected as MTs in the WP in each condition. Red boxes indicate the distribution of each protein between WP and WS fractions per condition. The extent of each variant's association with the microtubule polymer (% WP) was quantified by densitometry as in Figure 1 and is displayed to the right of each immunoblot. For tubulin a range of values is shown in the %WP quantitation reflecting each of the eight reactions performed. Actin was used as a negative control for microtubule association and is found in the supernatant fractions (WS) in all conditions.
ARv567, but not ARv7, nuclear translocation is impaired by microtubules targeting drugs
The distinct pattern of microtubule association exhibited by the two AR variants suggested potentially distinct mechanisms of nuclear translocation. To test this hypothesis, we perturbed the microtubule network by 2 hr treatment with drugs that either stabilize (docetaxel) or depolymerize (nocodazole) microtubules and assessed AR variant nuclear accumulation in cells microinjected with GFP-tagged ARv567 or ARv7. Live cell confocal microscopy was then used to image the dynamics of AR variant nuclear accumulation by obtaining z-stack images every 10 minutes for a total of 120 min.
Representative images from each condition are shown in Figure 3 and reveal that the nuclear accumulation of ARv567 was significantly impaired following microtubule perturbation with either drug, while ARv7 remained largely unaffected in the nucleus (see movies in Supplement). Interestingly, and in agreement with published reports (17-19, 33) the two variants were found predominantly in the nucleus of untreated cells as soon as the GFP-tagged protein was expressed following microinjection (time 0). Despite their initial nuclear localization at baseline, the two variants exhibited entirely distinct responses to drug-induced microtubule disruption. Quantitation of the extent of nuclear ARv567 revealed a significant decrease in its nuclear localization following microtubule perturbation at all time points tested (Figure 3C and Table S1 p<0.01 at baseline and p<0.001 at all other time points). The integrity of the microtubule cytoskeleton was assessed in each condition prior to time lapse image acquisition and is shown in the right panels of the figure indicating effective drug-target engagement for each of the conditions, microtubule bundling with docetaxel (Figures 3A,B middle row, arrowhead) and depolymerized tubulin with nocodazole treatment (Figures 3A,B, third row). In contrast and despite effective drug-target engagement, drug treatment had no effect on the other clinically relevant and constitutively active AR splice variant, ARv7 (18). Treatment of the ARv7-microinjected cells with microtubule targeting drugs did not impact this variant's nuclear localization at any time point (Figures 3B,D and Table S1). Taken together, these data suggested that ARv567 but not ARv7 is dependent on microtubules for effective nuclear accumulation.
Figure 3. Microtubule targeting drugs inhibit ARv567 nuclear trafficking but have no effect on ARv7 nuclear accumulation.
PC3:mCh-tub cells were microinjected with GFP-tagged ARv567 or GFP-tagged ARv7 and incubated for 2 hr post-microinjection with either 1 μM docetaxel (TXT) or 10 μM nocodazole (Noc). Time lapse images were obtained with a spinning disk confocal microscope by acquiring an entire Z-stack at 10 min intervals for 2 hr. Shown are maximum intensity projections of representative PC3:mCherry-Tub cells expressing GFP-ARv567 (panel A) or GFP-ARv7 (panel B) at the indicated time points (movies in supplement). The integrity of the microtubule cytoskeleton from untreated, TXT or Noc treated cells was visualized with mCherry-tagged tubulin at the beginning of the timelapse recording and is shown in the far right panels. Arrowhead points at microtubule bundles. (C and D) Graphic representation of ARv567 (panel C) or ARv7 (panel D) nuclear accumulation over time following treatment in control versus TXT- and Noc-pretreated cells. Quantitative analysis of nuclear of GFP-AR was performed on each focal plane (0.5 μm Z-sections through the entire cell depth) using integrated pixel intensity values from the sum projection and the percentage of nuclear AR was calculated using the following formula: % Nuclear AR = 100*Nuclear AR/Total AR. Number of individual cells imaged per condition (n) ARv567: Control 0, 10, 20 and 30 min: n=16; 40-120 min: n=15. TXT pretreated 0-80 min: n=12; 90-120 min: n=10. Noc pretreated 0-50 min: n=11; 60-110 min: n=10; 120 min: n=3. N values for ARv7: Control 0-120 min: n=9. TXT pretreated 0-120 min: n=9. Noc pretreated 0-100 min: n=21, 110-120 min: n=7. Statistical test: One-way ANOVA with equal variances followed by multiple comparisons with Bonferroni adjustments.
