Abstract
Fundamental questions remain unanswered about the longitudinal impact of blast-plus-impact complex traumatic brain injuries (TBI) from wars in Iraq and Afghanistan. This prospective, observational study investigated measures of clinical outcome in US military personnel evacuated to Landstuhl Regional Medical Center (LRMC) in Germany after such “blast-plus” concussive TBIs. Glasgow Outcome Scale-Extended assessments completed 6–12 months after injury indicated a moderate overall disability in 41/47 (87%) blast-plus TBI subjects and a substantial but smaller number (11/18, 61%, p=0.018) of demographically similar US military controls without TBI evacuated for other medical reasons. Cognitive function assessed with a neuropsychological test battery was not different between blast-plus TBI subjects and controls; performance of both groups was generally in the normal range. No subject was found to have focal neurological deficits. However, 29/47 (57%) of blast-plus subjects with TBI met all criteria for post-traumatic stress disorder (PTSD) versus 5/18 (28%) of controls (p=0.014). PTSD was highly associated with overall disability; 31/34 patients with PTSD versus 19/31 patients who did not meet full PTSD criteria had moderate to severe disability (p=0.0003). Symptoms of depression were also more severe in the TBI group (p=0.05), and highly correlated with PTSD severity (r=0.86, p<0.0001). Thus, in summary, high rates of PTSD and depression but not cognitive impairment or focal neurological deficits were observed 6–12 months after concussive blast-plus-impact complex TBI. Overall disability was substantially greater than typically reported in civilian non-blast concussive (“mild”) patients with TBI, even with polytrauma. The relationship between these clinical outcomes and specific blast-related aspects of brain injuries versus other combat-related factors remains unknown.
Key words: : blast, clinical outcomes, PTSD, TBI
Introduction
Blast-related traumatic brain injury (TBI) has been a common occurrence in US military personnel during the wars in Iraq and Afghanistan. Based on the Defense and Veterans Brain Injury Center website, there have been 266,810 physician diagnosed TBIs from 2000–2012, of which approximately 80% have been categorized as concussive or “mild” (http://www.dvbic.org/dod-worldwide-numbers-tbi). The RAND report survey1 indicated that the numbers could be substantially higher if the self-report measures used are accurate. Based on a survey of US Army soldiers injured in Iraq, approximately 75% of concussive (mild) TBIs are blast-related.2
Previous studies have reported that subjects with blast-related concussive (mild) TBI have impaired cognitive performance acutely after injury3 and substantial long-lasting symptoms,2,4–11 but generally normal cognitive performance at later times.7,12–16 U.S. military personnel with concussive (mild) TBI have also been reported to have a high rate of post-traumatic stress disorder (PTSD) and depression.2,6–9,11,15,17–21 It has been argued that PTSD symptoms may account for the mismatch between cognitive symptoms and performance.2,15 Many of these previous studies, however, have been based largely on self-report and screening tools,2,6,9,20 rather than direct clinical assessments in prospectively identified cohorts.
In addition, the previous studies of sub-acute to chronic clinical outcomes have been based on subjects enrolled after they have returned to the United States. Few of them needed evacuation from the combat theater for their injuries. There have been no previous reports to our knowledge on the clinical outcomes of US military personnel with injuries that met criteria for concussive (mild) TBI22 but nonetheless were substantial enough to necessitate evacuation. These more substantially injured US military personnel are typically evacuated from the theater to the Landstuhl Regional Medical Center (LRMC) in Landstuhl, Germany. LRMC has served as the sole level IV strategic evacuation hub for the wars in Iraq and Afghanistan, and has used a comprehensive TBI screening protocol for all evacuated casualties23 since 2006.
As part of an ongoing prospective study involving advanced magnetic resonance imaging (MRI)-based methods to evaluate acute military TBI,24 we enrolled US military personnel with blast-related TBI as well as blast-exposed controls with other injuries and illnesses at LRMC. We report clinical outcomes assessed in these subjects 6–12 months after enrollment at the time of their follow-up evaluations at Washington University in St. Louis.
Methods
Subjects
Inclusion criteria for the TBI group were as follows: (1) a positive screen for TBI at LRMC based on standard US military clinical criteria23 including self-report of blast exposure resulting in loss of consciousness, amnesia for the event, or change in neurological status; (2) injury from blast with or without additional mechanisms of injury within 90 days of enrollment; (3) US military; (4) ability to provide informed consent in person; (5) no contraindications to MRI such as retained metallic fragments; (6) no history of moderate to severe TBI based on Department of Defense (DoD) criteria; (7) no history of major psychiatric disorder; (8) agreement to communicate by telephone or e-mail monthly for 6–12 months and then travel to Washington University for in-person follow-up. Inclusion criteria for the control group were the same except for a negative screen for TBI at LRMC.
The research protocol was approved by the Human Research Protection Office at Washington University, the Institutional Review Board for LRMC at Brooke Army Medical Center, and the Clinical Investigation Regulatory and Human Research Protection Offices of the U.S. Army Medical Research and Materiel Command. Written informed consent was obtained from all subjects in person at LRMC; no surrogate consent was allowed by the funding agency. Competence to provide informed consent was assessed in a standardized fashion based on responses to questions regarding the purpose of the study, expected requirements for participation, and potential risks. Additional written consent was obtained from the subjects at the time of follow-up at Washington University. Active duty military subjects were not paid for participation, although travel expenses to St. Louis were covered. Subjects not on active military duty status at the time of follow-up in St. Louis were paid $240 plus travel expenses for participation.
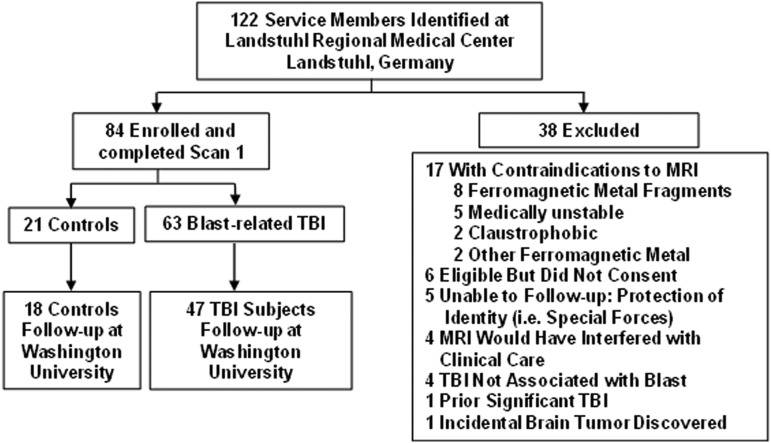
We enrolled 63 subjects with TBI and 21 controls at LRMC over 5 non-contiguous months during 2008–2009 (Fig. 1). Median time from injury to enrollment was 14 days (range 1–90 days). The demographics of the TBI subjects and controls were similar (Table 1). Most subjects were young, white, enlisted, soldiers in the US Army. All were male. We did not specifically exclude females but did not have an opportunity to enroll any during the period of this study.
FIG. 1.
Screening, enrollment, and exclusion characteristics of the study participants. TBI, tramautic brain injury; MRI, magnetic resonance imaging.
Table 1.
