Abstract
Objective
To describe the final adult height (FAH) and pubertal growth patterns in HIV-infected adolescents and to compare this to an age matched population of seroreverting HIV-exposed uninfected (HEU) adolescents. Further, to evaluate the interplay of proinflammatory cytokines (PIC) with IGF-1, IGFBP-3 and IGFBP-1 during the pubertal growth spurt.
Methods
HIV-infected adolescents (n=34) and 12 HEU patients, who had achieved FAH were evaluated. Auxologic data, viral load, CD4 count and HAART were obtained via a retrospective chart review. Serum IL-1 α, IL-6, TNF-α, IGFBP-1, IGFBP-3 and IGF-1 were assessed.
Results
The mean FAH for the HIV-infected group was SDS -0.78 (+/−1.1) compared to 0.05 (+/−0.78) for the HEU (p = 0.034). There was a positive correlation between CD4 count and FAH (p=0.019). The mean age and magnitude of peak growth velocity (GV) was within normal limits. IL-1 α, IL-6, TNF α, IGFBP-3 and IGF-1 were not significantly correlated with HIV RNA or height. IGFBP-1 was detectable in 100% of poorly controlled HIV-infected patients and 25% of the HEU cohort (p = 0.0003).
Conclusions
The FAH of HIV-infected patients was significantly shorter than HEU and positively correlated with CD4 T-lymphocyte count. Our cohort demonstrated normal timing and magnitude of peak GV during puberty.
Keywords: HIV, cytokines, IGFBP-1, growth hormone resistance, growth
Introduction
Since the recognition of the Human Immunodeficiency Virus (HIV) in pediatrics in 1983, the growth of infected children has been studied extensively. Failure to thrive was a feature of the earliest cases of pediatric Acquired Immunodeficiency Syndrome (AIDS) (1). Proposed etiologies included chronic illness, malnutrition, AIDS related enteropathy, increased tissue catabolism and pubertal delay (2). With the availability of highly active antiretroviral therapy (HAART) in children in 1996, the disease related morbidity and mortality decreased considerably. HIV infection changed from a life threatening illness to a chronic disease. Despite such progress, the height standard deviation score (SDS) of HIV-infected children remains relatively stable, and significantly below that of either age-matched controls or mid-parental height (3).
Prior to HAART, the Hemophilia Growth and Development Study, found a mean height SDS of −0.56 (+/−1.23) in those children with both HIV and hemophilia compared to a height SDS −0.01 (+/−1.18) in the HIV negative group (p=0.0001) (2). Multiple studies subsequently examined the effect of HAART on growth with mixed results. Guillen et al, examined 212 predominantly prepubertal (median age 6 years), perinatally infected HIV-infected children over a period of 5 years. HAART was associated with an increase in height SDS from −0.5 to −0.4 (p=0.008) (4). In contrast, Nachman et al, showed no statistically significant improvement in height or weight in 197 prepubertal patients (median age 7.2 years, 82%<10 years), after 96 weeks of HAART, despite adequate virologic control (5).
Much of the above data evaluated the growth of pre-pubertal HIV-infected children. However, the height achieved during the pubertal growth spurt can account for close to 20% of final adult height (6). Both the rate and duration of pubertal growth are important determinants of overall statural gain (7). Previous studies demonstrate pubertal delay in HIV-infected children with severe immunosuppression (8). However, data regarding puberty in perinatally HIV-infected children with relatively good immunologic control is limited. It is likely that non-genetic factors such as health, nutrition and body mass index influence the timing and duration of puberty (9). IGFBP-1, a binding protein produced in the liver in response to PIC, is increased in many catabolic states and has been shown to be inversely related to BMI in prepubertal HIV-infected children (10,11,12). Further, IGFBP-1 can induce a state of GH resistance by rendering IGF-1, a growth promoting polypeptide that mediates many of the anabolic actions of GH, unavailable to its receptors (11,12). Accordingly, IGFBP-1 inhibits both somatic linear growth and weight gain and has been described in association with growth failure in other chronic conditions, such as liver failure and Juvenile Rheumatoid Arthritis (13,14,15). Specifically, in the children with liver failure, liver transplant led to decreased IGFBP-1 and increased height (13).
