Abstract
Context
Older studies have shown that high doses of norepinephrine infused into human subjects can inhibit insulin secretion. Similar inhibition during electrical stimulation of sympathetic nerves in animals raises the possibility that the suppression of insulin secretion seen in humans could reflect a physiological effect of sympathetic nerves on islet β-cells. However, a direct test of the hypothesis that moderate and selective activation of these nerves is sufficient to inhibit insulin secretion in humans is lacking.
Objective
We sought to test this hypothesis by releasing moderate amounts of endogenous norepinephrine selectively from the sympathetic nerves of normal human subjects by infusing them with low doses of the indirect sympathomimetic agent tyramine.
Methods
During a single study visit, 11 healthy subjects received iv injections of arginine either alone or in combination with a low-dose tyramine infusion. Physiological (blood pressure) and biochemical (insulin, glucose, and norepinephrine) parameters were measured.
Results
The acute insulin response to arginine was significantly reduced during tyramine compared with that seen in the absence of tyramine (P = 0.036).
Conclusions
These data suggest that moderate and selective activation of sympathetic nerves inhibits insulin release in humans.
Porte et al. (1) first demonstrated that exogenous norepinephrine (NE) could inhibit insulin release in humans. In that study, NE infusions impaired the insulin response to either glucose or tolbutamide (1). Later measurements of the plasma NE levels achieved during similar NE infusions in humans made it clear that these plasma levels were seen only during severe exercise and pathophysiological stress (2). However, because NE is a neurotransmitter, synaptic levels achieved during activation of sympathetic nerves might approach the concentrations reached during these infusions. Thus, these studies suggested, but did not demonstrate, that physiological activation of sympathetic nerves might regulate the islet β-cell in humans.
Studies in animals, where it was possible to selectively activate these nerves electrically, did show the expected inhibition of insulin release (3–5), but to our knowledge, there are no analogous studies in humans. Thus, it remained unknown whether the inhibitory effect of exogenous NE on insulin secretion in humans, demonstrated decades ago, reflects a physiological effect of sympathetic nerves on the islet β-cells or simply the pharmacological effect of an endogenous compound. To answer this question finally, the present study sought to release moderate amounts of endogenous NE selectively from sympathetic nerves in normal human subjects.
To that end, we infused a low dose of tyramine and measured plasma insulin. Tyramine is an indirect sympathomimetic that releases vesicularly stored NE from sympathetic nerve terminals. It competes with axoplasmic NE for uptake into the synaptic vesicles within the nerve terminal. This in turn markedly elevates axoplasmic NE levels, inducing a carrier-mediated transport of NE out of the nerve terminal (6). Thus, tyramine elevates the synaptic levels of NE, yet has only a modest effect on circulating NE levels, thus permitting one to assess the role of sympathetic nerves (for example, in the pancreatic islet), independent of the effects of circulating NE.
Subjects and Methods
Experimental subjects
Eleven healthy individuals (eight men and three women) were recruited by advertisement for this study. The subjects had a mean age of 32 yr (range 18–44 yr) and a mean body weight of 70.9 kg (range 50–98 kg). Subjects were excluded if they had serious medical problems, elevated blood pressure (>130/80 mm Hg), or low hematocrit (<38%) or if they were significantly overweight (body mass index ≥ 28 kg/m2) taking monoamine oxidase inhibitors, or pregnant. Informed consent was obtained from each study participant, and the investigations were carried out in accordance with the guidelines in the Declaration of Helsinki.
Study design
After an overnight fast, an iv catheter was inserted into each arm of the study subject. One arm was used for the infusion of tyramine and arginine, and the other was used for collection of blood samples. First, a bolus dose of arginine was given (2.5 or 5 g; Pharmacia, New York, NY). Blood samples for the measurement of insulin, NE, epinephrine, and glucose were drawn every 2 min for the first 10 min and every 5 min thereafter. Vital signs (blood pressure and heart rate) were monitored on the same schedule. Thirty minutes after the arginine bolus, tyramine (Clinalfa, Laäufelfingen, Switzerland) was infused at a rate of 30 μg/ kg·min for 10 min. Our preliminary dose-response study had shown that the 30 μg/kg·min dose caused a clear but safe rise in systolic blood pressure (Δ = +33 ± 4 mm Hg), indicating significant neuronal NE release, accompanied by only a modest increase of plasma NE (Δ = +217 ± 28 pg/ml). Three minutes into the tyramine infusion, a second identical dose of arginine was given. We have previously shown that the insulin secretory response to repeated arginine injections is highly reproducible within individuals, indicating that repetitive arginine pulses neither exhaust nor prime the β-cell (7). Laboratory and vital sign measurements were repeated on the same schedule as described above.
