Abstract
Background
Chitinase 3-like 1 (CHI3L1) is an inducible molecule on intestinal epithelial cells (IECs) during the development inflammatory bowel disease (IBD).
Methods
To investigate the role of CHI3L1 in bacterial infectious colitis, we orally inoculated pathogenic Salmonella typhimurium and potentially pathogenic adherent-invasive Escherichia coli (AIEC) LF82 virulent strain, into C57Bl/6 wild-type (WT) or CHI3L1 knockout (KO) mice.
Results
Both S. typhimurium and AIEC LF82 were found to efficiently induce severe intestinal inflammation in WT but not CHI3L1 KO mice. These bacteria-infected CHI3L1 KO mice exhibit decreased cellular infiltration, bacterial translocation and productions of IL-6 and IL-22, as compared to those of WT mice. More importantly, CHI3L1 KO mice displayed aberrant STAT3 activation after bacterial infections. Co-stimulation of CHI3L1 and IL-6, but not IL-22, synergistically activates STAT3 signaling pathway in IECs in an NF-κB/MAPK dependent manner.
Conclusions
CHI3L1 promotes the onset of selected gram-negative bacterial infectious colitis through IL-6/STAT3 pathway.
Keywords: chitinase, cytokine, STAT3, intestinal epithelial cells
Introduction
Dysregulated host-microbial interactions have been identified as one of the crucial drivers of inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD) [1]. Bacterial infectious colitis can be caused by external acquisition of microbes, such as in the case Salmonella typhimurium that is present in contaminated food/water, or by opportunistic potentially pathogenic microbes that are normally present in healthy intestinal microflora, such as in the case of the adherent-invasive Escherichia coli (AIEC) [2, 3]. Understanding the mechanisms of bacterial infectious responses with host protein expression during inflammatory conditions is important to determine effective disease management and interventions.
S. typhimurium and AIEC are useful model pathogens for the study of gram negative bacterial infectious colitis in mice [2, 3]. S. typhimurium inoculation in antibiotics pre-treated mice results in its effective colonization from the intestinal lumen, followed by high density growth, thus triggering mucosal inflammation [4, 5]. One of the hallmarks of S. typhimurium virulence is systemic manifestation [3]. In contrast, AIEC is a residential enteric microbe present in healthy individuals but was found to colonize in increased number on intestinal epithelial cells (IECs) in acute and active IBD patients [2]. Following the adherence, AIEC efficiently invades into the human IECs and macrophages in the lamina proprial compartment and replicates massively, producing large amount of TNFα at the same time [6]. Therefore, identifying host factors, in which these pathogens exploit for their virulence advantages, is important to understand the pathogenesis of bacterial infectious colitis.
Chitinase 3-like 1 (CHI3L1; Brp39) has been previously shown in vitro to promote the adhesion and invasion of both S.typhimurium and AIEC on/into IECs [7, 8]. Negatively charged glutamic acid residue on bacterial chitin-binding protein has been shown to be one of the critical factors in interacting with CHI3L1 [9]. Physiological expression of CHI3L1 in the colon under steady state is almost undetectable, but is up-regulated in the IECs and macrophages under inflammatory conditions [7]. Many reports have investigated the important roles of CHI3L1 in mediating both inflammation and cell proliferation/tissue remodeling in autoimmune disorders, including asthma and rheumatoid arthritis [10]. CHI3L1 deficient mice showed suppressed Th2-immune responses, characterized by lower tissue inflammation, fibrosis and higher immune cell apoptosis [11]. In the colon, major signaling pathways in IECs have been shown to be directly triggered by CHI3L1 expression including the PI3K/AKT and NF-κB pathways [12, 13]. However, it has not been clearly understood whether colonic CHI3L1 expression is associated with STAT3 activation, another important inflammation-associated transcription factor.
In this study, we test the hypothesis that CHI3L1 is required to trigger the onset and/or perpetuation of inflammation during the course of bacterial infection in large intestine. We demonstrate that CHI3L1 knockout (KO) mice were protected from acute intestinal inflammation induced by either S. typhimurium or AIEC infection. This mode of protection involves diminishing STAT3 activation in CHI3L1 KO as compared to wild type (WT) mice. In the mice-derived colonic epithelial cells (CECs), we clearly demonstrate ex vivo that CHI3L1 synergistically cooperates with IL-6, but not IL-22, to activate STAT3 signaling pathway in NF-κB- as well as MAPK-dependent manner.
Materials and Methods
Bacterial strains and culture conditions
S. typhimurium strain (ATCC14028) and ampicillin-erythromycin-resistant AIEC LF82 strain, a kind gift from Dr. Arlette Darfeuille-Michaud (Clermont-Ferrand, France), were cultured as previously described [7].
Mice and in vivo bacterial infection
C57Bl/6 WT mice were originally purchased from the Jackson Laboratories (Bar Harbor, ME). CHI3L1 KO mice (C57BL/6 inbred), which were originally generated by Drs. Chun Geun Lee and Jack A. Elias (Yale University School of Medicine, New Haven, CT), were provided by Dr. Anthony J. Coyle (MedImmune LLC, Gaithersburg, MD) [11]. The CHI3L1 KO mice were backcrossed with the WT mice for more than 7 times and have been housed in the Massachusetts General Hospital specific pathogen free facility under an Institutional Animal Care and Use Committee approved protocol and compliance. For both infectious colitis models, WT and KO mice have been taken care in a same cage soon after weaning until euthanasia. In addition, before starting each experiment, beddings were mixed between all the experimental groups for at least 3–4 weeks. Eight- to ten-week old C57BL/6 WT or CHI3L1 KO mice weighing approximately 20 grams were used for the experiments. In S. typhimurium infection, mice were orally pretreated with 20 mg of the broad-spectrum antibiotics streptomycin for 24 hours and subsequently orally gavaged with 2×108 CFU suspended in 0.5% carboxylmethyl cellulose (CMC) for 48 hours. In AIEC infection, mice were treated with 1% (w/v) of dextran sulfate sodium (DSS; MP Biomedicals, Solon, OH) in the drinking water for 14 days and simultaneously orally inoculated daily with 108 AIEC LF82 strain suspended in CMC. Daily body weights were measured and clinical scores were determined based on stool in blood (0 or 1), diarrhea (0 or 1) and posture (0 or 1). Histological scores were determined based on previously reported criteria [7]. Spleen, mesenteric lymph node (MLN), liver and large intestine were homogenized in Sonifier® cell disruptor 185 (Branson/Emerson Industrial Automation, Dunbury, CT) and spreaded on LB agar plate (with 100 µg/mL ampicillin for AIEC samples) to determine the CFUs.
Immunohistochemical analysis
Frozen sections (4 µm thick) were stained with the respective specific primary antibodies and detected using avidin-biotin-complex method as previously described [15]. All primary antibodies were purchased from BD Biosciences (San Jose, CA) except anti-F4/80 (AbDSerotec, Oxford UK), anti-E. coli (Dako, Carpenteria, CA) and anti-Salmonella (GenWay, San Diego, CA) antibodies. Biotinylated secondary antibodies were purchased from Vector Laboratories (Burlingame, CA).
