Abstract
Co-delivery is a rapidly growing sector of drug delivery that aspires to enhance therapeutic efficacy through controlled delivery of diverse therapeutic cargoes with synergistic activities. It requires the design of carriers capable of simultaneously transporting to and releasing multiple therapeutics at a disease site. Co-delivery has arisen from the emerging trend of combination therapy, where treatment with two or more therapeutics at the same time can succeed where single therapeutics fail. However, conventional combination therapy offers little control over achieving an optimized therapeutic ratio at the target site. Co-delivery via inclusion of multiple therapeutic cargos within the same carrier addresses this issue by not only ensuring delivery of both therapeutics to the same cell, but also offering a platform for control of the delivery process, from loading to release. Co-delivery systems have been formulated using a number of carriers previously developed for single-therapeutic delivery. Liposomes, polymeric micelles, PLGA nanoparticles, and dendrimers have all been adapted for co-delivery. Much of the effort focuses on dealing with drugs having dissimilar properties, increasing loading efficiencies, and controlling loading and release ratios. In this review, we highlight the innovations in carrier designs and formulations to deliver combination cargoes of drug/drug, drug/siRNA, and drug/pDNA toward disease therapy. With rapid advances in mechanistic understanding of interrelating molecular pathways and development of molecular medicine, the future of co-delivery will become increasingly promising and prominent.
Introduction
Drug delivery is a constantly-evolving field that must address new challenges arising from handling of delicate drugs, targeting of inaccessible tissue, or fulfillment of unique release profiles. Many delivery systems have been custom-designed to meet these demands. As delivery vehicles become more sophisticated, they can offer more control while simultaneously introducing more variables to maximize functionality. One of the more recent developments in the field is the idea of co-delivering therapeutics to achieve a synergistically amplified effective treatment.
The concept of delivering more than one drug for treatment is not a new one; it has long been recognized that using two drugs with complementary effects can achieve a better result than the use of a single drug. The advantages of such combination therapy are various and typically application-specific. The majority of combination therapy is directed towards the treatment of cancer, and often involves targeting multi-drug resistance (MDR) pathways either through transporter inhibitors or targeting of MDR genes, while also delivering a chemotherapeutic. Many of these combination therapies have gone to clinical trials, but most have not, because of off-target inhibition of transporters1. Combination therapy is used in immunotherapy to amplify the immune response elicited by a weakly immunogenic antigen via co-delivery of an adjuvant2, and it can be used as a method of preventing developed resistance to cancer vaccines3.
However, the advantages of combination therapy can easily become nullified if the effects of both drugs are not experienced by the same cell. This is where co-delivery steps in, offering a carrier system that will deliver both therapeutic agents to the same cell, at the same ratio of loading, thereby ensuring the effectiveness of the combination therapy. Synergism in the context of co-delivery is typically identified as an increase in the level of the desired therapeutic effect when compared with delivery of only one drug, or when compared with the effect of the two drugs delivered in combination, but separately. To gain the optimal synergistic impact, cargo can be strategically chosen to exert a desired mechanistically-based synergistic effect on the target. Table 1 is a compilation of examples of therapeutics delivered simultaneously with the goal of producing a synergistic effect, as well as the mechanistic explanation of the synergism. As has been noted, the majority of these combinations are selected for their potential in the treatment of cancer, often aiming to increase the effect of a typically-used chemotherapeutic such as doxorubicin with the addition of a second therapeutic.
Table 1.
Synergism of co-delivery of therapeutics to enhance efficacy of cancer therapy.
| Therapeutics | Application | Explanation of synergistic effect | Ref |
|---|---|---|---|
| Drug/Drug | |||
| Doxorubicin and Combrestatin | Cancer | Combrestatin is a vascular disrupting agent that targets the tumor blood vessels, causing tumor vasculature shutdown, trapping DOX in the tumor. | 4 |
| Doxorubicin and Verapamil | Cancer | Verapamil is a calcium channel antagonist which also acts as a P-gp inhibitor. This increases the cell sensitivity and DOX accumulation within the cell. | 5 |
| Heparin with Taurocholate (LHT7) and Suberoylanilide hydroxamic acid (SAHA) | Cancer | LHT7 is an anti-angiogenic drug. SAHA causes cell cycle arrest, angiogenesis. Together they inhibit angiogenesis and cell proliferation. | 6 |
| Fluoroorotic acid and Irinotecan | Cancer | Irinotecan inhibits DNA re-ligation by inhibiting topoisomerase 1, and fluoroorotic acid inhibits DNA synthesis. | 7 |
| Cyclophosphamide and Doxorubicin | Cancer | Cyclophosphamide increases permeability of tumor micro vessels, leading to increased DOX accumulation in the tumor. | 8 |
| Vincristine and Topotecan | Cancer | Topotecan acts in the S-phase or G2-M phase by converting DNA topoisomerase 1 to a cellular toxin, and vincristine leads to mitotic arrest by depolarizing the microtubules. | 9 |
| Doxorubicin and Paclitaxel | Cancer | DOX bound to DNA prevents formation of tubulin, and paclitaxel degrades existing microtubules. | 10 |
| Drug/siRNA | |||
| Doxorubicin and Bcl-2 siRNA | Cancer | Bcl-2 induces an anti-apoptotic signal and DOX induces apoptosis. Knockdown of Bcl-2 with siRNA allows DOX to function more efficiently. | 11 |
| Doxorubicin and P-gp siRNA | Cancer | Knockdown of P-gp, a MDR-contributing gene, restores the sensitivity of the cancer cell to DOX. | 12 |
| Doxorubicin and Plk-1 siRNA | Cancer | Plk-1 is a regulator of mitotic progression in mammalian cells. Knockdown of the gene along with DOX delivery induces a clear synergistic effect. | 13 |
| Drug/Plasmid | |||
| Doxorubicin and pORF-hTRAIL gene | Cancer | TRAIL causes apoptosis by transmitting apoptotic signals through an extrinsic pathway. DOX causes DNA damage through intrinsic pathway. | 14 |
| Doxorubicin and Survivin mutant gene | Cancer | Survivin leads to increased resistance against DOX. The plasmid is a strong negative mutant of survivin which aims to reduce the resistance. | 15 |
| Paclitaxel and pEGFP-hTRAIL gene | Cancer | TRAIL targets cancer cells over normal cells but glioma gains resistance very quickly. PTX makes the cells more sensitive to TRAIL-induced apoptosis due to crosstalk between intrinsic and extrinsic pathways. | 16 |
| Doxorubicin and p53 antitumor gene | Cancer | DOX is a chemotherapeutic and p53 enhances sensitivity of cells to the chemotherapeutics. | 17 |
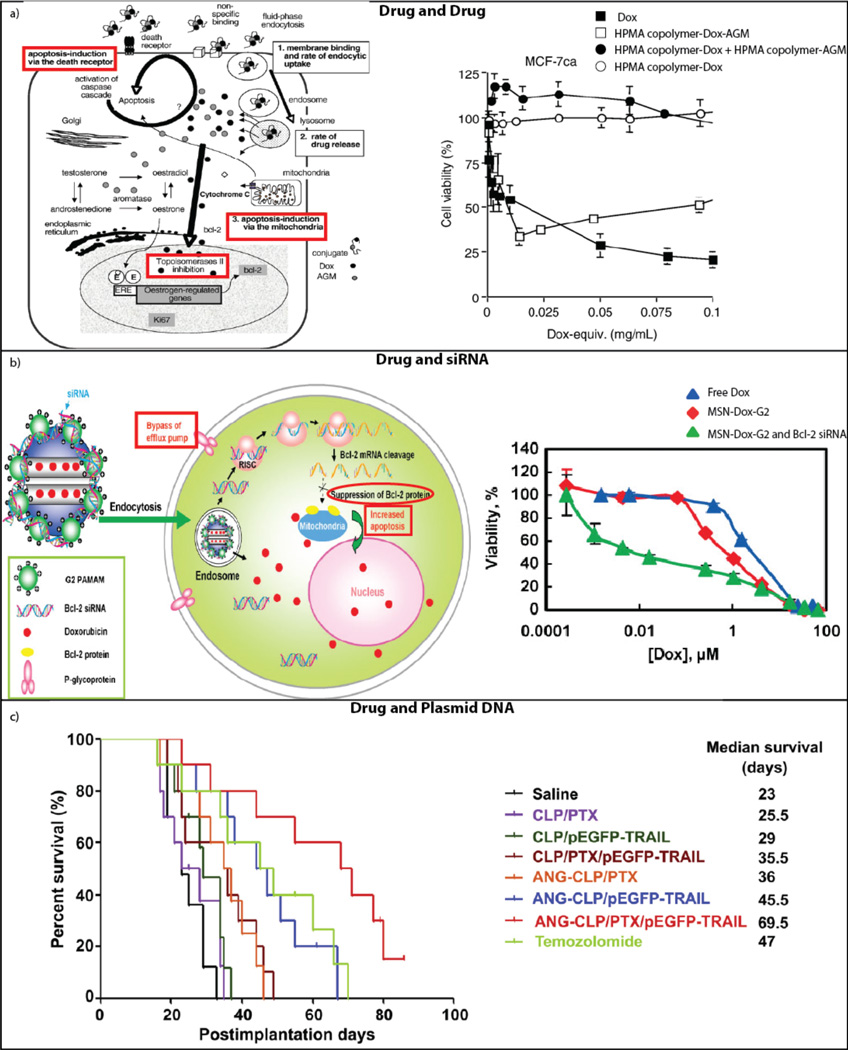
Three specific examples of synergism achieved in the context of co-delivery, along with graphical depiction of the mechanistic basis for the observed synergistic effect are provided in Figure 1. Figure 1a focuses on the co-delivery of DOX and aminoglutethimide (AGM). Here, the authors undertook a thorough study of what might be causing the synergistic effect shown by the graph on the right side of the panel. The data indicate highly decreased cell viability associated with the co-delivery of AGM and DOX on the same polymer backbone when compared to the two drugs delivered on separate polymer backbones. The schematic on the left depicts the proposed steps at which the drugs may be exerting their synergistic effect, focusing on endocytic uptake, rate of drug release, and induction of apoptosis. After investigation, the group determined that endocytic uptake was not the cause of the synergistic impact, leaving the possibility that either release kinetics or an effect on the anti-apoptotic protein bcl-2, or a combination of the two, was responsible for the synergism in the system18. The right side of Figure 1b shows a lower percent viability at intermediate concentrations of DOX using the synergistic co-delivery of DOX and siRNA knocking down the Bcl-2 gene. The left side of the panel shows a diagram of the mechanism of this synergism, emphasizing the suppression of Bcl-2 protein leading to an upswing in apoptosis, combined with the release of Dox within the cell11. Figure 1c demonstrates a clear example of synergism occurring with the co-delivery of paclitaxel (PTX) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). The median survival of the co-delivery system is at least 24 days longer than that of either of the two components delivered independently16. These examples clearly show the positive results that can be effected when co-delivery systems are implemented intelligently. In addition to a synergistic effect, co-delivery of drugs can actually decrease the likelihood of off-target effects due to the increased specificity of the joint system19.