Materials and Methods
Blood for the catecholamine assays was collected on EGTA/glutathione (2.3:1.5 mg/ml). Blood for insulin and glucose was collected on EDTA. Immediately after collection, blood samples were placed on ice and centrifuged, and plasma was stored at –80 C until assayed. Plasma epinephrine and NE concentrations were measured in duplicate using a radioenzymatic assay, as previously described (8). The intra- and interassay coefficients of variance in this laboratory are 6 and 10%, respectively. Plasma insulin concentrations were measured in duplicate using a modified double-antibody RIA technique (9). The intra- and interassay coefficients of variance in this laboratory are 6 and 12%, respectively. Plasma glucose concentrations were measured in duplicate using a Beckman glucose analyzer.
Data analyses
Statistical comparisons were made using paired Student's t tests. Results are expressed as mean ± SEM. All tests were two-tailed with significance set at P < 0.05.
Results
As expected, tyramine caused a significant but moderate increase in systolic blood pressure (Δ = +33 ± 4 mm Hg), suggesting a significant activation of sympathetic nerves coupled with a modest increase in circulating NE (Δ = +217 ± 28 pg/ml; baseline level = 274 ± 34). Epinephrine levels did not increase during tyramine (data not shown).
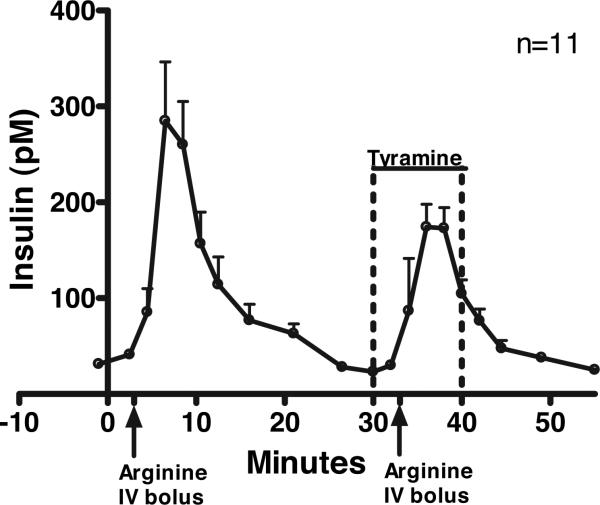
As shown in Fig. 1 and Table 1, the acute insulin response (AIR) to arginine was significantly reduced during tyramine, compared with the AIR to arginine alone (P = 0.036), despite no difference in the prearginine glucose levels (Δ = 0.3 ± + 1.6 mg/dl; P = 0.842) or the glucose response to arginine before vs. during tyramine (data not shown). The AIR to arginine before tyramine varied widely between individuals [range for area under the curve (AUC), 208-2812], but the decrement of the AIR induced by tyramine was closely correlated with the magnitude of the original response (r = 0.81). In contrast, there was no correlation (r = 0.03) between the decrement of the AIR to arginine and the increment of NE induced by tyramine.
FIG. 1.
AIR to arginine before and during a tyramine infusion. Total insulin was first measured in response to an arginine bolus (2.5 or 5 g). Thirty minutes later, total insulin was measured in response to the same dose of arginine administered 3 min after starting a 10-min tyramine infusion (30 μg/kg·min). Data are presented as mean ± SEM (n = 11).
TABLE 1.