Immunoblot analysis
Western blot analysis was performed as previously described [16]. Phospho- and total-STAT3 (Tyr705) antibodies were purchased from Cell Signaling Technology (Beverly, MA) and horseradish peroxidase (HRP)-conjugated goat-anti-rabbit IgG was purchased from Thermo Fisher Scientific (Rockford, IL). Four to five independent experiments were performed and the three representative results were analyzed by densitometry analysis (the National Institute of Health Scion image software β 4.03 for Windows XP (Frederick, MD).
CEC isolation and culture
Viable CECs were isolated using an EDTA-perfusion method as previously described [17]. Isolated CECs were cultured for 15 minutes or 1 hour in RPMI 1640 supplemented with 4% fetal calf serum containing a mixture of antibiotics (amphotericin B, penicillin G, and streptomycin). Human CHI3L1 protein was harvested from the culture supernatant of MG63 osteosarcoma cells as previously described (Quidel Corporation, San Diego, CA). Mouse recombinant-IL-6 and -IL-22 were purchased from R&D systems (Minneapolis, MN) and PeptoTec (Rocky Hill, NJ), respectively. NF-kB inhibitor (caffeic acid phenethyl ester; CAPE) and ERK1/2 inhibitor (U0126) were purchased from Santa Cruz Biotechnology (Dallas, TX) and Promega (Madison, WI), respectively.
Enzyme-linked immunosorbent assay (ELISA)
ELISA kits for mouse TNFα, IL-6 and IL-22 were purchased from R&D systems (Minneapolis, MN) and performed according to manufacturer’s protocol. Optical density at 450 nm was determined using an Auto-Reader Model 680 (Bio-Rad, Hercules, CA).
Statistics
Statistical significance was determined by Student t test or 1-way analysis of variance for multiple comparisons. Post-hoc Turkey’s honestly significant difference test was performed, where applicable, to analyze significant differences between groups.
Results
CHI3L1 promotes S. typhimurium infection and translocation in vivo
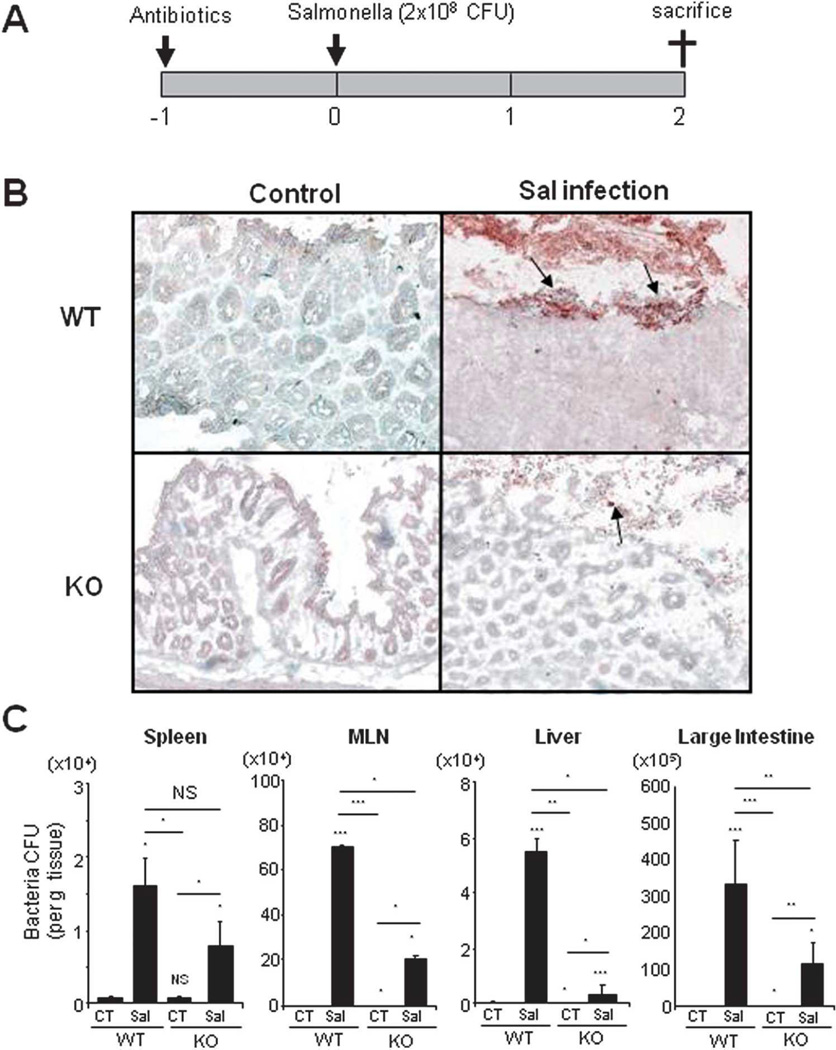
To examine the biological significance of CHI3L1 in bacterial infectious colitis, antibiotics-pretreated WT and CHI3L1 KO mice were orally inoculated with 2×108 CFU of S. typhimurium (ATCC14028) for 48 hours (Fig. 1A). Subsequent immunohistochemical staining of large intestinal sections using anti-Salmonella antibody displayed intensive staining at the surface colonic epithelium of the S. typhimurium-infected WT mice as compared to those of CHI3L1 KO mice (Fig. 1B). In contrast, both WT and CHI3L1 KO non-infected mice did not show appreciable Salmonella staining in large intestine including cecum and colon. Bacterial translocation assay also showed higher bacterial load in the MLNs, liver and large intestine-homogenized lysate of S. typhimurium-infected WT mice as compared to those of CHI3L1 KO mice (Fig. 1C). These results suggest that CHI3L1 is an important factor for S. typhimurium to attach and invade into the large intestine, as well as to translocate into other organs in vivo.
Fig. 1. CHI3L1-mediated enhancement of the Salmonella typhimurium translocation in vivo.
(A) Twenty-four hours after streptomycin treatment, C57Bl/6 WT and CHI3L1 KO mice (n=6 in each group) were orally inoculated with 2×108 CFU of S. typhimurium per mouse, and sacrificed after 48 hours post infection. (B) S. typhimurium in mucosa of proximal large intestine was detected by anti-Salmonella antibody 48 hours after infection in WT and CHI3L1 KO mice (10× objective). (C) Number of invading bacteria in spleen, mesenteric lymph nodes, liver and large intestine in WT or CHI3L1 KO mice with (Sal) or without (CT) S. typhimurium infection was shown. *P<.05, **P<.01, and NS, no significant difference (control vs. Salmonella-infected mice or between the indicated group).