Figure 1.
Synergism of co-delivery. A) Depiction of the synergistic mechanism of the co-delivered drugs DOX and AGM (left). Synergistic cytotoxic effect of linear HPMA copolymer-DOX-AGM when compared to conjugated DOX or combination delivery of separately-conjugated DOX and AGM (right). Reprinted18 with permission from Elsevier. B) Synergistic mechanism of the co-delivery of DOX and siRNA targeting the Bcl-2 gene (left). The synergistic impact on ovarian cancer cell viability of liposomal co-delivery of DOX and Bcl-2 siRNA (right). Reprinted11 with permission of John Wiley and Sons. C) Liposomal co-delivery of therapeutic plasmid pEGFP-hTRAIL and paclitaxel yields an increase in survival time for glioma-bearing mice. Reprinted16 with permission of Elsevier.
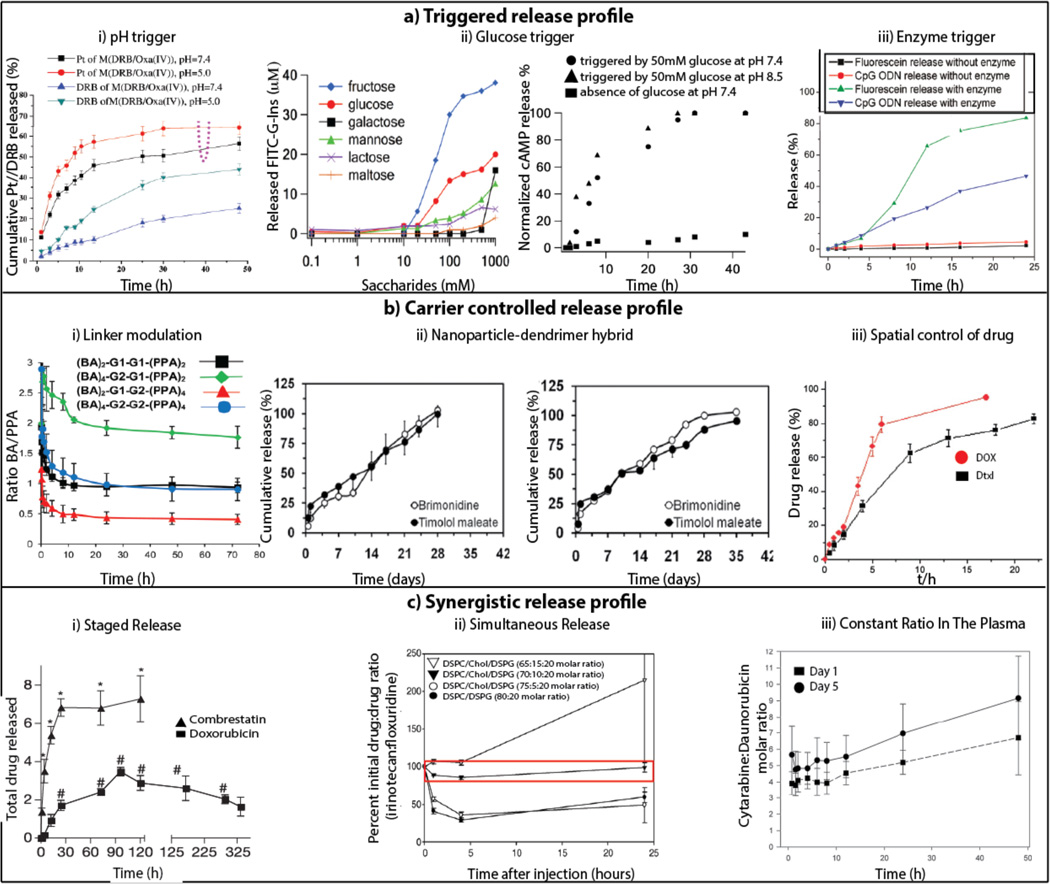
This review focuses on co-delivery via a variety of carriers, including the well-established liposomal, dendrimer, polymeric, and nanoparticle systems, as well as more unconventional carriers such as Janus particles and DNA nanogels. In particular, liposomes, dendrimers, linear polymers, polymeric micelles, and polymeric and inorganic nanoparticles have all been widely investigated in terms of their ability to delivery single-therapeutic payloads. However, several issues must be further addressed in these single-therapeutic systems to achieve success in the context of co-delivery. For instance, the addition of a second drug could impact the loading of the first drug, and there must be control over the ratio of the loaded co-encapsulated drugs. Once control over loading has been achieved, steps must be taken to ensure that the drugs do not interact unfavorably within the system, and that they can be released in an active form. Finally, the release of the drugs should be separately tunable and occur unhindered by the presence of the other. Often, some sort of release manipulation, such as staggered release of the drugs, is also desirable and must be controlled. In fact, the release kinetics in a two-therapeutic system is often of special importance, as demonstrated by the scenario in Figure 1a. Thus, it is necessary to devise methods of manipulating the release profiles of the therapeutics to achieve optimal results. Figure 2 compiles release kinetics data from co-delivery systems that have made use of diverse strategies to achieve control. Figure 2ai–iii shows the release kinetics of four co-delivery systems that are designed to release their cargo in the presence of an external trigger, such as a change in pH, the presence of glucose, or the presence of a specific enzyme. Figure 2bi–iii shows the release profiles of systems that have been designed such that the carrier itself allows control over the profile. Whereas the previous two boxes contain examples of controlled release profiles without an objective other than control, Figure 2ci–iii depicts release profiles that have been controlled specifically in a manner that will lead to a maximal synergistic effect.
Figure 2.
Diverse release profiles of co-delivery. ai) Increased release of platinum (Pt) and daunorubicin (DRB) at pH 5.0 compared to release at pH 7.4. Reprinted20 with permission from Elsevier. aii) Release of FITC-G-Ins triggered by glucose (Left). Release of cAMP triggered by glucose (Right). Reprinted21 with permission from The American Society. aiii) Increased release of CpG ODN and fluorescein in the presence of an enzyme, α-chymotrypsin. Reprinted22 with permission from American Chemical Society. bi) Modulating the release profile of BA and PPA based on the generation numbers. Reprinted23 with permission from American Chemical Society. bii) Release from PLGA nanoparticles (Left) compared to sustained release of brimonidine and timolol maleate from PLGA nanoparticles-dendrimer hybrid (Right). Reprinted24 with permission from American Chemical Society. biii) Spatially controlled release of DOX and Dtxl. Reprinted25 with permission from John Wiley and Sons. ci) Staged release demonstrated by fast release of combrestatin and slow release of DOX. Reprinted26 with permission from Nature Publishing Group. cii) Release of irinotecan and floxuridine at a constant synergistic 1:1 ratio for 25 hrs. Reprinted27 with permission from Elsevier. ciii) Release of cytarabine and daunorubicin at a constant synergistic ratio of 5:1 in the plasma. Reprinted28 with permission from Elsevier.
