The WRKY75 transcription factor is expressed in the pericycle and vascular tissues of the root and regulates root hair patterning in a non-cell-autonomous manner.
Abstract
In Arabidopsis (Arabidopsis thaliana), root hairs are formed in cell files over the cleft of underlying cortex cells. This pattern is established by a well-known gene regulatory network of transcription factors. In this study, we show that WRKY75 suppresses root hair development in nonroot hair files and that it represses the expression of TRIPTYCHON and CAPRICE. The WRKY75 protein binds to the CAPRICE promoter in a yeast one-hybrid assay. Binding to the promoter fragment requires an intact WRKY protein-binding motif, the W box. A comparison of the spatial expression of WRKY75 and the localization of the WRKY75 protein revealed that WRKY75 is expressed in the pericycle and vascular tissue and that the WRKY75 RNA or protein moves into the epidermis.
The establishment of a pattern of files forming root hairs and nonroot hairs in the root epidermis is a well-studied developmental model system (Schiefelbein et al., 2009; Tominaga-Wada et al., 2011; Grebe, 2012). Root hair cells develop from cell files over the cleft of two underlying cortical cells, whereas all other cells develop into nonroot hair cells (Dolan et al., 1993, 1994; Galway et al., 1994). The genetic dissection of the underlying pathway has enabled the isolation of essential genes and, subsequently, their molecular characterization (Schiefelbein, 2000; Pesch and Hülskamp, 2004; Ishida et al., 2008). Mutations in four genes lead to the formation of extra root hairs in nonroot hair cell positions, indicating their importance in the repression of root hair formation. These are TRANSPARENT TESTA GLABRA1 (TTG1), GLABRA3 (GL3), ENHANCER OF GLABRA3 (EGL3), and WEREWOLF (WER; Galway et al., 1994; Masucci and Schiefelbein, 1996; Lee and Schiefelbein, 1999; Bernhardt et al., 2003). In addition, a number of positive regulators of root hair formation have been identified, including CAPRICE (CPC), TRIPTYCHON (TRY), ENHANCER OF TRIPTYCHON AND CAPRICE1 (ETC1), ETC2, and ETC3 (Wada et al., 1997; Schellmann et al., 2002; Kirik et al., 2004a, 2004b; Simon et al., 2007; Wester et al., 2009). Current models explain root hair patterning by a lateral inhibition scenario with feedback loops: the R2R3MYB protein WER (Lee and Schiefelbein, 1999), the basic helix-loop-helix proteins GL3 and EGL3 (Bernhardt et al., 2003), and the WD40 domain protein TTG1 (Walker et al., 1999) are considered to form a trimeric complex (MBW complex) that activates GLABRA2 (GL2) expression in nonroot hair cells (Schiefelbein, 2003; Pesch and Hülskamp, 2004), which in turn represses the root hair cell fate (Masucci et al., 1996). In addition, the R3MYB genes CPC, TRY, ETC1, ETC2, and ETC3 are activated and are thought to suppress the activity of the MBW complex by binding to GL3/EGL3 protein, thereby replacing WER in a competitive manner (Esch et al., 2003; Kirik et al., 2004a, 2004b; Kurata et al., 2005; Ryu et al., 2005; Tominaga et al., 2007). Cross talk between the cells is mediated by the R3MYB proteins. These are expressed predominantly in the nonroot hair cell files and move to root hair cell file cells. Recently, it was shown that CPC can move freely in the root meristematic region and that it is trapped by EGL3 in root hair cell file cells (Kang et al., 2013). In addition, GL3 that is expressed in root hair cells moves in the opposite direction into the nonroot hair cells (Bernhardt et al., 2005). The position with respect to the underlying cortical cells is governed by the Leu-rich repeat receptor-like kinase, SCRAMBLED (SCM), and JACKDAW (JKD; Kwak et al., 2005; Hassan et al., 2010). It is postulated that JKD expression in the cortex differentially regulates SCM activity, because the sizes of the contact areas are different between epidermal cells overlying cortex cells and epidermal cells over the cleft of two underlying cortex cells (Hassan et al., 2010). SCM in turn represses WER in nonroot hair cells. Mathematical models have been developed to analyze the interaction schemes and highlight the importance of the reciprocal movement behavior of the R3MYBs and GL3 proteins (Savage et al., 2008). In addition to this gene regulatory network, the plant hormones ethylene and auxin are involved in root hair development. Genetic and expression analyses suggest that both hormones promote root hair formation downstream of TTG1 and GL2 (Masucci and Schiefelbein, 1996).
One additional regulator of root hair initiation is the WRKY75 gene. In WRKY75 RNAi, an RNA interference line, the number of root hairs is markedly increased, indicating that it is a negative regulator of root hair formation (Devaiah et al., 2007). A root hair-specific function of WRKY75 is unlikely, as it has been implicated also in phosphate starvation, pathogen responses, and senescence (Dong et al., 2003; Guo and Gan, 2004; Devaiah et al., 2007; Encinas-Villarejo et al., 2009). The WRKY75 gene encodes a WRKY protein containing a single WRKY domain and a C2H2 zinc finger motif at the C terminus. WRKY proteins act as transcription factors through direct interaction of the WRKY domain to the W box cis-regulatory elements in target promoter sequences (Rushton et al., 1995; Eulgem et al., 1999; Ciolkowski et al., 2008). Consistent with this, the WRKY75 protein localizes to the nucleus and regulates the expression of other phosphate-response genes (Devaiah et al., 2007).
Whether WRKY75-dependent regulation of root hair formation involves the root hair patterning genes or acts at the level of hormonal regulation has not been studied. In this work, we aimed to unravel in more detail the molecular mechanism by which WRKY75 regulates the initiation of Arabidopsis (Arabidopsis thaliana) root hairs.
RESULTS
WRKY75 Represses Root Hair Development
The previous finding that a WRKY75 RNAi line produces extra root hairs (Devaiah et al., 2007) raises the question of whether WRKY75 acts on the root hair patterning network, directly on GL2, or even later in the hormonal control of root hair formation. To address this question, we first analyzed the root hair pattern in wrky75 mutants and WRKY75 overexpression lines.
In our work, we used two mutant lines: the previously described WRKY75 RNAi line (Devaiah et al., 2007) and the transfer DNA (T-DNA) insertion line N121525, called wrky75-25 (Encinas-Villarejo et al., 2009). We confirmed that wrky75-25 is a null mutant by reverse transcription PCR (Supplemental Fig. S1). Next, we cloned the T-DNA flanking region to map the T-DNA insertion. The T-DNA insertion maps 143 bp downstream of the stop codon (Supplemental Fig. S2). This suggests that the 3′ region is essential for the expression of WRKY75.