Effect of tyramine on NE, systolic blood pressure, and the AIR to arginine
| Subject | Acute NE response to TYR (AUC) | Acute SBP response to TYR (AUC) | AIR ± TYR (AUC) |
|||
|---|---|---|---|---|---|---|
| – TYR | + TYR | Δ | %Δ | |||
| 1 | +2564 | +453 | +1372 | +689 | –683 | –50% |
| 2 | +1042 | +276 | +1147 | +961 | –186 | –16% |
| 3 | +1969 | +164 | +527 | +327 | –200 | –38% |
| 4 | +1227 | +265 | +1102 | +663 | –439 | –40% |
| 5 | +2002 | +123 | +1564 | +989 | –575 | –37% |
| 6 | +2523 | +152 | +2812 | +1007 | –1805 | –64% |
| 7 | +967 | +265 | +329 | +460 | +131 | +40% |
| 8 | +3649 | +316 | +1662 | +1767 | +105 | +6% |
| 9 | +2859 | +424 | +208 | +175 | –33 | –16% |
| 10 | +1304 | +140 | +925 | +488 | –437 | –47% |
| 11 | +2303 | +69 | +1000 | +802 | –198 | –20% |
| Mean | +2037 | +241 | +1150 | +757 | –392 | –26% |
| sem | 256 | 37 | 219 | 131 | 162 | 9% |
| P value | <0.001 | <0.001 | 0.036 | 0.016 | ||
SBP, Systolic blood pressure; TYR, tyramine.
Discussion
In this study, we used tyramine to activate the sympathetic innervation of the pancreatic β-cell and found that insulin secretion was inhibited. Although we believe that the sympathetic input to the β-cell is responsible for the observed inhibition of insulin secretion, we have not definitively proved this, but we have ruled out several other possible mechanisms. One alternative explanation for tyramine's suppressive effect on insulin secretion is that, in high doses, tyramine enhances glucose uptake into peripheral tissues (10), lowering plasma glucose and thereby its stimulation of insulin release. However, at the doses of tyramine used in the present study, the prearginine glucose levels, as well as those immediately after the arginine bolus, were identical before vs. during tyramine, ruling out differences in glycemia as responsible for the observed inhibition of insulin secretion.
Another alternative is that tyramine might exert its sympathomimetic effect directly on the β-cell, thereby inhibiting insulin secretion. However, other studies, using similar doses of tyramine in humans, have demonstrated that tyra-mine's action requires NE release. For example, the acute pressor effect of tyramine is inhibited by duloxetine, a compound that inhibits tyramine uptake into, and therefore subsequent NE release from, nerve terminals (11). Thus, in humans, tyramine's effect on blood pressure occurs via local release of NE from sympathetic nerve terminals rather than by a direct action of tyramine on the vasculature, and we suggest a similar mechanism of action at the pancreatic β-cell.
To our knowledge, this is the first study to demonstrate that selective activation of sympathetic nerves inhibits insulin secretion in humans. An earlier in vitro study using tyra-mine also showed an inhibition of insulin secretion, albeit from the perfused pancreas of the Zucker fatty rat (12). Additionally, because tyramine causes release of endogenous NE from nerve terminals, this approach can be viewed as similar to previous studies in animals, in which direct electrical stimulation of sympathetic nerves inhibited insulin release (5). Thus, the present study extends similar studies in animals but, more importantly, demonstrates that the previously described inhibitory effect of exogenous NE on insulin secretion in humans did, indeed, mimic the effect of sympathetic nerves on the human islet β-cells. In this regard, previous studies in exercising humans have also implicated sympathetic nerves as causing an inhibition of insulin secretion (13). For example, α-adrenergic blockade reduced the magnitude of the fall of insulin during exercise (13). Early during exercise the NE/epinephrine ratio was high, suggesting that the sympathetic innervation, rather than the adrenal medulla, was the mediator. Thus, both our current study employing tyramine and the study above employing exercise suggest that activation of sympathetic nerves can inhibit insulin secretion in humans.