Despite the intimidating array of requirements discussed above, the field is moving in the direction of making well-controlled co-delivery a reality. In this review, the aforementioned commonly-used drug carriers will be examined in the context of co-delivery and we will delve into what is being done to formulate systems capable of achieving controlled loading, delivery, and release of diverse therapeutics.
Liposomes
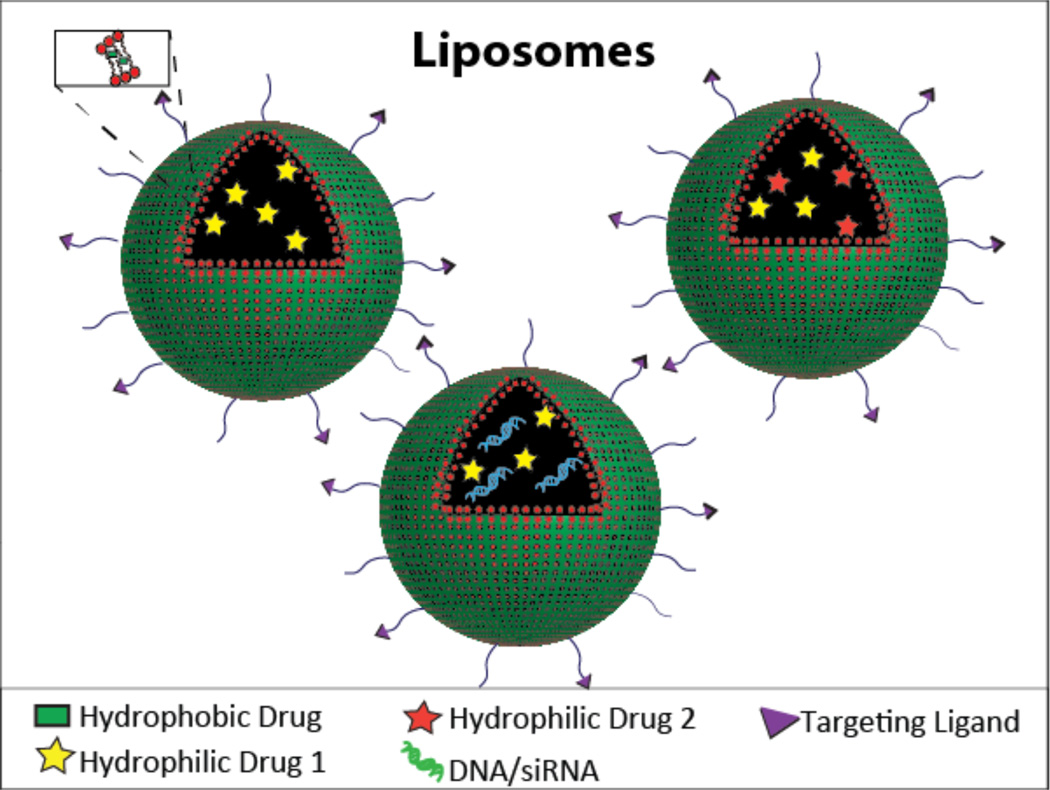
Liposomes are one of the most well-established nanoscale drug delivery systems, with several promising formulations now in clinical use. Doxil® is a liposomal product delivering doxorubicin for ovarian cancer, AIDS-related Kaposi’s sarcoma, and multiple myeloma. Comprised of amphiphilic phospholipids that self-assemble to form bilayers to enclose an aqueous phase, liposomes are an ideal candidate for dual drug delivery since they have the unique capability of entrapping both lipophilic and hydrophilic drugs. The lipid soluble drugs can be encapsulated within the lipid bilayer and the hydrophilic drug can be encapsulated within the inner aqueous compartment (Figure 3). Liposomes can protect the entrapped drug from the external environment, and represent a versatile and generally non-immunogenic system. As such, they are useful for co-delivery of therapeutics for vaccination and anti-microbial applications, as well as the treatment of cancer. In cancer-based applications, they make use of the EPR effect to passively target tumors, and are easily modified with targeting ligands, allowing them to actively target the tumor.
Figure 3.
Schematic of a liposomal drug carrier containing a hydrophilic drug in the inner aqueous compartment and a hydrophobic drug in the lipid bilayer (top left), two hydrophilic drugs in the inner compartment (top right), and a hydrophilic drug and DNA/siRNA in the inner compartment (bottom).
The drugs can be loaded either passively or remotely. In the case of passive loading, the drug is entrapped while the liposome is being prepared; when delivering hydrophobic drugs, the loading depends on the drug-lipid interaction, and when delivering hydrophilic drugs the loading depends on the trapped volume and drug solubility within the liposomes. The amount of drug encapsulated is generally lower with passive loading and there is a possibility of drug leakage. Remote drug loading addresses these deficiencies by loading drugs into preformed liposomes either through pH gradient or ammonium sulfate gradient across the membrane. However, this is limited only to drugs with ionizable groups29.
LipoViTo is a liposomal formulation containing vincristine and topotecan that demonstrates the advantages of remote loading. Topotecan is a water-soluble analogue of camptothecin targeting DNA topoisomerase 1, and vincristine leads to mitotic arrest by depolymerization of microtubules. The two drugs act on different targets to achieve a synergistic effect. Because the drugs were loaded using remote loading, they had similar physical stability and release profiles. LipoViTo showed superior therapeutic efficacy and reduced clearance of the drugs when compared to combination delivery. Careful characterization of the LipoViTo system using small-angle X-ray scattering (SAXS) analysis showed that the drugs did not interact with each other within the liposomes9, 30. Recently, fluoroorotic acid (FOA) was loaded and used to remotely load irinotecan (IRN) in a synergistic ratio, allowing for simultaneous release. Temperature and incubation time could be varied to adjust the ratio of FOA to IRN, the drug to lipid ratio.7
Delivery of a gene with a drug can increase the efficacy of the drug by acting on a different target and can also sensitize the cells to the drug by silencing pump and non-pump resistance. Delivery of nucleic acids (siRNA/plasmid) in conjunction with drugs is complicated by the wide differences in the properties of the cargo. Systems for the co-delivery of nucleic acids and drugs often make use of cationic liposomes, which have proven to be a superior non-viral gene delivery vehicle because the positive charge of the liposome allows for formation of stable complex with DNA/siRNA. While cationic liposomes are an excellent non-viral delivery system, their transfection efficiency is still lower than that of viral vectors. In the pursuit of higher transfection efficiencies, a trilysinoy oleylamide-based liposome was designed to co-deliver Mcl1-targeting siRNA and suberoylanilide hydroxamic acid, based on the hypothesis that the trilysinoy oleylamide would aid in transfection. The system showed a high silencing efficiency with minimal toxicity31. Cationic liposomes have also been used to co-deliver c-Myc siRNA and DOX32, as well as siMcl1 and MEK inhibitor33. In order to simultaneously silence pump and non-pump resistance, one group co-delivered DOX and siRNA (MRP 1 drug efflux pump and BCL2 antiapoptotic defense), which led to increased cytotoxicity against multidrug resistant cancer cells34.
In addition to delivering drugs and genes, cationic liposomes have also shown great efficacy in co-delivering multiple drugs. Due to their positive charge, cationic liposomes can passively target the exposed anionic phospholipids of the vasculature. This unique vasculature-targeting ability was exploited by one group to achieve an anti-angiogenic effect via cationic liposomes loaded with heparin-taurocholate conjugate and suberoylanilide hydroxamic6. However, cationic liposomes are prone to aggregation, inducing immunotoxicity, and poor entrapment efficiency. Their positive charge also leads to rapid elimination and non-specific cell binding. Some of these issues can be circumvented by using anionic liposomes; when used to co-deliver adenovirus and carmustine, anionic liposomes were able to reduce the immunogenicity of the adenoviral vectors35.
The release rate and target-site concentration of the therapeutics both play important roles in achieving optimal therapeutic activity. Some drug combinations require that an exact ratio be present at the tumor site, warranting simultaneous release. However, in other cases, a staggered controlled release rate is preferred. For example, temporal control over release is of the utmost importance during co-delivery of the drug combrestatin with chemotherapeutics. Combrestatin is a vascular disrupting agent that has shown promise in combination therapy with DOX and paclitaxel. However, the combination therapy can fail when combrestatin leads to vascular shutdown, preventing the accumulation of the chemotherapeutic in the tumor. As demonstrated in Figure 2ci, liposomal systems are capable of successfully addressing this need for carefully timed release26,36,4. For example, one group synthesized an RGD-modified liposome encapsulating combrestatin A-4 (CA-4) and doxorubicin that displayed staggered release of the drugs. Here, CA-4 was incorporated within the lipid bilayer and released quickly. DOX, on the other hand, was loaded with high encapsulation efficiency using an ammonium sulfate gradient where it was present in an aggregated gel-like state and released at a much slower rate. The system displayed a significant delay in the growth of subcutaneous B16F10 xenograft tumors in C57BL6 mice when compared to liposomes carrying single drug and combination of the free drug. This could be because CA-4 induced vascular shutdown with DOX entrapped within the tumor36.
