Abstract
A new phenyl-acetylene receptor containing a carbonaceous hydrogen bond donor activates anion binding in conjunction with two stabilizing ureas. The unusual CH···Cl– hydrogen bond is apparent in solution by large 1H NMR chemical shifts and by a short, linear contact in the solid state.
Numerous supramolecular hosts for anionic guests have been developed, including polymers, macrocycles and cryptands.1 Neutral hosts frequently combine a complementary geometry and strong hydrogen bond donors to selectively bind anionic guests.2 A range of hydrogen bond donors that exhibit drastically different pKas are found in supramolecular hosts. Some examples are amides (), sulfonamides (), phenols (), pyrroles () and ureas ().3 These examples of hydrogen bond donors all rely on protic N- or O-groups. Highly electronegative elements are favored, but are not the only hydrogen bond donors extant. Of the donors employed in molecular sensors, the phenyl C—H ()3b,c donor is relatively underappreciated by supramolecular chemists.
The C—H donor was recognized early on by molecular biologists as an important component in the secondary structures of biomolecules.4,5 Carbonaceous hydrogen bond donors have experienced a renaissance in recent years with the introduction of new functional groups, including imidazolium,6 triazole,7 diketopropylene-BF2,8 and benzene.9 The 1,2,3-triazole functionality is a promising anion binding moiety in neutral receptors,10 since the C—H hydrogen bond donor is activated by a strong dipole oriented through the nitrogen atoms.11 As previously observed in other selective hosts, the anion binding ability of 1,2,3-triazoles is optimized when the receptor is preorganized in an appropriately sized macrocycle.12
Aryl groups are used as the rigid linkers in supramolecular hosts to provide the desired anion receptor geometry. As a result, phenyl protons are a frequent feature in anion binding pockets.1 Despite their ubiquity, aryl protons play only a small role in the anion binding of most hosts. Contrary to this, calculations suggest phenyl protons can bind anions with association energies (ΔG) approaching –9.0 kcal mol–1 and volumes of crystallographic data exist for the general C—H···anion interaction.9,13 Recent solution studies have demonstrated the action of C—H hydrogen bonds in supramolecular hosts, yet crystal structures of these complexes are still rare.14
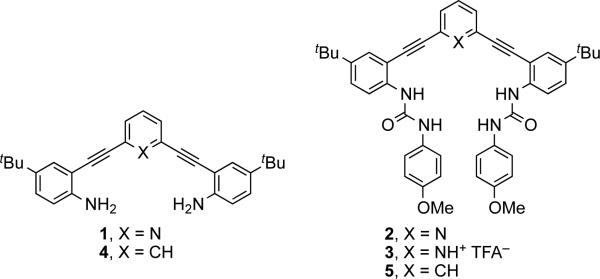
2,6-Bis(2-anilinoethynyl)pyridine (1, Fig. 1) is an easily functionalized fluorescent core. Addition of sulfonamides or ureas (e.g., 2) has illustrated the versatility of this scaffold for the construction of fluorescent sensors.15 Judicious modification of the substituents allows control of the fluorescent and coordinating properties.16 Previous work in our labs has demonstrated the increased anion binding ability by the pyridine-protonated receptors (3), showing an almost two order of magnitude increase in anion binding.17 The greater anion binding ability of the pyridinium host is limited by its pKa. While the exact pKa of 3 is not known, the pKa's for a series of similar ethynylpyridines have been measured in acetonitrile; the electron-withdrawing alkynes shift the pKa of 2,6-bis(ethynyl)pyridinium to 8.918 from 12.5 for pyridinium (MeCN).19 This complicates any attempts to apply a pyridinium-based anion sensor in an environment such as cells where a low pH cannot be maintained.18b Replacement of the pyridine with a phenyl moiety in the ethynyl core (e.g., 4, 5) will maintain the same geometry, while altering the electronic and anion binding properties. The phenyl C—H hydrogen bond offers an opportunity to expand the working conditions of this sensor and explore the nature of C—H hydrogen bonds. Herein, we introduce an aryl hydrogen bond donor in place of the pyridine/pyridinium moiety and report the anion binding characteristics of this new receptor.
Fig. 1.
Structures of 2,6-bis(2-anilinoethynyl)arene cores 1 and 4 and anion receptors 2, 3 and 5.