This is the first study to evaluate the pubertal growth patterns and final adult height in a predominantly non-Caucasian, inner city population of HIV-infected adolescents. We specifically evaluated both the timing and magnitude of peak growth velocity in this cohort. We hypothesized that worsening immunosuppression, suggesting increased viral mediated T-lymphocyte destruction, would lead to increased PIC, increased IGFBP-1 and subsequent growth hormone resistance, as evidenced by decreased IGF-1 and IGFBP-3. This would be associated with poor growth and possible pubertal delay.
Patients and Methods
Subjects
The Institutional Review Board of New York University School of Medicine approved this cross-sectional, retrospective analytical study. The study subjects consisted of 34 perinatally HIV-infected patients (19 males and 15 females) followed by the Pediatric Infectious Disease service and 12 HEU patients. The diagnosis of HIV was based on the detection of serum HIV antibodies and nucleic acid testing by polymerase chain reaction (PCR). The HEU patients were age matched siblings, born to HIV-infected mothers, but who were determined to be HIV negative based on negative HIV antibodies and no evidence of HIV nucleic acid. Exclusion criteria included growth hormone deficiency, non-compensated thyroid disorder, cerebral palsy, glucocorticoid treatment for > 5 days, chronic diseases such as diabetes mellitus, cerebral palsy or non-ambulating patients. All patients had either attained FAH or finished the pubertal growth spurt.
Database
This was a cross-sectional retrospective study consisting of a chart review to determine patient’s height, weight, BMI (kg/m2), pubertal Tanner staging, menarchal history, laboratory data including viral load (VL) and CD4+ T-lymphocyte count and use of HAART. Height, weight and BMI SDS was based on NHANES data. Data was obtained between 1995–2010. A database was created including demographic information (age, ethnicity, etc.) and the above data. Height was recorded annually beginning at 7 years of age. All height measurements were performed by consistent trained nursing staff using the same scale with each measurement. The degree of accuracy of height measurements is within 0.3 cm. All patients with a complete data set were included in the study. The time of peak growth velocity was identified. This was correlated with tanner staging, hormonal assays and menarchal history when available. VL, CD4 count and BMI were averaged over the period of peak growth. Final Adult Height was defined as a growth velocity of less than 1 cm/year for a minimum of a 6 month period following the pubertal growth spurt.
Serum Sample Selection
Serum samples were previously obtained by the pediatric infectious disease staff at routine office visits and not during time of intercurrent illness. Samples were stored in −70 to −120 degree F freezers with minimal freeze thaw cycles. A stored serum for each patient during the year of peak growth velocity was identified and analyzed for IL-1 α, IL-6, TNF, IGF-1, IGFBP-1 and IGFBP-3. A subset of patients (n = 5) had 2 serum samples analyzed, one during a period of good virologic control (VL log10 < 3 cps/ml) and one during a period of poor virologic control (VL log10 > 3 cps/ml). At least one sample was during the time of peak growth velocity in this subset.
Viral/Immunologic control
HIV-infected patients were divided into two groups based on VL. Group I had VL log10 < 3 cps/ml (<1000 cps/ml) and Group II had VL log10 > 3 cps/ml (>1000 cps/ml). Patients were considered to have severe immunosuppression with CD4 count: <200/mm3, 200–500/mm3 moderate immune suppression, and greater then >500/mm3 no immune suppression (15).
Laboratory Analysis
Viral load and lymphocyte subsets were determined by RNA PCR and flow cytometry, respectively. The quantification of the cytokines IL-1 α, IL-6, and TNF-α and the growth factors IGF-1, IGFBP-1 and IGFBP-3 were determined using human multiplexing bead immunoassays (Biosource, Camarillo, CA) that are based on a sandwich immunoassay that utilizes the Luminex® fluorescent-bead-based technology. Normative data for the analytes is presented in Table I.
TABLE I.