Characteristics of Study Participants
| Characteristic | Control (n=21) | Control follow-up (n=18) | TBI (n=63) | TBI follow-up (n=47) |
|---|---|---|---|---|
| Age in years: median (range) | 32 (19–53) | 32 (21–53) | 25 (19–58) | 25 (19–58) |
| Education in years: median (range) | N/A | 12.5 (11–17.5) | N/A | 12 (8–17) |
| Race/ethnicitya – no (%) | ||||
| White | 17 (80.9%) | 15 (83.3%) | 48 (76.2%) | 34 (72.3%) |
| African American | 3 (14.2%) | 2 (11.1%) | 7 (11.1%) | 5 (10.6%) |
| Hispanic/Latino | 1 (4.7%) | 1 (5.5%) | 9 (14.3%) | 6 (12.7%) |
| Asian | 0 | 0 | 2 (3.2%) | 2 (4.3%) |
| Branch of Service – no (%) | ||||
| US Army | 18 (85.7%) | 15 (83.3%) | 56 (88.9%) | 42 (89.3%) |
| US Air Force | 2 (9.5%) | 2 (11.1%) | 0 | 0 |
| US Marine Corps | 1 (4.8%) | 1 (4.5%) | 7 (11.1%) | 5 (10.7%) |
| US Navy | 0 | 0 | 0 | 0 |
| Military rank – no (%) | ||||
| Enlisted | 19 (90.5%) | 16 (88.9%) | 60 (95.2) | 44 (93.6) |
| Officer | 2 (9.5%) | 2 (11.1%) | 3 (4.7) | 3 (6.4) |
| Theater of operation – no (%) | ||||
| Iraq | 15 (71.4%) | 12 (66.7%) | 25 (39.7%) | 21 (44.7%) |
| Afghanistan | 6 (28.6%) | 6 (33.3%) | 38 (60.3%) | 26 (55.3%) |
N/A, not assessed in subjects that did not follow up.
Self-reported. Subjects were not limited to one choice.
TBI, traumatic brain injury.
All available clinical histories indicated blast exposure plus another mechanism of head injury such as a fall, motor vehicle crash, or being struck by a blunt object. None had an isolated blast injury. Thus, these subjects can be best described as having sustained blast-plus-impact complex TBIs. We refer to this type of injury as “blast-plus,” to distinguish it from isolated blast injury. Diagnosis of TBI was typically made based on self-report of blast exposures with alteration of neurologic function. Specifically, questions included the following23:
1. During this deployment, did you experience any of the following events? Blast (improvised explosive device, rocket-propelled grenade, landmine, grenades, etc), fall (striking head), other significant contact with blunt object (above the shoulders), bullet wound (above the shoulders), vehicular crashes (any vehicle including aircraft), fragment wound (above the shoulders).
2. If you answered yes to Question #1, did you experience any of these symptoms IMMEDIATELY afterward? Loss of consciousness (knocked out), being dazed, confused, or “seeing stars” (feeling disconnected from yourself or the environment), not remembering the injury.
Medical documentation regarding duration of loss of consciousness and post-traumatic amnesia was often not available or not reliable. All available clinical histories indicated change in level of consciousness or loss of consciousness for a few minutes and post-traumatic amnesia for less than 24 h. The requirement for in-person informed consent made patients with more severe TBI typically not eligible, and none were enrolled. No intracranial abnormalities were detected on non-contrast head computed tomography. Thus, all subjects with TBI met the DoD criteria for uncomplicated mild TBI. While previous literature has used the term mild to describe TBI on the lower end of the spectrum of severity, we now prefer the term concussive to describe these injuries, with the understanding that concussive and mild TBI are operationally defined identically.
All clinical histories were verified by study personnel by taking additional clinical history and review of medical records. Based on this review, four subjects were excluded because of TBI not associated with blast, and one was switched from the control group to the TBI group because of evidence of TBI on MRI. None who screened positive for TBI was determined not to have had a TBI.
Of these subjects, 47 with TBI and 18 controls were followed up at Washington University 6–12 months after enrollment. Reasons for inability of 19 subjects (3 controls and 16 with TBI) to follow up at Washington University included inability or unwillingness to travel to St. Louis (10 subjects), withdrawal of consent (4 subjects), inability to maintain telephone or e-mail contact (2 subjects), severe psychiatric illness (1 subject), redeployment overseas (1 subject), and other severe illness (1 subject). The TBI subjects not available for in-person follow-up did not differ from those who were available for follow-up in demographic characteristics (Table 1), Military Acute Concussion Evaluation (MACE) performance (p=0.54, Mann-Whitney U test), or most recent telephone-based Glasgow Outcome Scale-Extended (GOS-E) score (p=0.75, Mann-Whitney U test).
Clinical assessments
Initial records of clinical status in subjects with TBI assessed at LRMC using the MACE23 were reviewed. This brief cognitive test assesses orientation, immediate verbal memory, concentration, and short-term delayed verbal memory.
Overall clinical outcome was assessed using the GOS-E25,26 by telephone or e-mail monthly for 6–12 months. The GOS-E is scored from 1–8: 1=dead, 2=vegetative, 3–4=severe disability, 5–6=moderate disability, 7–8=good recovery. Moderate disability (GOS-E=5–6) is defined as one or more of the following: (1) inability to work to previous capacity; (2) inability to resume the majority of regular social and leisure activities outside the home; (3) psychological problems that have frequently resulted in ongoing family disruption or disruption of friendships. Severe disability is defined as reduced ability to perform activities of daily living such that supervision is needed. Standardized, structured interviews were performed according to published guidelines.25 The last assessment before in-person follow-up was considered the final outcome. Information was gathered separately from both the subject and a collateral source (typically a spouse, parent, or sibling) whenever possible. When information from the subject and the collateral source differed, the worse outcome was used.
The in-person clinical evaluations included a standardized neurological examination and structured interview designed for patients with TBI (Neurobehavioral Rating Scale-Revised27,28), a neuropsychological test battery (Table 2), and psychiatric assessments including the Clinician-Administered PTSD Scale for the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) (CAPS)29 plus the Montgomery-Asberg Depression Rating Scale.30 The CAPS was scored using the standard scoring rules from the National Center for Post-traumatic Stress Disorder, July 1998 revision, from Blake and associates. The standardized neurological examination and interview required approximately 1 h per subject. The psychiatric assessments needed approximately 2 h per subject, and the neuropsychological battery needed approximately 2 h per subject. Subjects took all medications as prescribed by their clinical providers. All tests were performed between 9 AM and 5 PM in private, quiet, well-lighted rooms. All examiners were blinded to other clinical information and imaging results, although in the course of the interviews, it often became clear whether the subjects were in the TBI or control group. All examiners were clinicians who underwent standardized training in administering the assessments.
Table 2.