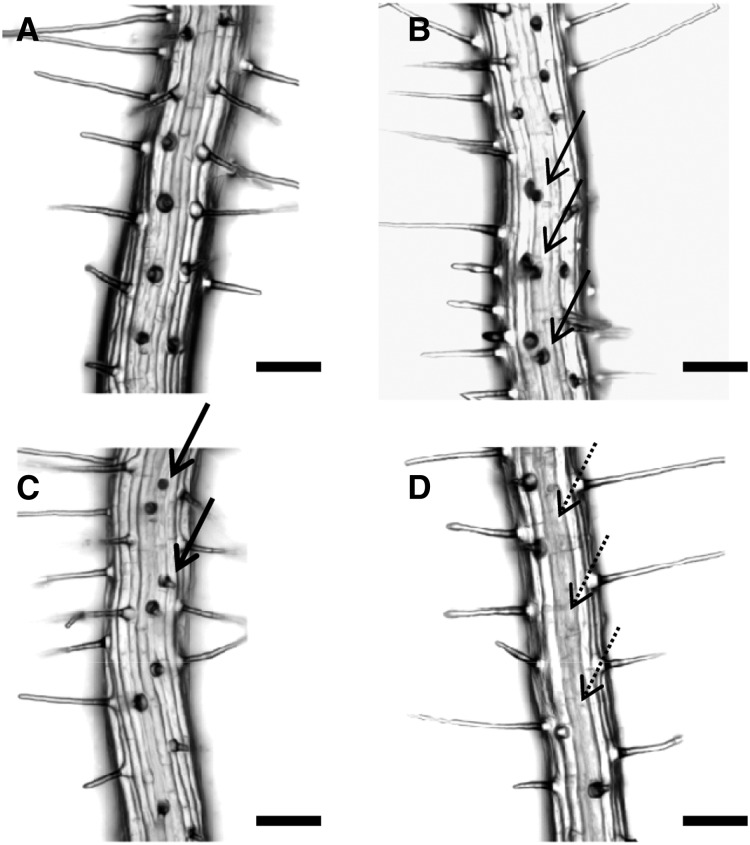
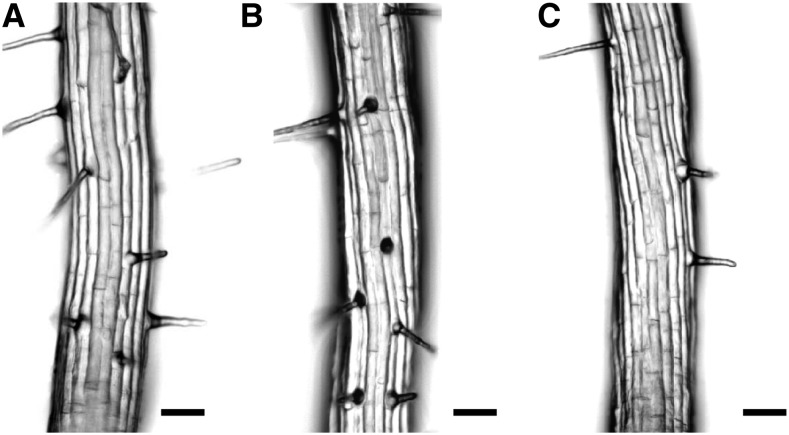
Our analysis of the root hair pattern showed that both wrky75 mutants exhibited ectopic root hairs in nonhair files (Fig. 1, B and C; Table I). This suggested to us that WRKY75 is a negative regulator of root hair formation. To test this, we generated lines expressing WRKY75 under the control of the ubiquitous 35S promoter (p35S:WRKY75). In these lines, significantly fewer root hairs were found in the hair files (Fig. 1D; Table I). In p35S:WRKY75, WRKY75 expression was 14.9 ± 3.9 times higher than the wild-type expression in quantitative real-time PCR experiments (Supplemental Fig. S3). Together, these phenotypes suggest that WRKY75 acts on the root hair patterning network by suppressing root hair fate in the nonhair files and by promoting root hair production in the root hair files.
Figure 1.
Root hair phenotypes in the wild type and mutants. A, Wild-type root. B, wrky75-25 root with ectopic root hairs in nonhair positions indicated by arrows. C, WRKY75 RNAi root displaying ectopic root hairs. D, p35S:WRKY75 root lacking root hairs in hair positions indicated by dashed arrows. Bars = 100 µm.
Table I. Root hair numbers in the patterning zone of the primary roots.
Ten 7-d-old Arabidopsis plants were analyzed for each treatment. Values represent three biological replicates (percentage ± sd). P < 0.05 (Mann-Whitney test). Col-0, Columbia-0.
| Root Hair Position |
Nonroot Hair Position |
|||
|---|---|---|---|---|
| Genotype | Root Hair Cell | Nonroot Hair Cells | Root Hair Cell | Nonroot Hair Cells |
| Wild type (Col-0) | 95 ± 6.0 | 5.0 ± 6.0 | 0.3 ± 1.5 | 99.7 ± 1.5 |
| wrky75-25 | 99 ± 3.6 | 1.0 ± 3.6 | 25 ± 9.6 | 75 ± 9.6 |
| WRKY75 RNAi | 96 ± 5.8 | 4.0 ± 5.8 | 29 ± 6.4 | 71 ± 6.4 |
| p35S:WRKY75 | 70 ± 9.7 | 30 ± 9.7 | 0.0 ± 100 | 100 ± 100 |
| cpc-2 | 34 ± 11.0 | 66 ± 11.0 | 0.0 ± 100 | 100 ± 100 |
| cpc-2 wrky75-25 | 39 ± 6.4 | 61 ± 6.4 | 0.0 ± 100 | 100 ± 100 |
| p35S:WRKY75 cpc-2 | 21 ± 8.5 | 79 ± 8.5 | 0.0 ± 100 | 100 ± 100 |
Transcriptional Regulation of Root Hair Patterning Genes by WRKY75
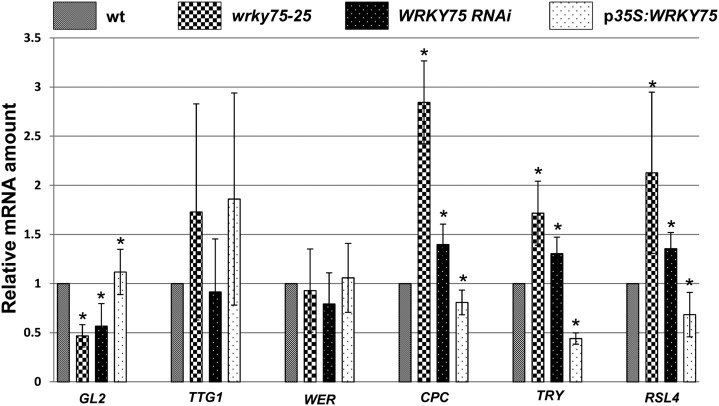
To determine whether WRKY75 regulates the expression of patterning genes, we studied the expression levels of selected candidates by real-time PCR (Fig. 2; Table 1; Supplemental Tables S1 and S2). We focused our expression analyses on the patterning process by isolating the RNA from the root hair initiation zone. We observed a significant increase in the expression levels of CPC and TRY in both wrky75 mutants and reduced expression levels in the p35S:WRKY75 line. Consistently, we found the corresponding changes in the expression levels of the nonroot hair marker GL2 (Szymanski et al., 1998) and the root hair marker ROOT HAIR DEFECTIVE6-LIKE4 (RSL4; Yi et al., 2010). In wrky75 mutants, the expression levels of RSL4 were increased, whereas GL2 expression levels were reduced. In the WRKY75 overexpression line, the RSL4 expression levels were reduced and those of GL2 were increased. Together, these data indicate that WRKY75 regulates the expression of the root hair patterning genes.