ANALYTE NORMATIVE DATA
| Analyte | Median | Range | Mean | Lower Limit of Sensitivity |
|---|---|---|---|---|
| IGF-1 | N/A | N/A | 217.3 ng/ml | 52 pg/ml |
| IGFBP-3 | 1214 pg/ml | 838 – 1557 pg/ml | 0.1453 pg/ml | |
| IGF BP-1 | 6.4 pg/ml | 0 – 24.8 pg/ml | 0.013 pg/ml | |
| IL-1 a | 16.5 pg/ml +/− 26.47 pg/ml |
1.02 – 78.9 pg/ml | 3.5 pg/ml | |
| IL-6 | 1.71 pg/ml (+/− 11.40) | 0.15 pg/ml – 39 pg/ml | 0.3 pg/ml | |
| TNF-a | 1.61 pg/ml | 0.47 pg/ml–6.42 pg/ml | 0.1 pg/ml |
Statistical Analysis
Statistical analysis was done using SPSS. Viral load data was converted into log10 data. Analytes below the limit of detection were assigned the following values; IL-1α 3.0 pg/ml, IL-6 - 0.2 pg/ml, IGF-1 5 ng/ml and 0.012 pg/ml for IGFBP-1. Undetectable viral load were assigned a value of 49 cps/ml. All non-parametric values are reported as median and all parametric values are reported as mean. Demographic, clinical, and biochemical factors were compared between patient groups using the Fisher’s exact test (categorical data) or Mann-Whitney U test (continuous data) for non-normally distributed variables and student’s T-test or Pearson Chi-square test for normally distributed variables.
Results
Patient Characteristics
Among the HIV-infected cohort, there were 15 females and 19 males (Table II). Of this cohort, 47% were Hispanic, 47% were Black, 2% were mixed Black/Hispanic and 4% were Caucasian. The average BMI in Group I (VL log10 < 3 cps/ml) was 19.7 kg/m2 (+/−3.38), and in Group II (VL log10> 3 cps/ml) was 20.1 kg/m2 (+/−3.76) (Table II). There was no statistically significant difference in BMI between Group I and II. The mean VL log 10 of group I and II was 1.77 (+/− 1.5) copies/ml and 4.00 (+/−0.6), respectively (Table II). Severe immunosuppresion (CD4 <200/mm3) was found in 3/34 (8%) of patients, moderate immunosuppression (CD4 200–500/mm3) in 12/34 (35%) and no immunosuppression (CD4 >500/mm3) in 19/34 (56%). There was no significant difference in CD4 + T-lymphocyte count between Group I and II. In Group I, 8 out of 11 (73%) of patients were on HAART compared to 2 out of 23 patients (8%) in Group II (Table II) (p = <0.001).
TABLE II.
DEMOGRAPHIC AND CLINICAL CHARACTERSTICS OF TWO GROUPS OF HIV POSITIVE ADOLESCENTS AND CONTROLS
| GROUP I | GROUP II | HEU | P-VALUE | |
|---|---|---|---|---|
| VL<log10 3 (n=11) | VL>log103 (n=23) | |||
| Age at Peak GV * (years) | 11.9 (+/−2.3) | 12.35(+/1.8) | 12.9 | (p = 0.58) |
| Male | 13.3 (1.6) | 13.6 (1.17) | (p = 0.63) | |
| Female | 9.9 (1.5) | 11.1 (1.1) | (p = 0.17) | |
| Gender | ||||
| Male number (%) | 6(54.5%) | 13(56.5%) | ||
| Female number (%) | 5(45.5 %) | 10(43.4%) | ||
| Magnitude of Peak GV (cm/yr) | 9.6 (2.7) | 8.6 (1.4) | (p = 0.26) | |
| Male | 8.80(2.4) | 9.35 (1.30) | (p = 0.60) | |
| Female | 10.64 (2.97) | 7.62 (0.92) | (p = 0.08) | |
| VL log 10 *(cps/ml) | 1.77 (1.5) | 4.00 (0.6) | ||
| CD4 * mm3 | 753 (452) | 572 (242) | (p = 0.24) | |
| FAH SDS * | −0.67 (1.1) | −0.83 (1.1) | 0.05(0.78) | (p = 0.034) |
| Number on HAART | 8 | 2 | ||
| BMI (kg/m2)* | 19.7(3.38) | 20.08(3.76) | 25.5 (6.9) | (p = 0.013) |
Value reported as mean
HEU; HIV exposed uninfected, VL; viral load, GV; growth velocity, FAH; final adult height, HAART; highly active antiretroviral therapy, BMI; body mass index
Among the HEU (n = 12), 9 were female and 3 were male, 42% were Hispanic, 50% were black and 8% were white. The average BMI of the HEU was 25.5 kg/m2 (+/−6.9). The BMI of the HEU cohort was significantly higher than the HIV-infected patients (p = 0.013).
