Abstract
Traumatic brain injury (TBI) is an international health concern with a complex pathogenesis resulting in major long-term neurological, neurocognitive, and neuropsychiatric outcomes. Although neuroinflammation has been identified as an important pathophysiological process resulting from TBI, the function of specific inflammatory mediators in the aftermath of TBI remains poorly understood. Granulocyte-macrophage colony-stimulating factor (GM-CSF) is an inflammatory cytokine that has been reported to have neuroprotective effects in various animal models of neurodegenerative disease that share pathological similarities with TBI. The importance of GM-CSF in TBI has yet to be studied, however. We examined the role of GM-CSF in TBI by comparing the effects of a lateral fluid percussion (LFP) injury or sham injury in GM-CSF gene deficient (GM-CSF-/-) versus wild-type (WT) mice. After a 3-month recovery interval, mice were assessed using neuroimaging and behavioral outcomes. All mice given a LFP injury displayed significant brain atrophy and behavioral impairments compared with those given sham-injuries; however, this was significantly worse in the GM-CSF-/- mice compared with the WT mice. GM-CSF-/- mice given LFP injury also had reduced astrogliosis compared with their WT counterparts. These novel findings indicate that the inflammatory mediator, GM-CSF, may have significant protective properties in the chronic sequelae of experimental TBI and suggest that further research investigating GM-CSF and its potential benefits in the injured brain is warranted.
Key words: : animal studies, cytokine, inflammation, models of injury, MRI, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is one of the leading causes of death and chronic neurological dysfunction worldwide.1 To date, there is no known effective pharmacological intervention to mitigate against the long-term adverse neurological outcomes of TBI, which is, in large part, because of the poor understanding of the pathophysiological events that occur during the post-TBI disease process.1,2 Neuroinflammation has been identified as an important and complex cascade after TBI.1,3,4 For example, previous studies have reported that neuroinflammation is initiated within minutes after TBI, can evolve over several months, and has both neuroprotective and neurotoxic effects.2–8 A number of neuroinflammatory factors have been implicated in this cascade including astrocytes, microglia, invading peripheral leukocytes, and numerous inflammatory cytokines. The exact functions and interactions between these various mediators in TBI are not well known, however, while other potentially important inflammatory factors remain unstudied.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is an inflammatory cytokine that was initially described as a hematopoietic growth factor produced by peripheral immune cells that promotes the generation, differentiation, and maturation of myeloid lineage cells.9,10 GM-CSF, however, has since been demonstrated to also have a function in the central nervous system because GM-CSF can cross the blood–brain barrier11,12 and both GM-CSF and its receptor are expressed by various brain cells.13,14 GM-CSF has also been reported to have neuroprotective effects in animal models of Alzheimer disease,15 Parkinson disease,16 stroke,14,17 spinal cord injury,18–20 and stab wound-induced brain injury,21 all of which bear pathological similarities to TBI. Furthermore, a post-mortem study of human brain tissue taken from acute TBI victims reported elevated levels of inflammatory cytokines including GM-CSF,22 and the modulation of GM-CSF in animal studies has been shown to alter other inflammatory cytokines involved in TBI, including tumor necrosis factor-alpha and interleukin-1 beta.23,24
Despite this implicating evidence, the role of GM-CSF in TBI has yet to be studied. Therefore, we examined the effect of experimental TBI in GM-CSF gene deficient (GM-CSF-/-) mice. GM-CSF-/- and wild type (WT) control mice were administered either a lateral fluid percussion (LFP) injury or sham injury. Considering that TBI is a progressive condition associated with long-term neurological disabilities and that inflammatory mechanisms evolve into chronic stages post-TBI, in this study, mice were given a 3-month recovery period to allow these long-term changes to manifest before undergoing neuroimaging, behavioral, and post-mortem assessment. We found that GM-CSF-/- mice given a LFP injury had reduced astrogliosis, worsened brain damage, and more severe behavioral impairments compared with their WT counterparts. These findings indicate that GM-CSF has an overall neuroprotective role against the long-term adverse outcomes after experimental TBI.
Methods
Mice
GM-CSF-/- mice were backcrossed onto the C57BL/6 background for 12 generations. C57BL/6 mice served as wild-type (WT) controls.25 A total of 42 male mice (20 GM-CSF-/- and 22 C57BL/6) were obtained from our on-site breeding colony at the Royal Melbourne Hospital Research Facility for use in this study. Mice were 8–12 weeks of age at the time of LFP injury, were housed individually under a 12 h light/dark cycle, and were given access to food and water ad libitum for the duration of the experiment. All experimental procedures were approved by the University of Melbourne Animal Ethics Committee.
Experimental groups
GM-CSF-/- and C57BL/6 mice were randomly assigned to receive either a sham injury or a LFP injury. Four mice died immediately post-LFP injury (two WT, two GM-CSF-/-). Thus, the study consisted of four experimental groups: WT mice+sham injury (WT+Sham, n=10); GM-CSF-/- mice+sham injury (GM-/-+Sham, n=9); WT mice+LFP injury (WT+LFP, n=10); GM-CSF-/- mice+LFP injury (GM-/-+LFP, n=9).
LFP injury
LFP and sham injury procedures were based on standard protocols previously described.26–29 Under isoflurane anesthesia, a 3-mm craniotomy positioned laterally over the parietal cortex was performed to create a circular window exposing the intact dura of the brain. A hollow injury cap was secured over the craniotomy window by dental acrylic, the mouse was removed from anesthesia, and attached to the fluid percussion device via the injury cap, and a fluid pulse (1–1.5 atm) generated by the fluid percussion device was delivered to the brain via the injury cap once the mouse responded to a hind-paw pinch. On resumption of breathing, the dental acrylic cap was removed and the wound sutured closed. Sham-injury mice underwent the same procedures as LFP-injury mice, with the exception that the fluid pulse was not given. The duration of apnea, unconsciousness (hind-paw withdrawal), and latency to occurrence of the self-righting reflex were all monitored immediately after each sham injury or LFP injury to assess acute injury severity.26,30 Body temperature was maintained at 37°C throughout surgical procedures using a heat mat and rectal thermometer. A heat mat was also placed under a portion of the recovery cage for 24 h post-injury.