Figure 2.
Transcriptional regulation of root hair patterning genes by WRKY75. The relative expression of patterning genes was compared by real-time PCR (three biological samples, each with three technical repeats). The wild-type (wt) level was set to 1, and the expression changes in the mutants and overexpression line were plotted. Changes significantly different from the wild type are marked with asterisks (Student’s t test, P < 0.05). Error bars represent sd.
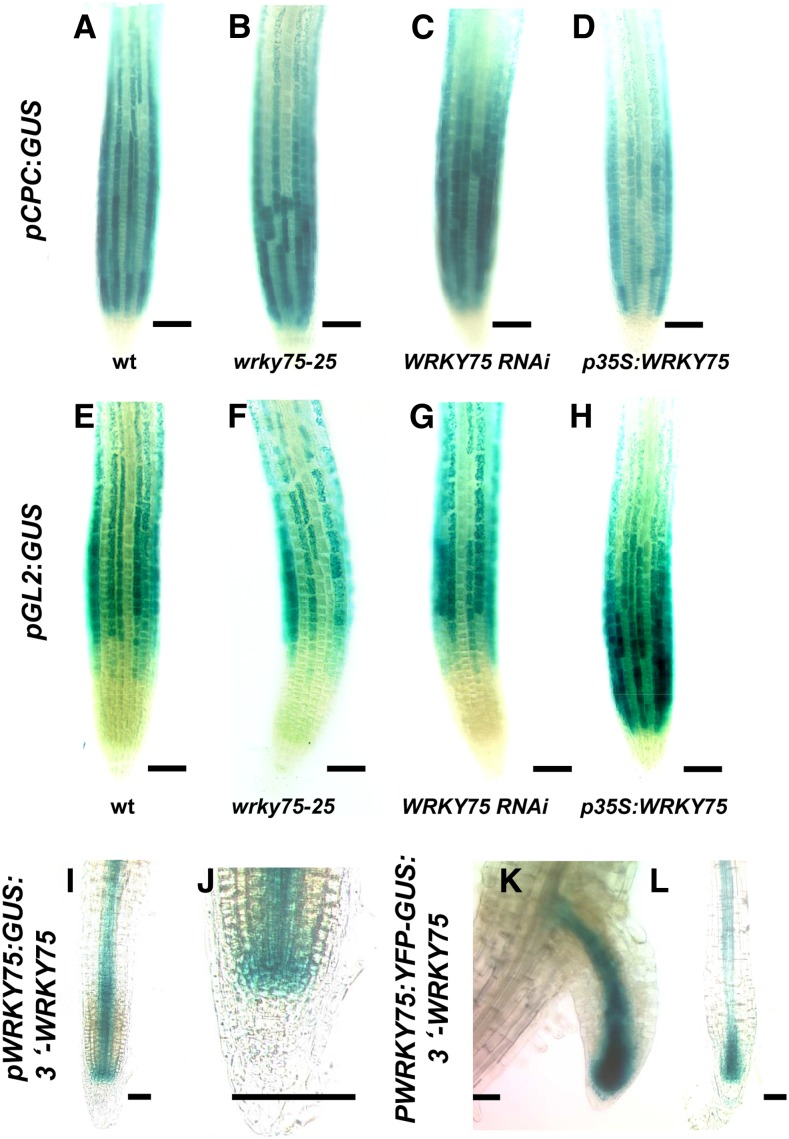
Changes in the expression levels can be explained by higher but spatially correct expression or by temporal/spatial changes. To distinguish between these two possibilities, we focused on the spatial expression of CPC and GL2 in roots using established promoter:GUS marker lines (Fig. 3). In the wild type, CPC was expressed in the nonroot hair files that can be recognized as continuous GUS-labeled stretches of cells and weakly expressed in the stele (Wada et al., 2002). In wrky75 mutants, we noted three differences (Fig. 3). First, we found up to four adjoining cell files with CPC expression, indicating that CPC is expressed in hair and nonroot hair files. Second, we noted that nonroot hair files showed discontinuous labeling, indicating that not all cells in nonroot hair cell positions express CPC. Third, CPC expression in the stele was increased in wrky75 mutants (Supplemental Fig. S4). Together, these data suggest that CPC expression is affected in both epidermal cell files and the stele. In p35S:WRKY75 lines, we found frequently that the CPC-expressing cell files were not continuous. CPC expression in the stele was barely detectable (Supplemental Fig. S4). The analysis of pGL2:GUS revealed discontinuous GUS-expressing cell files in wrky75 mutants (Fig. 3, F and G). In p35S:WRKY75, we also found pCPC:GUS expression in up to four immediately neighboring cell files (Fig. 3H).
Figure 3.
Histochemical GUS expression analysis of CPC, GL2, and WRKY75 in roots. The expression of CPC and GL2 is monitored in pCPC:GUS (A–D) and pGL2:GUS (E–H) transgenic plants. The genetic background is indicated below each image. The expression of WRKY75 is monitored in the pWRKY75:GUS:3′-WRKY75 (I and J) and pWRKY75:YFP-GUS:3′-WRKY75 (K and L) wild-type (wt) Col-0 background. Bars = 50 μm.
WRKY75 Protein Binds to the CPC Promoter through the W Box
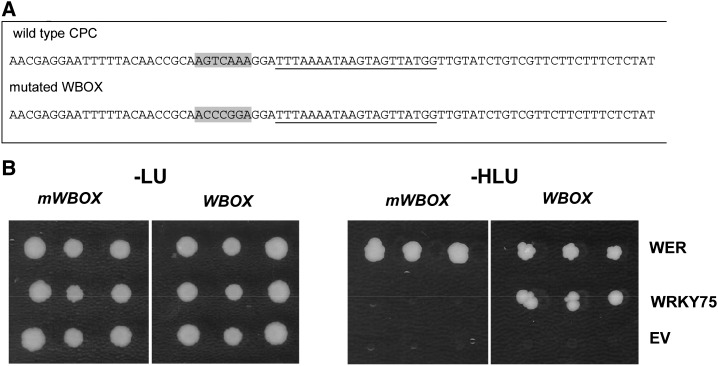
We tested the ability of WRKY75 protein to bind to the CPC promoter using a yeast one-hybrid assay. It is well established that WRKY proteins can bind to six-nucleotide-long consensus sequences (C/TTGACT/C) called the W box (Rushton et al., 1995; Eulgem et al., 1999; Ciolkowski et al., 2008). It was shown that the region between –425 and –406 of the CPC promoter contains a binding site of WER (Ryu et al., 2005). Therefore, we searched for W boxes near that region and chose an 80-bp fragment [pCPC(−459 to −378)] containing a W box and the WER-binding site (Fig. 4A).
Figure 4.
Yeast one-hybrid studies showing WRKY75 binding to a CPC promoter fragment. A, The 80-bp promoter sequence of CPC used for the yeast one-hybrid assay. The W box is highlighted in gray, and the WER-binding site is underlined. B, Yeast one-hybrid assay. Yeast strain YM4271 carrying a prey construct with three tandem repeats of 80 bp (−459 to −378) of the CPC promoter was cotransformed with WER (positive control) and WRKY75 fusions to the GAL4 activation domain or with an empty vector (EV; negative control). Binding to the wild-type sequence of the promoter fragment (WBOX) was compared with a fragment in which the W box was mutated (mWBOX). –LU, Medium lacking Leu and uracil; –HLU, medium lacking His, Leu, and uracil.
