Abstract
Purpose: To evaluate the effects of different drug-delivery techniques for levofloxacin (LVFX) in ocular penetration and the prevention of endophthalmitis using an aphakic rabbit model with posterior capsule rupture (PCR).
Methods: LVFX was administered to aphakic rabbit eyes with or without PCR using eye drops (EDs), subconjunctival injection (SCI), or intracameral (IC) injection. The concentration of the drug in the vitreous and aqueous humors was estimated at 2 h after injection. In another study, aphakic rabbit eyes with PCR were inoculated with Enterococcus faecalis, immediately followed by 0.5% LVFX ED, 0.5% moxifloxacin (MFLX) ED, LVFX IC (500 μg/0.1 mL), or IC saline. EDs were administered 0, 3, and 6 h after surgery. Changes on electroretinography (ERG) and intraocular bacterial growth were determined sequentially until 48 h after inoculation.
Results: The concentrations of LVFX at 2 h after IC were higher in the aqueous humor and the vitreous cavity of eyes with or without PCR, compared with EDs or SCI. Eyes treated with LVFX ED, MFLX ED, or IC saline showed a significantly greater reduction in b-wave amplitude on ERG at 48 h compared with eyes treated with LVFX IC. The number of bacteria recovered from the vitreous humor in eyes treated with IC LVFX at 48 h was significantly less than from eyes that received other treatments.
Conclusion: The LVFX IC was effective at suppressing endophthalmitis caused by E. faecalis in eyes with a PCR.
Introduction
Bacterial endophthalmitis is one of the most severe and sight-threatening complications of cataract surgery. The incidence of postoperative endophthalmitis has decreased (from 0.20% to 0.04%),1,2 mainly because of the introduction of new surgical techniques.3 However, an invariably sight-threatening infection is often resolved with substantial visual loss, and prophylaxis for endophthalmitis should be considered, given its pathogenesis. The external bacterial flora probably enters the anterior chamber through the surgical wound; in fact, contamination of the anterior chamber at the end of surgery has been noted in as many as 5.7%–21.1% of cases.4–7 Moreover, bacterial migration from the anterior chamber to the posterior chamber is a key event in the progression of postoperative endophthalmitis; in the posterior segment, severe retinal damages may result. Rupture of the posterior capsule during surgery leads to a significantly higher incidence of postoperative endophthalmitis8,9 because aqueous humor contaminants can readily access the vitreous cavity. Several procedures have been attempted to reduce bacterial contamination of the eye and to prevent endophthalmitis.10 Some reports have shown that intracameral (IC) antibiotics are effective in the prevention of endophthalmitis.11,12 Along with IC antibiotics, antibiotic eye drops (EDs) may be used to prevent endophthalmitis. Generally, topical antibiotics are administered immediately after surgery and on the following day as postoperative prophylaxis for endophthalmitis. Wallin et al.13 reported that starting topical antibiotic administration on the day after surgery rather than on the day of surgery was associated with an increased risk of endophthalmitis. These results suggest that bacteria proliferate and express virulence factors as early as 1 day after surgery. Indeed, we previously reported that immediate postoperative prophylaxis using a moxifloxacin (MFLX) ophthalmic solution reduced the risk of Enterococcus faecalis-caused endophthalmitis in a rabbit model.14 Further, Colleaux and Hamilton15 demonstrated that prophylactic subconjunctival antibiotic injections at the conclusion of cataract surgery decreased the incidence of postoperative endophthalmitis.
Thus, antibiotic administration at the end of cataract surgery could be effective for the prevention of endophthalmitis. However, little is known about the efficacy of antibiotics in eyes with posterior capsule rupture (PCR), which is a major risk factor for endophthalmitis. Moreover, the penetration of antibiotics using different drug-delivery techniques into the anterior or posterior segment of eyes with or without a ruptured posterior capsule has not been well documented.
This study was designed to investigate the pharmacokinetics of levofloxacin (LVFX) in the anterior chamber or vitreous cavity after the use of various drug-delivery techniques and the efficacy of antibiotics for the prophylaxis of postoperative endophthalmitis in eyes with PCR.
Materials and Methods
Antibiotics and bacteria
A 0.5% LVFX ophthalmic solution (Cravit; Santen Pharmaceutical, Co., Ltd., Osaka, Japan) and 0.5% MFLX ophthalmic solution (Vigamox; Alcon Japan Ltd., Tokyo, Japan) were purchased from their respective manufacturers. The laboratory strain of E. faecalis OG1S, which produces a secretory protease, was used.16 The minimum inhibitory concentrations (MICs) of LVFX and MFLX against OG1S were 2.0 and 0.5 μg/mL, respectively. The bacteria were grown in brain heart infusion (Difco Laboratories, Detroit, MI) broth for 18 h at 37°C and then washed twice with sterile physiological saline and resuspended in sterile physiological saline. The concentration of bacteria in the suspension was determined spectrophotometrically and then adjusted to ∼2×105 colony-forming units/mL (CFU/mL) with sterile physiological saline.