Final Adult Height (FAH) and Pubertal Parameters
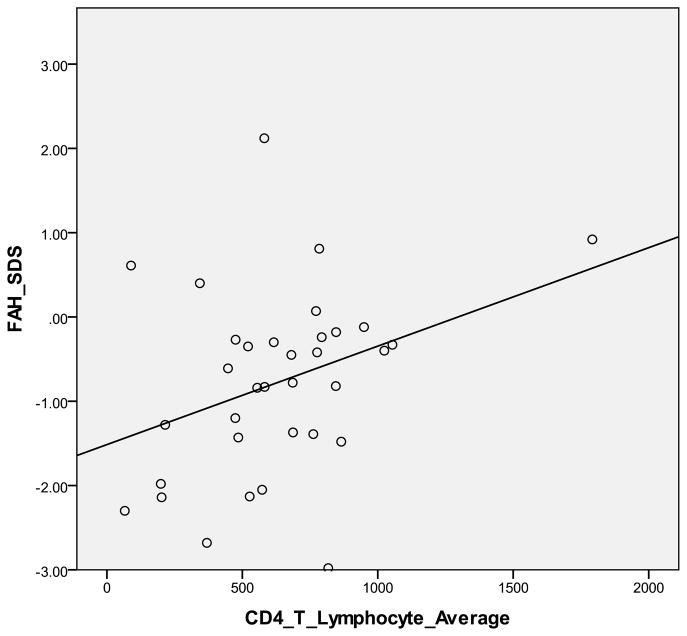
There was a statistically significant difference between the mean FAH SDS −0.78 (+/−1.1) for the 34 HIV-infected cohort and - 0.05 (+/−0.78) for the 12 HEU (p = 0.034), (Figure 1). Mean FAH SDS of the HIV-infected cohort was significantly correlated with CD4 + T-lymphocyte count (p = 0.019) (Figure II), but not VL (p = 0.37).
Figure I.
Mean Height, 25th % and 75th % for HIV-infected patients with “good control” (viral log10 < 3 cps/ml), “poor control” (viral log10 >3 cps/ml) and HIV-negative patients
Figure II.
Final Adult Height Standard Deviation Score vs. CD4 + T-Lymphocyte Count in HIV-infected patients
In the HIV-infected patients, the mean age of peak GV was 13.4 years (+/−1.9) and 10.7 years (+/−1.3) for males and females, respectively. The age of peak GV was not statistically different (p=0.58) based on VL (table II). The overall mean peak GV was 9.6 cm/year (+/−2.7) for Group I and 8.6 cm/year (+/−1.4) for Group II (p = 0.262). Males in Group I had a peak GV of 8.8 cm/year (+/−2.4), and Group II 9.35 cm/year (+/− 1.3) (p = 0.60). Among the females, pebak GV was 10.64 cm/year (+/−0.92) in Group I and 7.62 cm/year (+/−2.97) in Group II (p = 0.08). (Table II)
Cytokines
There were no significant differences in cytokines based on VL or HIV status (Table III). The median IL-1 α was below the limit of detection for Group I, Group II and the HEU. Of Group I patients, 18.1% had detectable IL-1 α, 18.18% of Group II had a detectable IL-1 α and none of the HEU had a detectable IL-1 α. The median IL-6 was also below the limit of detection for Group I, Group II and the HEU. The median TNF – α was 5.72 pg/ml for Group I, 8.3 pg/ml for Group II and 3.14 pg/ml for the HEU (p = 0.234) (Table III).
