Papacleovoulou and colleagues demonstrate that maternal cholestasis during pregnancy is associated with offspring adiposity and metabolic abnormalities, both in humans and in mice. This study reinforces the overwhelming evidence of the critical role of the in utero environment as a major determinant of adult health and disease.
Intrahepatic cholestasis of pregnancy (ICP), which is diagnosed by elevated maternal levels of serum bile acids and associated with pruritus, affects 0.5–2.0% of pregnant women and occurs usually in the third trimester of pregnancy.1 Papacleovoulou et al.2 used data from a birth cohort from Northern Finland and a mouse model of elevated serum levels of bile acid during pregnancy and showed that, in both, maternal ICP was associated with sex-specific increased offspring susceptibility to an obese, diabetic phenotype. Additionally, maternal ICP was associated with increased cholesterol concentrations in the umbilical cord, fetus and placenta of the offspring. These findings lead the authors to conclude that increased transplacental cholesterol transport probably contributes to the metabolic abnormalities in offspring of mothers with ICP.2
ICP is primarily a liver disease, with impaired flow of bile resulting in elevated blood bile acids levels. The incidence of ICP increases with parity, multiple pregnancies, maternal age (>35 years) and a family history of biliary disease, and varies dependent upon geographical location, ethnic group and climatic conditions.1,3 The cardinal symptom is itching, presumably as a result of bile acid deposition in the skin, which is often relieved with ursodeoxycholic acid (UDCA) treatment that decreases bile acid absorption.3,4 The pathogenesis of ICP is unclear, but genetic origin, specific mutations in bile transporter genes, hormonal milieu (estrogen, progesterone) and environmental factors probably influence the expression of the disease. Although essentially benign for the mother, evidence has associated ICP with a poor fetal prognosis, resulting from increased transfer of bile acids from mother to fetus. The resultant complications include meconium-stained amniotic fluid, fetal prematurity, stillbirth and an increased risk of neonatal respiratory distress syndrome. To reduce these complications, early delivery is advocated.1,4 In view of the recognized in utero programming effects on long-term health of an individual,5,6 it is possible that exposure to maternal cholestasis (increased bile acids) would adversely affect offspring metabolism (Figure 1).
Figure 1.
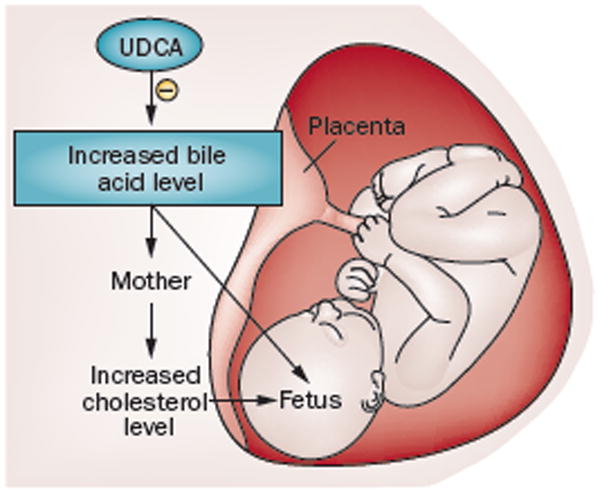
Maternal cholestasis and fetal lipid abnormalities. Maternal intrahepatic cholestasis of pregnancy (ICP) results in increased bile acid transfer from mother to fetus. As the fetus has reduced ability to eliminate bile acids across the placenta, the bile acids accumulate in the cord blood serum, meconium and amniotic fluid. Further, the resultant maternal elevated cholesterol levels, together with increased transplacental transport, lead to increased fetal cholesterol levels. Ursodeoxycholic acid (UDCA) treatment decreases bile acid levels in maternal serum and its passage to the fetus.
To understand the effect of maternal cholestasis on offspring, Papacleovoulou et al.2 undertook a comprehensive approach by investigating maternal, placental and fetal and/or neonate tissues in both humans and mice. Utilizing data from a cohort of babies born in Northern Finland between 1985 and 1986, they identified 7,808 control pregnancies (resulting in 4,034 male and 3,774 female babies) and 45 untreated ICP pregnancies (resulting in 27 male and 18 female babies); the mothers had no other metabolic complications such as diabetes mellitus or obesity. Birth and placental weights, including proportion of premature births were comparable between ICP and control pregnancies. However, at the age of 16 years, male children of ICP pregnancies had increased BMI and fasting insulin levels, whereas female children showed comparable BMI albeit with increased hip and waist girth and decreased HDL cholesterol levels compared with their respective counterparts from normal pregnancies.
Importantly, the investigators established a mouse model of maternal hypercholanaemia (elevated serum bile acid levels) to further investigate the association between maternal cholestasis and offspring susceptibility to metabolic abnormalities. Mice received either a normal chow diet or a diet supplemented with 0.5% cholic acid (a major hepatic bile acid) prior to and throughout pregnancy. Following delivery, all lactating mice were fed a normal diet, and the offspring were weaned (at 3 weeks of age) to a normal diet. At 12 weeks of age, offspring were either continued on a normal diet or given a Western (high-fat) diet for 6 weeks.
On gestational day 18, choline-supplemented pregnant mice had increased maternal serum and liver bile acid levels (cholestatic phenotype) and cholesterol levels, with no other abnormalities. Similar to the human findings, no differences were noted in length of gestation or birth weight between mice born to mothers with or without hypercholanaemia. Furthermore, in adult offspring from mothers with cholestasis fed a normal diet, no metabolic abnormalities or adiposity were evident in either male or female offspring at 18 weeks of age. However, when challenged with a Western diet, females exposed to maternal cholestasis exhibited obese and diabetic characteristics compared with females from normal pregnancies fed a Western diet. Microarray analysis of the liver and adipose tissue indicated an increased inflammatory response. In contrast to female mice, male mice exposed to maternal cholestasis and fed a Western diet showed no apparent metabolic abnormalities.