Behavioral testing
Three months after sham injury or LFP injury, all mice underwent well-validated assessments of spatial memory (Y-maze), motor ability (rotarod, open field), and anxiety-like behavior (elevated-plus maze) over the span of 3 consecutive days. Behavioral testing was conducted by an experimenter blinded to injury and strain.
As previously described,31 Y-maze testing was conducted in an apparatus consisting of three arms of equal dimension (length=38 cm, width=8 cm) that were enclosed by 13 cm high walls and adjoined in a Y-shape (San Diego Instruments). An exterior visual cue was placed above the distal end of each arm. Before Y-maze testing, mice were given a 15-min training trial. For the training trial, one arm (novel arm) was blocked. The mouse was then placed at the distal end of one of the remaining arms (start arm) and allowed to freely explore the start and other arm. After a 2 h intertrial interval, a 5-min test trial was conducted. For Y-maze testing, the novel arm was unblocked, and the mouse was placed in the same start arm and allowed to freely explore all three arms. The arms and visual cues were randomized between, but not within, rats. An overhead camera recorded each trial, and the time spent and number of entries into each of the arms was quantified using Ethovision tracking software (Noldus).
The elevated-plus maze was used to assess anxiety-like behavior as previously described.32 Briefly, the elevated-plus maze consisted of two elevated (height=38 cm) and intersecting arms, thereby creating four individuals arms each (length=30 cm, width=5 cm). Two opposing “closed” arms were enclosed by walls (height=15 cm; San Diego Instruments). Each trial consisted of the mouse being placed in the center of the maze facing an open arm and allowed to freely explore the maze for 5 min. An overhead camera recorded each session, and Ethovision tracking software was used to quantify the amount of time spent and the number of entries in each of the arms.
As previously described,33,34 open field testing was conducted in a circular arena (100 cm diameter, 20 cm high wall) to assess locomotor behavior. Each mouse was placed in the center of the open field and allowed to freely explore the arena for 10 min. An overhead camera recorded each session, and Ethovision tracking software was used to quantify total distance traveled, and the number of entries and time spent in the center of the arena (66 cm diameter).
The rotarod was used to assess motor function as previously described.32 The rotarod apparatus consisted of a rotating barrel (diameter=3 cm) that was separated into five equal lanes (width=5 cm) by dividing walls (height=10 cm; Harvard Apparatus). Rotarod assessment was performed over 2 consecutive days (training and testing), with each day consisting of three trials. For each trial, the mouse was placed on the rotating barrel, the speed was accelerated from 4 to 40 rpm over a period of 5 min, and the time that the mouse was able to maintain its balance was recorded.
Magnetic resonance imaging (MRI)
Following the completion of behavioral testing, in vivo MRI scanning was performed using a 4.7T Bruker Avance III scanner with 30 cm horizontal bore fitted with a BGA12S2 actively shielded gradient set and running Paravision 5.1 software (Bruker Biospec). Anesthetized mice were positioned supine on an animal cradle with stereotactic fixation and a nose cone to maintain anesthesia (2% isoflurane). Body temperature was maintained with a hot water circulation system built into the cradle.
The scanning protocol consisted of a three-plane localizer sequence followed by multislice axial, coronal, and sagittal scout images to accurately determine the position of the mouse brain. A T2-weighted image was acquired using a two-dimensional rapid acquisition with relaxation enhancement (RARE) sequence with the following imaging parameters: recovery time (TR)=8,000 ms, RARE factor=10, effective echo time (TEeff)=45 ms, field of view (FOV)=19.2×19.2 mm2, matrix size=160×160, number of slices=60, slice thickness=120 μm, and number of excitations (NEX)=6.35
All imaging analysis procedures followed those previously described.36,37 Briefly, T2-weighted MRI volumes of selected brain regions were quantified with manually drawn regions of interest (ROIs) using FSL (Analysis Group). A total of eight ROIs, including the cortex, hippocampus, corpus callosum, and lateral ventricles from both hemispheres, were drawn as previously described.36,37 ROIs were drawn on consecutive axial MRI slices containing hippocampus by an investigator blinded to experimental conditions. Volumetric analyses were performed using Matlab (Mathworks).
Immunofluorescence
After MRI, mice (n=4/group) were perfused transcardially with ice-cold phosphate-buffered saline (PBS; pH 7.2–7.4), followed by 4% paraformaldehyde in PBS. Brains were removed, post-fixed in 4% paraformaldehyde for 24 h at 4°C, then immersed in 70% ethanol until undergoing a 24-h paraffin-embedding cycle. Brains were then coronally sectioned at 8 μm on a paraffin microtome and mounted on slides.
For immunofluorescence staining, the sections were dewaxed in 100% xylene for 5 min and hydrated in decreasing graded ethanol to water (100%, 96%, 70% ethanol/water). Antigen retrieval was performed in a temperature- and pressure-controlled Decloaking Chamber Plus (BioCare Medical) in sodium citrate buffer (10 mM, 0.05% Tween 20, pH6) at 125°C for 10 min, followed by cooling under running tap water for 10 min. Sections were then immersed in PBS (pH 7.4) for 5 min, and then blocked in PBS blocking buffer (5% heat inactivated BSA, PBS at pH 7.4) for 1 h at room temperature. Sections were then incubated for 24 h at 4°C with goat polyclonal anti-glial fibrillary acidic protein (GFAP; 1:200; Abcam, Cambridge, MA) and rabbit polyclonal anti-neuronal nuclear antigen (NeuN; 1:1000; Abcam, Cambridge, MA) primary antibodies. Sections were then washed in PBS at room temperature and incubated with secondary antibodies (1:500, anti-rabbit for NeuN, anti-goat for GFAP) for 2 h.
