Abstract
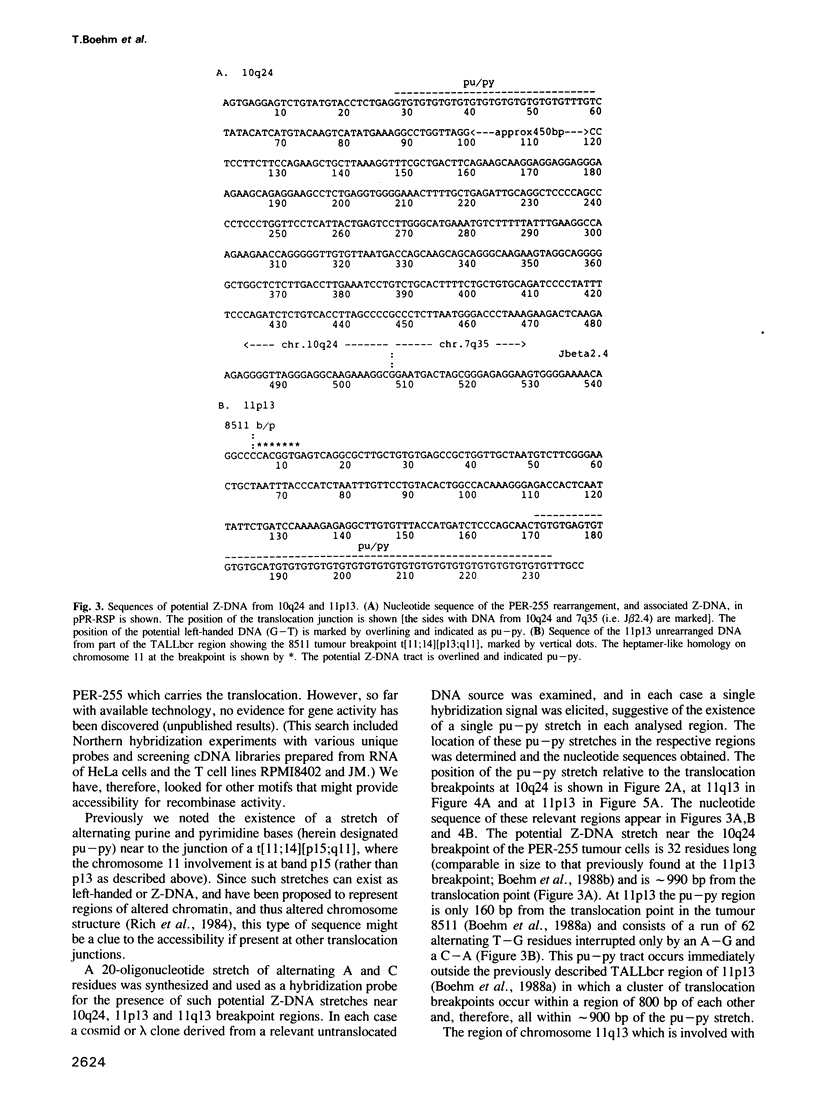
Chromosomal abnormalities which are prevalent in human lymphoid tumours are believed to be involved in tumour pathogenesis and their formation may be the result of erroneous activity by the V-D-J recombinase. Frequently, recombinase accessibility is provided by prior transcription of the chromosomal regions involved. However, this may not always be so and in those cases DNA structural features must be involved. Here we examine the breakpoints of three different tumour-specific translocations in the proximity of which we can detect no transcription; two of the translocations involve regions of chromosome 11, (t[11;14] [p13;q11] and t[11;14] [q13;q32]), and the third is a newly described translocation, t[7;10] [q35;q24], involving the T cell receptor beta-gene on chromosome 7. In each case, a purine--pyrimidine tract (potential Z-DNA) occurs near the translocation breakpoints. Four independent tumours with translocation t[11;14] [p13;q11] reveal a 2 kb breakpoint cluster region at 11p13 with an adjacent potential Z-DNA region of 62 bp in length; the analogous purine--pyrimidine tract at 10q24 is 32 bp long. The purine--pyrimidine tract at the 11q13 chromosome breakpoint, however, is very large as it covers approximately 800 bp. The position, surrounding sequence and potential Z-DNA tract of the human 11p13 TALLber is conserved in rodents. These results suggest that the purine--pyrimidine tracts, presumably in the Z-DNA form, can influence chromatin structure giving access for recombinase-mediated translocations. Such putative alterations of chromatin organization are supported by the observation of DNase I hypersensitive sites near to translocation breakpoints on 10q24 and 11p13.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Boehm T., Yssel H., Spits H., Rabbitts T. H. Complex rearrangements within the human J delta-C delta/J alpha-C alpha locus and aberrant recombination between J alpha segments. EMBO J. 1988 Jun;7(6):1661–1668. doi: 10.1002/j.1460-2075.1988.tb02993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Chen K. C., Smith S. D., Rabbitts T. H. Fusion of an immunoglobulin variable gene and a T cell receptor constant gene in the chromosome 14 inversion associated with T cell tumors. Cell. 1985 Dec;43(3 Pt 2):705–713. doi: 10.1016/0092-8674(85)90243-0. [DOI] [PubMed] [Google Scholar]
- Baer R., Forster A., Rabbitts T. H. The mechanism of chromosome 14 inversion in a human T cell lymphoma. Cell. 1987 Jul 3;50(1):97–105. doi: 10.1016/0092-8674(87)90666-0. [DOI] [PubMed] [Google Scholar]
- Bankier A. T., Weston K. M., Barrell B. G. Random cloning and sequencing by the M13/dideoxynucleotide chain termination method. Methods Enzymol. 1987;155:51–93. doi: 10.1016/0076-6879(87)55009-1. [DOI] [PubMed] [Google Scholar]
- Boehm T., Baer R., Lavenir I., Forster A., Waters J. J., Nacheva E., Rabbitts T. H. The mechanism of chromosomal translocation t(11;14) involving the T-cell receptor C delta locus on human chromosome 14q11 and a transcribed region of chromosome 11p15. EMBO J. 1988 Feb;7(2):385–394. doi: 10.1002/j.1460-2075.1988.tb02825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T., Buluwela L., Williams D., White L., Rabbitts T. H. A cluster of chromosome 11p13 translocations found via distinct D-D and D-D-J rearrangements of the human T cell receptor delta chain gene. EMBO J. 1988 Jul;7(7):2011–2017. doi: 10.1002/j.1460-2075.1988.tb03040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braaten D. C., Thomas J. R., Little R. D., Dickson K. R., Goldberg I., Schlessinger D., Ciccodicola A., D'Urso M. Locations and contexts of sequences that hybridize to poly(dG-dT).