CHI3L1 mediates proinflammatory cytokine production and STAT3 activation in S. typhimurium infectious acute intestinal inflammation
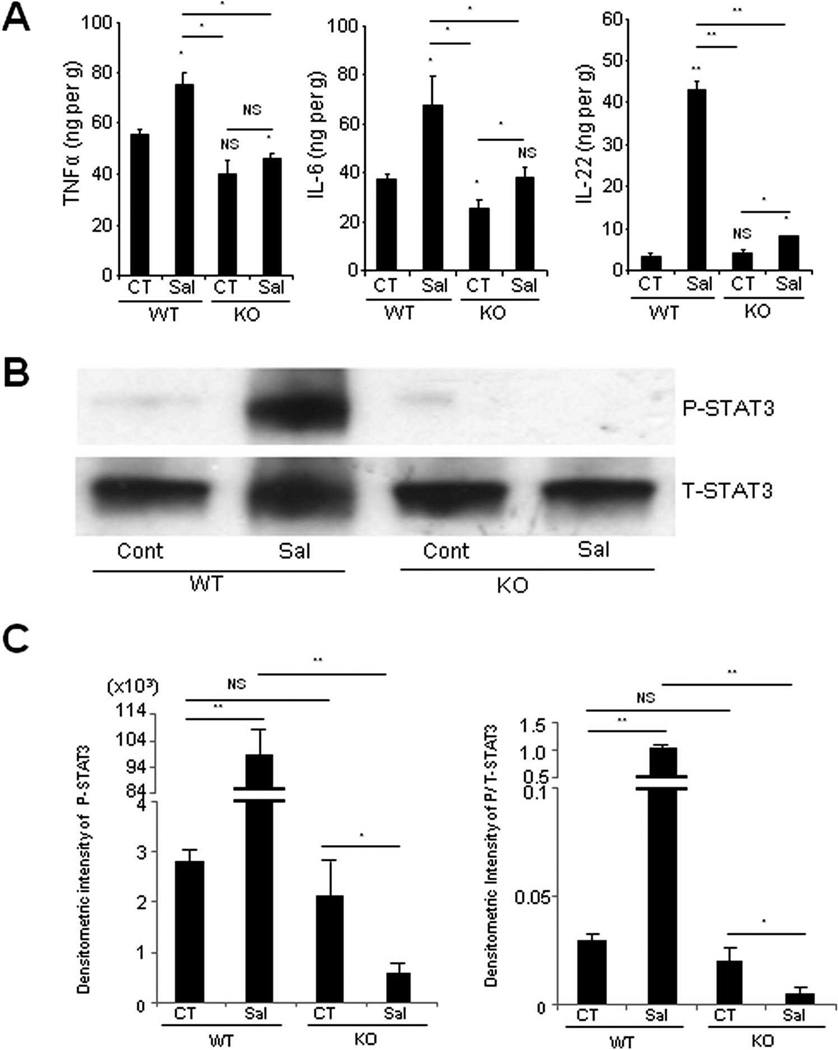
To investigate the differential cytokine milieu between S. typhimurium-infected- and uninfected- WT and CHI3L1 KO mice, the levels of TNFα, IL-6 and IL-22 in the large intestine were determined using ELISA. After S. typhimurium infection, significantly higher productions of all the above three cytokines were observed in WT mice as compared to CHI3L1 KO mice (Fig. 2A). Since both IL-6 and IL-22 are well-known activators of STAT3 signaling pathway, Western blot analyses were subsequently performed to determine STAT3 activation status in the large intestine with or without S. typhimurium infection. Surprisingly and unexpectedly, S. typhimurium-infected CHI3L1 KO mice did not show any evidence of STAT3 activation and the level was lower than the uninfected-WT and -CHI3L1 KO mice (Fig. 2B,C). In contrast, S. typhimurium-infected WT mice showed significantly higher levels of phosphorylated STAT3 as compared to the infected-CHI3L1 KO mice, suggesting that CHI3L1 is likely to be essential for STAT3 activation during S. typhimurium-mediated intestinal inflammation (Fig. 2B, C).
Fig. 2. CHI3L1 mediates STAT3 activation after Salmonella typhimurium infection.
(A) TNFα, IL-6, and IL-22 levels in large intestinal homogenates were analyzed by ELISA at 48 hours post-infection with (Sal) or without (CT) S. typhimurium. Each value represents the average of six mice in each group. *P<.05, **P<.01 (control vs. Salmonella-infected mice or between the indicated group). (B) Large intestinal samples, including cecum and colon, from the mice with or without S. typhimurium infection were lysed and 30 µg/lane of protein lysate was subjected to immunoblot using anti-phospho (P) or anti-total (T) -STAT3 antibodies. (C) Densitometry analysis of phospho-STAT3 signals (left) and phospho-STAT3 signals that were normalized to total-STAT3 signals (right) are shown. *P<.05, **P<.01 and NS, no significant difference (between the indicated groups).
CHI3L1 exacerbates AIEC-induced colitis with epithelial damage under low-dose DSS treatment
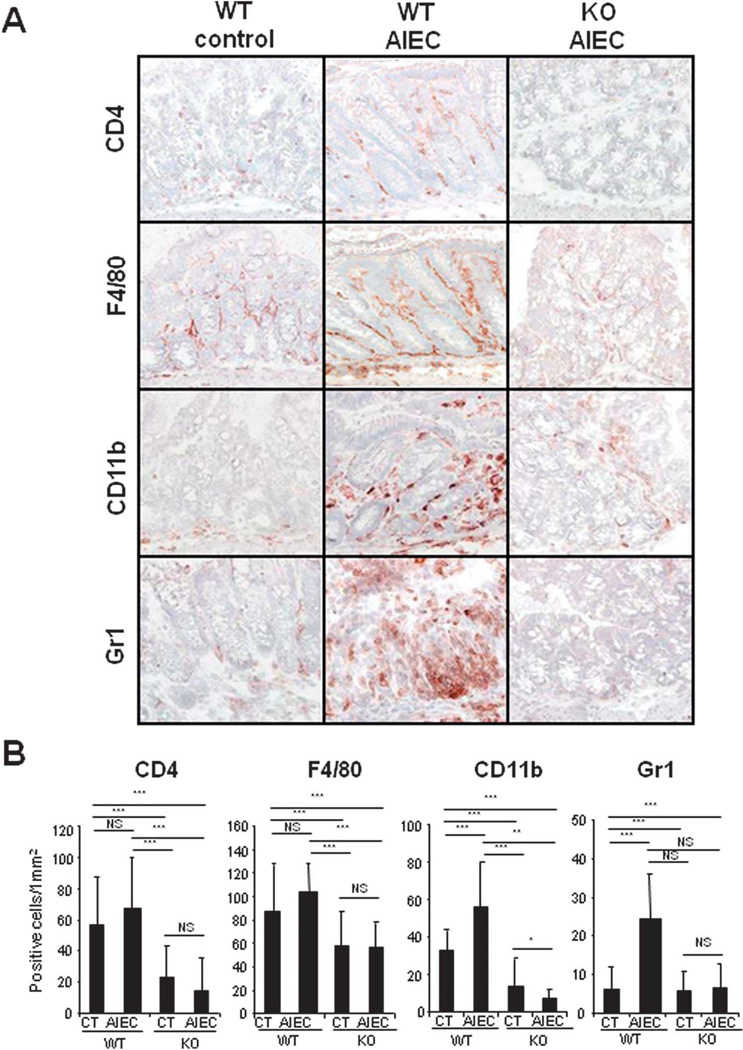
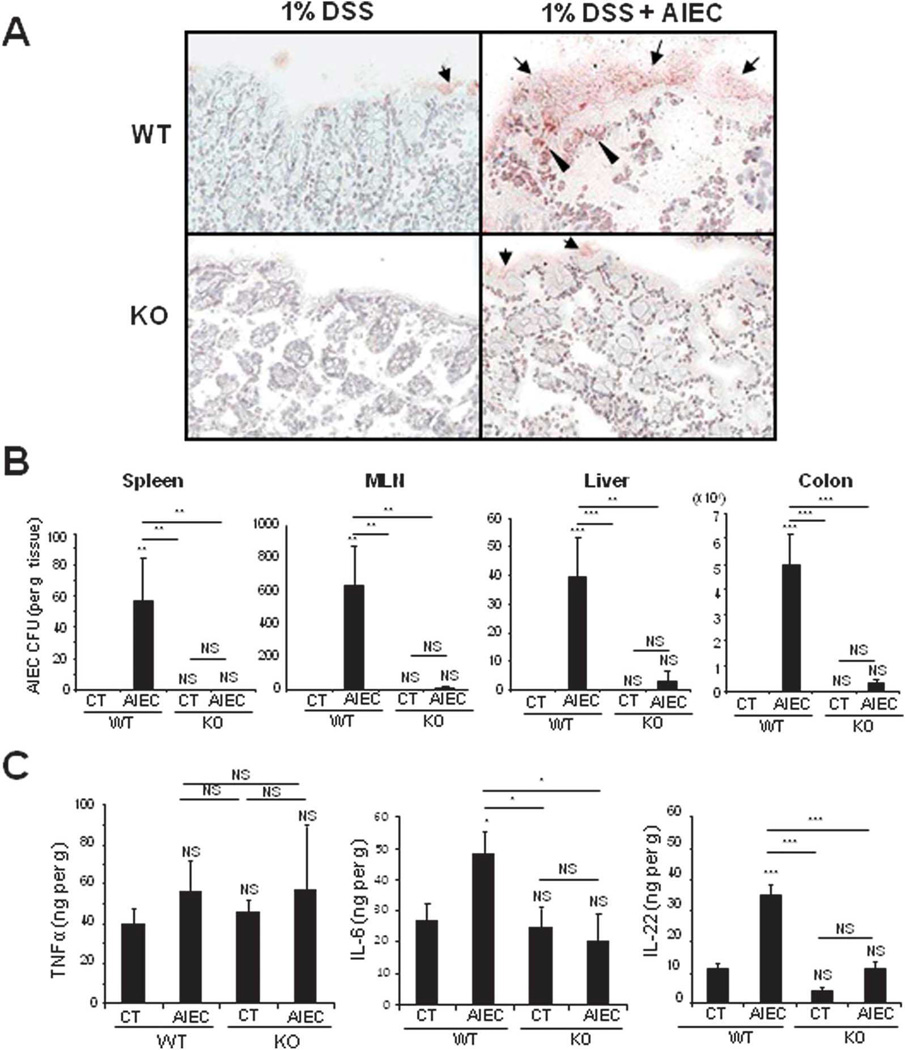
To further validate and confirm the essential role of CHI3L1 in bacterial infectious colitis, WT and CHI3L1 KO mice were subjected to 1% DSS treatment in the drinking water and orally inoculated with AIEC LF82 strain (108 CFU) daily for consecutive 14 days (Fig. 3A). AIEC-infected WT mice showed significant changes after day 7 in clinical scores, but only marginal changes in percent body weight loss, as compared to uninfected WT mice (Fig. 3B,C). In contrast, both uninfected and AIEC-infected CHI3L1 KO mice