As previously described,38,39 for semi-quantitative analysis of reactive astrogliosis (GFAP) and neuronal loss (NeuN), FOVs (20×) were captured from coronal sections at the level of injury by a researcher that was blinded to the experimental conditions. Using ImageJ software (National Institutes of Health), color thresholds were adjusted to detect immunopositive cells. GFAP immunoreactivity was assessed using photomicrographs captured from tissue adjacent to the lesion that included the area of highest immunoreactivity, and was measured as the percent of immunoreactive area within the FOV.38,39 Neuronal counts were conducted within an area of interest that included the end of the CA2 and beginning of CA3 regions of the ipsilateral hippocampus.38,39
Statistical analyses
All outcomes were analyzed using two-way analysis of variance with strain (GM-CSF-/- and WT) and injury (LFP and sham injury) as the between-subject variables. Bonferroni post hoc comparisons were performed when appropriate. Analyses were performed using SPSS 21.0 software (IBM Corp). Statistical significance was set at p<0.05.
Results
Acute injury severity
As reported in Table 1, LFP worsened all acute injury measures as indicated by a significant effect of injury on apnea (F1,39=187.396, p<0.001), unconsciousness (F1,39=485.392, p<0.001), and self-righting reflex times (F1,39=846.292, p<0.001). There was no significant effect of strain or a significant interaction between injury and strain on any acute injury measures (all p>.05).
Table 1.
Body Weight and Acute Injury Measures
| WT+Sham | GM-/-+Sham | WT+LFP | GM-/-+LFP | |
|---|---|---|---|---|
| Weight (g) | 26.4±0.6 | 26.6±0.6 | 26.5±0.5 | 26.8±0.5 |
| Apnea (s) | 0 | 0 | 55.9±5.6a | 53.5±5.4a |
| Unconsciousness (s) | 0 | 0 | 298.9±16.4a | 283.9±14.2a |
| Self-righting (s) | 54±2.4 | 50.9±1.3 | 494.4±16.4a | 483.7±23.9a |
Mice administered LFP display significantly worse acute injury outcome compared with sham-injured mice as indicated by increased apnea, unconsciousness, and self-righting reflex times. There were no significant differences in body weight. See Results for additional statistical details.
Significantly greater than sham-injured groups, p<0.05.
WT, wild type; GM, granulocyte-macrophage; LFP, lateral fluid percussion.
Behavioral outcomes
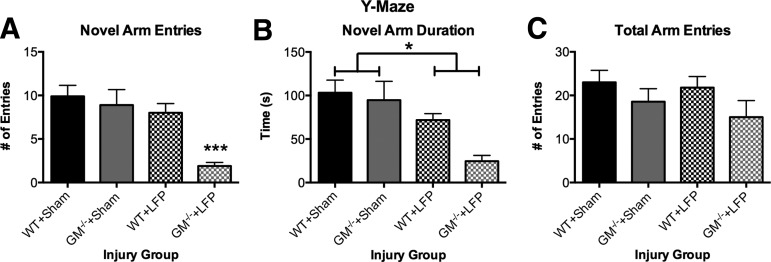
As indicated by a significant strain X injury interaction (F1, 37=4.28, p<0.05), GM-/-+LFP mice made significantly fewer entries into the novel arm of the Y-maze compared with all other groups (p<0.05; Fig. 1A), suggesting greater cognitive deficits. Further, LFP-injured mice spent significantly less time in the novel arm of the Y-maze, as indicated by a significant effect for injury (F1,37=15.38, p<0.001). There was also a significant effect for strain, indicating that GM-CSF-/- mice spent less time in the novel arm of the Y-maze (F1,37=5.01, p<0.05). There were no significant effects on the measure of closed arm entries (p>0.05, Fig. 1C), suggesting that motor abnormalities were not a confounding factor in the Y-maze.
FIG. 1.
Granulocyte-macrophage gene deficient+lateral fluid percussion (GM-/-+LFP) mice display more severe cognitive impairments in Y-maze. GM-/-+LFP mice displayed fewer entries into the novel arm of the Y-maze compared with all other groups (A). LFP mice spent less time in the novel arm of the Y-maze compared with sham-injured mice (B). There were no significant differences in total arm entries, suggesting motor abnormalities were not a confounding factor (C). ***Significantly different than all other groups, p<0.05. *Significant injury effect, p<0.05. See Results for additional statistical details. WT, wild type.
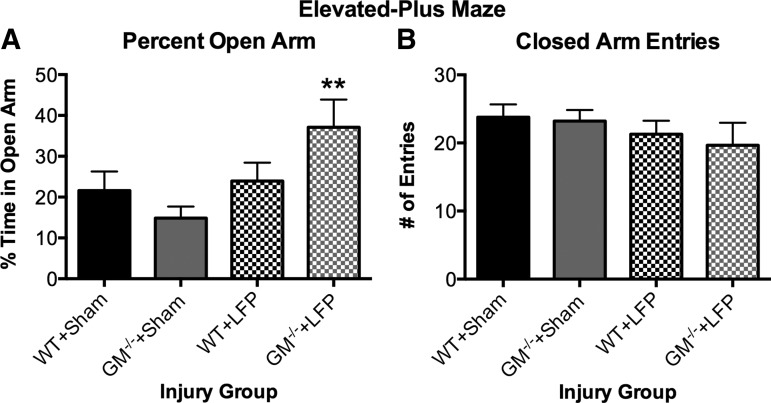
As indicated by a significant strain X injury interaction (F1, 37=4.161, p<0.05), GM-/-+LFP mice spent significantly more time in the open arm of the elevated-plus maze compared with both sham-injury groups (p<0.05; Fig. 2A), suggesting less anxiety-like behavior. There were no significant effects on the measure of closed arm entries (p>0.05, Fig. 2B), suggesting that motor abnormalities were not a confounding factor in the elevated-plus maze.
FIG. 2.
Granulocyte-macrophage gene deficient+lateral fluid percussion (GM-/-+LFP) mice display abnormal behavior in elevated-plus maze. GM-/-+LFP mice spend significantly more time on the open arm of the elevated-plus maze compared with sham-injury groups (A), with no significant differences on the number of closed arm entries (B), suggesting increased impulsivity. **Significantly different than wild type (WT)+Sham and GM-/-+Sham groups, p<0.05. See Results for additional statistical details.