Neuropsychological Test Performance
| Test | Control (n=18) | TBI (n=47) | p value | TBI GOS-E 7–8 (n=6) | TBI GOS-E<7 (n=41) | p value | TBI No PTSD (n=18) | TBI+PTSD (n=29) | p value |
|---|---|---|---|---|---|---|---|---|---|
| 25-foot walk (sec) (motor strength, balance, coordination) | 5.2±2.1 | 4.7±1.0 | 0.37 (U) | 4.2±1.2 | 4.8±1.0 | 0.18 (U) | 4.6±0.9 | 4.7±1.0 | 0.41 (t) |
| Conners Continuous Performance Test II | |||||||||
| Omission errors: (attention lapses) | −0.45±2.1 | −0.14±1.3 | 0.47 (U) | 0.36±0.4 | −0.21±1.3 | 0.15 (t) | 0.32±0.6 | −0.42±1.5 | 0.04 (U) |
| Commission errors: (impulsivity) | −0.1±1.1 | −0.17±1.0 | 0.38 (t) | −0.09±1.0 | −0.19±1.0 | 0.41 (t) | 0.11±0.9 | −0.35±1.0 | 0.06 (t) |
| Hit rate: (reaction time) | 0.06±1.1 | 0.23±0.9 | 0.26 (t) | 0.10±1.4 | 0.25±0.8 | 0.36 (t) | −0.06±0.9 | 0.41±0.9 | 0.04 (t) |
| Hit rate block change: (sustained vigilance) | −0.26±1.0 | −0.22±1.1 | 0.33 (U) | 0.06±0.5 | −0.26±1.1 | 0.34 (U) | −0.12±1.1 | −0.28±1.1 | 0.20 (U) |
| Wisconsin Card Sorting Test: total errors (concept formation, mental flexibility) | 0.58±0.8 | 0.66±0.9 | 0.38 (t) | 0.62±0.6 | 0.66±1.0 | 0.46 (t) | 0.63±0.7 | 0.67±1.1 | 0.43 (t) |
| Rey-Osterrieth Complex Figure Test: Delayed recall (visual memory) | 0.03±1.3 | −0.55±1.7 | 0.10 (t) | −0.32±1.5 | −0.58±1.7 | 0.36 (t) | −0.84±2.0 | −0.37±1.5 | 0.18 (t) |
| Wechsler Test of Adult Reading (estimate of pre-injury verbal intelligence) | −0.24±1.3 | −0.18±1.2 | 0.40 (U) | 0.07±1.4 | −0.22±1.1 | 0.27 (t) | −0.36±1.5 | −0.07±0.9 | 0.26 (U) |
| California Verbal Learning Test II | |||||||||
| Long-delay free recall (Verbal memory) | 0.0±0.9 | −0.13±0.9 | 0.35 (U) | −0.7±1.0 | −0.05±0.9 | 0.13 (U) | −0.11±0.9 | −0.14±1.0 | 0.46 (t) |
| Total intrusions (falsely recalled items) | −0.44±1.5 | −0.15±1.0 | 0.31 (U) | −0.42±0.5 | −0.11±1.1 | 0.15 (U) | 0.00±0.8 | −0.24±1.1 | 0.32 (U) |
| List B vs. Trial 1 List A (proactive memory interference) | 0.11±1.1 | −0.34±1.1 | 0.07 (t) | −0.08±0.9 | −0.39±1.1 | 0.26 (t) | −0.36±1.0 | −0.33±1.2 | 0.46 (t) |
| Grooved pegboard (motor speed & coordination) | |||||||||
| Dominant hand time | −1.4±0.6 | −1.1±0.8 | 0.10 (t) | −1.35±0.5 | −1.06±0.9 | 0.22 (t) | −1.27±0.7 | −0.99±0.9 | 0.13 (U) |
| Non-dominant hand time | −1.3±0.8 | −1.0±0.8 | 0.16 (t) | −0.68±0.6 | −1.07±0.8 | 0.15 (t) | −1.12±0.8 | −0.96±0.8 | 0.26 (t) |
| Trail making test | |||||||||
| Trails A time (visual scanning, coordination) | −0.09±0.9 | −0.29±1.1 | 0.25 (t) | −0.02±1.3 | −0.33±1.0 | 0.25 (t) | −0.01±1.1 | −0.46±1.0 | 0.08 (t) |
| Trails B time (Trails A+mental flexibility) | 0.02±0.9 | −0.23±1.1 | 0.20 (t) | −0.02±0.9 | −0.26±1.1 | 0.30 (t) | 0.00±1.13 | −0.38±1.0 | 0.12 (t) |
| Symbol digit modalities test (working memory) | 0.14±1.0 | −0.22±0.8 | 0.04 (U) | 0.08±0.5 | −0.27±0.8 | 0.24 (U) | −0.17±0.7 | −0.26±0.9 | 0.46 (U) |
| Controlled oral word association Total score: (verbal fluency) | −1.08±0.7 | −0.80±0.9 | 0.12 (t) | −0.75±1.0 | −0.81±0.9 | 0.44 (t) | −0.77±0.9 | −0.82±1.0 | 0.42 (t) |
Timed walk is reported in seconds. All other test results have been converted to Z-scores with higher scores representing better performance in all cases. The mean Z-scores for the US age and education-matched general male population are 0, and standard deviations are 1. Means±standard deviations are reported. All assessors were blinded to clinical and radiological information. GOS-E: Glasgow Outcome Scale-Extended. Scores of 7–8 represent good overall outcome, and scores <7 represent moderate to severe overall disability. PTSD: Post-traumatic stress disorder. PTSD was defined as meeting all criteria on the Clinician Administered PTSD Scale for DSM-IV. Note that many subjects in the “TBI no PTSD” group still have a substantial burden of PTSD symptoms, but did not meet all criteria for a categorical diagnosis of PTSD. The p values represent results of one-sided t tests (t) or one-sided Mann-Whitney U-tests (U); the prespecified hypotheses were that patients with traumatic brain injury (TBI) would perform worse than controls, subjects with poor overall outcome would perform worse than those with good overall outcome, and subjects with TBI+PTSD would perform worse than those with TBI and no PTSD. Results have not been corrected for multiple comparisons. None were significant after Bonferroni correction for 17 variables.
The neuropsychological test battery consisted of the Conners Continuous Performance Test II,31 a computer-based assessment of attention, impulsivity, reaction time, and vigilance; the Wisconsin Card Sorting Test,32 an assessment concept formation and mental flexibility; the Rey-Osterrieth Complex Figure Test,33 a paper and pencil test of visual memory; the California Verbal Learning Test II,34 an assessment of verbal declarative memory; the 25 hole grooved Peg-Board test,35 an assessment of upper extremity motor speed and coordination; a timed 25 foot walk; the Trail Making test,36 an assessment of visual scanning, coordination, and mental flexibility; the symbol digit modalities test,37 an assessment of working memory and processing speed; the controlled oral word association test,38 an assessment of verbal fluency; and the Wechsler Test of Adult Reading,39 as an estimate of pre-injury verbal intelligence. A relatively easy forced choice test embedded in the California Verbal Learning Test was used to assess adequacy of effort.
Safety and data monitoring
Subjects were assigned a random four-digit code number to protect confidentiality, and all research data were identified by code number only. A board-certified psychiatrist (ECN) was immediately available in case the CAPS examination exacerbated PTSD symptoms. No exacerbations necessitating medical intervention occurred, although additional support from study staff was needed on several occasions.
For clinical evaluations, the principal investigator audited 1 in 10 randomly selected subjects' data sets to ensure that data were scored and entered correctly. These audits revealed only minor discrepancies in scoring criteria that were then corrected across the entire cohort of subjects.
Statistical analyses
All data was analyzed using Statistica 6.0 (Statsoft Inc). Chi-square analyses were used to assess the relationships between categorical variables. Continuous variables have been summarized as mean±standard deviation unless otherwise specified. The normal distribution of each continuous variable was assessed using the Shapiro-Wilk test. For normally distributed variables, unpaired Student t tests were used to compare groups. For non-normally distributed variables, Mann-Whitney U tests were used. We prespecified that subjects with TBI would have worse outcomes than controls. One-sided tests were used when hypotheses were prespecified, and two-sided tests were used otherwise. For correlations between continuous variables, Pearson product moment correlations were used when correlations were approximately linear and residuals were normally distributed based on Shapiro-Wilk testing. When these criteria were not met, Spearman non-parametric correlations were used. Uncorrected p values have been reported, but only considered significant if p<0.05 after Bonferroni correction for multiple comparisons within each class of variables.
Clinical trials identifier
The study was registered at clinicaltrials.gov (NCT00785304).