Animals
Female Japanese albino rabbits, weighing 2 kg each (Kitayama Labes Co. Ltd., Nagano, Japan), were maintained in accordance with Institutional Animal Care and Use Committee guidelines and the Association for Research in Vision and Ophthalmology Statement for the Use of Laboratory Animals in Ophthalmic and Vision Research. All procedures involving rabbits were approved by the Committee of Animal Experimentation, Ehime University School of Medicine (Matsuyama, Japan).
The rabbits were anesthetized with an intramuscular injection of an equal mixture of 5% ketamine (Ketalar intramuscular, 500 mg; Sankyo Co., Ltd., Tokyo, Japan) and 2% xylazine (Selactar; Bayer Ltd., Tokyo, Japan) at 1 mL/kg for all procedures. The rabbits were euthanized with an overdose of pentobarbital sodium.
Lensectomy
A lensectomy was performed on both eyes as described previously.16 A clear corneal incision was performed, and the lens was extracted with a Phacompo Phacoemulsificator (Santen Pharmaceutical Co., Ltd.) using balanced salt solution (BSS plus; Alcon, Fort Worth, TX) for irrigation, and the incision was sutured with 10-0 nylon. Intentional rupture of the lens capsule was made in some cases using phaco tips.
Intraocular penetration of LVFX
After lensectomy with or without intentional rupture of the posterior capsule, a single dose of 0.5% LVFX ophthalmic solution was administered as ED (50 μL), a subconjunctival injection (SCI; 100 μL), or an IC injection (100 μL). The pharmacokinetics of LVFX were then investigated. The eyes (n=5 per group) were enucleated 0 or 2 h after the lensectomy. The aqueous humor was collected with a 23-gauge needle. The eyeball was rinsed in sterile saline, immediately dipped in liquid nitrogen, and frozen. The regions from the corneal limbus to the posterior segment of the frozen eyes were dissected and separated into 3 equal parts using a razor. After dissection, only the vitreous humor was collected to avoid contamination with other tissues, weighed, and stored at −80°C. The concentrations of LVFX in the aqueous and vitreous humors were determined by high-performance liquid chromatography (HPLC). Briefly, for the assay of LVFX, internal standard (lomefloxacin) and 0.2% acetic acid solution were added to the sample, and the mixture was extracted by solid-phase extraction (Oasis MAX; Waters Corporation, Milford, MA). The extract was injected into an HPLC system equipped with an analytical column (ACQUITY UPLC BEH Phenyl 1.7 μm, 100×2.1 mm i.d.; Waters Corporation) and the fluorescence intensity of LVFX was detected. The concentrations in the aqueous humor (μg/mL) and vitreous humor (ng/g) were calculated using a calibration curve.
Prevention of experimental E. faecalis-induced endophthalmitis
After lensectomy with intentional rupture of the posterior capsule, 0.1 mL of OG1S strain was inoculated into the anterior chamber using a blunt needle on a 1-mL tuberculin syringe. The rabbits were then divided into 4 groups and treated with 0.5% LVFX ED, 0.5% MFLX ED, IC LVFX, or IC saline. In the LVFX or MFLX ED group, 0.5% LVFX or 0.5% MFLX ED (50 μL) was administered 0, 3, and 6 h after surgery. The IC injection of 0.5% LVFX or saline (100 μL) was performed at the end of surgery in the IC LVFX and saline groups, respectively. The course of infection in the eyes of each group was monitored using clinical scores, electroretinography (ERG), and the quantification of bacteria recovered from samples collected as described previously.16 Animals in which the study drug was not administered correctly or a sample was not collected accurately were excluded from the analysis.
Statistical analysis
Differences between the 2 groups were analyzed using Student's t-test. The Tukey–Kramer test was used for multiple comparisons. A P value of<0.05 was considered to indicate statistical significance.