In the presence of WRKY75, the HIS3 reporter gene driven by three tandem repeats of pCPC(−459 to −378) was transcribed as indicated by yeast growth on selective medium lacking His (Fig. 4B). To test whether binding occurs through the W box, we performed this experiment with a promoter fragment in which the W box was mutated. This promoter fragment failed to mediate WRKY75 binding. Binding of WER was not impaired, indicating that the promoter fragment was generally accessible. Together, our data show that WRKY75 protein can bind directly to the CPC(−459 to −378) promoter fragment through the W box motif.
Genetic Analysis of WRKY75
If extra root hair formation in wrky75 mutants is caused by the derepression of CPC, one would expect that the cpc mutant root hair phenotype would be epistatic to wrky75. To test this hypothesis, we created the cpc-2 wrky75-25 double mutant. This double mutant showed only a few root hairs, as observed in the cpc-2 mutant (Fig. 5; Table I). Overexpression of WRKY75 in cpc-2 caused a further reduction in root hair numbers. This is essentially an additive phenotype, suggesting that WRKY75 overexpression can further reduce root hair number through additional targets. One possible target is the TRY gene, as we observed a reduced expression of TRY in p35S:WRKY75 lines.
Figure 5.
Genetic analysis of WRKY75. Light micrographs of roots in the wild type (A), cpc-2 wrky75-25 (B), and cpc-2 p35S:WRKY75 (C) are shown. Bars = 50 μm.
WRKY75 Is Expressed in the Inner Part of the Root
As WRKY75 represses CPC, we expected it to be coexpressed with CPC in nonroot hair files. As the T-DNA insertion in the wrky75-25 allele was found in the 3′ region of the WRKY75 gene, we created a promoter:GUS construct containing a 2.2-kb upstream region and a 627-bp 3′ region (called pWRKY75:GUS:3′-WRKY75). The characterization of five lines in a Col-0 background consistently revealed expression in the inner region of the root (Fig. 3, I and L). Typically, the highest expression was observed close to the root tip and weaker expression in the stele in upper root regions. GUS staining for 9, 12, and 24 h revealed that WRKY75 is not expressed in the early epidermal cells in the root cap (Fig. 3J; Supplemental Fig. S5). The same pattern was observed in lateral roots (Fig. 3K). Plants transformed with constructs containing only the 2.2-kb upstream region (pWRKY75:GUS) showed no GUS expression in 20 T1 seedlings.
WRKY75 Protein/RNA Moves from the Inner Part of the Root to the Epidermis
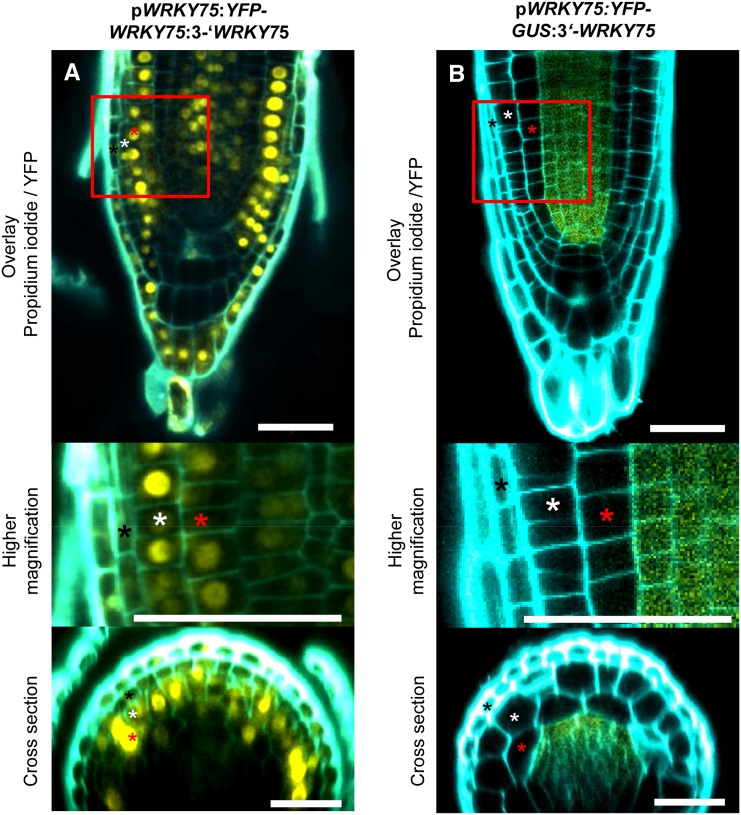
As WRKY75 expression does not coincide with its biological function in the epidermis, we tested the possibility that WRKY75 RNA or WRKY75 protein moves from the center of the root to the epidermis. To test whether WRKY75 is non cell autonomous, we created two constructs: pWRKY75:YFP-GUS:3′-WRKY75 and pWRKY75:YFP-WRKY75:3′-WRKY75. pWRKY75:YFP-GUS:3′-WRKY75 was used to precisely locate the expression of WRKY75 by yellow fluorescent protein (YFP) fluorescence and to avoid artificial GUS staining caused by GUS diffusion. The pWRKY75:YFP-WRKY75:3′-WRKY75 construct was used to study WRKY75 protein localization (Fig. 6). This construct rescued the wrky75 mutant root hair phenotype, indicating that the promoter sequences are sufficient for rescue and that the YFP-WRKY75 fusion protein is functional (Supplemental Table S3). The WRKY75 transcript levels of plants carrying this construct in the wrky75-25 background are lower than in Col-0 (Supplemental Fig. S6). This excludes the possibility that artificially high expression levels lead to the rescue. The expression of this construct in the wild-type Col-0 background did not cause a root hair phenotype (Supplemental Table S4). In pWRKY75:YFP-GUS:3′-WRKY75 plants, YFP fluorescence (and GUS staining; Fig. 3L) was found in the pericycle and the vascular tissue of the root (Fig. 6B; Supplemental Fig. S7), as observed with the pWRKY75:GUS:3′-WRKY75 construct. In contrast, the YFP signal was also found in the neighboring cell layers, including the epidermis in pWRKY75:YFP-WRKY75:3′-WRKY75 plants (Fig. 6A). This indicates that YFP-WRKY75 moves from the center to the epidermis of the primary root.
Figure 6.
Intercellular motility of WRKY75. A, Root of a wrky75-25 plant carrying pWRKY75:YFP-WRKY75:3′-WRKY75. B, Root of a Col-0 plant carrying pWRKY75:YFP-GUS:3′-WRKY75. Squares mark the region shown at higher magnification. Black stars mark lateral root cap cells, white stars label epidermal cells, and red stars mark cortical cells. Note that high laser intensity and high-brightness/contrast modifications were applied on the images to allow the visualization of the signal, resulting in overexposure to the outer layers (lateral root cap cells). Bars = 40 µm.