To extend these observations we used the M12 prostate cancer cell line, a tumorigenic cell line representative of the metastatic stage of prostate cancer, to engineer isogenic cell lines stably expressing GFP-tagged AR-wt AR, ARv567 or ARv7. We then investigated the effects of docetaxel treatment on the nuclear localization of each of the GFP-tagged receptors, as seen by GFP fluorescence (Figure 4A,B) or antibody-based detection (Figure S2). In agreement with our previous data (12) docetaxel inhibited ligand-induced AR-wt nuclear accumulation downstream of microtubule stabilization (Figure 4A, arrowhead for microtubule bundles, arrows for cytoplasmic AR). Docetaxel treatment also inhibited the nuclear localization of the ligand-independent ARv567 variant (Figure 4B). Similar results were obtained in the presence of R1881, which did not induce any further nuclear accumulation of ARv567 (Figure S3A). However, as shown in Figure 4C, docetaxel treatment failed to alter the nuclear localization of ARv7 variant in the absence or presence of R1881 (Figure S3B). In addition, we quantified the extent of AR nuclear localization for each condition by calculating the ratio of nuclear to total cellular AR for each individual cell, as described in the methods (Figure 4D). This analysis revealed that docetaxel treatment resulted in a significant decrease of nuclear localization in AR-wt and ARv567, but had no effect on ARv7 confirming and corroborating the live cell imaging results that showed that ARv7 does not depend on microtubules for its nuclear trafficking. To assess the effect of docetaxel treatment on AR transcriptional activity we performed quantitative real-time PCR using transcriptional targets previously shown to be differentially induced by AR-wt and AR variants. Specifically TMPRSS2 was identified as a target specific for AR-wt while FKBP51 was transcriptionally activated by the AR variants (18, 34). In this assay we used M12 cells expressing inducible AR-wt or variants. As seen in Figure 4E in M12 cells the AR-wt increased TMPRSS2 expression and docetaxel treatment significantly inhibited it, consistent with the drug's effects on AR cytoplasmic sequestration. In the case of the AR variants, TMPRSS2 is no longer regulated while FKBP51 transcription is induced by both ARv7 and ARv567. Here we see that docetaxel treatment significantly inhibited ARv567 mediated induction of FKBP51 but had no effect on ARv7 transcriptional activity, in agreement with the differential effects of docetaxel on each variant's nuclear localization (Figure 4B,C). These data confirm that the AR variants have a distinct transcriptome compared to AR-wt but that nuclear localization is necessary for AR-V and AR-wt activity.
Figure 4. Taxane treatment inhibits AR-wt AR and ARv567, but not ARv7, trafficking to the nucleus in M12 cells.
(A-C) M12 cells stably expressing GFP-tagged AR-wt AR (A), ARv567 (B) and ARv7 (C) were treated with 1 μM docetaxel (TXT) for 4 h either alone or followed by 10 nM R1881 for 2 h as indicated in the figure. Cells were then fixed, immunostained for tubulin and imaged for GFP-AR and tubulin by confocal microscopy. AR is shown in green, tubulin in red. Representative, high-magnification images from each condition are shown. Arrows point to cytoplasmic AR; arrowheads point to microtubule bundles. Scale bar: 10 μm. (D) For each condition, at least 20 representative cells expressing either AR-wt or each AR variant, were analyzed. Box plots indicate the 25th percentile (bottom boundary), median (middle line), 75th percentile (top boundary), nearest observations within the interquartile range (whiskers); whiskers extend to 1.5× the interquartile range; red dots indicate outliers beyond this range. Notched boxes indicate uncertainty of the median. *** Denotes significance of the difference between the two conditions with P < 0.005. * Denotes significance of the difference between the two conditions with P < 0.05. (E) M12 cells expressing inducible AR-wt or AR variants were treated with 1 μM docetaxel (TXT) for 4 h either alone or followed by 10 nM R1881 overnight as indicated in the figure. Relative mRNA expression of TMPRSS2 and FKBP51 was assessed by qPCR. Values were corrected to GAPDH and normalized to untreated M12 cells expressing control lentivirus (M12 lenti). Bar graphs represent the average of three independent experiments. * p<0.05.