TABLE III.
CYTOKINES AND GROWTH FACTORS IN TWO GROUPS OF HIV INFECTED ADOLESCENTS AND CONTROLS DURING GROWTH SPURT
| Group I | Group II | HEU | p-value | |
|---|---|---|---|---|
| VL<log10 3 (n=11) | VL>log10 3 (n=23) | |||
| IL-1α pg/ml (+/−SD) ** | 3 | 3 | 3 | p = 0.66 |
| IL-1 percent detectable | 18.2% | 18.18% | 0% | P = 0.99 |
| IL-6 pg/ml (+/−SD) ** | 0.2 | 0.2 | 0.2 | p = 0.37 |
| TNF-α pg/ml (+/−SD)** | 5.72 | 8.3 | 3.14 | p = 0.23 |
| IGFBP1 pg/ml (+/−SD) ** | 1.22 | 2.47 | 0.012 | p = 0.067 |
| IGFBP1 percent detectable | 63.3% | 100% | 25% | p = 0.0003 |
| IGFBP3 pg/ml (+/−SD) * | 1290 (400) | 1547 (412) | 1746 (172) | p = 0.096 |
| IGF-1 ng/ml (+/−SD) * | 159.6 (185) | 210.1 (154) | 168.0 (130) | p= 0.60 |
Value reported as mean
Value reported as median
IL; interleukin, TNF; tumor necrosis factor, IGFBP; insulin-like growth factor binding protein, IGF; insulin like growth factor
Growth factors
The mean IGF-1 was 159.6 ng/ml (+/− 185) for Group I, 210.1 ng/ml (+/−154) for Group II and 168.0 ng/ml (+/− 130) for the HEU (p = 0.60). There was no significant difference for IGF-1 based on viral load, however, IGF-1 trended toward significance with FAH SDS, with a Pearson correlation coefficient of 0.305, (p = 0.079). IGFBP-3 did not have a significant correlation with viral load (p =0.096). The median IGFBP-1 for Group I was 1.22 pg/ml, Group II was 2.47 pg/ml and HEU was 0.012 pg/ml. These results approached significance (p = 0.067). IGFBP-1 was detectable in 63.6% of patients in Group I, 100% of patients in Group II, and 25% of the HEU (p = 0.0003) (Table III). IGFBP-1 had a negative correlation with BMI (Pearson correlation coefficient of 0.299, p = 0.09).
Of the subgroup with one serum sample during good control and one during poor control, the mean IGFBP-1 was 1.09 pg/ml during good control and 8.01 pg/ml during poor control. This was not statistically significant. In one patient (patient X) with a dramatic shift in the viral load, the IGFBP-1 was 26 pg/ml when the viral load was >100,000 copies/ml and decreased to 1.275 pg/ml with effective HAART decreasing the viral load to <50 copies/ml. At the same time, the IGF-1 increased from 5 ng/ml to 200 ng/ml and the growth velocity improved from 3 cm/year to 10 cm/year. There was no significant difference between the cytokines IL-1 α, IL-6 or TNF-α, IGF-1 or IGFBP-3 in this subgroup between the sample with good control and that with poor control.
Discussion
This is the first study to evaluate final adult height (FAH) and growth patterns in a predominantly non-caucasian population of perinatally infected HIV-infected adolescents. Much of the research to date has been done with prepubertal children. We specifically evaluated the pubertal growth spurt, to determine if alterations in this key growth parameter contributed to a shorter adult height in those with HIV infection. We confirm the persistently decreased FAH compared to controls. The mean FAH SDS in our cohort was −0.78 (+/−1.1) compared to a final height SDS score of −0.05 (+/−0.78) in the HEU group (p = 0.034). That patients with HIV are significantly shorter than HEU controls, suggests that the HIV itself, rather than environmental factors negatively affects height (Figure I). The FAH was significantly correlated with CD4+T-lymphocyte count, emphasizing the importance of good immunologic control in order to improve FAH.