One criticism of earlier NE infusion studies in humans is that the plasma NE levels required to achieve suppression of the β-cell were supraphysiological (2, 14). Thus, in the present study, we sought to achieve a moderate activation of sympathetic nerves in our human subjects. The increment of systolic blood pressure during tyramine is evidence that this goal was achieved. For example, studies in humans show that similar increments of systolic blood pressure occur during moderate exercise (15). Likewise, the modest increment of circulating NE we observed during tyramine (~200 pg/ ml) argues for a moderate activation of sympathetic nerves because about 5-fold larger increments of plasma NE (~1000 pg/ml) are observed during moderate exercise (16, 17), and about 9-fold larger increments of circulating plasma NE (~1800 pg/ml) are required to inhibit insulin secretion (2) in the absence of activated sympathetic nerves. Thus, both the systolic blood pressure and the plasma NE response to tyra-mine in this study argue that we achieved an activation of sympathetic nerves within the physiological range, albeit using a pharmacological agent to do so.
The importance of this sympathetic inhibition of insulin secretion may be related to the suppression of endogenous insulin that occurs during insulin-induced hypoglycemia in humans, in addition to that that occurs during exercise (see above). Although the falling glucose certainly decreases the direct stimulation of insulin secretion, some studies have suggested active inhibition via sympathetic nerves as well (18). Evidence that pancreatic sympathetic nerves are activated during hypoglycemia comes from animal studies (19), although there is evidence that hypoglycemia also activates sympathetic nerves in humans, albeit those to muscle (20). Thus, the inhibitory effect of sympathetic nerves upon insulin secretion that we demonstrate here in humans may contribute to suppression of endogenous insulin secretion during insulin-induced hypoglycemia. Such suppression would limit the depth and duration of that hypoglycemia.
Acknowledgments
We acknowledge the contribution of the following individuals: Aryana Zavosh, Jira Wade, Emily Haines, and Robin Vogel for technical assistance and Thomas Mundinger for scientific input.
Support for this study was provided by a Research Enhancement Award Program funded by the Medical Research Service of the Veterans Administration and by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK50,154).The clinical studies were conducted at the General Clinical Research Center at the University of Washington and supported by the National Institutes of Health, Grant M01RR-00037.
Abbreviations
- AIR
Acute insulin response
- AUC
area under the curve
- NE
norepinephrine
Footnotes
Disclosure Statement: L.K.G., J.P.P., and G.J.T. have nothing to disclose.
References
- 1.Porte D, Jr, Williams RH. Inhibition of insulin release by norepinephrine in man. Science. 1966;152:1248–1250. doi: 10.1126/science.152.3726.1248. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg A, Shah S, Haymond M, Cryer P. Norepinephrine: hormone and neurotransmitter in man. Am J Physiol Endocrinol Metab. 1978;234:E252–E256. doi: 10.1152/ajpendo.1978.234.3.E252. [DOI] [PubMed] [Google Scholar]
- 3.Bloom S, Edwards A. The release of pancreatic glucagon and inhibition of insulin in response to stimulation of the sympathetic innervation. J Physiol. 1975;253:157–173. doi: 10.1113/jphysiol.1975.sp011185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holst JJ, Schwartz TW, Knuhtsen S, Jensen SL, Nielsen OV. Autonomic nervous control of the endocrine secretion from the isolated, perfused pig pancreas. J Auton Nerv Syst. 1986;17:71–84. doi: 10.1016/0165-1838(86)90045-7. [DOI] [PubMed] [Google Scholar]