did not show any significant changes in body weight loss or clinical scores throughout the entire course of treatment (Fig. 3B,C). In addition, colonic histological evaluation also reflects high inflammation status of AIEC-infected WT mice as compared to AIEC-infected- or uninfected-CHI3L1 KO mice, characterized by massive cellular infiltration in lamina propria and disruption of colonic epithelial architecture (Fig. 3D,E). Furthermore, colonic immunohistochemical analysis with the quantification of CD4-, F4/80-, CD11b- and Gr1- positive cellular infiltration confirmed higher influx of these immune cells in AIEC-infected WT mice as compared to AIEC-infected/uninfected CHI3L1 KO mice or uninfected-WT mice (Fig. 4A,B and data not shown). Immunohistochemical staining of colonic sections using anti-E. coli LPS antibody showed high bacterial load in both CECs and lamina propria compartments of the AIEC-infected WT mice (Fig. 5A). In contrast, only a minute amount of E. coli was detected in the CHI3L1 KO AIEC-infected mice, whereas no apparent E. coli adhesion/invasion was detected in the either uninfected-WT or -CHI3L1 KO mice (Fig. 5A). The extent of AIEC translocation to the spleen, MLN, liver and colon was only observed in the AIEC-infected WT mice, but not in the other groups of mice (Fig. 5B). To characterize the differential cytokine milieu between AIEC-infected WT and CHI3L1 KO mice, total colonic TNFα, IL-6 and IL-22 levels were measured using ELISA. Although no significant difference in TNFα production was observed among the mice groups, IL-6 and IL-22 levels were significantly increased in AIEC-infected WT mice as compared to the AIEC-infected or non-infected CHI3L1 KO mice (Fig. 5C). All these evidence strongly suggest that the presence of CHI3L1 expression is paramount to confer AIEC-induced infectious colitis.
Fig. 3. CHI3L1 KO mice showed milder form of AIEC infectious colitis as compared to WT mice.
(A) Eight- to ten-week-old C57Bl/6 WT or CHI3L1 KO mice (n=6 in each group) were treated with 1% DSS in drinking water for 14 days, and were orally challenged daily with 108 AIEC in 0.5% carboxymethyl cellulose (CMC) during the same period. (B, C) Percentage of body weight (B) and clinical score (C) of mice orally challenged with CMC alone (open diamonds for WT, open circles for KO) or CMC containing 108 AIEC LF82 strain (red diamonds for WT, closed circles for KO) are shown. #P<.05, WT-AIEC group as compared to WT-control group; *P<.05, **P<.001, WT-AIEC group as compared to KO-AIEC group. (D, E) Representative H&E images (10× objective) (D) or histological score (E) of the colon obtained from 1% DSS-treated non-infected (control) or AIEC LF82-infected (AIEC) WT and CHI3L1 KO mice (n=6 in each group). *P<.01, **P<.001 and NS, no significant difference.