In the case of cancer, active targeting plays a crucial role in minimizing off-target toxicity. There are a few targeted co-delivery liposomal systems that show superior therapeutic effects. For example, co-delivery of pEGFP-hTRAIL and paclitaxel to brain glioma was achieved using a cationic liposome conjugated to angiopep. In this case, two drugs were expected to increase the efficacy because the paclitaxel would sensitize the cells to TRAIL-induced apoptosis. The targeting liposomal system showed both a spatial increase in apoptosis and a 34 day increase in the median survival rate when compared to the untargeted system (Figure 1c)16. Another study used basic fibroblast growth factor peptide tbFGF and cationic liposomes to co-deliver doxorubicin and plasmid (Msurvivin T34A) to the same cell. This led to a significant increase in apoptotic cells in Lewis lung carcinoma (LLC)-bearing C57BL/6N mice. Mice treated with tbFGF-LPs-DOX-MsurvivinT34A had tumors smaller than 4000 mm3 for longer than 15 days, whereas mice treated with tbFGF–LPs–DOX, tbFGF–LPs–MsurvivinT34A and DOX, had tumors of that size at 9, 6, and 6 days, respectively15. In cases where polar and macromolecular drugs cannot cross the cell membrane, receptor-mediated endocytosis is one approach that can achieve intracellular delivery. For instance, transferrin receptor has often been exploited for targeted delivery, as exemplified by the co-delivery of doxorubicin and verampil or that of anti-BCR-ABL siRNA and imatinib mesylate5, 37.
The antitumor effect of drug combinations relies heavily on the drug ratios, as only some ratios are synergistic and others may be merely additive or even antagonistic. Hence, it is imperative that the correct ratio reaches the target site. Usually the half-life and pharmacokinetics of drugs are unique to the system, which could contribute to suboptimal or antagonistic ratios at the site of interest. With this in mind, Mayer and colleagues have developed a series of liposomal formulations that have drugs encapsulated in a synergistic ratio. CPX-351, a liposomal formulation currently in Phase III clinical trial for patients with acute myeloid leukemia (AML), contains a synergistic 5:1 molar ratio of cytarabine and daunorubicin. Preclinical data, as well as a Phase I trial, demonstrated that this synergistic ratio of the drugs could be maintained in the plasma and bone marrow for more than 24 hours, in addition to prolonged half-life and bioavailability of the drug (Figure 2ciii). Two different Phase II clinical trials have also been conducted to demonstrate the efficacy of the system. In newly diagnosed older secondary AML patients, CPX-351 was compared to the conventional “7+3” regime (7days of cytarabine infusion and 3 days of anthracycline IV push). CPX-351 showed a 25% higher response rate and a 23% higher aplasia rate. In addition, CPX-351 showed only 6% early mortality rate compared to 32% by “7+3” regime. Following the success of Phase II clinical trials, this system is currently recruiting patients for Phase III clinical trials28, 38–45. CPX-1 is another liposomal formulation currently in Phase II clinical trials for the treatment of advanced solid tumors and colorectal cancer. It consists of a 1:1 ratio of irinotecan and floxuridine, a formulation found to have higher efficacy than irinotecan and floxuridine combination therapy in the preclinical models. As seen in Figure 2cii, the authors were able to modulate the composition of the liposome to achieve a synergistic 1:1 ratio for more than 24hrs in vivo27. It also demonstrated a synergistic ratio in plasma for up to 12 hours, one of the first such co-delivery studies to do so46–49.
One of the earliest-developed applications of liposomes still in use is vaccine delivery. Co-delivery offers the advantage of delivering the adjuvant and the antigen together to the same immune cells where antigen presentation takes place. Many antigens alone elicit poor immunity, so an adjuvant is required to augment the antigen presentation process. The adjuvant activity is enhanced when it is physically associated with the antigen, but because chemical conjugation of the two is difficult, a co-delivery system is an appealing option. CpG is an immunostimulatory agent used widely as an adjuvant. However, its inability to enter the cells and poor stability hinders its utility. A liposomal formulation co-delivering CpG and antigens delivers them to the same antigen-presenting cells, eliciting a quicker, more long-lasting immunity. Liposomal formulations with CpG have been designed for the treatment of many applications, including breast cancer using HER-2/neu derived peptide50, hepatitis C using NS351, leishmaniasis with leishmania major stress-induced protein 152, tetanus toxoid53 and pseudorabies IE18054. In all of these cases, CpG is encapsulated within the liposome where it is less exposed to TLR-9 in the endosome both spatially and temporally. Recently, in order to enhance the likelihood of CpG binding to TLR-9, CpG was covalently linked to the lipid via an irreversible thioester bond. A robust immunostimulatory response was also seen upon incorporating OVA and listeriolysin into the liposomal formulation55.
Liposomes have also found use in antibacterial treatment. Bacteria are known to resist drug entry by changing their outer membrane permeability, which can be combated by incorporation of multiple drugs to target the changing bacteria. Liposomes are thought to fuse with the bacterial membrane, enhancing the anti-bacterial effect. By incorporating gallium and gentamicin into the liposomal formulation, the minimum inhibitory concentration (MIC) was 2 mg/L, which is significantly lower than that seen with free gentamicin (264mg/L), Ga-Gen combination (128mg/L) and liposomal gentamicin (8mg/L)56. Various other approaches have been taken to optimize the efficacy of existing antibiotics; liposomes containing bismuth-thiol and tobramycin were designed to reduce the cytotoxicity of the bismuth-thiol and at the same time inhibit growth of biofilm57. It was also shown that while the free tobramycin and bismuth killed and detached bacteria, the liposomal formulation of the dual drug was capable of penetrating and killing the bacteria in the core of the biofilm more efficiently58.
Liposomes are the only formulation currently in clinical trials for combination therapy, but controlling the colloidal stability and release profile is still a challenge. Although liposomes are most suitable for encapsulating hydrophobic drugs due to their interaction with the lipid bilayer, these drugs tend to be released quite quickly in vivo. Optimizing the loading and co-delivery of both hydrophobic and hydrophilic drugs by liposomal formulations is therefore a barrier that must be addressed prior to translation.
Linear polymer conjugates
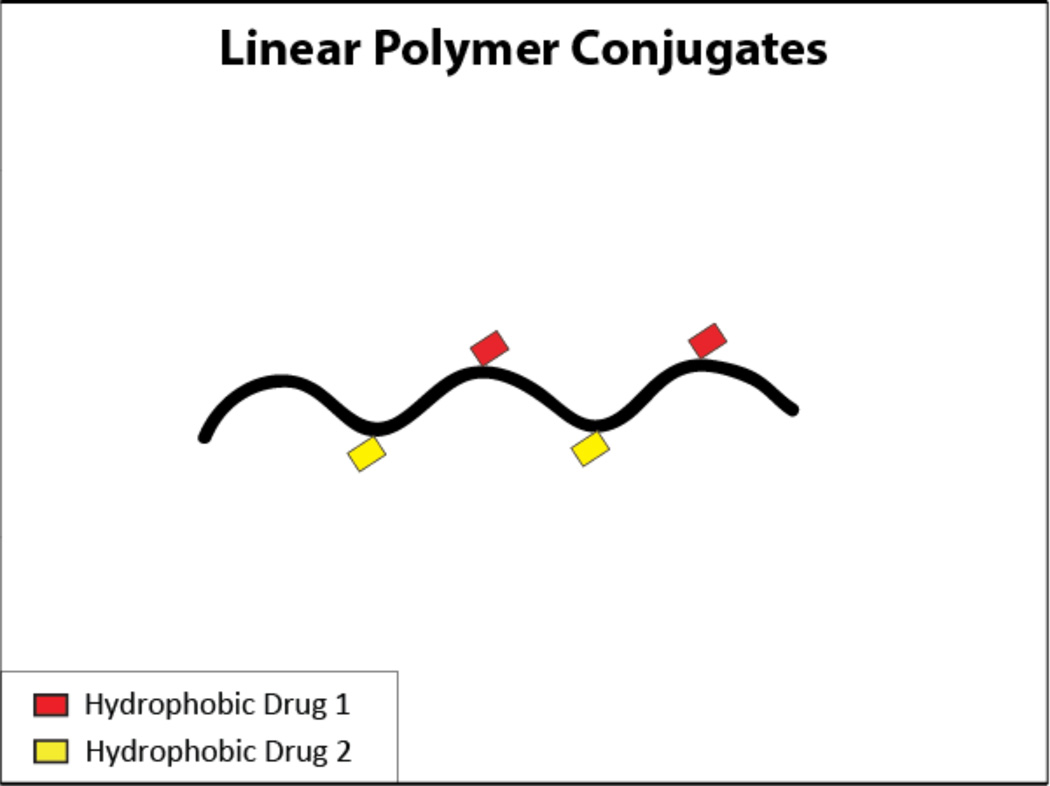
One of the most thoroughly studied systems of polymer drug delivery vehicles comprises a linear, water soluble polymer with an active agent, such as a low molecular weight drug, conjugated to it18, 59–65 (Figure 4). These active agents are attached via a linker that allows for the release of the active agent at the appropriate location, such as intracellularly. The linkers are sensitive to conditions that are unique to the target site (i.e. the presence of a specific enzyme, or a difference in pH) which increases the specificity of the drug delivery and allows for some control over release kinetics of the individual drugs66. One of the most commonly studied polymers for this purpose is N-(2-hydroxypropyl)methacrylamide (HPMA). HPMA copolymers are used because of their known non-immunogenicity and non-toxicity, as well as known stability in the blood66.