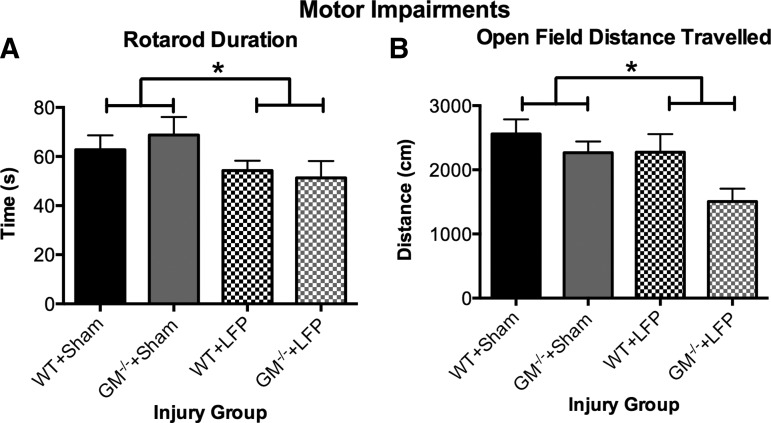
As indicated by a significant effect for injury (F1,37=4.613, p<0.05; Fig. 3A), LFP-injured mice spent significantly less time on the rotarod compared with sham-injured mice during the test session, suggesting motor impairments. There were no significant effects on rotarod during the training session (p>0.05, data not shown).
FIG. 3.
Mice given lateral fluid percussion (LFP) display chronic motor impairments. LFP mice remained on the rotarod for a shorter duration (A) and traveled less distance in the open field (B) compared with sham-injured mice. *Significant effect of injury, p<0.05. See Results for additional statistical details.
LFP-injured mice traveled significantly less distance in the open field compared with the sham-injured mice as indicated by a significant effect for injury (F1,37=5.162, p<0.05; Fig. 3B). GM-CSF-/- mice also traveled less distance compared with WT mice, as indicated by a significant effect for strain (F1,37=5.3198, p<0.05). There were no significant effects on the open field measures of time spent or entries into the middle of the field (p>0.05, data not shown).
MRI brain structure volumetrics
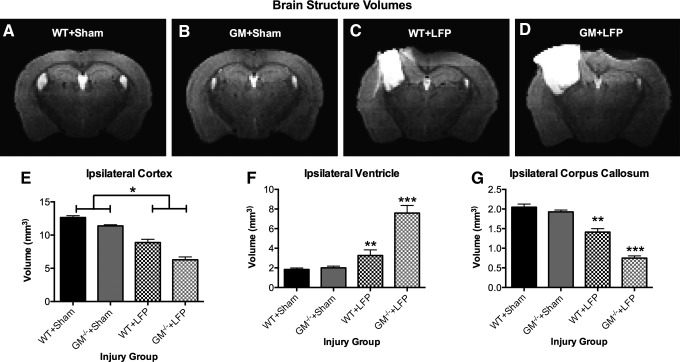
LFP-injured mice displayed a significant decrease in ipsilateral cortex volume compared with sham-injured mice, as indicated by a significant effect for injury (F1,37=117.162, p<0.001; Fig. 4E). There was also a significant effect for strain on ipsilateral cortex volume (F1,37=19.473, p<0.001). Significant injury X strain interactions, however, indicated that GM-/-+LFP mice displayed a significant decrease in corpus callosum volume (F1,37=4.222, p<0.005; Fig. 4G) and had significantly larger ventricles (F1,37=5.724, p<0.001; Fig. 4F) compared with all other groups (p<0.05). WT+LFP mice also displayed a significant decrease in corpus callosum volume, and had significantly larger ventricles compared with both sham-injury groups (p<0.05). There was no significant volumetric loss in the ipsilateral hippocampus in LFP-injured mice (p>0.05, data not shown), although clear morphological changes are qualitatively apparent. There were no significant effects in any of the contralateral structures (p>0.05, data not shown).
FIG. 4.
Granulocyte-macrophage gene deficient+lateral fluid percussion (GM-/-+LFP) mice display more severe brain atrophy, as demonstrated by representative images from each group of the injury groups (A–D). Mice given LFP displayed a significant decrease in ipsilateral cortex volume compared with sham-injured mice (E). Both groups of mice given LFP display significant increases in ipsilateral ventricle size compared with both sham-injured groups; however, GM-/-+LFP mice also have significantly larger ventricles than wild type (WT)+LFP mice (F). Both groups of mice given LFP display significant decreases in corpus callosum volume compared with both sham-injured groups; however, GM-/-+LFP mice also have significantly less corpus callosum than WT+LFP mice. *Significant effect of injury, p<0.05. **Significantly different than WT+Sham and GM-/-+Sham groups, p<0.05. ***Significantly different than all groups, p<0.05. See Results for additional statistical details.
Immunofluorescence
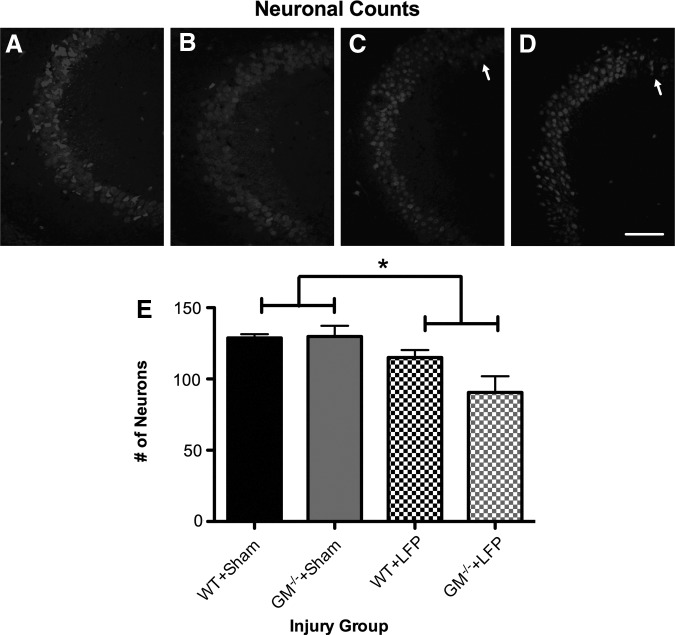
As shown in Figure 5, LFP-injured mice had significantly fewer neurons in the CA2/CA3 region of hippocampus compared with sham-injured mice, as indicated by a significant effect for injury (F1,12=12.464, p<0.01; Fig. 5).