Results
Intraocular penetration of LVFX
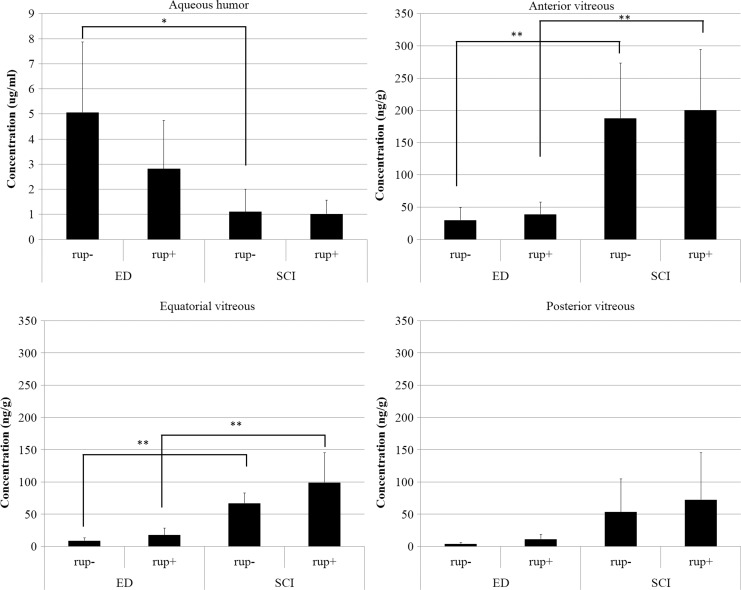
The concentrations of LVFX 2 h after the application of ED or SCI were measured and compared. In the aqueous humor of eyes treated with ED, the concentration of LVFX in the eyes without capsule rupture was higher than in the eyes with capsule rupture, although the difference was not statistically significant (P=0.18; Fig. 1). LVFX in the SCI group could similarly penetrate to the aqueous humor in eyes with or without capsule rupture (P=0.87; Fig. 1). The LVFX concentration in the ED group was significantly higher in the aqueous humor in eyes without capsule rupture than in the SCI group (P<0.05; Fig. 1). The LVFX concentration in the ED group, but not in the SCI group, could exceed the MIC against OG1S. In each part of the vitreous body, the LVFX concentration in the SCI group was significantly higher in eyes with or without PCR than in the ED group, except in the posterior vitreous (Fig. 1). No difference in LVFX concentration was found in eyes with and without rupture in the ED or SCI group (Fig. 1). However, the LVFX concentration in neither the ED nor the SCI group reached the MIC against OG1S in the vitreous.
FIG. 1.
Concentrations of LVFX in the aqueous and trisected vitreous (mean±standard deviation, n=5) at 2 h after a single instillation of ED or SCI. *P<0.05 **P<0.01. ED, eye drop; SCI, subconjunctival injection; rup, rupture. −, minus; +, positive.
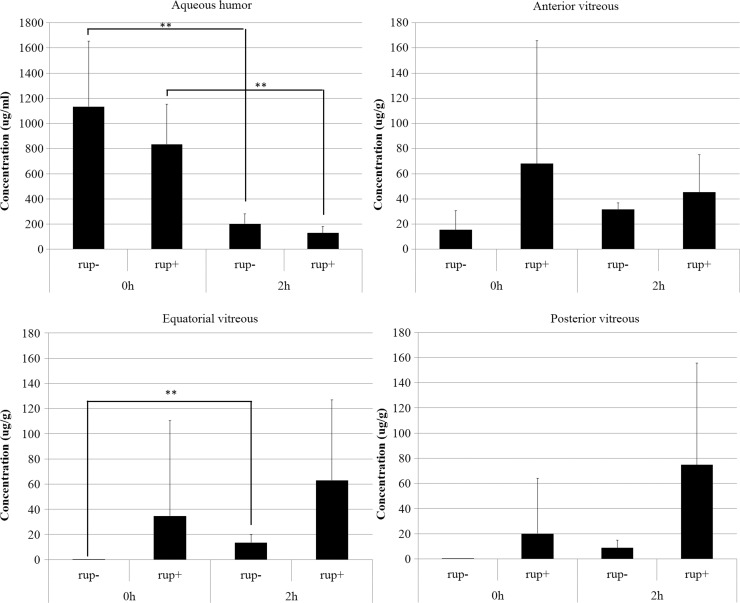
Next, we examined LVFX penetration in eyes treated with an IC injection of LVFX. In the aqueous humor, the LVFX concentration in eyes with or without capsule rupture was decreased from 0 to 2 h (P<0.05; Fig. 2). In each part of the vitreous, the LVFX concentration in eyes with rupture was higher than in eyes without rupture, although the differences were not statistically significant. The vitreous sustained high LVFX concentrations that greatly exceeded the MIC of OG1S for 2 h.
FIG. 2.
Concentrations of LVFX in the aqueous and trisected vitreous (mean±standard deviation, n=5) at 0 and 2 h after a single IC injection. **P<0.01. IC, intracameral. −, minus; +, positive.