A possible explanation for this direct correlation between immunologic control and FAH could be that increased viral mediated CD4+ T-lymphocyte destruction leads to elevated cytokines and/or a catabolic state, both resulting in increased IGFBP-1. We demonstrated an inverse correlation between IGFBP-1 and both FAH and BMI. The HEU were taller than the HIV-infected patients and only 25% had detectable IGFBP-1. In contrast, the HIV-infected patients with the highest viral load, were the shortest and 100% had detectable IGFBP-1 (p =0.0003). Further, we found an inverse relationship between IGFBP-1 and BMI. Possibly due to small sample size, this did not reach significance. As noted in patient X striking elevations in IGFBP-1 may only be seen in patients with considerable viremia leading to severe immunosuppression. Modest elevations in IGFBP-1, however, may be seen in patients with lower VL and better immune function. These modest elevations of IGFBP-1 may still have important physiologic implications, however, by inducing a certain level of GH resistance relative to controls.
We did not detect elevated serum cytokines, however there may be others we did not test for. As IGFBP-1 is produced in the liver in response to cytokines, it may be a relatively more stable indirect indication of insidious cytokine elevation.
Our findings suggest normal timing of pubertal onset. A hallmark of puberty is the pubertal growth spurt; girls typically experience peak growth at breast Tanner stage 3 and boys at testicular Tanner stage 4 (17). In a large multicenter study of 859 U.S. adolescents, the mean age of Tanner 3 breast was 10.7 years (range 9.4–12.7 years) in black girls and Tanner 4 testes was 12.8 years (range 10.7 – 15.4 years) in black boys (18). Accordingly, in our study, the mean age of peak growth was 10.7 (+/−1.9) years for girls and 13.4 (+/−1.3) years for boys, which suggests physiologic pubertal timing. De Martino, et al evaluated the time of pubertal onset in 212 perinatally infected, exclusively Caucasian, Italian patients. In contrast to our results, puberty was found to be delayed by approximately 2 years in girls and one year in boys (19). Notably, the average BMI in our study was 19.7 kg/m2 (+/− 3.38) for girls and 20.1 kg/m2 (+/− 3.76) for boys compared to a median BMI of 16.5 kg/m2 (95% CI 14.0 – 19.2) for HIV- infected girls and 16.0 kg/m2 (95% CI 13.6–19.7) for HIV-infected boys in the de Martino study (19). Evidence suggests that the amount of body fat and the hormonal events of puberty are closely related (9). Further, in the Italian cohort, 40.1% of girls and 56.1% of the boys were noted to have severe immunosuppression compared to <8% in our cohort (19).
Data regarding the peak growth velocity in HIV-infected patients is limited. As noted in the results section, we found a peak growth velocity for boys of 8.8 cm/year (+/−2.4) in Group I and 9.35 cm/year (+/−1.3) in Group II. This is consistent with peak height velocity longitudinal data by Tanner and Whitehouse for boys of 8.65 cm/year for the 25th%, 9.46 cm/year (50th%) and 10.27 cm/year (75th %) (20). For girls, we found a peak growth velocity of 10.64 cm/year (+/− 2.97) in Group I and 7.62 cm/year (+/− 0.92) in Group II. Again, this is consistent with the findings of Tanner and Whitehouse which found a peak velocity of 7.59 cm/year (25th%), 8.33 cm/year (50th%), and 9.07 cm/year (75th %). This suggests that the magnitude of the peak growth velocity is preserved. Our data did not permit analysis of the duration of the pubertal growth acceleration in our population.
Our study was limited by a relatively small sample size and the retrospective design as sibling heights, mid parental height data, birth weight and Tanner staging were often not available. We were not able to obtain data on the timing and magnitude of the pubertal growth spurt in the HEU cohort. Further, there are no age/gender specific normative data for our analytes with the Luminex technology.