FIG. 5.
Mice given lateral fluid percussion (LFP) have neuronal loss in hippocampus. (A–D) Representative photomicrographs of wild type (WT)+Sham (A), granulocyte-macrophage (GM)-/-+Sham (B), WT+LFP (C), and GM-/-+LFP (D) mice taken from coronal hippocampal sections in the CA2/CA3 region and immunostained with an antibody to neuronal nuclear antigen (NeuN). Mice given LFP had a significant decrease in the number of neurons counted in the CA2/CA3 region of the hippocampus compared with sham-injured mice (E). *Significant effect of injury, p<0.05. Scale bar=100 μm. See Results for additional statistical details.
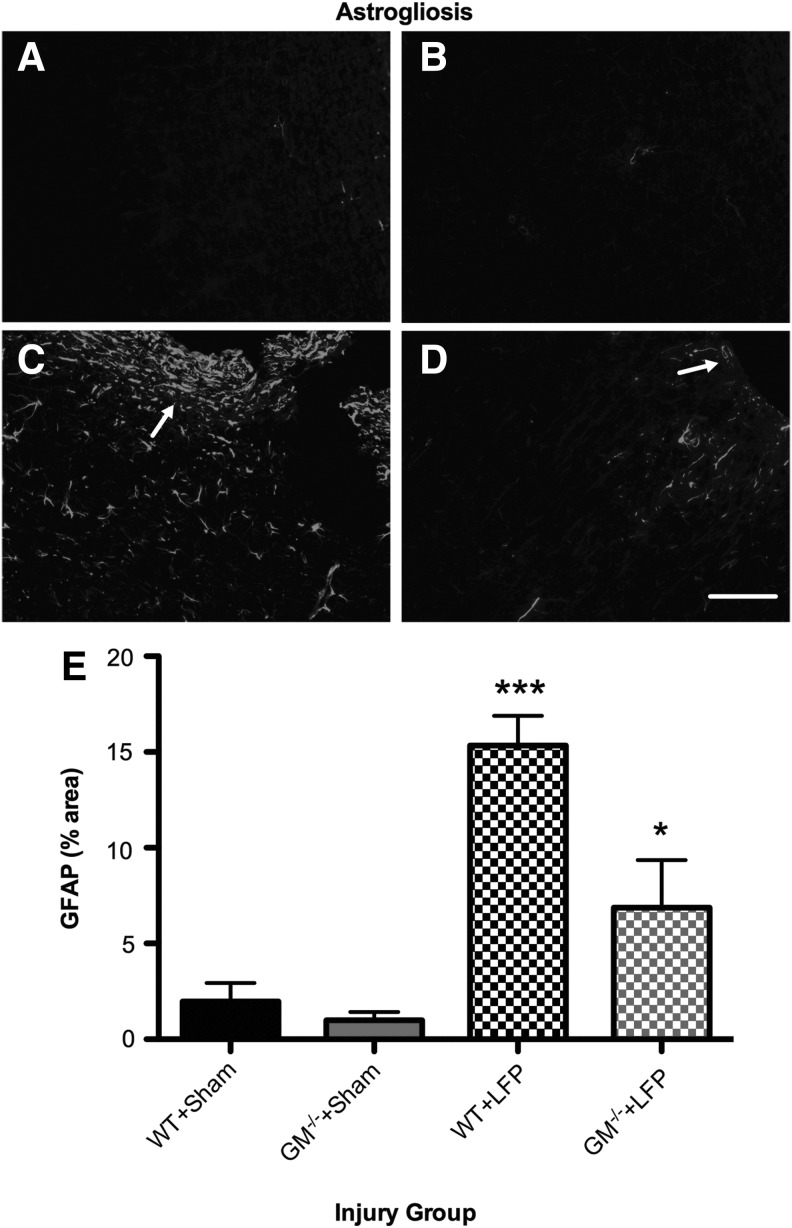
As shown in Figure 6, a significant injury X strain interaction (F1,12=5.693, p<0.05; Fig. 6) indicated that while both LFP groups displayed increased astrogliosis compared with their sham-injured counterparts (p<0.05), GM-/-+LFP mice displayed significantly less astrogliosis than the WT+LFP mice (p<0.05). Significant effects for injury (F1,12=37.987, p<0.001) and strain (F1,12=9.177, p<0.05) were also found.
FIG. 6.
Granulocyte-macrophage gene deficient+lateral fluid percussion (GM-/-+LFP) mice have reduced astrogliosis. (A–D) Representative photomicrographs of wild type (WT)+Sham (A), GM-/-+Sham (B), WT+LFP (C), and GM-/-+LFP (D) mice taken from cortical tissue adjacent to the injury lesion and immunostained with an antibody to glial fibrillary acidic protein (GFAP). Although both WT+LFP (C) and GM-/-+LFP (D) mice display an increased area of GFAP immunoreactivity compared with their sham-injured counterparts, GFAP immunoreactivity is significantly reduced in GM-/-+LFP mice compared with the WT+LFP group (E). ***Significantly different than all groups, p<0.05. *Significantly different than GM-/-+Sham group, p<0.05. Scale bar=100 μm. See Results for additional statistical details.
Discussion
We investigated the importance of the inflammatory cytokine GM-CSF in the chronic outcome after experimental TBI by administering either LFP or sham injury to GM-CSF-/- or WT mice. We found that while all mice displayed significant brain damage and behavioral impairments 3 months after a LFP injury compared with sham-injured mice, the GM-/-+LFP mice displayed significantly worse outcomes compared with the WT+LFP mice.