Fig. 4. Higher inflammatory cells infiltration in WT-AIEC infected mice compared CHI3L1 KO AIEC infected mice.
(A) Representative immunohistochemical images of colonic cryo-sections obtained from 1% DSS-treated non-infected or AIEC LF82 infected WT and CHI3L1 KO mice (n=6 in each group) stained with anti-CD4, anti-F4/80, anti-CD11b, and anti-Gr1 antibodies are shown (10× objective) (B) Number of positively stained cells were quantified per mm2 at 40× objective. *P<.05, **P<.01, ***P<.001 and NS, no significant difference.
Fig. 5. CHI3L1 KO mice are less susceptible to AIEC infection and produce lower amounts of IL-6 and IL-22.
(A) Colonic cryo-sections of 1% DSS treated non-infected or AIEC LF82 infected WT and CHI3L1 KO mice were stained with anti-Escherichia coli LPS-specific antibody at day 14 post-infection. (10× objective) (B, C) Quantification of ampicillin resistant AIEC LF82 bacteria in non-infected (CT) or infected (AIEC) WT and Brp39 KO mice was examined. Organ load of LF82 in spleen, mesenteric lymph nodes (MLN), liver, and colon (B) and productions of TNFα, IL-6, and IL-22 in colon (C) are shown among the above groups of mice (n=6 in each group). *P<.05, **P<.01 and NS, no significant difference.
CHI3L1 expression is essential for both DSS- and AIEC-induced STAT3 activation
Since both IL-6 and IL-22 are strong STAT3 activators, the status of colonic STAT3 phosphorylation in the AIEC-infected mice was next determined. Immunoblotting analyses using anti-phospho- and -total-STAT3 antibodies clearly display diminished STAT3 activation in AIEC-infected- and uninfected-CHI3L1 KO mice as compared to the WT mice groups (Fig. 6A,B), suggesting that CHI3L1 is directly or indirectly indispensable for STAT3 activation during the pathogenesis of AIEC-induced acute colitis. Although there is a significant difference (p<0.05) in phospho-STAT3 levels between AIEC-infected- and uninfected-WT mice, at least for the consecutive 14 days infection, AIEC infection is not so effective and strong activator for STAT3 signal in colon (Fig. 6B). Presumably, low dose DSS treatment may induce the nearly saturated level of STAT3 activation in colon (Fig. 6A). It is also interesting to note that the low-dose DSS-stimulated STAT-3 activation was also similarly abrogated in the absence of CHI3L1.
Fig. 6. Aberration of STAT3 activation in CHI3L1 KO mice.
(A) Colonic samples from WT (n=6 in each group) or CHI3L1 KO mice (n=6 in each group) without DSS treatment, shown as DSS(−), or with (AIEC)/without (CT) AIEC LF82 infection with treating low-dose DSS were lysed and 30 µg/lane of protein lysate was subjected to immunoblot with anti-phospho (P) or anti-total (T) -STAT3 antibodies. (B) Densitometry analysis of phospho-STAT3 signals (left) and phospho-STAT3 signals normalized to total STAT3 signals (right) is shown. *P<.05, **P<0.01, ***P<.001 and NS, no significant difference (between the indicated groups).
CHI3L1 and IL-6, but not IL-22, synergistically activates STAT3
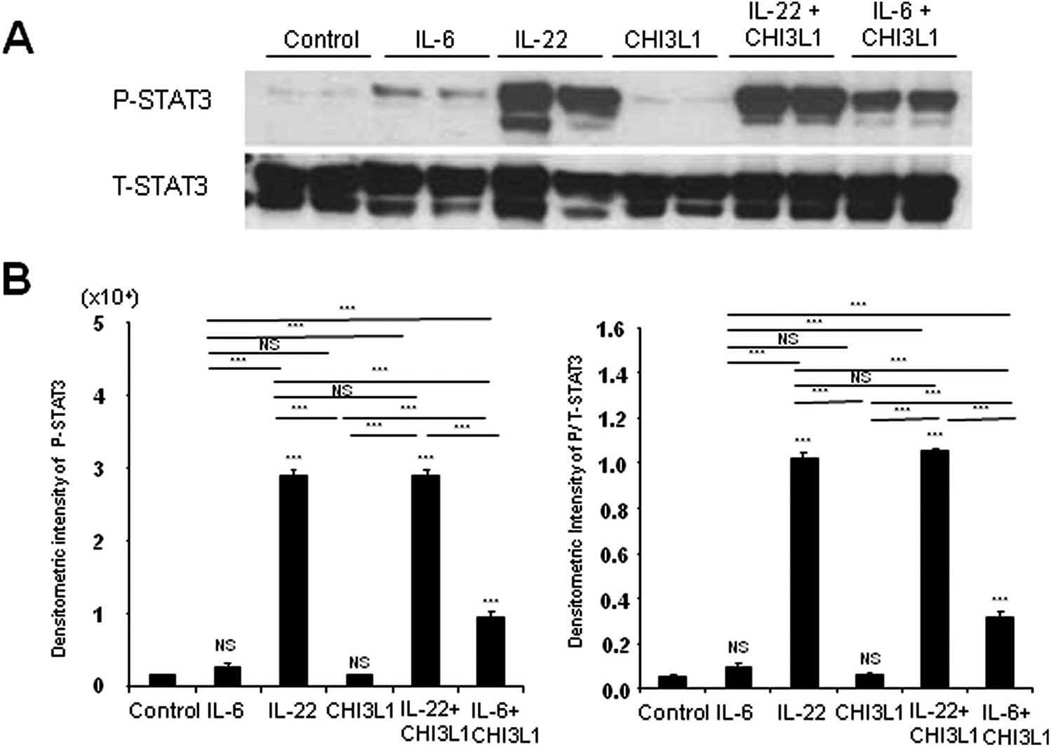
Since IL-6 and IL-22 both activate STAT3, and that this activation is impaired in the absence of CHI3L1, the role of these two cytokines in STAT3 activation, relative to CHI3L1, was next dissected. CECs that were isolated from WT and CHI3L1 KO mice were stimulated with IL-6, IL-22 or CHI3L1 independently. Results showed that IL-6 and IL-22, but not CHI3L1, confer the ability to activate STAT3 in both WT and CHI3L1 KO mice-derived CECs [Fig. 7A,B). In a dose dependent manner, IL-22 or IL-6 mono-stimulation enhances STAT3 phosphorylation in CHI3L1 KO-derived CECs (Supporting Fig. 1 and data not shown). However, co-stimulation of IL-6 and CHI3L1 resulted in a significantly increased STAT3 activation as compared with IL-6 mono-stimulated CHI3L1 KO-derived CECs (Fig. 7A,B). In contrast, co-stimulation of IL-22 and CHI3L1 did not yield significant differences when compared with mono-stimulation with IL-22 alone in those cells. Furthermore, IL-6 siRNA treatment efficiently inhibits the TNFα-mediated endogenous CHI3L1 mRNA expression in SW480 CEC line (Supporting Fig. 2). These data suggest that IL-6 and CHI3L1 have cooperative and synergistic effects in activating STAT3, whereas IL-22-mediated STAT3 activation is independent of CHI3L1 in CECs. Colonic epithelial-derived IL-6 is likely to be partially involved in the induction as well as activation of endogenous CHI3L1 expression in mucosal tissues during inflammatory conditions.