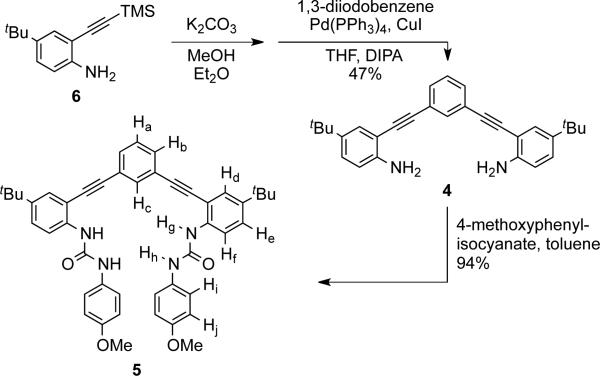
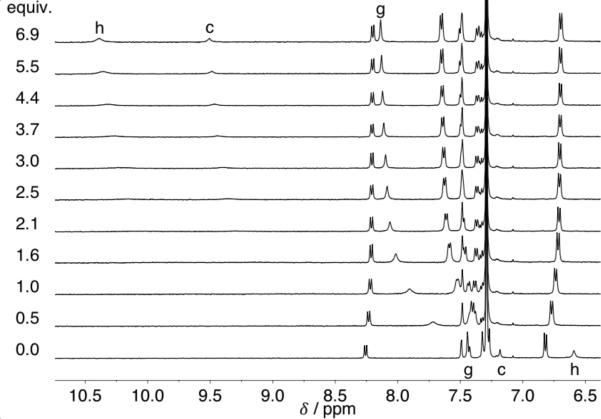
Phenyl core 4 was obtained by deprotection and subsequent Sonogashira cross-coupling of ethynylaniline 6 with 1,3-diiodobenzene in 47% yield (Scheme 1). Reaction of 4 with 4-methoxyphenyl isocyanate gave bisurea receptor 5 in 94% yield. The anion binding properties of 5 were analyzed using 1H NMR and UV-Vis spectroscopy. Titrations were performed in water-saturated chloroform with anions added as tetra-nbutylammonium (TBA) salts. A representative 1H NMR titration experiment with 5 and Cl– is shown in Fig. 2. The Δδ of urea proton Hg was fit using non-linear regression in MatLab to determine association constants (Ka).20 A 1:1 host:guest binding model was used to fit the data as this agrees with previous binding studies on similar systems.17 In addition, the model is supported by UV-Vis spectroscopic evidence and solid-state structures. Association constants for 5 and comparison with data for structurally similar hosts 2 and 3 are reported in Table 1.
Scheme 1.
Synthesis of receptor 5. Proton assignments determined via 1H-13C HSQC and 1H-1H ROSY NMR spectroscopy.
Fig. 2.
1H NMR titration of 5 with TBA+Cl– at 298 K; [5] = 0.69 mM in water saturated CDCl3. Peak assignments refer to Scheme 1.
Table 1.
Anion association constants (Ka) obtained by fitting titration data using MatLab for 1H NMR data or Hyperquad for UV-Vis data.a
| Host | Cl–/M–1 | Cl– ΔG/kcal mol–1 | Br–/M–1 | I–/M–1 |
|---|---|---|---|---|
| 2 | 700b | –3.88 | — | — |
| 5 | 6700c | –5.21 | 1600b | 150b |
| 3 | 40700c | –6.28 | — | — |
Anions added as tetrabutylammonium salts in water-saturated CHCl3 or CDCl3 at 298 K. Error is ca. ±10% and the values represent an average of three titrations.
Titrations performed using 1H NMR.
Titrations performed using UV-Vis.
Pyridine host 2, with only four hydrogen bond donors, has the lowest Ka for the tested anions. As previously reported, protonation of this class of receptor activates anion binding, increasing the Ka from 700 M–1 to 40700 M–1 for 3•Cl–. 17 The difference in Ka between 2 and 3 can be separated into three influencing factors: (1) the repulsive effect of the nitrogen lone pair on 2, (2) the additional hydrogen bond in 3, and (3) the electrostatic interaction of Cl– with 3. For the three hosts, receptor 2 has the lowest energy interaction due to the repulsion between the pyridine lone pair. The inclusion of an aryl C—H hydrogen bond donor in 5 increases the Ka by an order of magnitude for Cl– (Table 1). The difference in energy (ΔΔG = ΔG 2•Cl– — ΔG 5•Cl–) is 1.33 kcal mol–1. This ΔΔG is very close to the value (1.44 kcal mol–1) reported by Sessler et al. for strapped pyrroles containing benzene or furan.21 The additional electrostatic attraction in protonated receptor 3 leads to 1.07 kcal mol–1 added stabilization (ΔΔG). This value is perhaps smaller due to competition from the trifluoroacetate counter ion.