Prevention of endophthalmitis
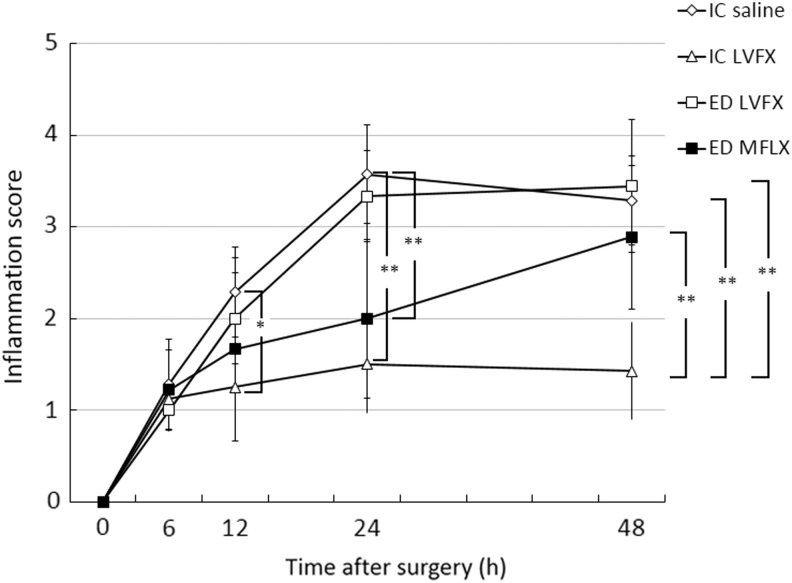
The eyes were examined at the designated times to assess their intraocular inflammation scores (Fig. 3). The mean scores in the IC LVFX group were significantly lower than those in the IC saline group at 12, 24, and 48 h (12 h: P<0.05; 24 and 48 h: P<0.001). There were significant differences in the intraocular inflammation scores between the IC LVFX group and ED group (LVFX and MFLX) at 48 h (P<0.001). The intraocular inflammation score in the MFLX ED group at 24 h was also significantly lower than that in the IC saline group (P<0.001).
FIG. 3.
Intraocular inflammation scores at the specified times after surgery and the induction of Enterococcus faecalis endophthalmitis. The data are the means±standard deviations (n=7–9). *P<0.05; **P<0.001. MLFX, moxifloxacin; LVFX, levofloxacin.
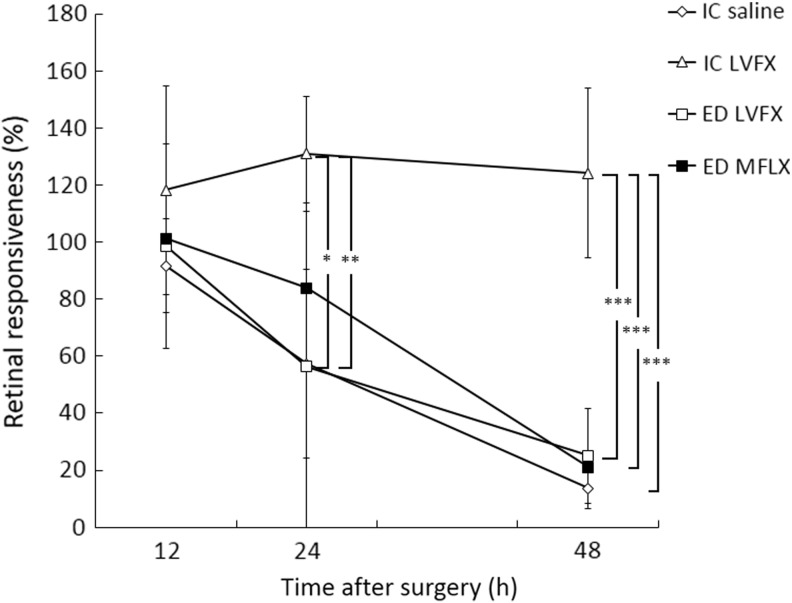
The retinal responsiveness of eyes infected with E. faecalis was determined by ERG (Fig. 4). Retinal function from 24 to 48 h was significantly greater in the IC LVFX group than in the IC saline (24 h: P<0.05; 48 h: P<0.001), LVFX ED (24 h: P<0.01; 48 h: P<0.001), and MFLX ED (48 h: P<0.001) groups, except in comparison to the MFLX ED group at 24 h. There was no significant difference in retinal responsiveness between the IC saline and ED groups (LVFX and MFLX) from 24 to 48 h.
FIG. 4.