Results
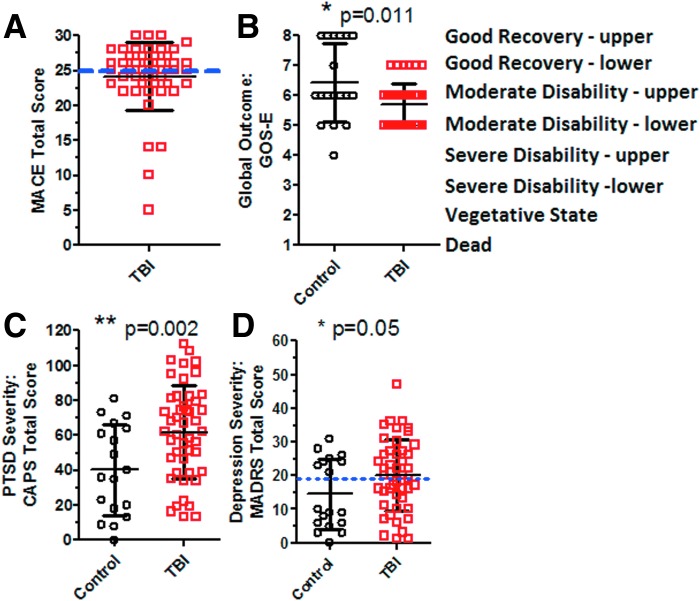
At LRMC, the MACE scores in the subjects with TBI (Fig. 2A) indicated that 19/47 (40.4%) fell below a score of 25 of 30 points, often used as a cutoff for an abnormal score.23 The MACE was not performed on the control subjects. MACE testing was part of clinical care for patients with TBI at LRMC and was not a prespecified part of the research protocol.
FIG. 2.
Clinical assessments in US military personnel with concussive “blast-plus” traumatic brain injury (TBI). (A) Military Acute Concussion Evaluation (MACE) scores in subjects with TBI 1–90 days after injury at Landstuhl Regional Medical Center. Maximum score is 30. Higher scores indicate better performance. A cutoff of below 25 (blue dashed line) is considered to represent poor performance. (B) Global clinical outcomes assessed using the Glasgow Outcome Scale-Extended (GOS-E) scores 6–12 months after enrollment. *Indicates one-tailed Mann-Whitney U test. (C) Post-traumatic stress disorder (PTSD) severity, based on the Clinician Administered PTSD scale (CAPS). Higher scores represent more severe PTSD; maximum score is 132. **Indicates two-sided Student t test. (D) Depression severity assessed based on the Montgomery-Asberg Depression Rating Scale (MADRS) structured interview. Dashed blue line indicated cutoff score of 19: >19 reflects moderate to severe depression. *Indicates one-sided Mann-Whitney U test. Color image is available online at www.liebertpub.com/neu
Global clinical outcomes were worse in subjects with TBI than controls (Fig. 2B). GOS-E scores 6–12 months after enrollment were significantly lower in the subjects with TBI (p=0.011, one-tailed Mann-Whitney U test). More subjects with TBI (41/47, 87%) than controls (11/18, 61%) had moderate to severe overall disability (p=0.019, chi-square), defined as a GOS-E score of 6 or less. Only one subject had severe disability; most subjects had moderate disability. The high rate of moderate disability in both groups is not typically observed in civilian polytrauma cases with concussive (mild) TBI (see Discussion).
Neuropsychological test results did not indicate substantial differences between the subjects with TBI and the controls; both groups generally performed within expectation for age and educational level on most tests (Table 2). All subjects performed well on a test of effort embedded in the California Verbal Learning Test. The psychometricians reported good apparent effort during testing. Likewise, performance did not differ between subjects with TBI with good outcomes (GOS-E=7–8) versus those with moderate to severe disability (GOS-E<7). This suggests that cognitive performance impairments may not account for the overall disability observed.
Performance on a standardized neurological interview and examination, the Neurobehavioral Rating Scale, similarly did not reveal major abnormalities, although subjects with TBI were slightly more impaired than controls (Table 3). Blast-plus subjects with TBI overall had marginally more severe neurobehavioral symptoms and deficits than control patients (p=0.03, one-sided Mann Whitney U test). The largest contributing sub-score was mood/affect abnormalities (p=0.03). Blast-plus subjects with TBI who met all criteria for PTSD had worse positive symptoms (p=0.005) and mood/affect abnormalities (p=0.02) compared with subjects with TBI who did not meet full PTSD criteria.
Table 3.
Neurobehavioral Rating Scale Results
| Rating | Control (n=18) | TBI (n=47) | p | TBI GOS-E 7–8 (n=6) | TBI GOS-E<7 (n=41) | p | TBI no PTSD (n=18) | TBI+PTSD (n=29) | p |
|---|---|---|---|---|---|---|---|---|---|
| Total score (max 87, higher scores worse) | 7.9±6.8 | 11.6±7 | 0.03* | 8.7±5.5 | 12.0±7.5 | 0.18 | 7.8±4.3 | 13.9±7.8 | 0.10 |
| Executive/cognitive dysfunction (max 24) | 3.1±2.6 | 3.8±2.8 | 0.23 | 2.5±1.8 | 4.0±2.9 | 0.11 | 3.1±1.9 | 4.3±3.2 | 0.10 |
| Positive symptoms (max 21) | 1.1±1.8 | 1.4±1.6 | 0.11 | 0.8±0.4 | 1.5±1.7 | 0.31 | 0.6±0.7 | 2.0±1.8 | 0.005* |
| Negative symptoms (max 12) | 0.8±1.0 | 1.1±1.3 | 0.23 | 1.3±1.6 | 1.1±1.2 | 0.43 | 0.8±0.9 | 1.4±1.4 | 0.09 |
| Mood/affect abnormalities (max 15) | 2.1±2.2 | 3.4±2.6 | 0.03 | 3.2±1.9 | 3.5±2.7 | 0.46 | 2.3±1.7 | 4.1±2.9 | 0.02 |
| Oral/motor dysfunction (max 12) | 0.1±0.3 | 0.7±1.0 | 0.02 | 0.5±0.5 | 0.7±1.0 | 0.50 | 0.4±0.6 | 0.8±1.1 | 0.13 |
| Worst single domain score (max 3) | 1.4±0.8 | 1.8±0.6 | 0.04 | 1.7±0.5 | 1.9±0.6 | 0.25 | 1.7±0.5 | 1.9±0.7 | 0.18 |
The Neurobehavioral Rating Scale score is based on a structured interview and neurological examination followed by clinician ratings across 29 domains. Each domain is rated 0 (no abnormalities) through 3 (severe, disabling abnormalities). The total score is the sum of the ratings across all 29 domains. The five sub-scores are based on previously published principal component analyses from a large group of civilian patients with TBI.28 Each sub-score is the sum of scores from 4–8 domains. The “worst single domain score” was also assessed because the total scores are not necessarily ordinal—i.e., a single high score (2 or 3) in one domain can represent impairing or disabling symptoms and deficits, while several scores of 1 in multiple domains may not represent as much overall impairment. The p values represent results of one-sided Mann-Whitney U tests. *Indicates statistical significance for the total score at p<0.05, or after Bonferroni correction for multiple comparisons at p<0.01 for the subscores. Means±standard deviations are reported.
TBI, traumatic brain injury; GOS-E, Glasgow Outcome Scale-Extended; PTSD, post-traumatic stress disorder.