(dC-dA) in mammalian ribosomal DNAs and two X-linked genes. Nucleic Acids Res. 1988 Feb 11;16(3):865–881. doi: 10.1093/nar/16.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buluwela L., Forster A., Boehm T., Rabbitts T. H. A rapid procedure for colony screening using nylon filters. Nucleic Acids Res. 1989 Jan 11;17(1):452–452. doi: 10.1093/nar/17.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghini S., Herbomel P., Jouanneau J., Saragosti S., Katinka M., Bourachot B., de Crombrugghe B., Yaniv M. Structure and function of the promoter-enhancer region of polyoma and SV40. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):935–944. doi: 10.1101/sqb.1983.047.01.107. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Nowell P. C. Molecular genetics of human B cell neoplasia. Adv Immunol. 1986;38:245–274. doi: 10.1016/s0065-2776(08)60008-5. [DOI] [PubMed] [Google Scholar]
- Croce C. M. Role of chromosome translocations in human neoplasia. Cell. 1987 Apr 24;49(2):155–156. doi: 10.1016/0092-8674(87)90552-6. [DOI] [PubMed] [Google Scholar]
- Denny C. T., Yoshikai Y., Mak T. W., Smith S. D., Hollis G. F., Kirsch I. R. A chromosome 14 inversion in a T-cell lymphoma is caused by site-specific recombination between immunoglobulin and T-cell receptor loci. Nature. 1986 Apr 10;320(6062):549–551. doi: 10.1038/320549a0. [DOI] [PubMed] [Google Scholar]
- Dubé I. D., Raimondi S. C., Pi D., Kalousek D. K. A new translocation, t(10;14)(q24;q11), in T cell neoplasia. Blood. 1986 Apr;67(4):1181–1184. [PubMed] [Google Scholar]
- Dyson P. J., Rabbitts T. H. Chromatin structure around the c-myc gene in Burkitt lymphomas with upstream and downstream translocation points. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1984–1988. doi: 10.1073/pnas.82.7.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards Y. H., Parkar M., Povey S., West L. F., Parrington J. M., Solomon E. Human myosin heavy chain genes assigned to chromosome 17 using a human cDNA clone as probe. Ann Hum Genet. 1985 May;49(Pt 2):101–109. doi: 10.1111/j.1469-1809.1985.tb01681.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Finger L. R., Harvey R. C., Moore R. C., Showe L. C., Croce C. M. A common mechanism of chromosomal translocation in T- and B-cell neoplasia. Science. 1986 Nov 21;234(4779):982–985. doi: 10.1126/science.3490692. [DOI] [PubMed] [Google Scholar]
- Fishel R. A., Detmer K., Rich A. Identification of homologous pairing and strand-exchange activity from a human tumor cell line based on Z-DNA affinity chromatography. Proc Natl Acad Sci U S A. 1988 Jan;85(1):36–40. doi: 10.1073/pnas.85.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. G., Lefranc M. P., Rabbitts T. H. Mechanisms of divergence and convergence of the human immunoglobulin alpha 1 and alpha 2 constant region gene sequences. Cell. 1984 Mar;36(3):681–688. doi: 10.1016/0092-8674(84)90348-9. [DOI] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T. A novel repeated element with Z-DNA-forming potential is widely found in evolutionarily diverse eukaryotic genomes. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6465–6469. doi: 10.1073/pnas.79.21.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman L., Steen M. L., Sundvall M., Pettersson U. A rapidly evolving region in the immunoglobulin heavy chain loci of rat and mouse: postulated role of (dC-dA)n.(dG-dT)n sequences. Gene. 1988 Aug 15;68(1):93–100. doi: 10.1016/0378-1119(88)90602-6. [DOI] [PubMed] [Google Scholar]