Effects of antibiotics on electroretinographic measurements of retinal responsiveness at the specified times after surgery and the induction of E. faecalis endophthalmitis. The data are the means±standard deviations (n=7–9). *P<0.05; **P<0.01; ***P<0.001.
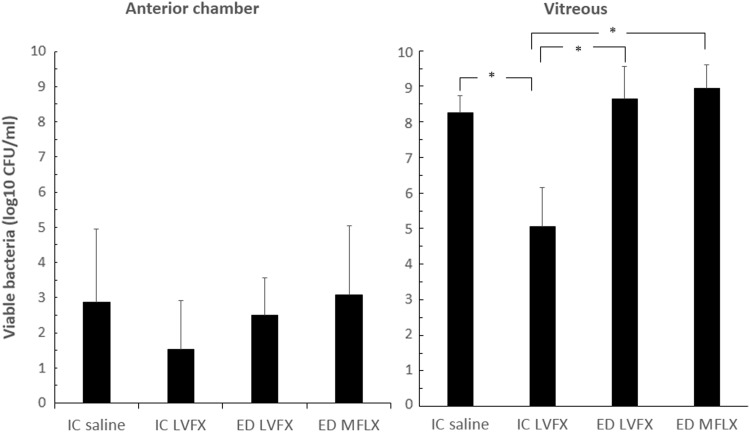
There was no significant difference in viable bacteria recovered from the anterior chamber among the 4 groups. In the vitreous, bacterial growth in the IC LVFX group (5.1±1.1 log10 CFU/mL) was significantly reduced compared with that in the IC saline (8.3±0.5 log10 CFU/mL; P<0.001), LVFX ED (8.7±0.9 log10 CFU/mL; P<0.001), and MFLX ED (8.9±0.7 log10 CFU/mL; P<0.001) groups (Fig. 5).
FIG. 5.
Effects of antibiotics on bacterial counts in the anterior chamber (left) and vitreous (right). The data are the means ±standard deviations (n=5–9). *P<0.001.
Discussion
Postoperative endophthalmitis is a severe complication of cataract surgery; however, it may be effectively prevented using antibiotics. Immediate postoperative antibiotic administration is essential to improve bacterial killing and prevent infection by blocking the rapid penetration of pathogens from the anterior chamber to the vitreous. Rupture of the posterior capsule can induce bacterial translocation from the anterior chamber to the vitreous. Some investigators have reported that cataract surgery without PCR does not allow substances in the aqueous humor to move into the vitreous humor because the posterior capsule17–19 and anterior vitreous membrane20 form a firm barrier. Thus, ocular penetration by postoperative antibiotics could differ between eyes with and without PCR. Because fluoroquinolone EDs have broad spectra of action and excellent ocular penetration, they are widely used for the postoperative prophylaxis of endophthalmitis.21 In our study, ocular penetration with different drug-delivery methods was compared using a 0.5% LVFX ophthalmic solution, which is widely used for surgical prophylaxis in Japan. This ophthalmic solution does not contain preservatives (e.g., boric acid and benzalkonium chloride) and the IC injection of LVFX was nontoxic in terms of the clinical toxicity score, corneal thickness, and cell viability.22 Although it is difficult to exactly compare drug penetration between ED and SCIs because of differences in the amount of drug administered (ED, 50 μL; SCI 100 μL), LVFX ED can penetrate the aqueous humor more efficiently than SCI. In contrast, the LVFX concentration following SCI in the vitreous was higher than that following ED delivery. This confirms previous data that show that a potential barrier to ED is diffusion through the cornea, and subconjunctival routes could use the permeability of the sclera to penetrate the posterior segment.23–28 Although not statistically significant, the LVFX concentration following ED delivery in the aqueous humor of eyes with PCR was lower than that in eyes without rupture. It is likely that the drug in the aqueous humor of eyes with PCR diffused to the posterior segment and therefore did not maintain a high concentration in the aqueous humor. In contrast, drug penetration with SCI would not be expected to be influenced by PCR because the drug could permeate through the sclera. The concentration of LVFX with ED or SCI at 2 h after administration was much lower than the MIC of E. faecalis (2 μg/mL). Because the 0.5% MFLX ophthalmic solution could penetrate the anterior chamber better than other fluoroquinolones,29–31 it may penetrate the vitreous in eyes with rupture. In our study, the IC injection of LVFX resulted in a high concentration of LVFX in the aqueous humor and vitreous of eyes with or without PCR. Although not statistically significant, LVFX could better penetrate the vitreous of eyes with rupture versus those without rupture following IC injection. This indicates that IC injection could be effective at inhibiting the proliferation of bacteria that translocate through the PCR to the vitreous cavity.