Nature of behavioral impairments
Consistent with previous findings,27,29 mice given a LFP injury displayed worsened motor and cognitive outcomes compared with sham-injured mice. This was evidenced in the current study by decreased time spent on the rotarod during testing (poorer sensorimotor performance), less distance traveled in the open field (decreased locomotor activity), and less time spent in the novel arm of the Y-maze (impaired spatial cognition). The neuroimaging findings that all mice given LFP injury displayed significant damage to the cortex and corpus callosum, as well as morphological changes and neuronal loss in the hippocampus, may have each contributed to the motor and cognitive deficits observed post-LFP injury.30,33,39,40
The GM-/-+LFP mice made significantly fewer entries into the novel arm of the Y-maze compared with the WT+LFP mice, indicating worsened cognitive deficits. The GM-/-+LFP mice also displayed abnormal behavior in the elevated-plus maze, spending significantly more time in the open arm compared with the sham-injured groups. The finding of increased time spent on the open arm is consistent with some previous LFP findings,30 and in the absence of hyperactivity has been interpreted as disinhibitory or impulsive behavior,30,41 which are symptoms observed in patients post-TBI. 42 Other studies, however, report that rats given a severe LFP injury display a hyperanxious phenotype post-TBI,33,39 whereas here, the WT+LFP mice did not. These contradictory findings may be related to interspecies differences or other methodological factors (e.g., injury severity), and warrant further investigation.
Considering that motor impairments might confound outcomes in the Y-maze and elevated-plus maze and that LFP mice had significant motor impairments in the rotarod and open field tasks, the cognitive and emotional findings here must be interpreted with caution. The GM-/-+LFP mice and WT+LFP mice, however, displayed similar motor impairments, the motor deficits were subtle, and there were no significant effects on the motor measures taken directly from the Y-maze and elevated-plus maze. Therefore, it seems unlikely that motor deficits account for the worsened GM-/-+LFP mice outcomes in the Y-maze and elevated-plus maze. Nonetheless, future studies might incorporate more sensitive sensorimotor measures, such as the beam task, or test at acute time points post-LFP injury when motor deficits are more pronounced, to better understand the extent of sensorimotor deficits induced by LFP, and how the depletion of GM-CSF might affect these outcomes.
In addition to the worsened behavioral phenotype, the GM-/-+LFP mice also exhibited significantly more damage to the ipsilateral corpus callosum and ventricle, as well as a trend suggesting worsened atrophy in the ipsilateral cortex. Thus, it follows that the worsened behavioral outcomes in GM-/-+LFP mice may be related to this exacerbated damage. 30,33,37 Future studies are needed, however, to better assess the exact neural mechanisms underlying the behavioral deficits observed here. Because the MRI-based volumetric analysis we used is limited in assessing smaller or deformed brain structures and cellular/molecular changes that might contribute to these deficits, additional imaging and biochemical/molecular methods that are sensitive to such changes could be used in future studies. Additional behavioral tasks, such as the water maze, could also be incorporated into future studies to provide a more comprehensive assessment of the functional deficits that occur.
Role of GM-CSF in TBI
The findings that GM-/-+LFP mice experienced worsened brain damage and behavioral deficits compared with WT+LFP mice indicate a neuroprotective role for GM-CSF against the chronic and progressive sequelae of TBI. Interestingly, the GM-/-+LFP mice displayed a significant reduction in astrogliosis within the injured cortex compared with WT+LFP mice. Consistent with previous reports,43 these findings suggest that GM-CSF may play an important role in the activation/proliferation of astrocytes. In the context of TBI, a lack of astrocyte activation might be detrimental because astrocyte-mediated scar formation is important in isolating damaged tissue and prevents the lesion from spreading.6 In addition, astrocytes might release neurotrophic factors in the aftermath of TBI.6 Thus, our findings of worsened outcomes in GM-/-+LFP mice may be in part because of reduced astrogliosis and consequent lack of scarring and/or neurotrophic factors.
Previous studies reporting a protective effect of GM-CSF in animal models of Alzheimer disease,15 Parkinson disease,16 stroke,14,17 spinal cord injury,18–20 and stab wound-induced brain injury21 have also proposed several other mechanisms by which GM-CSF may be neuroprotective including the alteration of other key neuroinflammatory mediators,15,16,21,23 anti-apoptotic effects,14 reduction of amyloid beta accumulation,15 increased production of trophic factors,16,18 neurogenesis,20,44 and angiogenesis.17 Because there are pathological similarities between these conditions and TBI, it is possible that these protective pathways might also be applicable here. Further, treatment with cytokines that may have similar properties to GM-CSF, such as granulocyte colony-stimulating factor and erythropoietin, have been reported to have beneficial effects in pre-clinical TBI.45,46 Taken together, these findings warrant further more clinically relevant investigations into the modulation of GM-CSF as a therapeutic intervention after TBI.
That the GM-CSF-/- mice used here were conventional knockouts should be considered when interpreting the current findings.47 Consistent with previous studies,48 the GM-CSF-/- and WT strains did not differ in body weight and appeared in good overall health, and there were no apparent differences between WT and GM-CSF-/- sham-injured groups. Nonetheless, it is possible that compensatory effects, particularly related to inflammatory and hematopoietic factors, might occur in this mouse strain. Further, the depletion of GM-CSF-/- might result in systemic effects, such as changes in body temperature or blood pressure, which could indirectly contribute to worsened outcomes after LFP. In light of these limitations, future studies might monitor such factors and incorporate more specific and controllable means to manipulate GM-CSF, such as conditional knockouts or GM-CSF specific antibodies.
Conclusions
We examined the importance of the inflammatory cytokine GM-CSF in mediating long-term neuropathological, motor, and behavioral outcomes after experimental TBI. GM-CSF-/- and WT mice were administered either LFP or sham injury, given a 3-month recovery, and assessed using neuroimaging, behavioral, and histological measures. Although all mice given LFP injury had some degree of brain damage and behavioral deficits, the GM-/-+LFP mice displayed significantly worse outcomes compared with their WT counterparts. These novel findings indicate an overall neuroprotective effect of GM-CSF in TBI and warrant future studies.