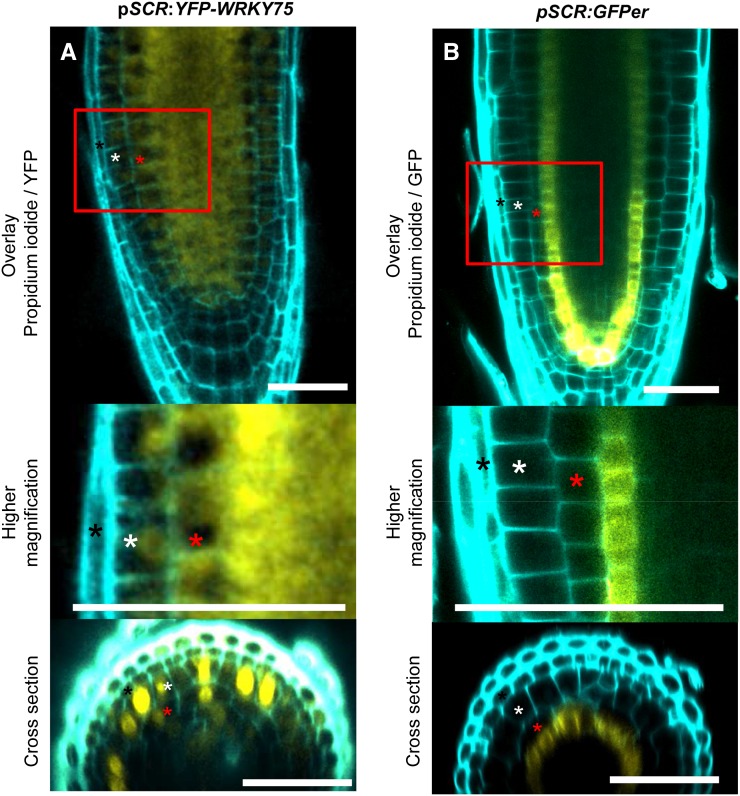
To independently confirm that WRKY75 can move between different tissue layers, we expressed the WRKY75 coding DNA sequence under the control of the SCARECROW (SCR) promoter in the wrky75-25 mutant background (Helariutta et al., 2000). As expected, pSCR:GFPer (Fig. 7B; Supplemental Fig. S8C) showed expression in the cortical/endodermis initial cells and in the endodermis (Di Laurenzio et al., 1996). In pSCR:YFP-WRKY75, the YFP signal was detected in all cell layers (Fig. 7A; Supplemental Fig. S8, A and B). Moreover, pSCR:YFP-WRKY75 was able to rescue the wrky75-25 phenotype (Supplemental Table S5). These results confirm that WRKY75 can move between different tissue layers.
Figure 7.
Movement of WRKY75 when expressed under the control of the SCR promoter. A, Root of a wrky75-25 plant carrying pSCR:YFP-WRKY75. B, Root of a Col-0 plant carrying pSCR:GFPer. Squares mark the region shown at a higher magnification. Black stars mark lateral root cap cells, white stars label epidermal cells, and red stars mark cortical cells. Bars = 40 µm.
DISCUSSION
The previous finding that suppression of WRKY75 leads to an increased number of root hairs (Devaiah et al., 2007) raised the question of how WRKY75 is tied in the root hair patterning or differentiation machinery.
WRKY75 Regulates the Root Hair Patterning System through Transcriptional Regulation of CPC
Our analysis of wrky75 mutants and WRKY75 overexpression lines revealed that WRKY75 acts as a repressor of root hair formation. These genetic results are supported by changes in the expression of the nonroot hair marker GL2 (Szymanski et al., 1998) and the root hair marker RSL4 (Yi et al., 2010), such that GL2 expression is decreased in wrky75 mutants and increased in p35S:WRKY75 lines whereas RSL4 expression shows the opposite behavior. Our data further indicate that these two markers are regulated by WRKY75 through the regulation of known root hair patterning genes. Our expression analysis of positive and negative regulators of root hair patterning pointed to a regulation of CPC and TRY. A temporal-spatial analysis of TRY was not considered, as the corresponding pTRY:GUS lines did not show expression in wild-type roots (Schellmann et al., 2002). The spatial analysis of CPC supported these findings. As compared with the wild type, the typical expression of pCPC:GUS in nonroot hair cells (Wada et al., 2002) was reduced in p35S:WRKY75 lines, and ectopic expression in root hair cell files was found in wrky75 mutants. The regulation of CPC is likely to be driven through direct binding of WRKY75 to the CPC promoter. This view is supported by our yeast one-hybrid data indicating that WRKY75 can bind to the CPC promoter. Binding specificity to the promoter is suggested by the finding that mutations of the W box abolish the binding.
Nonautonomous Regulation of Epidermal Fate by WRKY75
Our finding that WRKY75 is transcriptionally expressed in the inner part of the root and that YFP-WRKY75 is found in all tissue layers suggests that WRKY75 regulates root hair development in a non-cell-autonomous manner. The promoter regions chosen for these experiments are likely to reveal the correct expression pattern, as they are sufficient for rescue and as they contain the region in which a T-DNA insertion causes a wrky75 mutant phenotype. Thus, it is conceivable that WRKY75 RNA or protein travels from the inner part of the root to the epidermis. We confirmed this movement behavior by also showing that YFP-WRKY75 expressed under the control of an endodermis-specific promoter can move to the epidermis and that it can rescue the wrky75 root hair phenotype. As two transcriptomic approaches studying systematically the expression of genes in different root cell types found WRKY75 in the epidermis (Birnbaum et al., 2003; Lan et al., 2013), it is conceivable that WRKY RNA can move between cells.
How Does WRKY75 Fit in the Root Hair Patterning Models?
The current models explain the positional regulation of root hair formation by regulatory feedback loops of MBW proteins and their negative regulators, the R3MYBs (Schiefelbein et al., 2009; Tominaga-Wada et al., 2011; Grebe, 2012). The relative positions of root hair and nonroot hair cells with respect to the cortical cells are controlled by a repression of WER in root hair cell positions by SCM. In addition, the SCHIZORIZA gene had been implicated in epidermal differentiation (Mylona et al., 2002; ten Hove et al., 2010). How does WRKY75 fit into this network? Is WRKY75 involved in the positioning of root hair and nonroot hair cell files, possibly through JKD and SCM? We consider this to be unlikely, as the corresponding mutants show distortions of the overall radial pattern in the root. Our finding that WRKY75 expression is rather unspecific in the inner part of the root and the movement of WRKY75 RNA/WRKY75 protein to the epidermal layer provides no hint toward a biased regulation of root hair or nonroot hair cell files. The recent report by Kang et al. (2013) showing that expression of CPC in the stele can rescue the cpc mutant phenotype suggests that epidermal cell differentiation can be controlled by the inner part of the root through the known patterning genes. It is possible that WRKY75 operates in an analogous manner by regulating gene expression in the epidermis in a non-cell-autonomous manner. In this scenario, WRKY75 regulates CPC expression at two levels. First, in the stele, where both genes are expressed, and second, in the epidermis, through moving RNA/protein. WRKY75 seems to regulate root hair patterning by regulating only a subset of the patterning genes. CPC and TRY are clearly repressed, whereas other tested patterning genes remain unaffected. Moreover, the binding of WRKY75 to the CPC promoter in yeast one-hybrid assays suggests a direct regulation rather than an indirect control through JKD/SCM. This idea is supported by our finding that the levels of WER expression are not changed in wrky75 mutants or WRKY75 overexpression lines. We speculate that WRKY75 functions as a modulator of root hair formation through the direct regulation of CPC and possibly GL2. In the wild type, WRKY75 contributes to the balance of the expression levels of the two genes and, thereby, the number of root hairs. In wrky75 mutants, the balance is shifted and the number of root hairs increases in nonroot hair cell files. Also, the reduction of root hairs in root hair cell files in p35S:WRKY75 plants can be easily explained by a reduction of CPC (and TRY) expression. Given that WRKY75 mediates various environmental responses, including pathogen attack and phosphate starvation, this mechanism provides a simple and effective way to modulate root hair number in response to environmental stimuli.