Previous studies have shown the efficacy of antibiotics for preventing endophthalmitis in animal models. Kowalski et al.32 demonstrated that ofloxacin and MFLX were more effective at preventing endophthalmitis than nonfluoroquinolone antibacterial agents in phakic rabbit eyes in which the anterior chambers were inoculated with Staphylococcus aureus. The SCI of a combination of triamcinolone and ciprofloxacin hydrochloride was useful for preventing endophthalmitis caused by S. aureus in phakic rabbit eyes.33 Moreover, IC MFLX was effective in preventing endophthalmitis in a phakic rabbit model after an S. aureus intravitreal challenge.34 However, these animal models may differ from actual postoperative endophthalmitis, where there is an anatomical barrier between the anterior chamber and vitreous cavity. Our model was established by inoculating pathogens into the anterior chamber with PCR after lensectomy, similar to an actual endophthalmitis case, especially those occurring after surgical complications (e.g., rupture). In this study, E. faecalis was used to create a model of endophthalmitis. Although E. faecalis is rarely a causative agent of clinical endophthalmitis, it can rapidly induce postoperative endophthalmitis, often within 2–4 days, and can cause substantial vision loss upon infection.35–37 Indeed, patients with E. faecalis-related endophthalmitis had the worst visual outcome in the Endophthalmitis Vitrectomy Study.38
We found that the IC injection of LVFX significantly reduced inflammation scores and bacterial counts in the vitreous humor and maintained retinal function in our aphakic rabbit model, compared with LVFX ED, MFLX ED, and IC saline treatment. These results indicate that the IC injection of antibiotics could be effective for preventing endophthalmitis in cataract surgery with PCR. We used E. faecalis, for which the MICs of MFLX and LVFX are 0.5 and 2.0 μg/mL, respectively. The MICs of MFLX against ocular isolates, including staphylococci and streptococci, were lower than those of LVFX and gatifloxacin.39,40 Thus, MFLX ED could be more effective in preventing endophthalmitis. Indeed, MFLX ED reduced the inflammation scores significantly at 24 h compared with IC saline. Because EDs were not administered from 6 h after surgery in this study, EDs could not inhibit bacterial growth in the anterior chamber or vitreous at 24 or 48 h. However, even EDs using MFLX could be less effective in preventing endophthalmitis in eyes with PCR because of reduced penetration to the vitreous and the existence of drug-resistant bacteria. We did not check the effect of IC MFLX for preventing endophthalmitis because we would like to reduce numbers of animal for experiment. Since IC MFLX can penetrate not only to the aqueous humor but also the vitreous in aphakic rabbit eyes,41 it could be effective in preventing experimental endophthalmitis in complex phacoemulsification surgery. Moreover Matsuura et al. demonstrated that IC MFLX administration in clinical study decreased the risk for endophthalmitis by 3-fold. Thus, IC MFLX could prevent endophthalmitis in our model as well as IC LVFX.42
The results of our study should be interpreted with care, considering its limitations. First, this study was conducted in a rabbit model with experimentally induced E. faecalis endophthalmitis. Although this closely resembles the clinical situation, it is not identical to it. Second, the formulations of MFLX and LVFX used in vivo were the same as those used in clinical practice. Thus, the effective intraocular concentrations in this animal model may exceed those achieved in humans, considering the differences in eye size.
In conclusion, the results of this study indicate that the IC injection of antibiotics is effective in preventing endophthalmitis in complex phacoemulsification surgery compared with antibiotic ED. Prospective clinical studies are needed to confirm the potency, efficacy, and safety of IC injections for the prevention and treatment of bacterial endophthalmitis.
Acknowledgments
This study was supported, in part, by a Grant-in-Aid for scientific research (KAKENHI) from the Japan Society for the Promotion of Science (KAKENHI: Grants-in-Aid for Young Scientists B, 24791858). Technical expertise for measurement of drug concentrations was supported by Santen Pharmaceutical Co., Ltd.
Author Disclosure Statement
The authors have no commercial or financial interests associated with this article.