Dynamitin overexpression impairs ARv567 nuclear translocation, while it has no effect on ARv7
To further dissect the mechanism regulating the cytoplasmic to nuclear translocation of each variant we next investigated the involvement of the minus-end directed microtubule motor protein dynein, since we have shown that it mediates nuclear translocation (12). Dynein works in concert with several accessory proteins to drive subcellular motile functions including dynactin, which is an adapter that mediates the binding of dynein to cargo structures enhancing dynein's motor function. Overexpression of the dynactin associated protein, dynamitin, which disrupts dynein-cargo interactions (35), was used to dissect the involvement of dynein in the transport of the AR splice variants to the nucleus.
M12 cells stably expressing untagged ARv567 (M12-ARv567) or ARv7 (M12-ARv7) were transiently transfected with a c-myc-tagged p50-dynamitin vector and processed for double-labeling immunofluorescence with anti-AR and anti-c-myc antibodies. Overexpression of dynamitin impaired nuclear accumulation of ARv567 (Figure 5A, dashed arrows point to cytoplasmic AR) but had no effect on the nuclear accumulation of ARv7 (Fig 5B arrows point to nuclear AR). Quantitation of the extent of AR nuclear accumulation revealed a significant decrease of ARv567 in the nucleus of dynamitin expressing cells as compared with dynamitin-overexpressing M12-ARv7 cells where no effect on nuclear ARv7 was observed (Figure 5C).
Figure 5. Dynein associates with and mediates the nuclear translocation of ARv567, but not ARv7.
M12 cells stably expressing untagged AR variants, M12-ARv567 (A) and M12-ARv7 (B) were transiently transfected with pCMVH50myc (encoding a c-myc tagged human dynamitin). Cells were fixed, processed for double immunoflourescence labeling with anti-AR (green) and c-myc (red) antibodies and analyzed by confocal microscopy. Dashed arrows point to cytoplasmic AR and solid arrows to nuclear AR. (C) Quantitation of the amount of each of the variants in the nucleus was performed using MetaMorph image analysis software and shows a marked decrease of nuclear ARv567 after dynein-cargo disruption. ARv7, however, remains unaffected by dynamitin overexpression. The number of cells examined for each experimental condition is as follows: ARv567 CTL= 9 cells, ARv567 p50-dynamitin= 18 cells ARv7 CTL= 28 cells, ARv7 p50-dynamitin = 10 cells. (D) 1 mg input whole cell lystate from 293T cells transiently transfected with GFP-AR(wt), GFP-ARv567 and GFP-ARv7 were immunoprecipitated with a GFP antibody or control IgG and immunoblotted for dynein and AR. 50 μg of WCL was loaded as input. 1 μl of GFP antibody was loaded onto the gel as a control for the size of heavy chains in the samples.
To further investigate the role of the dynein microtubule-based motor protein on AR variant trafficking we performed a co-immunoprecipitation (co-IP) experiment in HEK293T cells transiently transfected with GFP-tagged AR-wt AR, ARv567 or ARv7 to determine their putative association with dynein. Co-precipitation using an antibody against GFP revealed that both the AR-wt AR and ARv567, but not ARv7, associated with dynein (Figure 5D). Taken together these data support a model whereby AR-wt AR and ARv567 utilize microtubules and dynein-dependent transport for their nuclear accumulation and subsequent activity. On the other hand, ARv7 does not utilize this mechanism of transport and hence remains insensitive to taxane treatment.
Docetaxel treatment inhibits ARv567-mediated subcutaneous tumor growth in SCID mice
Our recent (12, 14) and current data suggest that inhibition of AR nuclear accumulation and transcriptional activity mediates, as least in part, the clinical activity of taxanes. In order to determine the impact of AR variant expression on taxane sensitivity in vivo, we compared the effects of docetaxel on the growth of two LuCaP xenograft tumors grown subcutaneously in SCID mice as described in methods. The tumors were LuCaP 86.2, a human xenograft tumor in which the majority of the AR is ARv567, and LuCaP 23.1, a human xenograft expressing both AR-wt and ARv7 (20). All xenografts were grown in non-castrate SCID mice. There were 15 mice in each group. As shown in Figure 6A, LuCaP 86.2, which is resistant to castration and driven by ARv567, has its growth markedly suppressed by a low dose of docetaxel of 5 mg/kg (P< 0.01 control vs docetaxel treated), a dose that we have previously shown to be ineffective on the growth of LuCaP 35 xenograft expressing primarily AR-wt (29). In contrast, there was no effect of docetaxel on the growth of LuCaP23.1 tumors (p = NS control vs docetaxel treatment). The effect of docetaxel treatment on AR nuclear accumulation was further assessed by immunohistochemistry on explanted LuCaP tumors, as previously described (30). Quantitation of AR nuclear accumulation in tumors from untreated versus treated animals revealed that TXT treatment resulted in a statistically significant reduction of nuclear AR following in LuCaP86.2 tumors, while it had a minimal effect on LuCaP 23.1 tumors (Figure 6B and C). These data support the hypothesis that drug-induced inhibition of AR nuclear accumulation underlies taxane antitumor activity and show that AR variant expression can determine taxane sensitivity in vivo.