2-D HSQC and ROSY NMR spectroscopy were used to assign the urea and aryl peaks in the proton NMR spectrum of 5 (see Fig. S20-S22 in the Supporting Information). The urea protons (Hh and Hg) shift downfield upon addition of Cl–. A close examination of the aryl peak Hc reveals a large downfield shift, Δδ >2 ppm. The magnitude of Δδ suggests a strong aryl hydrogen bond, but may also be influenced by structural changes induced by anion binding. The other aromatic peaks shift very little or slightly upfield likely as a result of weak CH—π interactions. The lack of a significant Δδ for Ha or Hb contraindicates the influence of alternative binding conformations, although multiple conformations have been previously observed in 2 and 3 by rotation about the ethynyl linkers.16a,17,22 The stronger urea hydrogen bond to Cl– is formed with the more distant proton from the core, Hh, which is apparent in the large shift (Δδ 3.8 ppm) as compared to the interior urea Hg. Similar shifts are observed upon titration with Br– and I– (Fig. S4 and S7).
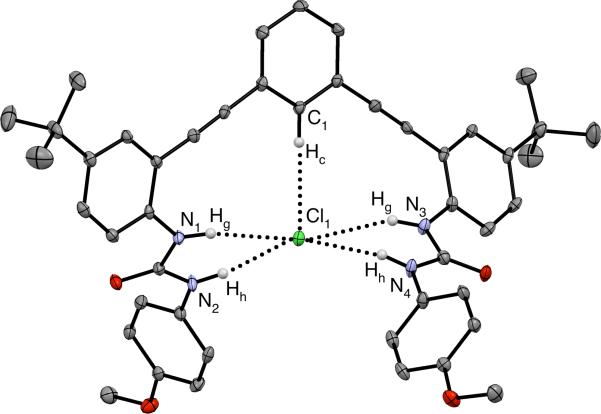
Evidence of hydrogen bonding was also observed in the X-ray crystal structure of 5•Cl–, which is shown in Fig. 3.23 The chloride resides within a binding pocket created by the aryl proton and the urea arms with a TBA+ cation in close proximity. The central phenylacetylene carbons (C1-C9, C27-C29) form a plane (± 0.033 Å), which the chloride sits slightly above (0.257(4) Å). The urea arms are twisted with one slightly above the plane and the other slightly below the plane. The C(Hc)···Cl distance is 3.579(3) Å and the C(Hc)···Cl angle is 169°. The N(H)···Cl distances vary greatly with the shortest distance at 3.212(3) Å and the longest distance measuring 3.732(3) Å. The N(H)···Cl angles vary from 146° to 170°. The C(H)···Cl total distance is less than the sum of the van der Waals radii for the component elements and is shorter than previously reported examples of arene C(H)···Cl contacts in anion hosts (3.538—3.793 Å).14 The large angle and short distance fall well within previously defined criteria for an aryl hydrogen bond (θ > 140°, d < 3.86 Å)4,24 and add credence for the importance of this C—H hydrogen bond in anion binding.
Fig. 3.
X-ray crystal structure of 5•Cl– shown as ORTEP representation. Hydrogen bond interactions are shown as dashed lines. Non-coordinating hydrogens, TBA+ counter cation and solvent have been omitted for clarity. Ellipsoids drawn at 50% probability level.
The urea hydrogen bond distances provide further evidence for the binding conformation observed in solid-state studies to exist in solution. The urea hydrogen bonds are divided into two asymmetric groups, where N2/N4 are on average 0.41 Å closer to Cl than N1/N3 (Hh and Hg, respectively). The average distance for N(Hg)···Cl is 3.637 Å and the average distance for N(Hh)···Cl is 3.213 Å. A longer N(Hg)···Cl distance would account for the smaller downfield shift observed by 1H NMR spectroscopy. The solution and solid state experiments provide a relative rank of the hydrogen bond lengths to Cl–, and perhaps strengths as follows: N(Hh) > C(Hc) > N(Hg).