Figure 4.
A schematic of a linear polymer conjugate carrying two hydrophobic drugs.
The vast majority of HPMA copolymers have been investigated for their anti-cancer potential. As with other pendant drug delivery systems, HPMA copolymers are of a size that allows them to engage in passive targeting of tumor tissue through the enhanced permeation and retention (EPR) effect, a result of the leaky vasculature and poor lymphatic clearance at cancer sites67. One of the earlier examples of using HPMA copolymers to simultaneously deliver two therapeutics aimed to combat breast cancer by co-delivering aminoglutethimide (AGM) and doxorubicin (DOX)65. AGM is a first-generation aromatase inhibitor used to prevent estrogen biosynthesis, and DOX is a commonly-used chemotherapeutic. The drugs were attached to the polymer using a tetrapeptide linker that can be cleaved by the lysosomal enzyme cathepsin B. The drug release studies showed that total drug release after 5 hours was highest in the formulations with the lowest DOX loading. Presumably, higher drug loading resulted in a polymer-drug conjugate structure that introduced steric hindrance, thereby inhibiting efficient cleaving of the linkers by the enzyme. These data support the idea that the conformation of linear polymers in solution is impacted by the drug loading of the polymer, which in turn impacts efficiency of drug release. The co-delivery concept was tested in vitro on two cell types, and the findings indicated that polymer-drug conjugates containing both DOX and AGM linked to the same carrier demonstrated an approximately tenfold more potent cytotoxic effect than that of either DOX- or AGM-equivalents. This effect was not observed when polymers containing only DOX were mixed with polymers containing only AGM and delivered to the cells.
In an effort to elucidate the mechanism of this synergism, the group carried out a more in-depth study of the process18 (Figure 1a). The study was able to definitively demonstrate the synergistic ‘whole is greater than the sum of the parts’ result of delivering two therapeutic agents attached to the same carrier. It was hypothesized that enzymatic cleavage of the linker was the major contributor to the synergism. No significant differences were seen in either cell binding or endocytic uptake when the drugs were co-delivered, but it was apparent that the release kinetics of the drugs differed when both were conjugated to the same backbone. The release of co-delivered drugs from the carrier was unexpectedly non-linear, and AGM was released more quickly than DOX over the span of the first 5 hours of release, at which point both release profiles plateaued. The possibility exists that certain portions of the polymer may have contained higher amounts of conjugated drug due to uneven distribution of linkers along the length of the polymer, which affected enzymatically-activated drug release.
Another cancer-targeting study was carried out using an HPMA copolymer conjugated to both DOX and dexamethasone (Dex), an anti-inflammatory drug59. In this formulation, the drugs were conjugated to the backbone via a hydrazone bond, which is susceptible to hydrolytic degradation. These bonds are stable in the blood, but are hydrolyzed at low pH typical of intracellular lysosomal compartments. In contrast to the aforementioned study, release studies of this system showed that the addition of a second drug to the backbone had no effect on the drug release kinetics when compared to single drug conjugates.
In summary, linear polymer-drug conjugates offer a method of co-delivering drugs with release in response to specific conditions (i.e. the presence of an enzyme or an acidic environment) in a manner that elicits a synergistic therapeutic effect. However, they are limited in their functionality simply because of the factors discussed above; high drug loading is difficult to achieve due to lack of functional groups68 and may inhibit release due to hindrance of linker cleavage. In addition, it is difficult to predict the interactions of the drugs with each other and the backbone, and variations in drug loading may occur even between batches of conjugates containing the same drugs69.
Polymeric micellar nanoparticles
While linear polymers represent a subset of the earliest carriers for multiple therapeutics, additional types of polymer systems have gained momentum in the field. Although problems similar to those experienced with linear polymer carriers can be encountered in certain formulations of polymeric micellar nanoparticles, as in one study that found undesirable interactions between the cholesterol chains and drugs prevented high drug loading70, polymeric micelles have the versatility to deal with the challenges13, 20, 69–87.
Polymeric micellar nanoparticles are self-assembled carriers that form spontaneously in an aqueous solution. Typically, the micelles are formed from diblock copolymers which contain both hydrophilic and hydrophobic segments; the hydrophilic portion of the copolymer forms a shell or corona, while the hydrophobic portion forms the core region (Figure 5, top row). Self-assembly is driven by hydrophobic interactions and the increased entropy of the solvent associated with the formation of a hydrophobic core protected from the surrounding aqueous environment88. This spontaneous assembly can also occur as a result of electrostatic interactions, host/guest interactions, or hydrogen bonding89. Drugs can be loaded during the self-assembly process by including them in the solvent with the polymer; when self-assembly occurs, the drug is essentially trapped within the hydrophobic core. Similarly to linear polymers, these are of a size that allows them to take advantage of the enhanced permeation and retention effect in cancer treatment, and the hydrophilic corona often offers protection from phagocytic activity, resulting in prolonged stability in circulation88. The formation of the block copolymers themselves as well as the methods of loading polymeric micelles with drugs have been reviewed elsewhere88, 90, so we will focus instead on progress that has been made in the area of specifically modifying carriers to make them ideal for co-delivery of two or more therapeutics.
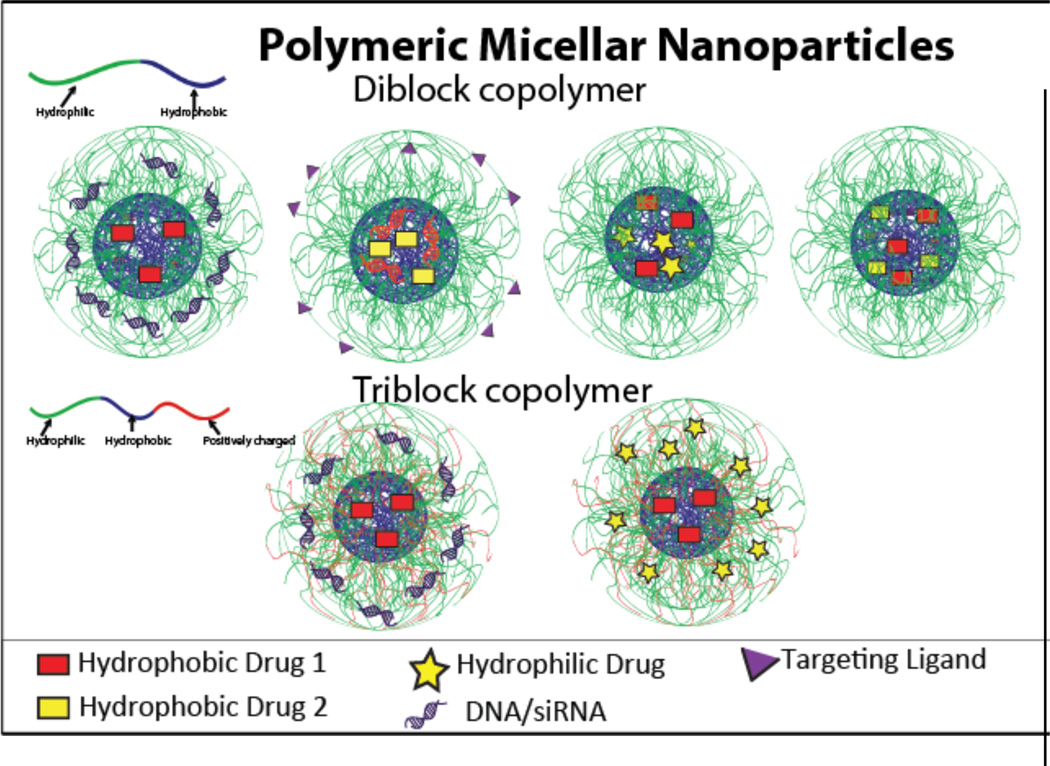
Figure 5.