- Kagan J., Finan J., Letofsky J., Besa E. C., Nowell P. C., Croce C. M. Alpha-chain locus of the T-cell antigen receptor is involved in the t(10;14) chromosome translocation of T-cell acute lymphocytic leukemia. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4543–4546. doi: 10.1073/pnas.84.13.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Matthes H. W., Gait M. J., Brenner S. A new selective phage cloning vector, lambda 2001, with sites for XbaI, BamHI, HindIII, EcoRI, SstI and XhoI. Gene. 1984 Dec;32(1-2):217–224. doi: 10.1016/0378-1119(84)90049-0. [DOI] [PubMed] [Google Scholar]
- Kmiec E. B., Holloman W. K. Synapsis promoted by Ustilago rec1 protein. Cell. 1984 Mar;36(3):593–598. doi: 10.1016/0092-8674(84)90338-6. [DOI] [PubMed] [Google Scholar]
- LeFranc M. P., Forster A., Baer R., Stinson M. A., Rabbitts T. H. Diversity and rearrangement of the human T cell rearranging gamma genes: nine germ-line variable genes belonging to two subgroups. Cell. 1986 Apr 25;45(2):237–246. doi: 10.1016/0092-8674(86)90388-0. [DOI] [PubMed] [Google Scholar]
- Leder P., Battey J., Lenoir G., Moulding C., Murphy W., Potter H., Stewart T., Taub R. Translocations among antibody genes in human cancer. Science. 1983 Nov 18;222(4625):765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- Leith I. R., Hay R. T., Russell W. C. Detection of Z DNA binding proteins in tissue culture cells. Nucleic Acids Res. 1988 Sep 12;16(17):8277–8289. doi: 10.1093/nar/16.17.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenhaupt K., Rich A., Pardue M. L. Nonrandom distribution of long mono- and dinucleotide repeats in Drosophila chromosomes: correlations with dosage compensation, heterochromatin, and recombination. Mol Cell Biol. 1989 Mar;9(3):1173–1182. doi: 10.1128/mcb.9.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengle-Gaw L., Albertson D. G., Sherrington P. D., Rabbitts T. H. Analysis of a T-cell tumor-specific breakpoint cluster at human chromosome 14q32. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9171–9175. doi: 10.1073/pnas.85.23.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Lafer E. M., Peck L. J., Wang J. C., Stollar B. D., Rich A. Negatively supercoiled plasmids contain left-handed Z-DNA segments as detected by specific antibody binding. Cell. 1982 Dec;31(2 Pt 1):309–318. doi: 10.1016/0092-8674(82)90124-6. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Lowenhaupt K., Rich A., Nordheim A. (dC-dA)n.(dG-dT)n sequences have evolutionarily conserved chromosomal locations in Drosophila with implications for roles in chromosome structure and function. EMBO J. 1987 Jun;6(6):1781–1789. doi: 10.1002/j.1460-2075.1987.tb02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicci P. G., Knowles D. M., 2nd, Magrath I., Dalla-Favera R. Chromosomal breakpoints and structural alterations of the c-myc locus differ in endemic and sporadic forms of Burkitt lymphoma. Proc Natl Acad Sci U S A. 1986 May;83(9):2984–2988. doi: 10.1073/pnas.83.9.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts T. H., Boehm T., Mengle-Gaw L. Chromosomal abnormalities in lymphoid tumours: mechanism and role in tumour pathogenesis. Trends Genet. 1988 Nov;4(11):300–304. doi: 10.1016/0168-9525(88)90106-0. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H., Forster A., Hamlyn P., Baer R. Effect of somatic mutation within translocated c-myc genes in Burkitt's lymphoma. Nature. 