Fig. 7. CHI3L1 synergistically enhances the IL-6-mediated STAT3-phosphorylation in CHI3L1 KO mice-derived colonic crypt cells.
(A) Colonic crypt cells were freshly isolated from 8–10 weeks old CHI3L1 KO mice and stimulated with recombinant mouse IL-6 (100 ng/ml), recombinant mouse IL-22 (10 ng/ml) and/or purified human CH3L1 (80 ng/ml) for 15 minutes at 37°C with 5% CO2. After stimulations, the samples were lysed and 30 µg/lane of protein lysate was subjected to immunoblot with anti-phospho (P) or anti-total (T) -STAT3 antibodies. (B) Densitometry analysis of phospho-STAT3 signals (left) and phospho-STAT3 signals normalized to total STAT3 signals (right) is shown. *P<.05, **P<.01, and NS, no significant difference (versus control or between the indicated groups).
NF-κB is required for IL-6 and CHI3L1-mediated STAT3 synergistic activation
Since NF-κB is an important master regulator of inflammatory processes, its potential role of both IL-6- and CHI3L1-mediated STAT3 activation was next examined. CHI3L1 KO-derived CECs that were treated with NF-κB inhibitor CAPE, but not ERK1/2 inhibitor U0126, resulted in the suppression of basal phospho-STAT3 level (Fig. 8A,B). Strikingly, IL-6- and CHI3L1-mediated STAT3 phosphorylation was significantly suppressed by both CAPE and U0126, indicating that NF-κB and MAPK are paramount mediators of IL-6- and CHI3L1-mediated synergistic activation of STAT3 signaling pathway (Fig. 8A,B).
Fig. 8. CHI3L1-IL-6 mediated STAT3 activation is efficiently suppressed by NF-kB or ERK1/2 inhibitors in CHI3L1 KO-derived CECs.
(A) Freshly isolated colonic crypt cells from 8–10 week old CHI3L1 KO mice (n=6) were treated with or without NF-kB inhibitor (CAPE) or ERK1/2 inhibitor (U0126) followed by the stimulations with CHI3L1 and IL-6 proteins for 15 minutes at 37°C with 5% CO2. After stimulations, the samples were lysed and 30 µg/lane of protein lysate was subjected to immunoblot with anti-phospho (P) or anti-total (T) -STAT3 antibodies. (B) Densitometry analysis of phospho-STAT3 signals (left) and phospho-STAT3 signals normalized to total STAT3 signals (right) is shown. *P<.05, **P<.01, ***P<.001 and NS, no significant difference (versus untreated control or between the indicated groups between the indicated groups).
Discussion
The onset of bacterial infectious colitis first requires the pathogen to efficiently adhere on the surface of the mucosal epithelium, followed by the invasion into the lamina propria and submucosal compartments, thus triggering the initial flare of inflammation. We previously reported that CHI3L1 is an important inducible molecule that facilitates the adhesion and invasion of certain genre of bacteria, including S. typhimurium and AIEC [7]. In this report, we demonstrate that CHI3L1 deficient mice are more resistant towards acute infection of S. typhimurium- and AIEC-mediated intestinal inflammation, providing the most direct evidence of CHI3L1 involvement in the initiation and/or exacerbation of bacterial infectious colitis.
Following the bacterial adhesion and invasion, the manifestation of subsequent inflammation involves numerous host molecules in both the innate (including IECs and macrophages) and adaptive immune system which coordinates three major responses. The first mechanism is to recruit and activate immune cells to attack and clear the invading pathogen by releasing of a variety of factors. The second is to block apoptosis of host cells to protect them from the toxic environment as a result of the inflammatory process. The third mechanism is to heal and regenerate the colonic mucosa and finally terminates the inflammatory responses. CHI3L1 appears to participate in all three levels of the above described responses, functioning at the intersection of inflammation and tissue remodeling [10]. During inflammatory conditions, CHI3L1 expression is essential for mounting Th2-type immune responses, stimulating dendritic cells accumulation/activation and recruitment of macrophages in local inflammatory sites [11, 18]. CHI3L1 deficient mice also showed diminished IL-13-induced airway tissue inflammation [11]. In this current study, CHI3L1 KO mice developed less severe inflammatory responses at the acute phase of bacterial infectious colitis, characterized by milder histological scores, reduced immune cellular infiltrates and lower production of pro-inflammatory cytokines after S. typhimurium- or AIEC-infection.
CHI3L1 also appears to be highly involved in host cell protection against apoptosis and wound healing [10, 11]. This is likely achieved through the activation of NF-κB and PI3K/AKT pathways during IBD manifestation [12, 13]. In this study, we show that in the presence of IL-6, CHI3L1 efficiently activates colonic epithelial STAT3 signaling pathway. Like CHI3L1, STAT3 has been shown to possess both pathogenic and regulatory functions during the pathogenesis of IBD (Fig. 9). For instance, IL-6-mediated activation of STAT3 in CD4+ T cells has a primarily pathogenic role by preventing T cell apoptosis and thus perpetuating chronic intestinal inflammation [19]. In contrast, IL-22-mediated STAT3 activation in IECs induces regulatory cytokine IL-10 as well as mucin productions, which efficiently suppress acute colitis and exert regulatory control to the disease [20, 21]. Therefore, it is tempting to hypothesize that the synergistic STAT3 activation by IL-6 and CHI3L1 in IECs, demonstrated in our current study, also serves to enhance immune regulation under the harsh inflammatory environment. Interestingly, we also found that CHI3L1 has no additive effect on IL-22-mediated STAT3 activation. This result suggests that healing process in colonic epithelium-mediated by IL-22, including mucin production and mucosal repair, may be independent of CHI3L1.