No subjects had focal neurological deficits detected during the neurological examination, performed by a board-certified neurologist (DLB). Specifically, none of the patients with TBI had impairing dysarthria, aphasia, neglect, hemianopsia, cranial nerve deficits, hemiparesis, parkinsonism, ataxia, dystonia, sensory loss, or neurological gait disorders.
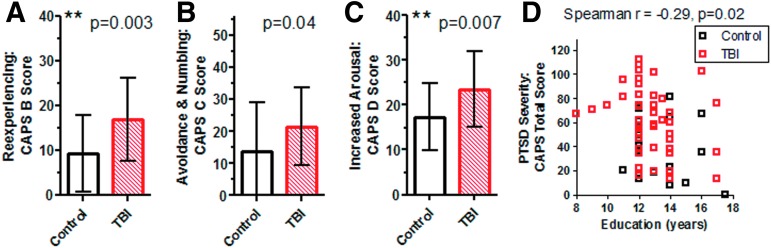
Psychiatric assessments revealed substantially more frequent and more severe PTSD in the subjects with TBI. Specifically, 61% (29/47) of subjects with TBI and 28% (5/18) of controls met DSM-IV criteria for PTSD (p=0.0143, chi-square) as assessed using the CAPS. The severity of PTSD was also significantly greater in the TBI group (Fig. 2C, p=0.002, t test). All evaluated subjects with TBI and 17/18 controls met PTSD criterion A: “one or more traumatic events that involved actual or threatened death or serious injury and a reaction that included intense fear, helplessness or horror.” PTSD severity was significantly increased in the subjects with TBI across all three major sub-domains (Fig. 3A–C): “Reexperiencing” (CAPS B), “Avoidance and Numbing” (CAPS C), and “Increased Arousal” (CAPS D). The largest difference between TBI and control subjects was in reexperiencing (CAPS B), and the greatest overall burden of PTSD symptoms was in increased arousal (CAPS D).
FIG. 3.
Post-traumatic stress disorder (PTSD) severity assessed using Clinician Administered PTSD scale (CAPS subscales A–C). Subjects with traumatic brain injury (TBI) had more severe PTSD symptoms in all three sub-domains. The sub-domains were based on the DSM-IV criteria for PTSD. The maximum scores are CAPS B: 40, CAPS C: 56, CAPS D: 40. Bars represent mean and standard deviation. **Indicates one-sided Student t tests <0.017 after Bonferroni correction for multiple comparisons. Inverse correlation between self-reported years of formal education and PSTD severity (D). Color image is available online at www.liebertpub.com/neu
Symptoms of depression were also more severe in subjects with TBI compared with controls (Fig. 2D). Depression severity based on Montgomery-Asberg Depression Rating Scale scores were 19±11 in subjects with TBI and 14±10 in controls (p=0.05, one-sided Mann-Whitney U test). Depression, however, did not differentiate as strongly as PTSD between TBI and control subjects. Using a total score >19 on the Montgomery-Asberg Depression Rating Scale as the criterion, significant depression was present in 24/47 (51%) of subjects with TBI and 8/18 (44%) of controls (p=0.63).
Montgomery-Asberg Depression Rating Scale scores were highly correlated with CAPS total scores for PTSD in the entire cohort (Pearson r=0.86, p<0.001), in the subjects with TBI (Pearson r=0.82, p<0.001), and in the control subjects (Pearson r=0.95, p<0.001).
PTSD was strongly associated with overall adverse outcomes. Across both TBI and control groups, 33/34 (97%) of subjects who met all criteria for PTSD had moderate to severe overall disability versus 19/31 (61%) who did not meet full PTSD criteria (p=0.0003, chi-square). A similar relationship held for the TBI subjects in isolation (28/29 vs. 13/19, p=0.015). CAPS scores were 62±25 in subjects with moderate to severe disability versus 31±24 in subjects with good outcomes (p=0.0001, t test). Depression was also strongly associated with overall adverse outcomes: 31/32 (97%) of subjects with depression versus 21/33 (64%) of subjects without depression had moderate to severe overall disability (p=0.0008, chi-square).
There were no differences in neuropsychological test performance in subjects with TBI with PTSD versus subjects with TBI who did not meet all criteria for PTSD (Table 2). Likewise, there were no apparent demographic differences between TBI study participants with versus without PTSD (Table 4). Subjects with PTSD had higher positive symptoms (p=0.005) and mood/affect abnormalities (p=0.02) on the neurobehavioral rating scale (Table 3). Neurobehavioral Rating Scale was performed by a different investigator than the CAPS interview, and these two measures were scored blinded to the other results.
Table 4.
Characteristics of Traumatic Brain Injury Study Participants with and without Post-Traumatic Stress Disorder
| Characteristic | TBI No PTSD (n=18) | TBI+PTSD (n=29) | p value |
|---|---|---|---|
| Age in years: median (range) | 23.5 (21–58) | 27 (19–45) | 0.44 (U) |
| Education in years: median (range) | 13 (10−17) | 12 (8−17) | 0.25 (U) |
| Race/ethnicity* – no (%) | 0.49 (C) | ||
| White | 12 (66.7%) | 22 (75.9%) | |
| African American | 3 (16.6%) | 2 (6.9%) | |
| Hispanic/Latino | 2 (11.1%) | 4 (13.8%) | |
| Asian | 1 (5.6%) | 1 (3.4%) | |
| Branch of Service – no (%) | 0.04 (C) | ||
| US Army | 14 (77.8%) | 28 (95.6%) | |
| US Air Force | 0 | 0 | |
| US Marine Corps | 4 (22.2%) | 1 (3.4%) | |
| US Navy | 0 | 0 | |
| Military rank – no (%) | 0.30 (C) | ||
| Enlisted | 16 (88.9) | 28 (95.6) | |
| Officer | 2 (11.1) | 1 (3.4) | |
| Theater of operation – no (%) | 0.98 (C) | ||
| Iraq | 8 (44.4%) | 13 (44.9%) | |
| Afghanistan | 10 (55.5%) | 16 (55.1%) |
TBI, traumatic brain injury; PTSD, post-traumatic stress disorder; (U), Mann-Whitney U test, (C) chi square. For race/ethnicity, this comparison was for white versus other.
There was a modest negative correlation between self-reported level of education and overall PTSD severity (Spearman r=−0.29, p=0.02, Fig. 3D). The tight clustering of educational between 12–14 years made this difficult to fully interpret, however.
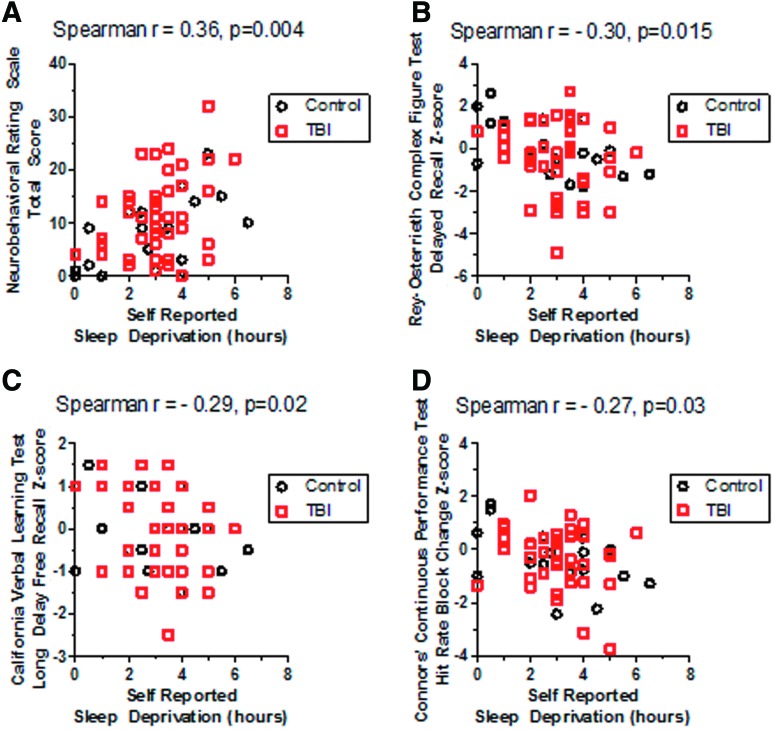
Sleep deprivation was correlated with Neurobehavioral Rating Scale scores (Fig. 4) and performance on several neuropsychological test measures (Fig. 4B–D). Sleep deprivation was self-reported as part of CAPS item D-1 and defined as desired number of hours of sleep per night minus total number of hours of sleep per night. Within the Neurobehavioral Rating Scale, the strongest correlations were with the mood/affect (Spearman r=0.31) and executive/cognitive subscales (Spearman r=0.28).