References
- 1.Miller J.J., Scott I.U., Flynn H.W., Jr., Smiddy W.E., Newton J., and Miller D.Acute-onset endophthalmitis after cataract surgery (2000–2004): incidence, clinical settings, and visual acuity outcomes after treatment. Am. J. Ophthalmol. 139:983–987, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Oshika T., Hatano H., Kuwayama Y., Ogura Y., Ohashi Y., Oki K., Uno T., Usui N., and Yoshitomi F.Incidence of endophthalmitis after cataract surgery in Japan. Acta Ophthalmol. Scand. 85:848–851, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Yu C.Q., and Ta C.N.Prevention of postcataract endophthalmitis: evidence-based medicine. Curr. Opin. Ophthalmol. 23:19–25, 2012 [DOI] [PubMed] [Google Scholar]
- 4.John T., Sims M., and Hoffmann C.Intraocular bacterial contamination during sutureless, small incision, single-port phacoemulsification. J. Cataract Refractive Surg. 26:1786–1791, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Manners T.D., Chitkara D.K., Marsh P.J., and Stoddart M.G.Anterior chamber aspirate cultures in small incision cataract surgery. Br. J. Ophthalmol. 79:878–880, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sobaci G., Tuncer K., Tas A., Ozyurt M., Bayer A., and Kutlu U.The effect of intraoperative antibiotics in irrigating solutions on aqueous humor contamination and endophthalmitis after phacoemulsification surgery. Eur. J. Ophthalmol. 13:773–778, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Tervo T., Ljungberg P., Kautiainen T., Puska P., Lehto I., Raivio I., Jarvinen E., Kuusela P., and Tarkkanen A.Prospective evaluation of external ocular microbial growth and aqueous humor contamination during cataract surgery. J. Cataract Refractive Surg. 25:65–71, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Wong T.Y., and Chee S.P.The epidemiology of acute endophthalmitis after cataract surgery in an Asian population. Ophthalmology. 111:699–705, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Hatch W.V., Cernat G., Wong D., Devenyi R., and Bell C.M.Risk factors for acute endophthalmitis after cataract surgery: a population-based study. Ophthalmology. 116:425–430, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Vazirani J., and Basu S.Role of topical, subconjunctival, intracameral, and irrigative antibiotics in cataract surgery. Curr. Opin. Ophthalmol. 24:60–65, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Barry P., Seal D.V., Gettinby G., Lees F., Peterson M., and Revie C.W.ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery: Preliminary report of principal results from a European multicenter study. J. Cataract Refractive Surg. 32:407–410, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Romero-Aroca P., Mendez-Marin I., Salvat-Serra M., Fernandez-Ballart J., Almena-Garcia M., and Reyes-Torres J.Results at seven years after the use of intracamerular cefazolin as an endophthalmitis prophylaxis in cataract surgery. BMC Ophthalmol. 12:2, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallin T., Parker J., Jin Y., Kefalopoulos G., and Olson R.J.Cohort study of 27 cases of endophthalmitis at a single institution. J. Cataract Refractive Surg. 31:735–741, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Tasaka Y., Suzuki T., Kawasaki S., Uda T., Mito T., Uno T., and Ohashi Y.Moxifloxacin as postoperative prophylaxis for Enterococcus faecalis-induced endophthalmitis after cataract surgery in Aphakic rabbits. J. Ocul. Pharmacol. Ther. 29:403–409, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Colleaux K.M., and Hamilton W.K.Effect of prophylactic antibiotics and incision type on the incidence of endophthalmitis after cataract surgery. Can. J. Ophthalmol. 35:373–378, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Suzuki T., Wada T., Kozai S., Ike Y., Gilmore M.S., and Ohashi Y.Contribution of secreted proteases to the pathogenesis of postoperative Enterococcus faecalis endophthalmitis. J. Cataract Refractive Surg. 34:1776–1784, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki S., Suzuki T., Yamaguchi M., Tasaka Y., Shiraishi A., Uno T., Sadamoto M., Minami N., Naganobu K., and Ohashi Y.Disruption of the posterior chamber-anterior hyaloid membrane barrier during phacoemulsification and aspiration as revealed by contrast-enhanced magnetic resonance imaging. Arch. Ophthalmol. 127:465–470, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Ohrloff C., Schalnus R., Rothe R., and Spitznas M.Role of the posterior capsule in the aqueous-vitreous barrier in aphakic and pseudophakic eyes. J. Cataract Refractive Surg. 16:198–201, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Smith R.T., Campbell C.J., Koester C.J., Trokel S., and Anderson A.The barrier function in extracapsular cataract surgery. Ophthalmology. 97:90–95, 1990 [PubMed] [Google Scholar]