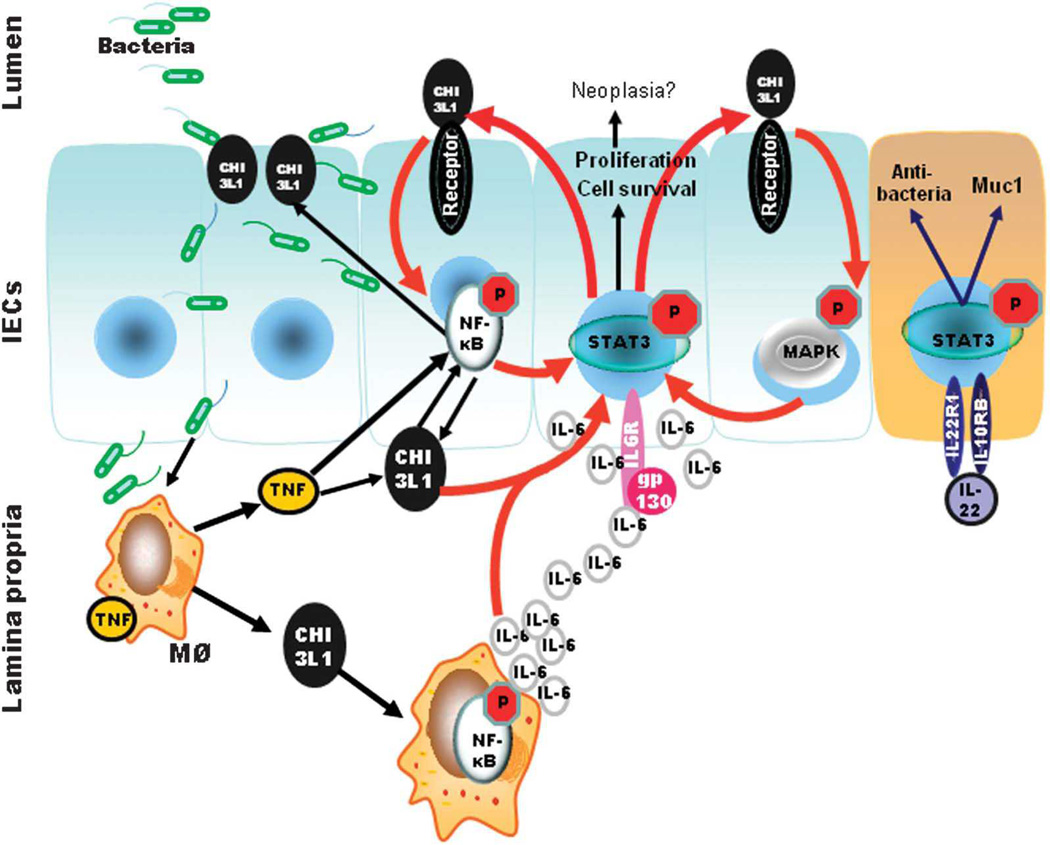
Fig. 9. Proposed schematic representation of CHI3L1 and IL-6-mediated STAT3 activation during gram-negative bacteria-induced intestinal inflammation.
Initial onset of gram-negative S. typhimurium and AIEC infection triggers CHI3L1 expression on IECs. Bacterial exploits the N-glycosylation residue on CHI3L1 to adhere to IECs and invade into the lamina propria. Exposure of bacterial stimulates macrophages to produce TNFα which further enhances CHI3L1 expression on both IECs and macrophages in an NF-kB dependent manner. IL-6 then activates STAT3 in adaptive immune cells (e.g. T cells) to confer pro-inflammatory roles to attack the invading pathogen, or in IECs to promote cell survival and anti-apoptotic effect. At this time, IL-6 mediated STAT3 activation in IECs induces CHI3L1 expression. CHI3L1 then activates NF-κB pathway (presumably via Myd88/IRAK) and MAPK pathway through specific surface receptor(s), which enhances STAT3 activation to further exert cell-survival and -proliferation. IL-6 also stimulates Th17 cells to produce IL-22, which also activates STAT3 to produce epithelial recovery, but in a CHI3L1 independent manner. These concerts of action orchestrate both bacterial clearance and wound healing during the pathogenesis of bacterial infectious colitis.
Another crucial factor in the understanding of IBD pathogenesis is how CHI3L1 expression is induced in the intestinal mucosa under inflammatory conditions. Major transcriptional factors, including SP1, C/EBP, ETS, AML-1 and CBF family transcription factors, have been identified to regulate CHI3L1 promoter and transcriptional activities [22]. In addition, NF-κB has been also shown to bind to CHI3L1 promoter cis elements in a TNFα specific response manner [23]. Inflammation-induced CHI3L1 efficiently activates the NF-κB signaling pathway and enhances the secretion of IL-8 and TNFα, suggesting an existence of positive feedback loop of pro-inflammatory cascade [13]. Interestingly, STAT3 has also been shown to directly activate CHI3L1 promoter and expression [24, 25]. Our current data showed that CHI3L1 activates STAT3, indicating that positive feedback loop also exists along this pathway. In mammalian epithelium, the STAT3-mediated induction of CHI3L1 appears to be restricted during the initial phase where inflammation predominates, but is upregulated during the secondary phase where wound healing is initiated [25]. Therefore, it is possible that the initial induction of CHI3L1 during disease onset is triggered through TNFα-mediated NF-κB activation, with the intention of activating immune responses. Following the NF-κB activation, STAT3 phosphorylation then may induce CHI3L1 expression to promote wound healing and epithelial restitution.
One has to be extremely cautious when defining CHI3L1 pathogenic or regulatory role during intestinal inflammation with bacterial infection. In this study, we demonstrate that CHI3L1 KO mice developed significantly less severe intestinal inflammation as compared to WT mice upon two types of gram negative bacterial infection. This is supported by previous report showing how CHI3L1 facilitates bacterial adhesion and invasion through specific CHI3L1 post-translational modification, as well as by reducing Th2-type inflammatory responses [7, 10]. However, in other bacterial genre infectious studies, CHI3L1 seemed to play a regulatory role. For instance, CHI3L1 KO mice developed exaggerated lung injury and inflammation when infected with gram positive Streptococcus pneumonia [26]. Therefore, the different mode of pathogenesis by different genre of pathogens may be critical when defining CHI3L1 roles. It is also possible that the lack of CHI3L1 function physiologically affects tissue repair and damage protection, resulting in an exacerbation of inflammatory condition in the long term. This hypothesis is also supported by a study on an experimental autoimmune encephalomyelitis model, in which inmunized-CHI3L1 KO mice developed no obvious phenotype at the initial stages of Mycobacterium tuberculosis infection, but showed more sever clinical disease as compared to the WT controls after 14 days of infection [27].
Finally, since pathogen-, cell-type- and temporal- specification appears to be crucial factor to resolve and dissect the precise role of CHI3L1, we propose the following simplified mechanism based on our current study as well as previous consolidated reports (Fig. 9). During the onset of inflammation by specific type of pathogen, CHI3L1 expression is induced on the IECs and facilitates the adhesion and invasion of these bacteria through the N-glycosylated residue on CHI3L1 [28]. The bacteria then invade into the lamina proprial compartment, where it activates macrophages to produce TNFα. This proinflammatory factor activates NF-κB, which then binds with CHI3L1 promoter to further regulate its expression in mucosal cells [23]. The CHI3L1 secreted by macrophages then activates NF-kB on the IECs, as well as macrophages to produce IL-6, which activates STAT3 signaling pathway on the IECs to promote cell survival and proliferation. At the same time, during the course of bacterial infection, Th17 cells produce IL-22 that exerts regulatory effects through anti-bacterial protein and mucin productions. Simultaneously, IL-6 activates STAT3 in the immune cells, particularly CD4+ T cells, to further mediate pro-inflammatory response to attack and clear the invading pathogen. The activated STAT3 in the IECs produce a positive feedback loop by activating CHI3L1 promoter and expression to further promote healing. As a result of successful epithelial healing and restitution, fewer bacteria can reach to the lamina proprial and submucosal compartments. The immune cells then clear off the invading pathogen(s), concluding the infection episode.