1984 Jun 14;309(5969):592–597. doi: 10.1038/309592a0. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H., Hamlyn P. H., Baer R. Altered nucleotide sequences of a translocated c-myc gene in Burkitt lymphoma. Nature. 1983 Dec 22;306(5945):760–765. doi: 10.1038/306760a0. [DOI] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Russo G., Isobe M., Pegoraro L., Finan J., Nowell P. C., Croce C. M. Molecular analysis of a t(7;14)(q35;q32) chromosome translocation in a T cell leukemia of a patient with ataxia telangiectasia. Cell. 1988 Apr 8;53(1):137–144. doi: 10.1016/0092-8674(88)90495-3. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Hennighausen L., Battey J., Leder P. Chromatin structure and protein binding in the putative regulatory region of the c-myc gene in Burkitt lymphoma. Cell. 1984 Jun;37(2):381–391. doi: 10.1016/0092-8674(84)90368-4. [DOI] [PubMed] [Google Scholar]
- Sims J. E., Tunnacliffe A., Smith W. J., Rabbitts T. H. Complexity of human T-cell antigen receptor beta-chain constant- and variable-region genes. Nature. 1984 Dec 6;312(5994):541–545. doi: 10.1038/312541a0. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Solomon E., Bobrow M., Goodfellow P. N., Bodmer W. F., Swallow D. M., Povey S., Noël B. Human gene mapping using an X/autosome translocation. Somatic Cell Genet. 1976 Mar;2(2):125–140. doi: 10.1007/BF01542626. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. The current status and portability of our sequence handling software. Nucleic Acids Res. 1986 Jan 10;14(1):217–231. doi: 10.1093/nar/14.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow D. M., Solomon E., Pajunen L. Immunochemical analysis of the N-acetyl hexosaminidases in human-mouse hybrids made using a double selective system. Cytogenet Cell Genet. 1977;18(3):136–148. doi: 10.1159/000130758. [DOI] [PubMed] [Google Scholar]
- Thomas B. J., Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989 Feb 24;56(4):619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Jaffe E., Cossman J., Gorham J., Nowell P. C., Croce C. M. Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with the t(11;14) chromosome translocation. Nature. 1985 May 23;315(6017):340–343. doi: 10.1038/315340a0. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Yunis J., Onorato-Showe L., Erikson J., Nowell P. C., Croce C. M. Molecular cloning of the chromosomal breakpoint of B-cell lymphomas and leukemias with the t(11;14) chromosome translocation. Science. 1984 Jun 29;224(4656):1403–1406. doi: 10.1126/science.6610211. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weinreb A., Katzenberg D. R., Gilmore G. L., Birshtein B. K. Site of unequal sister chromatid exchange contains a potential Z-DNA-forming tract. Proc Natl Acad Sci U S A. 1988 Jan;85(2):529–533. doi: 10.1073/pnas.85.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Bingham P. M., Livak K. J., Holmgren R., Elgin S. C. The chromatin structure of specific genes: I. Evidence for higher order domains of defined DNA sequence. Cell. 1979 Apr;16(4):797–806. doi: 10.1016/0092-8674(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- van Heyningen V., Bobrow M., Bodmer W. F., Gardiner S. E., Povey S., Hopkinson D. A. Chromosome assignment of some human enzyme loci: mitochondrial malate dehydrogenase to 7, mannosephosphate isomerase and pyruvate kinase to 15 and probably, esterase D to 13. Ann Hum Genet. 1975 Jan;38(3):295–303. doi: 10.1111/j.1469-1809.1975.tb00613.x. [DOI] [PubMed] [Google Scholar]