Placental studies in humans and the mouse model revealed that maternal cholestasis potentiates lipid biosynthesis and transport in the fetoplacental unit. Overall findings suggest that maternal cholestasis mainly increases maternal, placental and fetal cholesterol levels, and that female offspring exposed to maternal cholestasis have greater susceptibility to metabolic abnormalities on a high-calorie and high-fat diet. The adverse effect of maternal cholestasis on offspring is consistent with the findings of a previous study demonstrating persistent altered biliary lipid levels and bile acid secretion in 4-week old rat pups.7
To further investigate the effect of maternal cholestasis on the epigenome of the offspring, Papacleovoulou et al. used the viable yellow agouti (Avy) mouse model, in which coat colour variation is correlated to epigenetic marks. The silent allele is hypermethylated and produces a wild-type agouti-coloured coat (pseudoagouti), whereas the active allele is hypomethylated and produces a completely yellow coat. A cryptic promoter in the proximal end of the Avy IAP promotes constitutive ectopic Agouti transcription not only in hair follicles, but throughout all cells, leading to yellow fur, as well as adult-onset obesity and diabetes. Papacleovoulou et al. showed that Avy mice fed a cholic-acid-supplemented diet prior to and throughout gestation produced female offspring with brown coat and male offspring with yellow coat and that the coat colour was consistent with the methylation status of the cryptic IAP promoter.2
Despite the interesting results, the study by Papacleovoulou et al. has a number of limitations. The authors imply that the putative programming factor in either model is fetal cholesterol or bile acid levels. Maternal cholesterol levels rise markedly during pregnancy, serving a critical role in placental and fetal steroid production.8 The mechanism by which an increase in fetal cholesterol levels would program the metabolic health of offspring is unclear. Moreover, genes regulating cholesterol biosynthesis and bile acid homeostasis pathways appear unaltered (transcriptome profile) in adult female offspring exposed to maternal cholestasis and receiving a Western diet. With regards to bile acids, the researchers report fetal mouse bile acid levels in mmol/l whereas normal or elevated (that is, ICP) human levels are measured in μmol/l.9 Thus, the comparatively high concentrations of bile acids in the mouse model raise an important issue as to whether the mouse model is physiologic or pharmacologic. Nevertheless, the epigenetic results indicate a pathway(s) by which elevated bile acids might alter key metabolic parameters.
In the human study, the investigators do not provide data on maternal bile acid levels or on the relationship between the duration, gestational period and level of bile acids to the offspring metabolic abnormalities. In humans, boys were more susceptible to metabolic perturbations than girls, whereas female mice were more susceptible than male mice. ICP usually occurs in the third trimester; however, in the mouse model, bile supplementation started prior to pregnancy. Although UDCA treatment alleviates maternal symptoms, reduces bile acids levels in umbilical cord blood and amniotic fluid and can improve fetal outcome,4 no studies have been undertaken to determine whether UDCA therapy can prevent or reverse the potential metabolic programming in humans.
In summary, these studies provide a novel insight into the diversity of potential in utero factors that might affect offspring programming. Additional studies of mechanisms, basis for sex-specific effects, treatments and offspring perturbations (for example, the influence of milk bile acids10) will contribute importantly to our understanding of ICP effects and pathways of metabolic programming.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Pathak B, Sheibani L, Lee RH. Cholestasis of pregnancy. Obstet Gynecol Clin North Am. 2010;37:269–282. doi: 10.1016/j.ogc.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Papacleovoulou G, et al. Maternal cholestasis during pregnancy programs metabolic disease in offspring. J Clin Invest. 2013;123:3172–3181. doi: 10.1172/JCI68927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacq Y, et al. Efficacy of ursodeoxycholic acid in treating intrahepatic cholestasis of pregnancy: a meta-analysis. Gastroenterology. 2012;143:1492–1501. doi: 10.1053/j.gastro.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Azzaroli F, Turco L, Lisotti A, Calvanese C, Mazzella G. The pharmacological management of intrahepatic cholestasis of pregnancy. Curr Clin Pharmacol. 2011;6:12–17. doi: 10.2174/157488411794941313. [DOI] [PubMed] [Google Scholar]
- 5.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 6.Ross MG, Desai M. In: Obstetrics: Normal and Problem Pregnancies. Gabbe SG, editor. Churchill Livingstone; Elsevier: 2012. pp. 84–95. [Google Scholar]
- 7.El-Mir MY, et al. Effect of maternal cholestasis on biliary lipid and bile acid secretion in the infant rat. Hepatology. 1997;26:527–536. doi: 10.1002/hep.510260301. [DOI] [PubMed] [Google Scholar]
- 8.Baardman ME, et al. The role of maternal-fetal cholesterol transport in early fetal life: current insights. Biol Reprod. 2013;88:1–9. doi: 10.1095/biolreprod.112.102442. [DOI] [PubMed] [Google Scholar]
- 9.Egan N, et al. Reference standard for serum bile acids in pregnancy. BJOG. 2012;119:493–498. doi: 10.1111/j.1471-0528.2011.03245.x. [DOI] [PubMed] [Google Scholar]
- 10.Brites D, Rodrigues CM. Elevated levels of bile acids in colostrum of patients with cholestasis of pregnancy are decreased following ursodeoxycholic acid therapy. J Hepatol. 1998;29:743–751. doi: 10.1016/s0168-8278(98)80255-9. [DOI] [PubMed] [Google Scholar]