FIG 4.
Correlations between self-reported sleep deprivation and test performance. (A) Positive correlation with Neurobehavioral Rating Scale total score, where higher scores indicate worse performance. (B) Negative correlation with visual memory performance on the delayed recall portion of the Rey-Osterrieth Complex Figure Test, where lower Z-scores indicate worse performance. (C) Negative correlation with verbal memory performance on the long delay free recall portion of the California Verbal Learning Test, where again lower Z-scores indicate worse performance. (D) Negative correlation with sustained vigilance, assessed using the hit rate block change measure from the Conners Continuous Performance Test, where similarly lower Z-scores indicate worse performance. TBI, traumatic brain injury. Color image is available online at www.liebertpub.com/neu
Discussion
We found that overall disability 6–12 months after concussive blast-plus TBI in US military personnel evacuated to LRMC was surprisingly high. Overall outcomes based on the GOS-E were considerably worse than have been reported in civilian cohorts with concussive (mild) TBI,40–46 and higher even than civilian polytrauma patients with concussive (mild) TBI.40,41 The most directly comparable civilian studies40,41 reported that 33–36% of polytrauma patients with concussive (mild) TBI had GOS-E scores of <7, indicating moderate to severe disability. In contrast, 87% of subjects in our cohort had GOS-E scores of <7.
Importantly, while outcomes were worse in the subjects with TBI than in the controls, moderate disability was common in the control group as well. This suggests that common aspects experienced by US military personnel injured or ill enough to be evacuated from theater also contributed substantially to outcomes. This is not surprising, because subjects with less substantial health concerns were typically treated in theater and not evacuated to LRMC.
We found that cognitive performance, however, as assessed using standardized neuropsychological testing was generally normal. Control subjects, subjects with TBI without PTSD, and subjects with TBI with PTSD all performed equally well, and all three groups performed essentially as expected for their ages and educational levels. Likewise, none had focal neurological deficits.
Performance on the grooved Peg-Board, a test of motor speed and coordination, was borderline to mildly deficient in both TBI and control subjects. This did not differ between groups and did not differ as a function of global outcome (Table 2). Also, there was low average performance in both TBI and control subjects on the Controlled Oral Word Association test, an assessment of verbal fluency (Table 2). It was not clear why performance on the grooved Peg-Board and Controlled Oral Word Association were worse than expected in both groups. The subjects did not have focal neurological deficits, and neither grooved Peg-Board nor Controlled Oral Word Association scores were correlated with PTSD measures, depression, or self-reported sleep loss.
The modest correlations of self-reported sleep deprivation with cognitive performance are not surprising. Future investigations with larger samples sizes will be needed to determine whether there is an interaction between TBI and sleep deprivation, such that, for example, patients with TBI might be more sensitive to the effects of sleep deprivation than controls. Further, objective measurements of sleep quantity and quality would likely improve the accuracy of these correlations.
The disability appeared to be most closely related to PTSD and depression. Most subjects who reported being unable to work at all (GOS-E of 5), work at reduced capacity (GOS-E of 6), or reported significant impairments in social or family life (GOS-E of 6) also had substantial PTSD, depression, or both. As noted above, no cognitive impairments or focal neurological deficits that could account for this level of disability were detected. It was clearly understood by the subjects that the clinical assessors and research staff had no role in any disability determinations and none of the research data would become part of their medical records. Thus, secondary gain issues were unlikely to have played a role. In general, validity ratings for all assessments were quite high. Because of the close correlation between PTSD and depression severities, the relative contribution of PTSD versus depression could not be determined.
Strengths of this study include prospective identification of a relatively homogenous cohort of subjects and standardized, blinded, clinician evaluations of outcomes. Limitations include a modest sample size, all male subjects, no predeployment testing,16 no direct comparison with identically assessed non–blast-related subjects with TBI, no formal assessment of combat exposure intensity, and absence of genetic data. We cannot rule out deficits in cognitive or behavioral domains not tested,47,48 nor early cognitive impairments that resolved before follow-up evaluation. Likewise, we did not address the question of pure blast-related TBI versus blast plus other mechanisms. All of our subjects had blast plus another injury mechanism indicating that the incidence of pure blast-related TBI47,49,50 may be low in US military personnel injured seriously enough to be evacuated to LRMC. It should also be noted that this cohort, although representative of medically evacuated personnel, may not be generalizable to those sustaining injuries who remain in theater. Further, the diagnoses of TBI were largely based on self-report; thus, we cannot rule out the possibility that some patients with TBI and controls were miscategorized. At present, there are no validated objective tests for concussive TBI, so this reflects a limitation not just of these results but of the entire field of concussive (mild) TBI research.
We did not systematically assess all potential factors contributing to depression and PTSD other than TBI. Based on three lines of evidence, however, major physical disabilities did not appear to play a substantial role in the greater severity of depression and PTSD in the subjects with TBI compared with the controls: First, analysis of timed 25 foot walk performance (Table 2) revealed no difference between TBI and control groups, no difference between subjects with TBI with good outcomes versus poor outcomes, and no difference between subjects with TBI with PTSD versus without PTSD. All subjects completed the 25 foot walk. Second, oral/motor dysfunction as assessed by the Neurobehavioral Rating Scale (Table 3) was the least substantially affected sub-score, and again did not differ by group, by outcome, or by PTSD status. Third, there were no substantial differences in the extent of self-reported sleep deprivation in the two groups of subjects, as can be seen from the scatter plots in Figure 4. We did not collect Injury Severity Scores, however, nor did we systematically assess pain or medications to treat pain in this study.
Further research will be needed to determine the underlying explanation for the higher rate of PTSD and depression in the concussive subjects with TBI than in controls and whether this directly translates to patients who are not medically evacuated from combat. Possibilities include more intense combat experiences, intrinsic genetic or environmental factors leading to both higher risk of TBI and vulnerability to PTSD and depression, blast-related hormonal abnormalities,51 and blast-related injuries to specific parts of the brain causing impaired emotional resilience and thereby increasing the incidence or severity of disorders of mood regulation. Of note, repetitive blast injuries in anesthetized rats caused chronic PTSD-like behavioral traits.52 Ongoing human imaging and genetic studies will be needed to begin to address these possibilities. It remains to be determined whether specific treatments for PTSD and depression will effectively improve outcomes in blast-related concussive subjects with TBI.