A schematic of polymeric micellar nanoparticles, with diblock copolymer shown in the top row and triblock copolymers shown in the bottom row. These are capable of carrying a variety of combinations of cargo.
There are several challenges uniquely associated with the delivery of two therapeutics by polymeric micelles; one is to handle two therapeutics which have different properties. The micellar system is particularly suited to delivering hydrophobic drugs because of the ease of encapsulation via interactions with the hydrophobic core of the micelle. Therefore, some focus has been placed on using polymeric micelles to co-deliver two or more hydrophobic drugs69, 72, 81. However, many co-delivery applications involve both hydrophobic and hydrophilic drugs, which presents a challenge of efficient loading20, 71, 74, 76, 79. Often, this requires conjugation of the hydrophilic drug to the copolymer, followed by self-assembly and encapsulation of the hydrophobic drug.
One approach to delivering drugs with different properties involves modifying the hydrophobic portion of an amphiphilic copolymer with pendant functionality for the attachment of the hydrophilic molecule74, 76. For example, one group linked the hydrophilic molecule to the hydrophobic random copolymer of vinyl benzyl chloride (VBC) and pentafluorophenyl acrylate (PFP-A) via a thiol-disulfide exchange reaction. The amphiphilic copolymer with attached hydrophilic drug then self-assembled while simultaneously incorporating the hydrophobic drug in the core. The core was stabilized with a crosslinker that degrades in acidic conditions, releasing the hydrophobic molecules. In this manner, it is possible to not only create a polymeric micelle containing both hydrophobic and hydrophilic molecules, but also to produce controlled release in reducing and acidic environment74.
In line with the challenge of delivering drugs with different properties, there has also been focus on the development of carriers that are able to deliver both a therapeutic drug and a therapeutic nucleic acid70, 73, 75, 77, 78, 80, 82, 83. Carriers for co-delivery of a gene and a drug are often conceived for the purposes of overcoming multidrug-resistance in cancer cells. Typically, these carriers consist of a hydrophobic core capable of encapsulating a hydrophobic drug and a positively charged, hydrophilic shell capable of complexing the negatively-charged nucleic acids70, 75, 78, 80, 83. One group created PEI-PCL nanoparticles that contained doxorubicin in the hydrophobic core and siRNA targeting the BCL-2 gene complexed to PEI. These cationic micelles were then coated with a folic acid-targeted PEG-PGA shell, a process occurring via ionic interactions between the cationic micelle and the anionic shell components. This particular system made use of the known efficacy of PEI as a gene transfection agent, with the unique hierarchical assembly of the particles enabling siRNA to be complexed without hindrance from the PEG shell, which was added after siRNA complexation. The co-delivery of these two therapeutic agents yielded a synergistic effect of increased cell death in Bel-7402 cells, due to knockdown of the anti-apoptotic BCL-2 gene combined with DOX-induced toxicity. The micelle containing DOX and BCL-2 siRNA had an IC50 value two orders of magnitude lower than the same system contained scrambled siRNA73. Another method of incorporating therapeutics with different properties resulted in a carrier with both siRNA and a hydrophobic drug conjugated to the polymer via acid-cleavable bonds within the hydrophobic core82.
Whereas diblock copolymers can form micelles, triblock copolymers offer more versatility in adjusting the length of the individual segments to form smaller and more stable micelles. The majority of triblock copolymers used for co-delivery are used for the co-delivery of a drug and a gene13, 85, 86. Similarly to diblock copolymers used for the co-delivery of a gene and a drug, triblock copolymer carriers typically contain a hydrophobic drug encapsulated within the hydrophobic core and the nucleic acid complexed to one of the hydrophilic blocks (Figure 5, bottom row). However, these triblock copolymers have an additional cationic shell block which aids in the protection of the genetic material while in circulation. One such system made use of a PEG-b-PCL-b-PPEEA triblock copolymer to encapsulate paclitaxel within the hydrophobic PCL core and complex siRNA to the cationic PPEEA block. The hydrophilic PEG block then formed a protective shell around the particle. Micelleplexes carrying polo-like kinase 1 (PLK1) targeting siRNA and paclitaxel were delivered to a MDA-MB-435s xenograft murine model. The system achieved the same degree of cell death with co-delivery as that achieved with paclitaxel only, but at a one thousand-fold lower paclitaxel dosage. This synergistic effect was attributed to the action of the siRNA in knocking down the mitotic regulatory gene PLK1, a gene that is upregulated in cancer cells, thereby rendering cancer cells more vulnerable to the cytotoxic effects of paclitaxel13.
A second challenge is to exercise control over the loading ratios of the two therapeutics. For synergistic purposes, it is important to be able to achieve an optimal drug ratio, which can be done only with controlled flexibility during the loading process. Several groups have investigated the concept of mixed polymeric micelles, or composite micelles as a method for attaining controlled loading20, 69, 72. These mixed polymeric micelles can be formed two ways; in one method, only one type of drug is conjugated to each of the amphiphilic diblock copolymers20, 72, yielding a population of drug-conjugated amphiphilic diblock copolymers for each of the two drugs. The two populations of conjugates are then mixed in the desired ratio to obtain micelles containing a controlled ratio of the two drugs. The second method involves the formation of a chemically mixed micelle containing both types of drugs conjugated to a single polymer. The group found that physically mixed micelles, which bypass the difficulty of attaching two different drugs to the same polymer backbone with limited functional groups available, did not perform significantly differently than chemically mixed micelles. The group also characterized the final drug concentration in each micelle and found that it corresponded well with the initial concentration in the reaction, thus providing ratiometric control over the final product72.
Another group used the same concept, but instead of conjugating the drugs to amphiphilic copolymers, they conjugated the drugs, DOX and camptothecin (Cpt), to the hydrophobic polymer PLA (with only one drug type per polymer chain). The PLA-drug mixture then self-assembled into a PEGylated lipid-coated polymeric nanoparticle via nanoprecipitation. Again, the initial molar ratio of the DOX-PLA and Cpt-PLA drug-polymer conjugates was mirrored closely in the final drug loading composition of the particles, indicating that mixed or composite micelles formed at ratios corresponding to those in the initial mixture. A study comparing the cytotoxicity of the composite micelles with a physical mixture of DOX-PLA nanoparticles and Cpt-PLA nanoparticles found that composite nanoparticles were reliably more potent than the mixture of single-drug nanoparticles. At a DOX-PLA:Cpt-PLA ratio of 3:1, the composite nanoparticles were 3.5 times more effective than the single-drug nanoparticle mixture. The authors attributed this at least in part to the synergistic effect of the two drugs reaching the same cells at the same time with the composite system69.
The micellar design described above draws from the advantages of linear polymers, which include control over the spatial loading and temporal release of the drug through the use of cleavable linkers, with the added advantage of ratiometric control between the two drugs and fewer concerns about undesirable interactions between two drugs linked to the same backbone. However, as of now, these composite micelle systems are still limited to co-delivery of hydrophobic drugs.
Polymeric micelles offer the advantage of a modifiable polymeric building block, which enables flexibility in the type of therapeutics included in the micellar formulation. However, as with other carriers, achieving control over the loading ratio and subsequent release ratio has proven to be a non-trivial issue, and remains the focus of much of the ongoing research. In addition to the obstacles facing all drug delivery systems, future obstacles include increasing the ease of production and reducing variability of the self-assembly process.
PLGA nanoparticles
In addition to self-assembled polymeric micelles, other forms of polymeric nanoparticles, namely PLGA nanoparticles, can be used for the co-delivery of therapeutic cargos10, 25, 26, 91–102(Figure 6). PLGA is an optimal material to use for delivery because it is both biodegradable and biocompatible. It allows for extended, targeted release, and PLGA nanoparticles are particularly useful for immunotherapy because their size range makes them inherently targeted to dendritic cells for uptake and their properties can be modulated to allow for a variety of cargo types92. PLGA nanoparticles for co-delivery are typically formed via a double emulsion method. The initial oil/water (O/W) emulsion contains PLGA and the hydrophobic drug(s) dissolved in the oil phase, and any hydrophilic components dissolved in the water phase. This primary emulsion is then further emulsified in another water phase to form the particle, after which the solvents are removed via evaporation or lyophilization92, 98.