- 5.Ahren B, Veith RC, Taborsky GJ., Jr Sympathetic nerve stimulation versus pancreatic norepinephrine infusion in the dog: 1). Effects on basal release of insulin and glucagon. Endocrinology. 1987;121:323–331. doi: 10.1210/endo-121-1-323. [DOI] [PubMed] [Google Scholar]
- 6.Graefe KH, Bossle F, Wolfel R, Burger A, Souladaki M, Bier D, Dutschka K, Farahati J, Bonisch H. Sympathomimetic effects of MIBG: comparison with tyramine. J Nucl Med. 1999;40:1342–1351. [PubMed] [Google Scholar]
- 7.Palmer JP, Walter RM, Ensinck JW. Arginine-stimulated acute phase of insulin and glucagon secretion. I. In normal man. Diabetes. 1975;24:735–740. doi: 10.2337/diab.24.8.735. [DOI] [PubMed] [Google Scholar]
- 8.Evans MI, Halter JB, Porte D., Jr Comparison of double- and single-isotope enzymatic derivative methods for measuring catecholamines in human plasma. Clin Chem. 1978;24:567–570. [PubMed] [Google Scholar]
- 9.Morgan C, Lazarow A. Immunoassay of insulin: two antibody systems. Diabetes. 1963;12:115–126. [Google Scholar]
- 10.Morin N, Visentin V, Calise D, Marti L, Zorzano A, Testar X, Valet P, Fischer Y, Carpene C. Tyramine stimulates glucose uptake in insulin-sensitive tissues in vitro and in vivo via its oxidation by amine oxidases. J Pharmacol Exp Ther. 2002;303:1238–1247. doi: 10.1124/jpet.102.040592. [DOI] [PubMed] [Google Scholar]
- 11.Vincent S, Bieck PR, Garland EM, Loghin C, Bymaster FP, Black BK, Gonzales C, Potter WZ, Robertson D. Clinical assessment of norepinephrine transporter blockade through biochemical and pharmacological profiles. Circulation. 2004;109:3202–3207. doi: 10.1161/01.CIR.0000130847.18666.39. [DOI] [PubMed] [Google Scholar]
- 12.Hirose H, Maruyama H, Kido K, Ito K, Koyama K, Tashiro Y, Saruta T. α- and β-cell function in obese Zucker (fa/fa) rats: a study with the isolated perfused pancreas. Clin Sci (Lond) 1994;86:311–316. doi: 10.1042/cs0860311. [DOI] [PubMed] [Google Scholar]
- 13.Galbo H, Christensen NJ, Holst JJ. Catecholamines and pancreatic hormones during autonomic blockade in exercising man. Acta Physiol Scand. 1977;101:428–437. doi: 10.1111/j.1748-1716.1977.tb06026.x. [DOI] [PubMed] [Google Scholar]
- 14.Clutter WE, Cryer PE. Plasma dose-response studies with noradrenaline and adrenaline in man. Prog Biochem Pharmacol. 1980;17:84–89. [PubMed] [Google Scholar]
- 15.Singh JP, Larson MG, Manolio TA, O'Donnell CJ, Lauer M, Evans JC, Levy D. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension: the Framingham heart study. Circulation. 1999;99:1831–1836. doi: 10.1161/01.cir.99.14.1831. [DOI] [PubMed] [Google Scholar]
- 16.Galbo H, Holst JJ, Christensen NJ. Glucagon and plasma catecholamine responses to graded and prolonged exercise in man. J Appl Physiol. 1975;38:70–76. doi: 10.1152/jappl.1975.38.1.70. [DOI] [PubMed] [Google Scholar]
- 17.Winder WW, Hickson RC, Hagberg JM, Ehsani AA, McLane JA. Training-induced changes in hormonal and metabolic responses to submaximal exercise. J Appl Physiol. 1979;46:766–771. doi: 10.1152/jappl.1979.46.4.766. [DOI] [PubMed] [Google Scholar]
- 18.Luzi L, Battezzati A, Perseghin G, Bianchi E, Vergani S, Secchi A, La Rocca E, Staudacher C, Spotti D, Ferrari G, Di Carlo V, Pozza G. Lack of feedback inhibition of insulin secretion in denervated human pancreas. Diabetes. 1992;41:1632–1639. doi: 10.2337/diab.41.12.1632. [DOI] [PubMed] [Google Scholar]
- 19.Havel PJ, Veith RC, Dunning BE, Taborsky GJ., Jr Pancreatic noradrenergic nerves are activated by neuroglucopenia but not by hypotension or hypoxia in the dog. Evidence for stress-specific and regionally selective activation of the sympathetic nervous system. J Clin Invest. 1988;82:1538–1545. doi: 10.1172/JCI113763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis SN, Shavers C, Costa F, Mosqueda-Garcia R. Role of cortisol in the pathogenesis of deficient counterregulation after antecedent hypoglycemia in normal humans. J Clin Invest. 1996;98:680–691. doi: 10.1172/JCI118839. [DOI] [PMC free article] [PubMed] [Google Scholar]