Acknowledgments
We would like to thank Thanh Nguyen for assistance with immunofluorescence experiments. This study was funded by grants to T.O.B. from the National Health and Medical Research Council (NHMRC #1006077), the Victorian Transport Accident Commission (Victorian Neurotrauma Initiative Grant #DNP13), and the Royal Melbourne Hospital Neuroscience Foundation, a fellowship from the Canadian Institute of Health Research to S.S., and a Senior Principal Research Fellowship from NHMRC to J.A.H.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Blennow K., Hardy J., and Zetterberg H. (2012). The neuropathology and neurobiology of traumatic brain injury. Neuron 76, 886–899 [DOI] [PubMed] [Google Scholar]
- 2.Kumar A. and Loane D.J. (2012). Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav. Immun. 26, 1191–1201 [DOI] [PubMed] [Google Scholar]
- 3.Johnson V.E., Stewart J.E., Begbie F.D., Trojanowski J.Q., Smith D.H., and Stewart W. (2013). Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136, 28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziebell J.M., and Morganti-Kossman M.C. (2010). Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 7, 22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadhim H.J., Duchateau J., and Sebire G. (2008). Cytokines and brain injury: invited review. J. Intensive Care Med. 23, 236–249 [DOI] [PubMed] [Google Scholar]
- 6.Laird M.D., Vender J.R., and Dhandapani K.M. (2008). Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals 16, 154–164 [DOI] [PubMed] [Google Scholar]
- 7.Lu J., Goh S.J., Tng P.Y.L., Deng Y.Y., Ling E.A., and Moochhala S. (2009). Systemic inflammatory response following acute traumatic brain injury. Front. Biosci 14, 3795–3813 [DOI] [PubMed] [Google Scholar]
- 8.Masel B.E., and DeWitt D.S. (2010). Traumatic brain injury: a disease process, not an event. J. Neurotrauma 27, 1529–1540 [DOI] [PubMed] [Google Scholar]
- 9.Armitage J.O. (1998). Emerging applications of recombinant human granulocyte-macrophage colony-stimulating factor. Blood 92, 4491–4508 [PubMed] [Google Scholar]
- 10.Metcalf D. (1989). The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature 339, 27–30 [DOI] [PubMed] [Google Scholar]
- 11.Franzen R., Bouhy D., and Schoenen J. (2004). Nervous system injury: focus on the inflammatory cytokine 'granulocyte-macrophage colony stimulating factor'. Neurosci. Lett. 361, 76–78 [DOI] [PubMed] [Google Scholar]
- 12.McLay R.N., Kimura M., Banks W.A., and Kastin A.J. (1997). Granulocyte-macrophage colony-stimulating factor crosses the blood–brain and blood–spinal cord barriers. Brain 120, 2083–2091 [DOI] [PubMed] [Google Scholar]
- 13.Krieger M., Both M., Kranig S.A., Pitzer C., Klugmann M., Vogt G., Draguhn A., and Schneider A. (2012). The hematopoietic cytokine granulocyte-macrophage colony stimulating factor is important for cognitive functions. Sci. Rep. 2, 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schäbitz W.R., Krüger C., Pitzer C., Weber D., Laage R., Gassler N., Aronowski J., Mier W., Kirsch F., Dittgen T., Bach A., Sommer C., and Schneider A. (2008). A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GM-CSF). J. Cereb. Blood Flow Metab. 28, 29–43 [DOI] [PubMed] [Google Scholar]
- 15.Boyd T.D., Bennett S.P., Mori T., Governatori N., Runfeldt M., Norden M., Padmanabhan J., Neame P., Wefes I., Sanchez-Ramos J., Arendash G.W., and Potter H. (2010). GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J. Alzheimers Dis. 21, 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangano E.N., Peters S., Litteljohn D., So R., Bethune C., Bobyn J., Clarke M., and Hayley S. (2011). Granulocyte macrophage-colony stimulating factor protects against substantia nigra dopaminergic cell loss in an environmental toxin model of Parkinson's disease. Neurobiol. Dis. 43, 99–112 [DOI] [PubMed] [Google Scholar]
- 17.Schneider U.C., Schilling L., Schroeck H., Nebe C.T., Vajkoczy P., and Woitzik J. (2007). Granulocyte-macrophage colony-stimulating factor-induced vessel growth restores cerebral blood supply after bilateral carotid artery occlusion. Stroke 38, 1320–1328 [DOI] [PubMed] [Google Scholar]
- 18.Bouhy D., Malgrange B., Multon S., Poirrier A.L., Scholtes F., Schoenen J., and Franzen R. (2006). Delayed GM-CSF treatment stimulates axonal regeneration and functional recovery in paraplegic rats via an increased BDNF expression by endogenous macrophages. FASEB J. 20, 1239–1241 [DOI] [PubMed] [Google Scholar]
- 19.Ha Y., Kim Y.S., Cho J.M., Yoon S.H., Park S.R., Yoon D.H., Kim E.Y., and Park H.C. (2005). Role of granulocyte-macrophage colony-stimulating factor in preventing apoptosis and improving functional outcome in experimental spinal cord contusion injury. J. Neurosurg. Spine 2, 55–61 [DOI] [PubMed] [Google Scholar]
- 20.Hayashi K., Ohta S., Kawakami Y., and Toda M. (2009). Activation of dendritic-like cells and neural stem/progenitor cells in injured spinal cord by GM-CSF. Neurosci. Res. 64, 96–103 [DOI] [PubMed] [Google Scholar]
- 21.Nishihara T., Ochi M., Sugimoto K., Takahashi H., Yano H., Kumon Y., Ohnishi T., and Tanaka J. (2011). Subcutaneous injection containing IL-3 and GM-CSF ameliorates stab wound-induced brain injury in rats. Exp. Neurol. 229, 507–516 [DOI] [PubMed] [Google Scholar]
- 22.Frugier T., Morganti-Kossmann M.C., O'Reilly D., and McLean C.A. (2010). In situ detection of inflammatory mediators in post mortem human brain tissue after traumatic brain injury. J. Neurotrauma 27, 497–507 [DOI] [PubMed] [Google Scholar]
- 23.Cook A.D., Braine E.L., and Hamilton J.A. (2004). Stimulus-dependent requirement for granulocyte-macrophage colony-stimulating factor in inflammation. J. Immunol. 173, 4643–4651 [DOI] [PubMed] [Google Scholar]
- 24.Hamilton J.A. (2008). Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8, 533–544 [DOI] [PubMed] [Google Scholar]