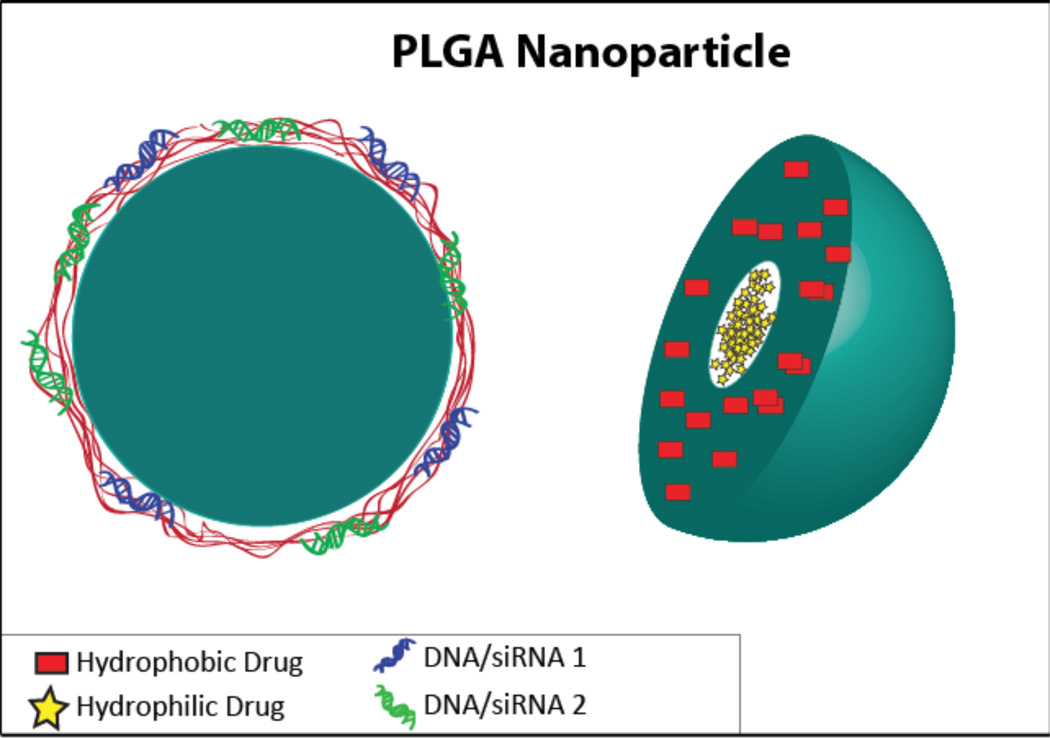
Figure 6.
A schematic of a PLGA nanoparticle, shown on the left with an outer layer complexed to two types of DNA/siRNA, and on the right with hydrophilic drug in the core and a hydrophobic drug in the PLGA layer.
The double emulsion technique allows PLGA nanoparticles to easily carry both hydrophilic and hydrophobic cargo. For example, one group formed PLGA nanoparticles containing the melanoma antigen tyrosinase-related protein 2 (TRP-2), which does not have a strong immunogenic effect, and Toll-like receptor ligand (7-acyl lipid A), in order to produce a strong anti-tumor immune response. In this formulation, TRP2 peptide, dissolved in water, was first emulsified in a chloroform solution of PLGA/7-acyl lipid A, followed by secondary emulsification in water/PVA and solvent evaporation. Despite a high degree of variability within test groups, it was concluded that the co-delivery of the antigen with 7-acyl lipid A was superior to separate delivery based on the slower tumor growth, smaller tumor size, and overall survival associated with co-delivery92. Another group co-delivered hydrophilic DOX and hydrophobic paclitaxel using amphiphilic copolymers, but the nanoparticles were formed using a double emulsion instead of self-assembly to encapsulate both the polar and non-polar molecules10.
PLGA microspheres have been developed containing both hydrophilic platelet-derived growth factor (PDGF)-BB, to stimulate wound healing, and hydrophobic chlorhexidine, an antimicrobial for the combined effect of more efficient wound healing. Inclusion of the hydrophobic CHX actually increased the encapsulation efficiency of the hydrophilic PDGF-BB, and the combination effectively promoted infection-free wound healing94. Another group formed PLGA nanoparticles containing both a hydrophobic and a hydrophilic chemotherapeutic drug as well as embedded magnetic nanoparticles for the purpose of simultaneous cancer therapy and imaging capabilities98.
An alternative route to achieving the efficient loading of both hydrophobic and hydrophilic drugs into a nanoparticle involves modifying the drugs themselves. In one system, hydrophobic paclitaxel (PTXL) and hydrophilic gemcitabine hydrochloride (GEM) were conjugated to each other using a hydrolyzable linker, allowing the drugs to be released by hydrolysis as therapeutically-active individual drugs in the target cell. The free drug-conjugate was tested in vitro with a human pancreatic cancer cell line, with resulting toxicity at nearly the same levels as a free drug mixture after 72 hours. These linked drugs were then encapsulated into a lipid-coated PLGA nanoparticle using nanoprecipitation, a feat which could have been accomplished only with high costs in terms of encapsulation efficiency of hydrophilic GEM if the drugs were not conjugated to each other. The encapsulated conjugates resulted in a decrease in the IC50 value by a factor of 200 when compared to that of the free PTXL-GEM conjugates, indicating the benefit of a nanoparticle carrier system71.
PLGA nanoparticles can also be modified after nanoparticle formation to make composite carriers. For example, one group formulated a carrier that delivered intercalated hydrophilic DOX within an aptamer, which was attached to the surface of a PEG-PLGA nanoparticle containing hydrophobic docetaxel25 (Figure 2biii), and another group used a PLGA nanoparticle carrier containing a hydrophobic drug in a self-assembly process with a folate-coated, PEGylated lipid shell to which DNA was bound100. Other variations include modifying the PLGA nanoparticle surface with PEI and using the PEI to complex genes coding for factors to induce chondrogenesis in mesenchymal stem cells93, or the use of chitosan to decrease the hydrophilic nature of CpG and subsequent application of Total Recirculation One-Machine System (TROMS) to form a nanoparticle encapsulating both an antigen and the adjuvant CpG97. Although PLGA nanoparticles can serve as a platform upon which further modification can be made to increase the flexibility of the carrier, one concern is the difficulty of completely removing the residual organic solvent during the formulation103. Another concern is the process used to load the second drug, which often requires a chemical reaction or physical adsorption in aqueous solvent, and may prematurely release the first drug.
Dendrimers
Although liposomes have garnered much success in clinical trials, they are usually prepared from multiple components and may be harder to synthesize and stabilize. Dendrimers, on the other hand, are polymeric macromolecules with a high degree of functionality and versatility, and as such are gaining greater prominence in the field of drug delivery. Dendrimers have a unique tree-like architecture and contain a core, repeating units and surface functionalized groups (Figure 7). They are well defined, monodisperse, and relatively biocompatible with numerous surface groups, which can be easily modified. Their unique structure offers the potential for high drug loading. Precise loading of drugs can also be obtained based on the generation number of the dendrimer.
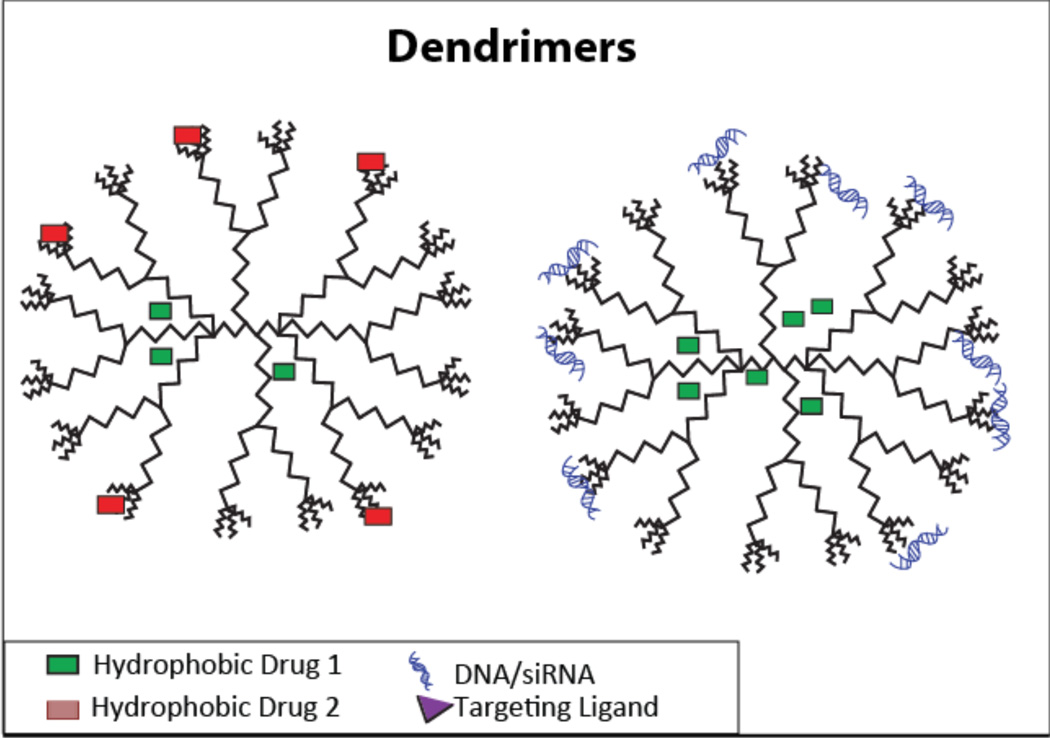
Figure 7.