- 20.De Groot V., Hubert M., Van Best J.A., Engelen S., Van Aelst S., and Tassignon M.J.Lack of fluorophotometric evidence of aqueous-vitreous barrier disruption after posterior capsulorhexis. J. Cataract Refractive Surg. 29:2330–2338, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Fintelmann R.E., and Naseri A.Prophylaxis of postoperative endophthalmitis following cataract surgery: current status and future directions. Drugs. 70:1395–1409, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Choi J.A., and Chung S.K.Safety of intracameral injection of gatifloxacin, levofloxacin on corneal endothelial structure and viability. J. Ocul. Pharmacol. Ther. 25:425–431, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Barza M.Antibacterial agents in the treatment of ocular infections. Infect. Dis. Clin. of North Am. 3:533–551, 1989 [PubMed] [Google Scholar]
- 24.Behrens-Baumann W., and Martell J.Ciprofloxacin concentration in the rabbit aqueous humor and vitreous following intravenous and subconjunctival administration. Infection. 16:54–57, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Clements D.B., and Tailor V.A study of aqueous and serum levels of ceftazidime following subconjunctival administration. Br. J. Ophthalmol. 71:433–435, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geroski D.H., and Edelhauser H.F.Transscleral drug delivery for posterior segment disease. Adv. Drug Deliv. Rev. 52:37–48, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Prausnitz M.R., and Noonan J.S.Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J. Pharm. Sci. 87:1479–1488, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Starr M.B., and Lally J.M.Antimicrobial prophylaxis for ophthalmic surgery. Surv. Ophthalmol. 39:485–501, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Kim D.H., Stark W.J., O'Brien T.P., and Dick J.D.Aqueous penetration and biological activity of moxifloxacin 0.5% ophthalmic solution and gatifloxacin 0.3% solution in cataract surgery patients. Ophthalmology. 112:1992–1996, 2005 [DOI] [PubMed] [Google Scholar]
- 30.McCulley J.P., Caudle D., Aronowicz J.D., and Shine W.E.Fourth-generation fluoroquinolone penetration into the aqueous humor in humans. Ophthalmology. 113:955–959, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Solomon R., Donnenfeld E.D., Perry H.D., Snyder R.W., Nedrud C., Stein J., and Bloom A.Penetration of topically applied gatifloxacin 0.3%, moxifloxacin 0.5%, and ciprofloxacin 0.3% into the aqueous humor. Ophthalmology. 112:466–469, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Kowalski R.P., Romanowski E.G., Shanks R.M., and Mah F.S.The comparison of fluoroquinolones to nonfluoroquinolone antibacterial agents for the prevention of endophthalmitis in a rabbit model. J. Ocul. Pharmacol. Ther. 28:604–608, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardillo J.A., Paganelli F., Melo L.A., Jr., Silva A.A., Jr., Pizzolitto A.C., and Oliveira A.G.Subconjunctival delivery of antibiotics in a controlled-release system: a novel anti-infective prophylaxis approach for cataract surgery. Arch. Ophthalmol. 128:81–87, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Kowalski R.P., Romanowski E.G., Mah F.S., Yates K.A., and Gordon Y.J.Intracameral Vigamox (moxifloxacin 0.5%) is non-toxic and effective in preventing endophthalmitis in a rabbit model. Am. J. Ophthalmol. 140:497–504, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Chen K.J., Lai C.C., Sun M.H., Chen T.L., Yang K.J., Kuo Y.H., Chao A.N., and Wu W.C.Postcataract endophthalmitis caused by Enterococcus faecalis. Ocul. Immunol. Inflamm. 17:364–369, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Rishi E., Rishi P., Nandi K., Shroff D., and Therese K.L.Endophthalmitis caused by Enterococcus faecalis: a case series. Retina. 29:214–217, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Scott I.U., Loo R.H., Flynn H.W., Jr., and Miller D.Endophthalmitis caused by Enterococcus faecalis: antibiotic selection and treatment outcomes. Ophthalmology. 110:1573–1577, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Microbiologic factors and visual outcome in the endophthalmitis vitrectomy study. Am. J. Ophthalmol. 122:830–846, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Kowalski R.P., Dhaliwal D.K., Karenchak L.M., Romanowski E.G., Mah F.S., Ritterband D.C., and Gordon Y.J.Gatifloxacin and moxifloxacin: an in vitro susceptibility comparison to levofloxacin, ciprofloxacin, and ofloxacin using bacterial keratitis isolates. Am. J. Ophthalmol. 136:500–505, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Mather R., Karenchak L.M., Romanowski E.G., and Kowalski R.P.Fourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibiotics. Am. J. Ophthalmol. 133:463–466, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Asena L., Akova Y.A., Goktas M.T., Bozkurt A., Yasar U., Karabay G., and Demiralay E.Ocular pharmacokinetics, safety and efficacy of intracameral moxifloxacin 0.5% solution in a rabbit model. Curr. Eye Res. 38:472–479, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Matsuura K., Miyoshi T., Suto C., Akura J., and Inoue Y.Efficacy and safety of prophylactic intracameral moxifloxacin injection in Japan. J. Cataract Refractive Surg. 39:1702–1706, 2013 [DOI] [PubMed] [Google Scholar]