- 25.Campbell I.K., Rich M.J., Bischof R.J., Dunn A.R., Grail D., and Hamilton J.A. (1998). Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J. Immunol. 161, 3639–3644 [PubMed] [Google Scholar]
- 26.Alder J., Fujioka W., Lifshitz J., Crockett D.P., and Thakker-Varia S. (2011). Lateral fluid percussion: model of traumatic brain injury in mice. J. Vis. Exp. 54, 3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carbonell W.S., Maris D.O., McCall T., and Grady M.S. (1998). Adaptation of the fluid percussion injury model to the mouse. J. Neurotrauma 15, 217–229 [DOI] [PubMed] [Google Scholar]
- 28.Shultz S.R., MacFabe D.F., Foley K.A., Taylor R., and Cain D.P. (2012). Sub-concussive brain injury in the Long-Evans rat induces acute neuroinflammation in the absence of behavioral impairments. Behav. Brain Res. 229, 145–152 [DOI] [PubMed] [Google Scholar]
- 29.Spain A., Daumas S., Lifshitz J., Rhodes J., Andrews P.J., Horsurgh K., and Fowler J.H. (2010). Mild fluid percussion injury in mice produces evolving selective axonal pathology and cognitive deficits relevant to human brain injury. J. Neurotrauma 27, 1429–1438 [DOI] [PubMed] [Google Scholar]
- 30.Shultz S.R., MacFabe D.F., Foley K.A., Taylor R., and Cain D.P. (2011). A single mild fluid percussion injury induces short-term behavioral and neuropathological changes in the Long-Evans rat: support for an animal model of concussion. Behav. Brain Res. 224, 326–335 [DOI] [PubMed] [Google Scholar]
- 31.Conrad C.D., Galea L.A.M., Kuroda Y., and McEwen B.S. (1996). Chronic stress impairs rat spatial memory on the Y-maze, and this effect is blocked by tianeptine pretreatment. Behav. Neurosci. 110, 1321–1334 [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi H., Iba M., Inoue H., Higuchi M., Takao K., Tsukita K., Karatsu Y, Iwamoto Y., Miyakawa T., Suhara T., Trojanowski J.Q., Lee V.M., and Takahashi R. (2011). P301S mutant tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating. PLoS One 6, e21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones N.C., Cardamone L., Williams J.P., Salzberg M.R., Myers D. and O'Brien T.J. (2008). Experimental traumatic brain injury induces a pervasive hyperanxious phenotype in rats. J. Neurotrauma 25, 1367–1374 [DOI] [PubMed] [Google Scholar]
- 34.Shultz S.R., Bao F., Omana V., Chiu C., Brown A., and Cain D.P. (2012). Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J. Neurotrauma 29, 281–294 [DOI] [PubMed] [Google Scholar]
- 35.Hennig J., Nauerth A., and Friedburg H. (1986). RARE imaging: a fast method for clinical MR. Magn. Reson. Med. 3, 823–833 [DOI] [PubMed] [Google Scholar]
- 36.Liu Y.R., Cardamone L., Hogan R.E., Gregoire M.C., Williams J.P., Hicks R.J., Binns D., Koe A., Jones N.C., Myers D.E., O'Brien T.J., and Bouilleret V. (2010). Progressive metabolic and structural cerebral perturbations after traumatic brain injury: an in vivo imaging study in the rat. J. Nucl. Med. 51, 1788–1795 [DOI] [PubMed] [Google Scholar]
- 37.Shultz S.R., Cardamone L., Liu Y.R., Hogan R.E., Maccotta L., Wright D.K., Zheng P., Koe A., Gregoire M.C., Williams J.P., Hicks R.J., Jones N.C., Myers D.E., O'Brien T.J., and Bouilleret V. (2013). Can structural or functional changes following traumatic brain injury in the rat predict epileptic outcome? Epilepsia 54, 1240–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shultz S.R., Bao F., Weaver L.C., Cain D.P., and Brown A. (2013). Treatment with an anti-CD11d antibody reduces neuroinflammation and improves outcome in a rat model of repeated concussion. J. Neuroinflammation 10, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bao F., Shultz S.R., Hepburn J.D., Omana V., Weaver L.C., Cain D.P., and Brown A. (2012). A CD11d monoclonal antibody treatment reduces tissue injury and improves neurological outcome after fluid percussion brain injury in rats. J. Neurotrauma 29, 2375–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wahl S., Barth H., Ciossek T., Aktories K., and Mueller B.K. (2000). Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J. Cell Biol. 149, 263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bortolato M., Godar S.C., Davarian S., Chen K., and Shih J.C. (2009). Behavioral disinhibition and reduced anxiety-like behaviors in monoamine oxidase B-deficient mice. Neuropsychopharmacology 34, 2746–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim E. (2002). Agitation, aggression, and disinhibition syndromes after traumatic brain injury. NeuroRehabilitation 17, 297–310 [PubMed] [Google Scholar]
- 43.Guillemin G., Boussin F.D., Le Grand R., Croitoru J., Coffigny H., and Dormont D. (1996). Granulocyte macrophage colony stimulating factor stimulates in vitro proliferation of astrocytes derived from simian mature brains. Glia 16, 71–80 [DOI] [PubMed] [Google Scholar]
- 44.Krüger C., Laage R., Pitzer C., Schäbitz W.R., and Schneider A. (2007). The hematopoietic factor GM-CSF (granulocyte-macrophage colony-stimulating factor) promotes neuronal differentiation of adult neural stem cells in vitro. BMC Neurosci. 8, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shelbani N., Grabowski E.F., Schoenfeld D.A., and Whalen M.J. (2004). Effect of granulocyte colony-stimulating factor on functional and histopathological outcome after traumatic brain injury. Crit. Care Med. 32, 2274–2278 [DOI] [PubMed] [Google Scholar]
- 46.Xiong Y., Lu D., Qu C., Goussev A., Schallert T., Mahmood A., and Chopp M. (2008). Effects of erythropoietin on reducing brain damage and improving functional outcome after traumatic brain injury in mice. J. Neurosurg. 109, 510–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thyagarajan T., Totey S., Danton M.J., and Kulkarni A.B. (2003). Genetically altered mouse models: the good, the bad, and the ugly. Crit. Rev. Oral Biol. Med. 14, 154–174 [DOI] [PubMed] [Google Scholar]
- 48.Hamilton J.A., Davis J., Pobjoy J., and Cook A.D. (2012). GM-CSF is not essential for optimal fertility or for weight control. Cytokine 57, 30–31 [DOI] [PubMed] [Google Scholar]