Conclusion
Our study demonstrates shorter FAH in HIV patients compared to controls, despite normal timing and magnitude of the pubertal growth spurt. Thus, any negative impact of HIV-infection on height likely occurs in the pre-pubertal period. We found a significant correlation between CD4+ T-lymphocyte count and FAH, suggesting deteriorating immune function adversely affects height. Larger, prospective trials should be done to further evaluate the timing, magnitude and duration of the pubertal growth spurt in HIV infected adolescents. Further, the relationship of IGFBP-1 with growth and BMI should be further explored, as this could lead to alternative treatments, such as anti-cytokine therapy to improve the growth of perinatally infected children.
Acknowledgments
Supported in part by grant 1UL1RR029893 from the National Center for Research Resources, National Institutes of Health.
Abbreviations
- HIV
human immunodeficiency virus
- HAART
highly active antiretroviral therapy
- IL
interleukin
- TNF
tumor necrosis factor
- IGF-1
insulin like growth factor 1
- IGFBP-1
insulin like growth factor binding protein 1
- GV
growth velocity
- FAH
final adult height
- SDS
standard deviation score
- PCR
polymerase chain reaction
- VL
Viral load
- PIC
Proinflammatory cytokines
- HEU
HIV-exposed, uninfected
- CD4
CD4+ T-lymphocyte
Contributor Information
Marion Kessler, Email: marion.kessler@gmail.com.
Aditya Kaul, Email: aditya.kaul@nyumc.org.
Claritsa Santos-Malavé, Email: claritsa.santos-malave@nyumc.org.
William Borkowsky, Email: Borkowsky.william.borkowsky@nyumc.org.
Jason Kessler, Email: Jason.Kessler@nyumc.org.
Bina Shah, Email: bina.shah@nyumc.org.
12. Bibliography
- 1.Arpadi S. Growth Failure in Children with HIV Infection. J Acquir Immune Def Syndrome. 2000;25:537–542. doi: 10.1097/00042560-200010001-00006. [DOI] [PubMed] [Google Scholar]
- 2.Gertner JM, Kaufman FR, Donfield SM, Sleeper LA, Shapiro AD, Howard C, et al. Delayed Somatic Growth and Pubertal Development in human immunodeficiency infected Hemophiliac Boys: Hemophilia Growth and Development Study. J Peds. 1994;124:896–902. doi: 10.1016/s0022-3476(05)83177-4. [DOI] [PubMed] [Google Scholar]
- 3.Stagi S, et al. Final Height in Patients Perinatally Infected with the Human Immunodeficiency Virus. Horm Res Paediatr. 2010;74 (3):165–71. doi: 10.1159/000281018. [DOI] [PubMed] [Google Scholar]
- 4.Guillén S, Ramos JT, Resino R, Bellón JM, Muñoz MA. Impact on Weight and Height With the Use of HAART in HIV-infected Children. Ped Infect Dis Journal. 2007;26:334–338. doi: 10.1097/01.inf.0000257427.19764.ff. [DOI] [PubMed] [Google Scholar]
- 5.Nachman SA, Lindsey JC, Pelton S, Mofenson L, McIntosh K, Wiznia A, et al. Growth in Human Immunodeficiency Infected Children receiving Ritonovir-containing antiretroviral therapy. Arch Pediatr Adolesc Med. 2002;156:497–503. doi: 10.1001/archpedi.156.5.497. [DOI] [PubMed] [Google Scholar]
- 6.Spencer RP. Pubertal Height Gain, male-female and interpopulation comparisons. Med Hypothesis. 2002;59 (6):759–61. doi: 10.1016/s0306-9877(02)00326-2. [DOI] [PubMed] [Google Scholar]
- 7.Vizmanos B, Marti-Hennenberg C. Age of Pubertal Onset Affects the Intensity and Duration of Pubertal Growth Peak, But not Final Height. Am J Hum Biol. 2001;13:409–416. doi: 10.1002/ajhb.1065. [DOI] [PubMed] [Google Scholar]