UV-Vis and fluorescence spectroscopy experiments were performed to evaluate the sensing ability of compound 5 (see Fig. S11 and S15 in the Supporting Information). The color change of 5 was modest but allowed for the determination of binding constants. Cl– titrations of 5 have an isosbestic point at 312 nm indicating a clean transition from free host and guest to the final host:guest complex, which lends credence to the 1:1 host:guest model used for Ka determination. Crystallographic evidence points to a higher order complex (1:2 or 2:1 host:guest) being unlikely and these larger complexes would require an intermediate complex, which is not evident in solution. Receptors 2 and 3 were previously shown to be good fluorescent sensors16, and the conjugated core of 5 should also lend itself to this application. Excitation at 320 nm produced a fluorescence emission at 381 nm with a Stoke's shift of 5000 cm–1. Addition of one equivalent TBACl caused a marked decrease in the fluorescence. The turn-off fluorescent response for chloride is the same as previously observed with receptor 3. The fluorescence response of this class of sensors can be controlled by substitution at the para position of the phenylureas. In the pyridine sensors 2 and 3, an electron donating group (OMe) produced an “on-off” response; however, electron withdrawing groups (NO2) led to an “off-on” response.16
In summary, replacement of a pyridyl unit with a phenyl moiety in the bis(anilinoethynyl)arene class of anion receptors has provided a new avenue of inquiry into aryl C—H hydrogen bonding. The importance of the phenyl hydrogen bond donor has been demonstrated with solution and solid-state evidence for a strong C—H to Cl– contact. In addition, the structural modification has not negatively affected the selectivity or electronic properties of the host. Related computational modeling has shown that substitution with electron withdrawing groups can increase the hydrogen bond energy of benzene closer to that of pyrrole.9 Further experiments with the phenyl core may help to elucidate the mechanism of fluorescence in this class of sensor, especially the influence of the pyridine ring in the parent receptor. Work is underway to explore substituted phenyl cores with the goal of achieving Ka's on the same order or greater than found for 3.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01-GM087398, which also funded early stage intellectual property that was licensed by SupraSensor Technologies, a company co-founded by the PIs. C.N.C. acknowledges the National Science Foundation for an IGERT (DGE-0549503) fellowship. We also thank the NSF for support in the form of an instrumentation grant (CHE-0923589). The authors acknowledge the Biomolecular Mass Spectrometry Core of the Environmental Health Sciences Core Center at Oregon State University (NIH P30ES000210).
Footnotes
† Electronic Supplementary Information (ESI) available: experimental details, spectroscopic data and X-ray analysis of receptors and titration experiments. CCDC 929532. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b000000x/
Notes and references
- 1.Sessler JL, Gale PA, Cho W-S. Anion Receptor Chemistry. Royal Society of Chemistry; Cambridge: 2006. [Google Scholar]
- 2.Beer PD, Gale PA. Angew. Chem. Int. Ed. 2001;40:486–516. [PubMed] [Google Scholar]
- 3.Bordwell FG. Acc. Chem. Res. 1988;21:456–463. There appears to be discrepancy for the pKa of benzene in water, with values of 37 and 43 appearing most commonly. We chose to use the value reported in: Anslyn EV, Dougherty DA. Modern Physical Organic Chemistry. University Science Books; Sausalito, CA: 2006. p. 280.
- 4.Desiraju GR, Steiner T. The Weak Hydrogen Bond: in Structural Chemistry and Biology. Oxford University Press; Oxford; New York: 1999. [Google Scholar]
- 5.Schneider H-J. Angew. Chem. Int. Ed. 2009;48:3924–3977. doi: 10.1002/anie.200802947. [DOI] [PubMed] [Google Scholar]