Figure 6. Docetaxel treatment inhibits ARv567-mediated subcutaneous tumor growth in SCID mice.
(A) Human prostate cancer xenografts LuCap 86.2, expressing predominantly ARv567, and LuCap 23.12 expressing both AR(wt) and ARv7, were treated with docetaxel 5 mg/kg weekly i.p or vehicle control. Study was terminated when all mice in the LuCaP 23.1 5mg/kg group meet UW IACUC criteria for euthanasia. Y axis shows tumor volumes +/− SEM. The differences in tumor volume between LuCaP 86.2 treated with docetaxel 5mgm weekly were highly significant , (p<0.0001) at the 8 week time period. There were no differences in LuCaP23.1 between vehicle or docetaxel treatment at any time point, (p>0.05). (B) Western Blot for AR using α-ARN20 (upper panel) and α-ARv7 in LuCaP 23.1, LuCaP 35 and LuCaP 86.2 showing the differences in the expression of AR and AR variants in the used xenografts. (C) AR immunostaining in explanted LuCaP tumors showing the reduction in AR nuclear localization in LuCaP 86.2 xenograft. In the upper row are shown representative pictures from untreated tumors (LuCaP 86.2 left side, LuCaP 23,1 right side) and in the bottom row from TXT-treated tumors. (D) Percent of nuclear AR from explanted LuCaP tumors by AR immunostaining showing the statistically significant reduction of AR nuclear localization in LuCaP 86.2 xenograft when treated with TXT. *** p<0.01
Discussion
Prostate cancer, the most commonly diagnosed malignancy in males in the United States, depends on active AR signaling (36). Androgen deprivation therapy alone is effective in a subset of PC patients, however, many patients progress to CRPC. The discovery of AR splice variants has provided significant insight into mechanisms of disease progression and ADT resistance. However, the potential impact of AR splice variant expression on chemotherapy has not been investigated. Currently, the taxanes represent the standard of care in CRPC treatment. However, the therapeutic benefit of taxane treatment cannot be indefinitely sustained and currently we fail to understand the molecular basis of clinical taxane resistance. Herein, we provide evidence that the presence of AR splice variants may affect sensitivity to taxane treatment.
The clinical significance of these variants is highlighted by studies showing that elevated ARv7 expression is associated with more rapid disease recurrence following radical prostatectomy for localized disease (17, 18). In addition, it was recently shown that ARv7 is regulated by the transcriptional factor FOXO1 in a PTEN-PI3K-AKT dependent manner (37, 38), which is a pathway activated in 50% of prostate cancers.
The ARv567 variant, arising through skipping of exons 5, 6 and 7 was identified in a panel of 25 different prostate cancer xenografts, termed the LuCaP series, most of which derived from metastases obtained from men with CRPC after prolonged exposure to androgen-deprivation therapy (19). Unlike ARv7, ARv567 retains the hinge region, that contains the second and most important part of AR's bipartite nuclear localization signal (NLS) consisting of aa 629RKLKKL634 and responsible of AR/importin-interaction (23, 26, 39). The hinge region contains also some well-defined control sites for AR-activity, being target for acetylation, ubiquitylation and methylation (23, 25).
However, even though AR variants have been found to increase the expression of full-length AR and enhance its transcriptional activity in the absence of ligand (19) their expression regulation and functional relationships had not been fully dissected. Studies on the transcriptional programs induced by variant signaling have shown that an adaptive shift toward AR-variant-mediated signaling occurs in a subset of CRPC tumors, and thus suggesting that AR-variants have different gene signatures from full-length AR potentially contributing to drug resistance to AR-targeted therapies such as abiraterone and enzalutamide (20, 21, 34). However, a recent report argues that AR-variants induce genes that constitute a subset of the genes regulated by AR-full length, rather than having a distinct transcriptional signature, and therefore, AR-variants are constitutive and independent effectors of AR transcriptional program (21). Regardless of these discrepancies the data on AR variants strongly suggest that their expression arises following castration and plays a role in the progression of prostate cancer.