MATERIALS AND METHODS
Plant Material, Growth, and Root Hair and Cytological Analyses
The following Arabidopsis (Arabidopsis thaliana) lines were used in this study: wrky75-25 (Encinas-Villarejo et al., 2009), WRKY75 RNAi (Devaiah et al., 2007), and cpc-2 (Kurata et al., 2005). Double mutants were created by crossing. The homozygous lines were confirmed by PCR. The pGL2:GUS and pCPC:GUS lines (Pesch et al., 2013) in p35S:WRKY75, WRKY75 RNAi, and wrky75-25 backgrounds were created by crossing with the respective Col-0 reporter lines. All lines are in the Col-0 wild-type background. Plants were transformed by the floral dip method (Clough and Bent, 1998).
For root hair analysis, seeds were sterilized by adding 100% (v/v) ethanol followed by 4% (v/v) HCl and washed three times with water, vernalized at 4°C for 3 d in the dark, and planted on Murashige and Skoog plates supplemented with 1% (w/v) Suc. Seedlings were grown under long-day conditions (16 h of light/8 h of dark) for 7 d. Plates were positioned vertically to avoid root penetration in the medium. Seedlings were fixed (50% [v/v] methanol, 10% [v/v] acetic acid) at 4°C for 24 h, transferred to 80% (v/v) ethanol for 2 h, washed three times with water, and analyzed by transmission light microscopy. The files were defined by their relative positions to the cortical cells. For each seedling, the number of root hairs was determined for 10 cells in the root hair cell file and 10 cells in the nonroot hair cell position.
GUS staining was done as described previously (Vroemen et al., 1996).
Plasmid Construction
The coding DNA sequence (CDS) of WRKY75 was cloned into pDONR201 amplified from Col-0 CDS by Gateway technology (Invitrogen) using primers with attB sites (Supplemental Table S1). The coding sequence of WER was amplified from Landsberg erecta CDS and cloned with SalI and NotI restriction sites in pENTR1A. GUS-pDONR201 was obtained from Invitrogen. The constructs p35S:WRKY75 (pAMPAT-GW), pCPC:GUS (pCPC-pAMPAT), pGL2:GUS (pGL2-pAMPAT), WRKY75-pC-ACT2-attR, WER-pC-ACT2-attR, p35S:GUS (pAMPAT-GW), YFP-WRKY75 (pENSG-YFP), and YFP-GUS (pENSG-YFP) were created by Gateway LR recombination (Invitrogen) with the respective entry clones (Weinl et al., 2005; Wester et al., 2009).
For pAMPAT-GW-pWRKY75:GUS:3′-WRKY75, the WRKY75 promoter was amplified using Asc1-pWRKY75 F and Xho1-pWRKY75 R primers (Supplemental Table S1) and introduced in pJET1.2.b (Fermentas). pWRKY75 was recovered using AscI/XhoI and introduced to GUS-pAMPAT-GW to obtain pWRKY75:GUS (pAMPAT-GW). The downstream 627 bp of WRKY75 was isolated with 3′WRKY75 F and 3′WRKY75 R (Supplemental Table S1) and introduced to pWRKY75:GUS (pAMPAT-GW) using Mss1.
For pWRKY75:YFP-WRKY75:3′-WRKY75 (pENSG) and pWRKY75:YFP-GUS:3′-WRKY75 (pENSG), the same strategy was used to introduce pWRKY75 and 3′-WRKY75 in pENSG-YFP. The WRKY75 coding sequence and GUS coding sequence were introduced in a second step by LR reaction (Clontech).
For pSCR:GPFer (pAMPAT), first the SCR promoter was amplified using Asc1-pWRKY75 F and Xho1-pWRKY75 R primers (Supplemental Table S1) and introduced in pJET1.2.b (Fermentas). pSCR was recovered using AscI/XhoI and introduced to GFPer-pAMPAT-GW to obtain pSCR:GFPer (pAMPAT-GW).
pSCR:YFP-GUS (pENSG) and pSCR:YFP-WRKY75 (pENSG) were created in a similar way to pWRKY75:YFP-WRKY75:3′-WRKY75.
Quantitative Real-Time PCR
Using the stereomicroscope at 10× magnification, the root hair patterning zone (the area where the first root hairs appear in the differentiation zone) was determined. Seedlings were cut using a sharp blade to collect the root part containing the patterning zone including the root cap. RNA extraction and real-time PCR were performed as described previously (Kwon et al., 2006). Relative mRNA was normalized to the concentration of ELONGATION FACTOR α A4 mRNA. The expression levels of genes of wild-type plants were set to 1 and subsequently used to calculate the relative changes of genes in wrky75-25, WRKY75 RNAi, and p35S:WRKY75. The primers used are listed in Supplemental Table S1.
Yeast One-Hybrid Analysis
Vectors containing the CPC promoter fragments wild-type and mutated W box were created by sequentially cloning three equal promoter fragments differing in the attached restriction sites (EcoRI and KpnI, KpnI and BamHI, and BamHI and XbaI) in puc18. The fragments were either produced by PCR or ordered as long oligonucleotides. The trimers were introduced in the EcoRI and XbaI sites in pHISi (Invitrogen).
Yeast one-hybrid analyses were carried out using the MATCHMAKER One-Hybrid System (Clontech). First, the yeast strain YM4271 was transformed with pCPC(−459 to −378)-pHISi or pCPC(−459 to −378, mutated)-pHISi. Second, the selected vectors expressing the GAL4 activation domain fusion proteins were transformed into YM4271 carrying the promoter fragments and grown on medium without uracil and Leu. Finally, the binding activities were determined by the ability of yeast to grow on selective medium without His, Leu, and uracil. Three single colonies from each combination were tested. pC-ACT2-ccdB (empty vector) served as a negative control and was described before (Pesch et al., 2013).
Microscopy and Image Acquisition
Fluorescence microscopy and bright-field differential interference contrast microscopy were performed with a Leica DMRB 5500 microscope equipped with the Leica Application Software AF (Leica Microsystems). Images of unstained roots were generated by light microscopy as described previously (Failmezger et al., 2013), and GUS-stained roots were acquired using a Leica DMRA microscope (Leica Microsystems) equipped with DISKUS software (Carl H. Hilgers-Technisches Büro).