Acknowledgments
We would like to thank the participants, their families, commanding officers, and clinical providers for making this study possible. We are grateful for the assistance of the LRMC TBI screening team including Marcel Flores, Kathie Martin, Sgt. Shawn Nelson, Pam Nyman, Maj. Shawna Scully, MD, Karen Williams, and Janna Welch; LRMC support staff including Daniel Lovasz, Caroline Tuman, and Linda Wierzechowski; and the Washington University clinical assessment team including Vera Bonsi, Justin Hampton, Leslie French, PhD, Eric Shumaker, PhD, and Elaine Tamez, PhD.
The study was funded by grant W81XWH-08-2-0061 from the Congressionally Directed Medical Research Program (PI: Brody) with additional support from NIH F32NS062529 (Mac Donald) and 5K08NS49237 (Brody).
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of the Army, Department of Defense, or U.S. Government.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Tanielian T.L., and Jaycox L.H. (2008). Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. RAND Corporation: Santa Monica, CA [Google Scholar]
- 2.Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., and Castro C.A. (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463 [DOI] [PubMed] [Google Scholar]
- 3.Luethcke C.A., Bryan C.J., Morrow C.E., and Isler W.C. (2011). Comparison of concussive symptoms, cognitive performance, and psychological symptoms between acute blast-versus nonblast-induced mild traumatic brain injury. J. Int. Neuropsychol. Soc. 17, 36–45 [DOI] [PubMed] [Google Scholar]
- 4.Wilk J.E., Bliese P.D., Kim P.Y., Thomas J.L., McGurk D., and Hoge C.W. (2010). Relationship of combat experiences to alcohol misuse among U.S. soldiers returning from the Iraq war. Drug Alcohol Depend. 108, 115–121 [DOI] [PubMed] [Google Scholar]
- 5.Wilk J.E., Herrell R.K., Wynn G.H., Riviere L.A., and Hoge C.W. (2012). Mild traumatic brain injury (concussion), posttraumatic stress disorder, and depression in U.S. soldiers involved in combat deployments: association with postdeployment symptoms. Psychosom. Med. 74, 249–257 [DOI] [PubMed] [Google Scholar]
- 6.Lippa S.M., Pastorek N.J., Benge J.F., and Thornton G.M. (2010). Postconcussive symptoms after blast and nonblast-related mild traumatic brain injuries in Afghanistan and Iraq war veterans. J. Int. Neuropsychol. Soc. 16, 856–866 [DOI] [PubMed] [Google Scholar]
- 7.Scheibel R.S., Newsome M.R., Troyanskaya M., Lin X., Steinberg J.L., Radaideh M., and Levin H.S. (2012). Altered brain activation in military personnel with one or more traumatic brain injuries following blast. J. Int. Neuropsychol. Soc. 18, 89–100 [DOI] [PubMed] [Google Scholar]
- 8.Verfaellie M., Lafleche G., Spiro A., 3rd, Tun C., and Bousquet K. (2013). Chronic postconcussion symptoms and functional outcomes in OEF/OIF veterans with self-report of blast exposure. J. Int. Neuropsychol. Soc. 19, 1–10 [DOI] [PubMed] [Google Scholar]
- 9.Polusny M.A., Kehle S.M., Nelson N.W., Erbes C.R., Arbisi P.A., and Thuras P. (2011). Longitudinal effects of mild traumatic brain injury and posttraumatic stress disorder comorbidity on postdeployment outcomes in national guard soldiers deployed to Iraq. Arch. Gen. Psychiatry 68, 79–89 [DOI] [PubMed] [Google Scholar]
- 10.Terrio H., Brenner L.A., Ivins B.J., Cho J.M., Helmick K., Schwab K., Scally K., Bretthauer R., and Warden D. (2009). Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J. Head Trauma Rehabil. 24, 14–23 [DOI] [PubMed] [Google Scholar]
- 11.Kontos A.P., Kotwal R.S., Elbin R.J., Lutz R.H., Forsten R.D., Benson P.J., and Guskiewicz K.M. (2013). Residual effects of combat-related mild traumatic brain injury. J. Neurotrauma 30, 680–686 [DOI] [PubMed] [Google Scholar]
- 12.Brenner L.A., Terrio H., Homaifar B.Y., Gutierrez P.M., Staves P.J., Harwood J.E., Reeves D., Adler L.E., Ivins B.J., Helmick K., and Warden D. (2010). Neuropsychological test performance in soldiers with blast-related mild TBI. Neuropsychology 24, 160–167 [DOI] [PubMed] [Google Scholar]
- 13.Belanger H.G., Kretzmer T., Yoash-Gantz R., Pickett T., and Tupler L.A. (2009). Cognitive sequelae of blast-related versus other mechanisms of brain trauma. J. Int. Neuropsychol. Soc. 15, 1–8 [DOI] [PubMed] [Google Scholar]
- 14.Brenner L.A., Ladley-O'Brien S.E., Harwood J.E., Filley C.M., Kelly J.P., Homaifar B.Y. and Adler L.E. (2009). An exploratory study of neuroimaging, neurologic, and neuropsychological findings in veterans with traumatic brain injury and/or posttraumatic stress disorder. Mil. Med. 174, 347–352 [DOI] [PubMed] [Google Scholar]
- 15.Drag L.L., Spencer R.J., Walker S.J., Pangilinan P.H., and Bieliauskas L.A. (2012). The contributions of self-reported injury characteristics and psychiatric symptoms to cognitive functioning in OEF/OIF veterans with mild traumatic brain injury. J. Int. Neuropsychol. Soc. Society 18, 576–584 [DOI] [PubMed] [Google Scholar]
- 16.Vasterling J.J., Brailey K., Proctor S.P., Kane R., Heeren T., and Franz M. (2012). Neuropsychological outcomes of mild traumatic brain injury, post-traumatic stress disorder and depression in Iraq-deployed US Army soldiers. Br. J. Psychiatry 201, 186–192 [DOI] [PubMed] [Google Scholar]
- 17.Maguen S., Madden E., Lau K.M., and Seal K. (2012). The impact of head injury mechanism on mental health symptoms in veterans: Do number and type of exposures matter? J. Trauma. 25, 3–9 [DOI] [PubMed] [Google Scholar]
- 18.Bryant R. (2011). Post-traumatic stress disorder vs traumatic brain injury. Dialogues Clin. Neurosci. 13, 251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruff R.L., Riechers R.G., 2nd, Wang X.F., Piero T., and Ruff S.S. (2012). A case-control study examining whether neurological deficits and PTSD in combat veterans are related to episodes of mild TBI. BMJ Open 2, e000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanderploeg R.D., Belanger H.G., Horner R.D., Spehar A.M., Powell-Cope G., Luther S.L., and Scott S.G. (2012). Health outcomes associated with military deployment: mild traumatic brain injury, blast, trauma, and combat associations in the Florida National Guard. Arch. Phys. Med. Rehabil. 93, 1887–1895 [DOI] [PubMed] [Google Scholar]
- 21.Eskridge S.L., Macera C.A., Galarneau M.R., Holbrook T.L., Woodruff S.I., Macgregor A.J., Morton D.J., and Shaffer R.A. (2013). Influence of combat blast-related mild traumatic brain injury acute symptoms on mental health and service discharge outcomes. J. Neurotrauma 30, 1391–1397 [DOI] [PubMed] [Google Scholar]
- 22.Casscells S. (2007). Traumatic Brain Injury: Definition and Reporting, in: Defense, A.S.o. (ed). Department of Defense [Google Scholar]