For the variants to exert their transcriptional activity they have to be localized to the nucleus. Although the published literature highlights the strong nuclear presence of the variants, our results are the first to show a differential preference of AR variants for microtubule-mediated nuclear transport; ARv567 being microtubule- and dynein-dependent and ARv7 being microtubule independent (Figures 4, 5). Interestingly, we showed that the C-terminus of AR mediates microtubule-binding (Figure 1) and that the specific microtubule-binding domain comprises of the DNA-BD together with the adjacent hinge region (aa 559-663) (Figure 1C). This finding can readily explain the reduced association of ARv7 with microtubules, as ARv7 lacks the hinge region which mediates microtubule binding (Figure 1C) and is also involved in the regulation of AR nuclear translocation and intranuclear motility (40). We were surprised to find that none of the individual C-terminus truncation mutants displayed as extensive microtubule association as the entire C-ter AR (aa 540-919) (Figure 2B, C). These results suggest that perhaps non-sequential regions of AR may come together in 3D to generate the surface of microtubule interaction indicating a larger and globular surface required for protein-protein interaction.
Additional studies using cryo-electron microscopy and recombinant full-length AR and AR-variant proteins are currently ongoing in order to investigate the structural interaction between AR and its variants with microtubules. Our preliminary results have revealed that recombinant purified full-length AR protein binds directly to purified microtubules in vitro (data not shown), suggesting that AR may bind microtubules not only for the purpose of trafficking but also as a microtubule-associated protein potentially regulating polymer stability and dynamics.
We and others have previously shown that taxane treatment inhibits AR nuclear translocation and activity in prostate cancer cell lines and patient-derived CTCs (12, 13) and that this mechanism underlies at least in part clinical response to taxane treatment (14). While AR nuclear accumulation remains a critical factor responsible for AR transcriptional activity, the pathways regulating AR-variant nuclear translocation remain poorly understood. Our data support the requirement of the hinge region (aa 625-663) for AR microtubule association and downstream nuclear translocation, while other reports show that the NTD/DNA-BD core (aa 1-627) alone -in the absence of the hinge region- is sufficient for receptor nuclear translocation (28). At the same time mutation of three key residues of the hinge region, namely K630A, K632A and K633A, completely abrogates nuclear accumulation of full-length AR in agreement with our results. These data suggest that in addition to microtubule-mediated AR nuclear transport, there are additional pathways that operate in the absence of the hinge region, like in the case of ARv7.
Clinically, although 50% of men respond to docetaxel following progression after standard androgen ablation, some men clearly are sensitive to taxanes and have a prolonged response, for reasons not well understood. In this report we show that taxane-mediated microtubule stabilization differentially affects AR variant trafficking and transcriptional activity, raising the possibility that these variants may predict sensitivity or resistance to taxane chemotherapy. In agreement with this hypothesis, we show that LuCaP human xenografts expressing ARv567 or full-length AR co-expressed with ARv7 show differential sensitivity to docetaxel treatment (Figure 6). Interestingly, LuCaP 86.2 expressing predominantly ARv567 variant was the most sensitive to taxane treatment, consistent with the drug's ability to inhibit this variant's nuclear localization and activity. While our animal data do not indicate that AR inhibition is the only mechanism by which docetaxel is effective in prostate cancer, as we also showed that LuCaP xenografts that do not express high levels of ARv567 can respond to higher does of docetaxel (Figure S4), LuCaP 86.2 is clearly an outlier in the sensitivity and duration of response to docetaxel.
Taken together, these data suggest that tumors driven by ARv567 may be marked as those that, even if resistant to next generation AR-targeting drugs, will likely benefit most by taxane treatment. Conversely, our data suggest that tumors predominantly expressing ARv7 will likely be resistant to taxane chemotherapy and that alternate treatments able to inhibit ARv7 transcriptional activity might be beneficial. Although the clinical impact of ARv7 expression on patient response to next generation AR-targeting drugs is not yet determined, our findings suggest that ARv7 expression may constitute a common mechanism of resistance to both taxanes and AR inhibitors. In agreement with this hypothesis a recent clinical report showed that the activity of docetaxel in post- abiraterone treated patients was lower than expected while no responses to docetaxel were observed in abiraterone-refractory patients (15). Moreover a recent but not published clinical trial showed that men with hormone-sensitive metastatic prostate cancer who received docetaxel given at the start of ADT lived longer than patients who received hormone therapy alone (41). These recent findings are encouraging and strongly support the hypothesis that next-generation AR inhibitors and taxanes act on the same pathway (even if at a different level), and are synergistic in patients who presumably do not express significant amounts of AR variants since they are still responsive to ADT.