- 8.Buchacz K, Rogol AD, Lindsey JC, Wilson CM, Hughes MD, Seage GR, et al. Delayed Onset of Pubertal Development of Children and Adolescents with Perinatally Acquired HIV infection. Journal of Acquired Immunodeficiency Syndromes. 2003;33:56–65. doi: 10.1097/00126334-200305010-00009. [DOI] [PubMed] [Google Scholar]
- 9.Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier Onset of Puberty in Girls Relation to Body Mass Index and Race. Pediatrics. 2001;108:347–353. doi: 10.1542/peds.108.2.347. [DOI] [PubMed] [Google Scholar]
- 10.Chantry CJ, Hughes MD, Alvero C, Cervia JS, Hodge J, Borum P, et al. Insulin-Like Growth Factor-1 and Lean Body Mass in HIV-Infected Children. J Acquir Immune Defic Syndr. 2008;48:437–443. doi: 10.1097/QAI.0b013e31817bbe6d. [DOI] [PubMed] [Google Scholar]
- 11.Samstein B, Hoimes ML, Fan J, Frost RA, Gelato MC, Lang CH. IL-6 Stimulation of Insulin-like Growth Factor Binding Protein (IGFBP-1) Production. Biochem and Biophys Res Commun. 1996;228:611–615. doi: 10.1006/bbrc.1996.1705. [DOI] [PubMed] [Google Scholar]
- 12.Lang C, Nystrom GJ, Frost RA. Regulation of IGF binding protein-1 in Hep G2 cells by Cytokines and Reactive Oxygen Species. American Physiological Society. 1999;276:G719–726. doi: 10.1152/ajpgi.1999.276.3.G719. [DOI] [PubMed] [Google Scholar]
- 13.Holt R, Baker A, Jones J, Miell J. The insulin-like growth factor and binding protein axis in children with end-stage liver disease before and after orthotopic liver transplantation. Pediatr Transplant. 1998;2(1):76–84. [PubMed] [Google Scholar]
- 14.Davies UM, Jones J, Reeve J, Camacho-Hubner C, Charlett A, Ansell BM, et al. Juvenile Rheumatoid Arhtritis: Effects of Disease Activity and Recombinant Human Growth Hormone on Insulin-like Growth Factor 1, Insulin-Like Growth Factor Binding Proteins 1 and 3, Osteocalcin. Arthritis and Rheumatism. 1997;40:332–338. doi: 10.1002/art.1780400218. [DOI] [PubMed] [Google Scholar]
- 15.De Benedetti F, Meazza C, Martini A. Role of Interleukin-6 in Growth Failure: An Animal Model. Horm Res. 2002;58:24–27. doi: 10.1159/000064757. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. c2007 http://www.who.int/en/ [cited March 30, 2009]. Available from: www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf.
- 17.Rogol A, Clark P, Roemmich JN. Growth and Pubertal Development in Children and Adolescents; effects of Diet and Physical Activity. Am J Clin Nutr. 2000;72:521S–528S. doi: 10.1093/ajcn/72.2.521S. [DOI] [PubMed] [Google Scholar]
- 18.Susman E, Houts R, Steinberg L, Belsky J, Cauffman E, Dehart G, et al. Longitudinal Development of Secondary Sexual Characteristics of boys and girls between 9 ½ and 15 ½ years. Arch Pediatric Adolesc Med. 2010;164:166–73. doi: 10.1001/archpediatrics.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Martino M, Tovo PA, Galli L, Gabiano C, Chiarelli F, Zappa M, et al. Puberty in Perinatal HIV Infection: a Multicentre Longitudinal Study of 212 Children. AIDS. 2001;15:1527–34. doi: 10.1097/00002030-200108170-00010. [DOI] [PubMed] [Google Scholar]
- 20.Tanner JM, Whitehouse RH, Takaishi M. Standards from Birth to Maturity for Height, Weight, Height velocity and Weight velocity: British Children. Arch Dis Child. 1966;41:451–474. doi: 10.1136/adc.41.219.454. [DOI] [PMC free article] [PubMed] [Google Scholar]