- 23.Dempsey K.E., Dorlac W.C., Martin K., Fang R., Fox C., Bennett B., Williams K., and Flaherty S. (2009). Landstuhl Regional Medical Center: traumatic brain injury screening program. J. Trauma Nurs. 16, 6–7, 10–12 [DOI] [PubMed] [Google Scholar]
- 24.Mac Donald C.L., Johnson A.M., Cooper D., Nelson E.C., Werner N.J., Shimony J.S., Snyder A.Z., Raichle M.E., Witherow J.R., Fang R., Flaherty S.F., and Brody D.L. (2011). Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 364, 2091–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson J.T., Pettigrew L.E., and Teasdale G.M. (1998). Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. Journal of neurotrauma 15, 573–585 [DOI] [PubMed] [Google Scholar]
- 26.Pettigrew L.E., Wilson J.T. and Teasdale G.M. (2003). Reliability of ratings on the Glasgow Outcome Scales from in-person and telephone structured interviews. J. Head Trauma Rehabil. 18, 252–258 [DOI] [PubMed] [Google Scholar]
- 27.Levin H.S., High W.M., Goethe K.E., Sisson R.A., Overall J.E., Rhoades H.M., Eisenberg H.M., Kalisky Z., and Gary H.E. (1987). The neurobehavioural rating scale: assessment of the behavioural sequelae of head injury by the clinician. J. Neurol. Neurosurg. Psychiatry 50, 183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCauley S.R., Levin H.S., Vanier M., Mazaux J.M., Boake C., Goldfader P.R., Rockers D., Butters M., Kareken D.A., Lambert J., and Clifton G.L. (2001). The neurobehavioural rating scale-revised: sensitivity and validity in closed head injury assessment. J. Neurol. Neurosurg. Psychiatry 71, 643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weathers F.W., Keane T.M., and Davidson J.R. (2001). Clinician-administered PTSD scale: a review of the first ten years of research. Depress. Anxiety 13, 132–156 [DOI] [PubMed] [Google Scholar]
- 30.Montgomery S.A., and Asberg M. (1979). A new depression scale designed to be sensitive to change. Br. J. Psychiatry 134, 382–389 [DOI] [PubMed] [Google Scholar]
- 31.Conners C., and Staff M. (2000). Conners' Continuous Performance Test II: Computer Program for Windows Technical Guide and Software Manual. Multi-Health Systems: North Tonwanda, NY [Google Scholar]
- 32.Heaton R., Chelune G., Talley J., Kay G., and Curtiss G. (1993). Wisconsin Card Sorting Test Manual Revised and Expanded. Pychological Assessment Resources: Odessa, Fl [Google Scholar]
- 33.Taylor L. (1991). Scoring criteria for the ROCF, in: A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Spreen O., Strauss E. (eds). Oxford: New York [Google Scholar]
- 34.Delis D, Kramer J, Kaplan E and B O. (2000). California Verbal Learning Test Manual: Second Edition, Adult Version. Psychological Corporation: San Antonio, TX [Google Scholar]
- 35.Matthews C, and Kløve H. (1964). Instruction Manual for the Adult Neuropsychology Test Battery. University of Wisconsin Medical School: Madison, WI [Google Scholar]
- 36.Reitan R. (1992). Trail Making Test Manual for Administration and Scoring. Reitan Neuropsychology Laboratory: Tuscon, AZ [Google Scholar]
- 37.Smith A. (1991). Symbol Digit Modalities Test. Western Psychological Services: Los Angeles [Google Scholar]
- 38.Benton A, Hamsher K., and Sivan A.B. (1983). Multilingual Aphasia Examination (3rd ed.). AJA Associates: Iowa City, Ia [Google Scholar]
- 39.Wechsler D. (2001). Wechsler Test of Adult Reading (WTAR) Manual. Psychological Corporation: New York [Google Scholar]
- 40.Jacobs B., Beems T., Stulemeijer M., van Vugt A.B., van der Vliet T.M., Borm G.F., and Vos P.E. (2010). Outcome prediction in mild traumatic brain injury: age and clinical variables are stronger predictors than CT abnormalities. J. Neurotrauma 27, 655–668 [DOI] [PubMed] [Google Scholar]
- 41.Stulemeijer M., van der Werf S.P., Jacobs B., Biert J., van Vugt A.B., Brauer J.M. and Vos P.E. (2006). Impact of additional extracranial injuries on outcome after mild traumatic brain injury. J. Neurotrauma 23, 1561–1569 [DOI] [PubMed] [Google Scholar]
- 42.Mosenthal A.C., Livingston D.H., Lavery R.F., Knudson M.M., Lee S., Morabito D., Manley G.T., Nathens A., Jurkovich G., Hoyt D.B., and Coimbra R. (2004). The effect of age on functional outcome in mild traumatic brain injury: 6-month report of a prospective multicenter trial. J. Trauma 56, 1042–1048 [DOI] [PubMed] [Google Scholar]
- 43.Thornhill S., Teasdale G.M., Murray G.D., McEwen J., Roy C.W., and Penny K.I. (2000). Disability in young people and adults one year after head injury: prospective cohort study. BMJ 320, 1631–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexander M.P. (1995). Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology 45, 1253–1260 [DOI] [PubMed] [Google Scholar]
- 45.Benedictus M.R., Spikman J.M., and van der Naalt J. (2010). Cognitive and behavioral impairment in traumatic brain injury related to outcome and return to work. Arch. Phys. Med. Rehabil. 91, 1436–1441 [DOI] [PubMed] [Google Scholar]
- 46.Sigurdardottir S., Andelic N., Roe C., and Schanke A.K. (2009). Cognitive recovery and predictors of functional outcome 1 year after traumatic brain injury. J. Int. Neuropsychol. Soc. 15, 740–750 [DOI] [PubMed] [Google Scholar]
- 47.Mendez M.F., Owens E.M., Jimenez E.E., Peppers D., and Licht E.A. (2013). Changes in personality after mild traumatic brain injury from primary blast vs. blunt forces. Brain Inj. 27, 10–18 [DOI] [PubMed] [Google Scholar]
- 48.Mendez M.F., Owens E.M., Reza Berenji G., Peppers D.C., Liang L.J., and Licht E.A. (2013). Mild traumatic brain injury from primary blast vs. blunt forces: post-concussion consequences and functional neuroimaging. NeuroRehabilitation 32, 397–407 [DOI] [PubMed] [Google Scholar]
- 49.Mac Donald C., Johnson A., Cooper D., Malone T., Sorrell J., Shimony J., Parsons M., Snyder A., Raichle M., Fang R., Flaherty S., Russell M., and Brody D.L. (2013). Cerebellar white matter abnormalities following primary blast injury in US military personnel. PloS One 8, e55823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warden D.L., French L.M., Shupenko L., Fargus J., Riedy G., Erickson M.E., Jaffee M.S., and Moore D.F. (2009). Case report of a soldier with primary blast brain injury. NeuroImage 47, Suppl 2, T152–T153 [DOI] [PubMed] [Google Scholar]
- 51.Wilkinson C.W., Pagulayan K.F., Petrie E.C., Mayer C.L., Colasurdo E.A., Shofer J.B., Hart K.L., Hoff D., Tarabochia M.A., and Peskind E.R. (2012). High prevalence of chronic pituitary and target-organ hormone abnormalities after blast-related mild traumatic brain injury. Front. Neurol. 3, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elder G.A., Dorr N.P., De Gasperi R., Gama Sosa M.A., Shaughness M.C., Maudlin-Jeronimo E., Hall A.A., McCarron R.M., and Ahlers S.T. (2012). Blast exposure induces post-traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. J. Neurotrauma 29, 2564–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]