The data presented in this study provide insights into the regulation of AR-variant trafficking and activity in prostate cancer, link their expression with the therapeutic benefit of taxane treatment and suggest that the presence of AR variants may determine the clinical efficacy of taxane chemotherapy. Evaluating and discriminating AR-variants in prostate cancer tumor cells can help clinicians tailor treatment in CRPC patient by identifying patients who are most likely to benefit from taxane chemotherapy.
Supplementary Material
Acknowledgments
Financial Support: U.S. NIH (R01 CA137020-01 and U54 CA143876, P. Giannakakou), the NIH Physical Sciences Oncology Center at Cornell (P. Giannakakou and D.M. Nanus), NCI Pacific Northwest Prostate Cancer SPORE (2 P50 CA 097186-12, S.R. Plymate), PO1 CA85859 (S.R. Plymate), DOD-CDMRP (S.R. Plymate), the Prostate Cancer Foundation (S. R. Plymate), the Prostate Cancer Foundation (R.L. Vessella) and the Department of Veterans Affairs Research Service (S.R. Plymate). Additional support was received from the Weill Cornell Clinical and Translational Science Center (D.M. Nanus and P. Giannakakou), the Prostate Cancer Foundation (P. Giannakakou and D.M. Nanus), the Italian Association for Cancer Research (AIRC) to Luigi Portella, NCI F32CA177104 to Alex Matov and the Genitourinary Oncology Research Fund (D.M. Nanus).
Footnotes
Conflict of interest
The authors disclose no potential conflicts of interest
References
- 1.Hoffman-Censits J, Kelly WK. Enzalutamide: a novel antiandrogen for patients with castrate-resistant prostate cancer. Clin Cancer Res. 2013;19:1335–9. doi: 10.1158/1078-0432.CCR-12-2910. [DOI] [PubMed] [Google Scholar]
- 2.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson PS. Molecular states underlying androgen receptor activation: a framework for therapeutics targeting androgen signaling in prostate cancer. J Clin Oncol. 2012;30:644–6. doi: 10.1200/JCO.2011.39.1300. [DOI] [PubMed] [Google Scholar]
- 5.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–15. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 6.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 7.Nadiminty N, Gao AC. Mechanisms of persistent activation of the androgen receptor in CRPC: recent advances and future perspectives. World J Urol. 2012;30:287–95. doi: 10.1007/s00345-011-0771-3. [DOI] [PubMed] [Google Scholar]
- 8.Joseph JD, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D, et al. A Clinically Relevant Androgen Receptor Mutation Confers Resistance to Second-Generation Antiandrogens Enzalutamide and ARN-509. Cancer Discov. 2013 doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- 9.Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, et al. An F876L Mutation in Androgen Receptor Confers Genetic and Phenotypic Resistance to MDV3100 (Enzalutamide). Cancer Discov. 2013 doi: 10.1158/2159-8290.CD-13-0142. [DOI] [PubMed] [Google Scholar]
- 10.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr., Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 11.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 12.Darshan MS, Loftus MS, Thadani-Mulero M, Levy BP, Escuin D, Zhou XK, et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011;71:6019–29. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu ML, Horbinski CM, Garzotto M, Qian DZ, Beer TM, Kyprianou N. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 2010;70:7992–8002. doi: 10.1158/0008-5472.CAN-10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thadani-Mulero M, Nanus DM, Giannakakou P. Androgen receptor on the move: boarding the microtubule expressway to the nucleus. Cancer Res. 2012;72:4611–5. doi: 10.1158/0008-5472.CAN-12-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mezynski J, Pezaro C, Bianchini D, Zivi A, Sandhu S, Thompson E, et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann Oncol. 2012;23:2943–7. doi: 10.1093/annonc/mds119. [DOI] [PubMed] [Google Scholar]