In conclusion, here we show that CHI3L1 is required for mediating selected gram negative bacterial infectious colitis. In addition, we also demonstrate that CHI3L1 and IL-6, but not IL-22, synergistically enhances STAT3 phosphorylation that is associated with the activations of NF-κB- and MAPK-pathways. These data will provide further insights into the multi-functions of CHI3L1 in mediating inflammation and tissue remodeling.
Supplementary Material
Acknowledgement
The authors are grateful to Drs. Victoria Llado and Tsuyoshi Mishiro for helpful discussions, advices and/or data analyses and to Drs. Anthony J Coyle and Alexander Kozhick for providing us the CHI3L1 KO mice. The authors would like to thank to Mardoniya Moghimi, Cindy W. Lau, Jiang Zhang, Manasa Kanneganti and Radhika Santhanam for their technical assistance.
Sources of support: This work has been supported by National Institute of Health (R01-DK80070), and grants from the Broad Medical Foundation and American Gastroenterological Association Foundation to EM. DL has been awarded the fellowship grant supported by A*STAR Graduate Academy (Singapore) and IAL was supported by the National Research Foundation of Korea.
Footnotes
Disclosure
The authors declared no conflict of interest.
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Darfeuille-Michaud A. Adherent-invasive Escherichia coli: a putative new E. coli pathotype associated with Crohn's disease. Int J Med Microbiol. 2002;292:185–193. doi: 10.1078/1438-4221-00201. [DOI] [PubMed] [Google Scholar]
- 3.Eckmann L. Animal models of inflammatory bowel disease: lessons from enteric infections. Ann NY Acad Sci. 2006;1072:28–38. doi: 10.1196/annals.1326.008. [DOI] [PubMed] [Google Scholar]
- 4.Que JU, Hentges DJ. Effect of streptomycin administration on colonization resistance to Salmonella typhimurium in mice. Infect Immun. 1985;48:169–174. doi: 10.1128/iai.48.1.169-174.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthel M, Hapfelmeier S, Quintanilla-Martinex L, et al. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glasser AL, Boudeau J, Barnich N, et al. Adherent invasive Escherichia coli strains from patients with Crohn's disease survive and replicate within macrophages without inducing host cell death. Infect Immun. 2001;69:5529–5537. doi: 10.1128/IAI.69.9.5529-5537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizoguchi E. Chitinase 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology. 2006;130:398–411. doi: 10.1053/j.gastro.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Sancho-Shimizu V, Khan R, Mostowy S, et al. Molecular genetic analysis of two loci (Ity 2 and Ity3) involved in the host response to infection with Salmonella typhimurium using congenic mice and expression profiling. Genetics. 2007;177:1125–1139. doi: 10.1534/genetics.107.075523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawada M, Chen CC, Arihiro A, et al. Chitinase 3-like-1 enhances bacterial adhesion to colonic epithelial cells through the interaction with bacterial chitin-binding protein. Lab Invest. 2008;88:883–895. doi: 10.1038/labinvest.2008.47. [DOI] [PubMed] [Google Scholar]
- 10.Lee CG, Da Silva CA, Dela Cruz CS, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CG, Hartl D, Lee GR, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009;206:1149–1166. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CC, Llado V, Eurich K, et al. Carbohydrate-binding motif in chitinase 3-like 1 (CHI3L1/YKL-40) specifically activates Akt signaling pathway in colonic epithelial cells. Clin Immunol. 2011;140:268–275. doi: 10.1016/j.clim.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CC, Pekow J, Llado V, et al. Chitinase 3-like-1 expression in colonic epithelial cells as a potentially novel marker for colitis-associated neoplasia. Am J Pathol. 2011;179:1494–1503. doi: 10.1016/j.ajpath.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boudeau J, Glasser AL, Masseret E, et al. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect Immun. 1999;67:4499–4509. doi: 10.1128/iai.67.9.4499-4509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mombaerts P, Mizoguchi E, Grusby MJ, et al. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 16.Mizoguchi E, Hachiya Y, Kawada M, et al. TNF receptor type I-dependent activation of innate responses to reduce intestinal damage-associated mortality. Gastroenterology. 2008;134:470–480. doi: 10.1053/j.gastro.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 17.Mizoguchi E, Mizoguchi A, Takedatsu H, et al. Role of tumor necrosis factor receptor 2 (TNFR2) in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology. 2002;122:134–144. doi: 10.1053/gast.2002.30347. [DOI] [PubMed] [Google Scholar]
- 18.Kawada M, Seno H, Kanda K, et al. Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene. 2012;31:3111–3123. doi: 10.1038/onc.2011.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto K, Ogawa A, Mizoguchi E, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagalakshmi ML, Rascle A, Zurawski S, et al. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int Immunopharmacol. 2004;4:679–691. doi: 10.1016/j.intimp.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Rehli M, Niller HH, Ammon C, et al. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. J Biol Chem. 2003;278:44058–44067. doi: 10.1074/jbc.M306792200. [DOI] [PubMed] [Google Scholar]
- 23.Recklies AD, Ling H, White C, et al. Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J Biol Chem. 2005;280:41213–41221. doi: 10.1074/jbc.M510146200. [DOI] [PubMed] [Google Scholar]
- 24.Singh SK, Bhardwaj R, Wilczynska KM, et al. A complex of nuclear factor I-X3 and STAT3 regulates astrocyte and glioma migration through the secreted glycoprotein YKL-40. J Biol Chem. 2011;286:39893–39903. doi: 10.1074/jbc.M111.257451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes K, Wickenden JA, Allen JE, et al. Conditional deletion of Stat3 in mammary epithelium impairs the acute phase response and modulates immune cell numbers during post-lactational regression. J Pathol. 2012;227:106–117. doi: 10.1002/path.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dela Cruz CS, Liu W, He CH, et al. Chitinase 3-like-1 promotes Streptococcus pneumoniae killing and augments host tolerance to lung antibacterial responses. Cell Host Microbe. 2012;12:34–46. doi: 10.1016/j.chom.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonneh-Barkay D, Wang G, Laframboise WA, et al. Exacerbation of experimental autoimmune encephalomyelitis in the absence of breast regression protein 39/chitinase 3-like 1. J Neuropathol Exp Neurol. 2012;71:948–958. doi: 10.1097/NEN.0b013e31826eaee7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Low D, Tran HT, Lee IA, et al. Chitin-binding domains of Escherichia coli chiA mediate interactions with intestinal epithelial cells in mice with colitis. Gastroenterology. 2013;145:602–612. doi: 10.1053/j.gastro.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.