- 6.White NG, Carvalho S, Felix V, Beer PD. Org. Biomol. Chem. 2012;10:6951–6959. doi: 10.1039/c2ob25934f. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Flood AH. Angew. Chem. Int. Ed. 2008;47:2649–2652. doi: 10.1002/anie.200704717. [DOI] [PubMed] [Google Scholar]
- 8.a Maeda H, Haketa Y, Nakanishi T. J. Am. Chem. Soc. 2007;129:13661–13674. doi: 10.1021/ja074435z. [DOI] [PubMed] [Google Scholar]; b Dong B, Sakurai T, Honsho Y, Seki S, Maeda H. J. Am. Chem. Soc. 2013;135:1284–1287. doi: 10.1021/ja312214a. [DOI] [PubMed] [Google Scholar]; c Terashima Y, Takayama M, Isozaki K, Maeda H. Chem. Commun. 2013;49:2506–2508. doi: 10.1039/c3cc38494b. [DOI] [PubMed] [Google Scholar]
- 9.Bryantsev VS, Hay BP. J. Am. Chem. Soc. 2005;127:8282–8283. doi: 10.1021/ja0518272. [DOI] [PubMed] [Google Scholar]
- 10.Hua Y, Flood AH. Chem. Soc. Rev. 2010;39:1262–1271. doi: 10.1039/b818033b. [DOI] [PubMed] [Google Scholar]
- 11.McDonald KP, Hua Y, Flood AH. Top. Heterocycl. Chem. 2010;24:341–366. [Google Scholar]
- 12.Hua Y, Ramabhadran RO, Karty JA, Raghavachari K, Flood AH. Chem. Commun. 2011;47:5979–5981. doi: 10.1039/c1cc10428d. [DOI] [PubMed] [Google Scholar]
- 13.Bryantsev VS, Hay BP. Org. Lett. 2005;7:5031–5034. doi: 10.1021/ol0520119. [DOI] [PubMed] [Google Scholar]
- 14.a Juwarker H, Lenhardt JM, Pham DM, Craig SL. Angew. Chem. Int. Ed. 2008;47:3740–3743. doi: 10.1002/anie.200800548. [DOI] [PubMed] [Google Scholar]; b Lee C-H, Na H-K, Yoon D-W, Won D-H, Cho W-S, Lynch VM, Shevchuk SV, Sessler JL. J. Am. Chem. Soc. 2003;125:7301–7306. doi: 10.1021/ja029175u. [DOI] [PubMed] [Google Scholar]; c Szemes F, Hesek D, Chen Z, Dent SW, Drew MGB, Goulden AJ, Graydon AR, Grieve A, Mortimer RJ, Wear T, Weightman JS, Beer PD. Inorg. Chem. 1996;35:5868–5879. [Google Scholar]
- 15.a Carroll CN, Naleway JJ, Haley MM, Johnson DW. Chem. Soc. Rev. 2010;39:3875–3888. doi: 10.1039/b926231h. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Berryman OB, Johnson CA, II, Zakharov LN, Haley MM, Johnson DW. Angew. Chem. Int. Ed. 2008;47:117–120. doi: 10.1002/anie.200703971. [DOI] [PubMed] [Google Scholar]
- 16.a Engle JM, Carroll CN, Johnson DW, Haley MM. Chem. Sci. 2012;3:1105–1110. doi: 10.1039/C2SC00975G. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Carroll CN, Coombs BA, McClintock SP, Johnson CA, Berryman OB, Johnson DW, Haley MM. Chem. Commun. 2011;47:5539–5541. doi: 10.1039/c1cc10947b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carroll CN, Berryman OB, Johnson CA, Zakharov LN, Haley MM, Johnson DW. Chem. Commun. 2009:2520–2522. doi: 10.1039/b901643k. [DOI] [PubMed] [Google Scholar]
- 18.Heemstra JM, Moore JS. Org. Lett. 2004;6:659–662. doi: 10.1021/ol0363016. b It should be noted that the this value is determined in CH3CN; in water the bis(ethynyl)pyridiniums should still be more acidic than pyridinium and thus have pKa’s < 5.
- 19.Kaljurand I, Kütt A, Sooväli L, Rodima T, Mäemets V, Leito I, Koppel IA. J. Org. Chem. 2005;70:1019–1028. doi: 10.1021/jo048252w. [DOI] [PubMed] [Google Scholar]
- 20.Thordarson P. Chem. Soc. Rev. 2011;40:1305–1323. doi: 10.1039/c0cs00062k. [DOI] [PubMed] [Google Scholar]
- 21.Yoon D-W, Gross DE, Lynch VM, Sessler JL, Hay BP, Lee C-H. Angew. Chem. Int. Ed. 2008;47:5038–5042. doi: 10.1002/anie.200801426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engle JM, Lakshminarayanan PS, Carroll CN, Zakharov LN, Haley MM, Johnson DW. Cryst. Growth Des. 2011;11:5144–5152. doi: 10.1021/cg201074v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crystallographic data for 5 can be found in the ESI and as CCDC 929532. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 24.a Taylor R, Kennard O. J. Am. Chem. Soc. 1982;104:5063–5070. [Google Scholar]; b Hay BP, Bryantsev VS. Chem. Commun. 2008;21:2417–2428. doi: 10.1039/b800055g. [DOI] [PubMed] [Google Scholar]; c Wood PA, Allen FH, Pidcock E. CrystEngComm. 2009;11:1563–1571. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.