Confocal laser-scanning microscopy was performed using a Leica TCS-SP8 confocal microscope equipped with the Leica LAS AF software or a Zeiss Meta 510 equipped with ZEN 2012 software. For imaging, a Leica 1.2 numerical aperture, 63× water objective was used. The propidium iodide staining was excited using a white light laser at 561 nm, and the GFP/YFP signal was excited using an argon laser at 488/514 nm. Images were analyzed with Imaris 7.0 (Bitplane). For protein localization, seedlings were stained with 100 µg mL−1 propidium iodide for 1 min followed by washing the samples with water. The analyses were done by sequential scanning starting with the respective higher wavelength.
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: TTG1 (AT5G24520, The Arabidopsis Information Resource [TAIR] locus 2153914), TRY (AT5G53200, TAIR locus 2163766), GL2 (AT1G79840, TAIR locus 2017874), CPC (AT2G46410, TAIR locus 2005503), WER (AT5G14750, TAIR locus 2185470), WRKY75 (AT5G13080, TAIR locus 2179862), SCR (AT3G54220, TAIR locus 2080345), and RSL4 (AT1G27740, TAIR locus 2199221).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Agarose gel electrophoresis showing RT-PCR for the wrky75-25 mutant.
Supplemental Figure S2. Nucleotide sequence showing the position of T-DNA insertion in the wrky75-25 mutant.
Supplemental Figure S3. Quantitative real-time PCR of mRNA obtained from root hair-patterning zone of 7-d-old seedlings grown on Murashige and Skoog media.
Supplemental Figure S4. Expression analysis of CPC in the stele.
Supplemental Figure S5. Time series for pWRKY75:GUS:3'WRKY75 GUS staining.
Supplemental Figure S6. Quantitative real-time PCR of mRNA obtained from root hair-patterning zone of 7-d-old seedlings grown on Murashige and Skoog media.
Supplemental Figure S7. Three-dimensional reconstruction of pWRKY75:YFP-GUS:3′WRKY75.
Supplemental Figure S8. Movement of WRKY75 when expressed under the SCR promoter.
Supplemental Table S1. Primer list.
Supplemental Table S2. Real-time PCR results.
Supplemental Table S3. Summary of root hair phenotype of wrky75-25 T1 plants transformed with pWRKY75:YFP-WRKY75 3′-WRKY75.
Supplemental Table S4. Root hair numbers in the patterning zone of the primary roots; 7-d-old Arabidopsis plants were analyzed for each treatment.
Supplemental Table S5. Summary of root hair phenotype of wrky75-25 T1 plants transformed with pSCR:YFP-WRKY75.
Supplementary Material
Acknowledgments
We thank Birgit Kernebeck for excellent technical assistance.
Glossary
- T-DNA
transfer DNA
- Col-0
Columbia-0
- YFP
yellow fluorescent protein
- cDNA
complementary DNA
- TAIR
The Arabidopsis Information Resource
Footnotes
This work was supported by the Cluster of Excellence on Plant Sciences (grant no. EXC 1028 to M.H.) and International Max Planck Research Schools (fellowship to L.R.).
The online version of this article contains Web-only data.
References
- Bernhardt C, Lee MM, Gonzalez A, Zhang F, Lloyd A, Schiefelbein J. (2003) The bHLH genes GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root. Development 130: 6431–6439 [DOI] [PubMed] [Google Scholar]
- Bernhardt C, Zhao M, Gonzalez A, Lloyd A, Schiefelbein J. (2005) The bHLH genes GL3 and EGL3 participate in an intercellular regulatory circuit that controls cell patterning in the Arabidopsis root epidermis. Development 132: 291–298 [DOI] [PubMed] [Google Scholar]
- Birnbaum K, Shasha DE, Wang JY, Jung JW, Lambert GM, Galbraith DW, Benfey PN. (2003) A gene expression map of the Arabidopsis root. Science 302: 1956–1960 [DOI] [PubMed] [Google Scholar]
- Ciolkowski I, Wanke D, Birkenbihl RP, Somssich IE. (2008) Studies on DNA-binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function. Plant Mol Biol 68: 81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Devaiah BN, Karthikeyan AS, Raghothama KG. (2007) WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol 143: 1789–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Laurenzio L, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn MG, Feldmann KA, Benfey PN. (1996) The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 86: 423–433 [DOI] [PubMed] [Google Scholar]
- Dolan L, Duckett CM, Grierson C, Linstead P, Schneider K, Lawson E, Dean C, Poethig S, Roberts K. (1994) Clonal relationships and cell patterning in the root epidermis of Arabidopsis. Development 120: 2465–2474 [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. (1993) Cellular organisation of the Arabidopsis thaliana root. Development 119: 71–84 [DOI] [PubMed] [Google Scholar]
- Dong J, Chen C, Chen Z. (2003) Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol 51: 21–37 [DOI] [PubMed] [Google Scholar]
- Encinas-Villarejo S, Maldonado AM, Amil-Ruiz F, de los Santos B, Romero F, Pliego-Alfaro F, Muñoz-Blanco J, Caballero JL. (2009) Evidence for a positive regulatory role of strawberry (Fragaria × ananassa) Fa WRKY1 and Arabidopsis At WRKY75 proteins in resistance. J Exp Bot 60: 3043–3065 [DOI] [PubMed] [Google Scholar]
- Esch JJ, Chen M, Sanders M, Hillestad M, Ndkium S, Idelkope B, Neizer J, Marks MD. (2003) A contradictory GLABRA3 allele helps define gene interactions controlling trichome development in Arabidopsis. Development 130: 5885–5894 [DOI] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Schmelzer E, Hahlbrock K, Somssich IE. (1999) Early nuclear events in plant defence signalling: rapid gene activation by WRKY transcription factors. EMBO J 18: 4689–4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW. (1994) The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev Biol 166: 740–754 [DOI] [PubMed] [Google Scholar]
- Grebe M. (2012) The patterning of epidermal hairs in Arabidopsis: updated. Curr Opin Plant Biol 15: 31–37 [DOI] [PubMed] [Google Scholar]
- Guo Y, Gan S. (2004) Transcriptome of Arabidopsis leaf senescence. Plant Cell Environ 27: 521–549 [Google Scholar]
- Failmezger H, Jaegle B, Schrader A, Hülskamp M, Tresch A. (2013) Semi-automated 3D leaf reconstruction and analysis of trichome patterning from light microscopic images. PLoS Comput Biol 9: e1003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H, Scheres B, Blilou I. (2010) JACKDAW controls epidermal patterning in the Arabidopsis root meristem through a non-cell-autonomous mechanism. Development 137: 1523–1529 [DOI] [PubMed] [Google Scholar]
- Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser MT, Benfey PN. (2000) The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 101: 555–567 [DOI] [PubMed] [Google Scholar]
- Ishida T, Kurata T, Okada K, Wada T. (2008) A genetic regulatory network in the development of trichomes and root hairs. Annu Rev Plant Biol 59: 365–386 [DOI] [PubMed] [Google Scholar]