- 16.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–77. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–13. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–30. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–25. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–9. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hornberg E, Ylitalo EB, Crnalic S, Antti H, Stattin P, Widmark A, et al. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One. 2011;6:e19059. doi: 10.1371/journal.pone.0019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinckemalie L, Vanderschueren D, Boonen S, Claessens F. The hinge region in androgen receptor control. Mol Cell Endocrinol. 2012;358:1–8. doi: 10.1016/j.mce.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 24.Cutress ML, Whitaker HC, Mills IG, Stewart M, Neal DE. Structural basis for the nuclear import of the human androgen receptor. J Cell Sci. 2008;121:957–68. doi: 10.1242/jcs.022103. [DOI] [PubMed] [Google Scholar]
- 25.Haelens A, Tanner T, Denayer S, Callewaert L, Claessens F. The hinge region regulates DNA binding, nuclear translocation, and transactivation of the androgen receptor. Cancer Res. 2007;67:4514–23. doi: 10.1158/0008-5472.CAN-06-1701. [DOI] [PubMed] [Google Scholar]
- 26.Zhou ZX, Sar M, Simental JA, Lane MV, Wilson EM. A ligand-dependent bipartite nuclear targeting signal in the human androgen receptor. Requirement for the DNA-binding domain and modulation by NH2-terminal and carboxyl-terminal sequences. J Biol Chem. 1994;269:13115–23. [PubMed] [Google Scholar]
- 27.Giannakakou P, Sackett DL, Ward Y, Webster KR, Blagosklonny MV, Fojo T. p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nat Cell Biol. 2000;2:709–17. doi: 10.1038/35036335. [DOI] [PubMed] [Google Scholar]
- 28.Chan SC, Li Y, Dehm SM. Androgen receptor splice variants activate androgen receptor target genes and support aberrant prostate cancer cell growth independent of canonical androgen receptor nuclear localization signal. J Biol Chem. 2012;287:19736–49. doi: 10.1074/jbc.M112.352930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu JD, Haugk K, Coleman I, Woodke L, Vessella R, Nelson P, et al. Combined in vivo effect of A12, a type 1 insulin-like growth factor receptor antibody, and docetaxel against prostate cancer tumors. Clin Cancer Res. 2006;12:6153–60. doi: 10.1158/1078-0432.CCR-06-0443. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Morrissey C, Sun S, Ketchandji M, Nelson PS, True LD, et al. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS One. 2011;6:e27970. doi: 10.1371/journal.pone.0027970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosin P. Unimodal thresholding. Pattern Recognit. 2001;34:2083–96. [Google Scholar]
- 32.Aurenhammer FaK. R. Voronoi Diagrams. Handbook of Computational Geometry; Amsterdam: 2000. pp. 201–90. [Google Scholar]
- 33.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A. 2010;107:16759–65. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu R, Lu C, Mostaghel EA, Yegnasubramanian S, Gurel M, Tannahill C, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–62. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–84. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma NL, Massie CE, Ramos-Montoya A, Zecchini V, Scott HE, Lamb AD, et al. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell. 2013;23:35–47. doi: 10.1016/j.ccr.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Bohrer LR, Liu P, Zhong J, Pan Y, Angstman J, Brand LJ, et al. FOXO1 binds to the TAU5 motif and inhibits constitutively active androgen receptor splice variants. Prostate. 2013 doi: 10.1002/pros.22649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mediwala SN, Sun H, Szafran AT, Hartig SM, Sonpavde G, Hayes TG, et al. The activity of the androgen receptor variant AR-V7 is regulated by FOXO1 in a PTEN-PI3KAKT-dependent way. Prostate. 2013;73:267–77. doi: 10.1002/pros.22566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claessens F, Denayer S, Van Tilborgh N, Kerkhofs S, Helsen C, Haelens A. Diverse roles of androgen receptor (AR) domains in AR-mediated signaling. Nucl Recept Signal. 2008;6:e008. doi: 10.1621/nrs.06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanner TM, Denayer S, Geverts B, Van Tilborgh N, Kerkhofs S, Helsen C, et al. A 629RKLKK633 motif in the hinge region controls the androgen receptor at multiple levels. Cell Mol Life Sci. 2010;67:1919–27. doi: 10.1007/s00018-010-0302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sweeney C. NIH-funded study shows increased survival in men with metastatic prostate cancer who receive chemotherapy when starting hormone therapy. 2103 12/05/2013; Available from: http://www.cancer.gov/newscenter/newsfromnci/2013/E3805.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.