- Kang YH, Song SK, Schiefelbein J, Lee MM. (2013) Nuclear trapping controls the position-dependent localization of CAPRICE in the root epidermis of Arabidopsis. Plant Physiol 163: 193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik V, Simon M, Huelskamp M, Schiefelbein J. (2004a) The ENHANCER OF TRY AND CPC1 gene acts redundantly with TRIPTYCHON and CAPRICE in trichome and root hair cell patterning in Arabidopsis. Dev Biol 268: 506–513 [DOI] [PubMed] [Google Scholar]
- Kirik V, Simon M, Wester K, Schiefelbein J, Hulskamp M. (2004b) ENHANCER of TRY and CPC 2 (ETC2) reveals redundancy in the region-specific control of trichome development of Arabidopsis. Plant Mol Biol 55: 389–398 [DOI] [PubMed] [Google Scholar]
- Kurata T, Ishida T, Kawabata-Awai C, Noguchi M, Hattori S, Sano R, Nagasaka R, Tominaga R, Koshino-Kimura Y, Kato T, et al. (2005) Cell-to-cell movement of the CAPRICE protein in Arabidopsis root epidermal cell differentiation. Development 132: 5387–5398 [DOI] [PubMed] [Google Scholar]
- Kwak SH, Shen R, Schiefelbein J. (2005) Positional signaling mediated by a receptor-like kinase in Arabidopsis. Science 307: 1111–1113 [DOI] [PubMed] [Google Scholar]
- Kwon CS, Hibara K, Pfluger J, Bezhani S, Metha H, Aida M, Tasaka M, Wagner D. (2006) A role for chromatin remodeling in regulation of CUC gene expression in the Arabidopsis cotyledon boundary. Development 133: 3223–3230 [DOI] [PubMed] [Google Scholar]
- Lan P, Li W, Lin WD, Santi S, Schmidt W. (2013) Mapping gene activity of Arabidopsis root hairs. Genome Biol 14: R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MM, Schiefelbein J. (1999) WEREWOLF, a MYB-related protein in Arabidopsis, is a position-dependent regulator of epidermal cell patterning. Cell 99: 473–483 [DOI] [PubMed] [Google Scholar]
- Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, Marks MD, Schiefelbein JW. (1996) The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development 122: 1253–1260 [DOI] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW. (1996) Hormones act downstream of TTG and GL2 to promote root hair outgrowth during epidermis development in the Arabidopsis root. Plant Cell 8: 1505–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylona P, Linstead P, Martienssen R, Dolan L. (2002) SCHIZORIZA controls an asymmetric cell division and restricts epidermal identity in the Arabidopsis root. Development 129: 4327–4334 [DOI] [PubMed] [Google Scholar]
- Pesch M, Hülskamp M. (2004) Creating a two-dimensional pattern de novo during Arabidopsis trichome and root hair initiation. Curr Opin Genet Dev 14: 422–427 [DOI] [PubMed] [Google Scholar]
- Pesch M, Schultheiß I, Digiuni S, Uhrig JF, Hülskamp M. (2013) Mutual control of intracellular localisation of the patterning proteins AtMYC1, GL1 and TRY/CPC in Arabidopsis. Development 140: 3456–3467 [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Macdonald H, Huttly AK, Lazarus CM, Hooley R. (1995) Members of a new family of DNA-binding proteins bind to a conserved cis-element in the promoters of alpha-Amy2 genes. Plant Mol Biol 29: 691–702 [DOI] [PubMed] [Google Scholar]
- Ryu KH, Kang YH, Park YH, Hwang I, Schiefelbein J, Lee MM. (2005) The WEREWOLF MYB protein directly regulates CAPRICE transcription during cell fate specification in the Arabidopsis root epidermis. Development 132: 4765–4775 [DOI] [PubMed] [Google Scholar]
- Savage NS, Walker T, Wieckowski Y, Schiefelbein J, Dolan L, Monk NA. (2008) A mutual support mechanism through intercellular movement of CAPRICE and GLABRA3 can pattern the Arabidopsis root epidermis. PLoS Biol 6: e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellmann S, Schnittger A, Kirik V, Wada T, Okada K, Beermann A, Thumfahrt J, Jürgens G, Hülskamp M. (2002) TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO J 21: 5036–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein J. (2003) Cell-fate specification in the epidermis: a common patterning mechanism in the root and shoot. Curr Opin Plant Biol 6: 74–78 [DOI] [PubMed] [Google Scholar]
- Schiefelbein J, Kwak SH, Wieckowski Y, Barron C, Bruex A. (2009) The gene regulatory network for root epidermal cell-type patterning. J Exp Bot 60: 1515–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW. (2000) Constructing a plant cell: the genetic control of root hair development. Plant Physiol 124: 1525–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M, Lee MM, Lin Y, Gish L, Schiefelbein J. (2007) Distinct and overlapping roles of single-repeat MYB genes in root epidermal patterning. Dev Biol 311: 566–578 [DOI] [PubMed] [Google Scholar]
- Szymanski DB, Jilk RA, Pollock SM, Marks MD. (1998) Control of GL2 expression in Arabidopsis leaves and trichomes. Development 125: 1161–1171 [DOI] [PubMed] [Google Scholar]
- ten Hove CA, Willemsen V, de Vries WJ, van Dijken A, Scheres B, Heidstra R. (2010) SCHIZORIZA encodes a nuclear factor regulating asymmetry of stem cell divisions in the Arabidopsis root. Curr Biol 20: 452–457 [DOI] [PubMed] [Google Scholar]
- Tominaga R, Iwata M, Okada K, Wada T. (2007) Functional analysis of the epidermal-specific MYB genes CAPRICE and WEREWOLF in Arabidopsis. Plant Cell 19: 2264–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga-Wada R, Ishida T, Wada T. (2011) New insights into the mechanism of development of Arabidopsis root hairs and trichomes. Int Rev Cell Mol Biol 286: 67–106 [DOI] [PubMed] [Google Scholar]
- Vroemen CW, Langeveld S, Mayer U, Ripper G, Jurgens G, Van Kammen A, De Vries SC. (1996) Pattern formation in the Arabidopsis embryo revealed by position-specific lipid transfer protein gene expression. Plant Cell 8: 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Kurata T, Tominaga R, Koshino-Kimura Y, Tachibana T, Goto K, Marks MD, Shimura Y, Okada K. (2002) Role of a positive regulator of root hair development, CAPRICE, in Arabidopsis root epidermal cell differentiation. Development 129: 5409–5419 [DOI] [PubMed] [Google Scholar]
- Wada T, Tachibana T, Shimura Y, Okada K. (1997) Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science 277: 1113–1116 [DOI] [PubMed] [Google Scholar]
- Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, Blundell TL, Esch JJ, Marks MD, Gray JC. (1999) The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell 11: 1337–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinl C, Marquardt S, Kuijt SJ, Nowack MK, Jakoby MJ, Hülskamp M, Schnittger A. (2005) Novel functions of plant cyclin-dependent kinase inhibitors, ICK1/KRP1, can act non-cell-autonomously and inhibit entry into mitosis. Plant Cell 17: 1704–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester K, Digiuni S, Geier F, Timmer J, Fleck C, Hülskamp M. (2009) Functional diversity of R3 single-repeat genes in trichome development. Development 136: 1487–1496 [DOI] [PubMed] [Google Scholar]
- Yi K, Menand B, Bell E, Dolan L. (2010) A basic helix-loop-helix transcription factor controls cell growth and size in root hairs. Nat Genet 42: 264–267 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.