A shematic depicting a dendrimer carrying two types of hydrophobic drugs (left), and a hydrophobic drug with DNA/siRNA (right).
Dendrimers are usually synthesized either through convergent or divergent synthesis. Convergent synthesis starts at the surface and proceeds inwards before the attachment of the previously synthesized dendrons to the core.
This method allows precise placement of the functional groups on the surface. Divergent synthesis, on the other hand, grows outwards from a functional core. Dendrimers are superior to the currently available liposomal and linear polymer formulations because of their strict structural control, high drug loading, and precise control over loading and release of drugs, all of which are key criteria for co-delivery. Dendrimers are particularly useful for co-delivery because they can load poorly soluble drugs in their hydrophobic cores and their versatile surface groups allow for attaching multiple drugs to the surface14, 23, 24, 104–111. Furthermore, they can be complexed with nucleic acids and used for gene therapy. For example, in one study, 5Fu was loaded via membrane dialysis and miRNA21 was complexed with the dendrimer. In another instance, DOX was used to intercalate the pORF-TRAIL plasmid and this intercalated-gene complex was loaded onto the dendrimer14, 109. Both of these formulations displayed excellent transfection efficiency due to the small size and spherical nature of dendrimers. This is in contrast to linear polymers, which have a random coil-like structure and offer less structural control.
Dendrimers also have the capability to load hydrophobic and hydrophilic drugs simultaneously. One group used this property to conjugate hydrophobic paclitaxel and hydrophilic alendronate to dendritic poly(ethylene glycol). The high degree of homogeneity of this fixed ratio of PTX/ALN also presented better targeting and enhanced activity compared to the free drugs105.
High drug loading is one of the crucial parameters in drug delivery and it is even more prominent in co-delivery because two different drugs are loaded in the same system. Dendrimers offer high drug loading due to the hydrophobic core and multiple surface groups available for modification. One group took advantage of the multiple surface groups of dendrimers to achieve both high drug loading and pH sensitivity. This was done by conjugating DOX to MPEG PAMAM through an acid labile hydrazine bond. This conjugate self-assembled, with DOX acting as a hydrophobic core, after which 10-hydroxycamptothecin was loaded into the hydrophobic core. The system displayed a high drug loading of 41.2% for DOX and 19.2% for HCPT. In addition, the release was predominantly at lower pH due to the acid labile bond, making the system especially useful for cancer therapy111.
One of the challenges with encapsulating drugs in the hydrophobic core of dendrimers is their burst release. However, conjugating the drugs can lead to very slow release. To achieve an optimum release, one group loaded the drugs in PLGA nanoparticles and used a partially cross-linked dendrimer hydrogel as a dispersion agent. Brimonidine and timolol maleate were loaded into the system with the goal of treating glaucoma. This system displayed high drug loading, sustained delivery of drugs for up to a week and an anti-glaucoma effect for 4 days following a one-time topical administration, highlighting the importance of release and drug loading properties (Figure 2bii)24.
Targeting is a key attribute which must be conferred to dendrimers to successfully target and selectively deliver drugs to specific tissue with minimal off-target accumulation. Dendrimers have numerous functional groups on their surface, which can be easily manipulated to present targeting properties. Unlike linear polymers, dendrimers can present a high ligand density with an increase in generation number, which facilitates ligand-receptor binding. In order to confer targeting properties, one group conjugated an aptamer-oligodeoxynucleotide (Apt-ODN) to the dendrimer. DOX was then intercalated within the nucleic acids. The aptamer was designed to target the prostate-specific membrane (PSMA) antigen overexpressed in prostate cancer and the ODN acted as an immune stimulatory agent. Since the timing and scheduling plays a crucial role in synergism, incorporating both the immunostimulatory and chemotherapeutic drug should lead to a stronger therapeutic effect. The Apt-ODN complex was stable even after 24 hours, due to the steric protection offered by the dendrimer. In addition, the DNA-RNA hybrid stimulated both TLR7 and TLR9 and exhibited high drug loading107. However, such a design might be unique to the intercalating properties associated with DOX, indicating that effort needs to be focused towards developing a more robust platform system, which can be applied to any combination of drugs.
Although dendrimers offer an exciting platform for combination therapy, they are still in their infancy compared to other conventional carriers such as liposomes. Blood circulation half-life and biodegradability are some of the challenges that need to be overcome. Additionally, advances in dendritic chemistry with respect to ease and cost of synthesis will stimulate further development of dendrimers112.
Mesoporous silica nanoparticles (MSNP)
Mesoporous silica nanoparticles are inorganic particles with honeycomb-like pores. They are known to have excellent biocompatibility and are beginning to find increasing use as a drug delivery carrier. As an inorganic nanoparticle, they are durable and robust, which allows them to encapsulate drugs more efficiently than other systems. In addition, they display high drug loading capabilities due to their porous nature, which offers high surface area for drug adsorption, and they also have controllable size, shape, and porosity, with pore size varying from 2–30 nm. Furthermore, they are resistant to a wide range of pH, mechanical strain, organic solvents and can easily undergo surface modification, which can be used to functionalize them with polycations to load nucleic acids. The synthesis of MSNPs is a relatively simple process with very few purification steps, and is either achieved by solution routes or aerosol-based evaporation-induced self -assembly.
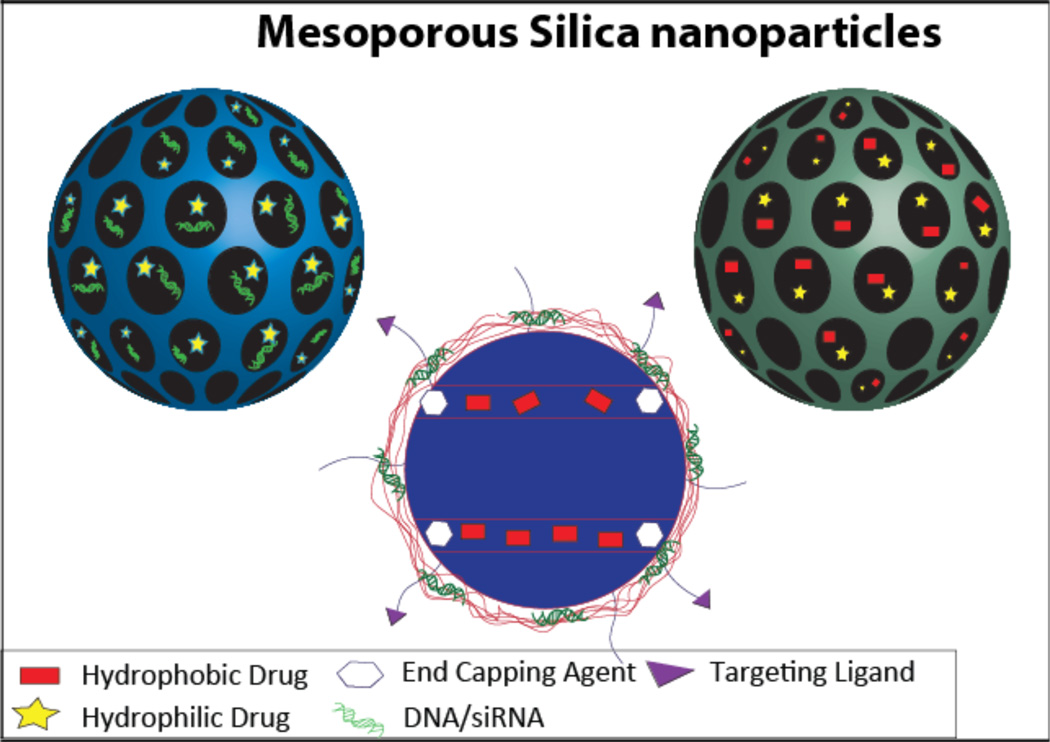
One of the unique features of silica nanoparticles is the presence of two functional surfaces; the inner pores and the outer surface (Figure 8). The properties of both these surfaces can be manipulated using a wide range of chemical moieties. The availability of multiple surfaces for functionalization has been exploited to load multiple drugs11, 12, 21, 113–117. The small molecules can be loaded within the pores and nucleic acids can be loaded on the modified surface. Recent co-delivery applications mostly focus on combating multidrug resistance in cancer through delivery of siRNA and drug or DNA and drug. One of the earliest approaches to loading multiple drugs was achieved by loading DOX within the pores and complexing Bcl-2 siRNA to the surface functionalized with amine-terminated PAMAM. The loaded DOX was released only in the presence of glutathione, achieving a preferential intracellular-triggered release. In addition, the co-delivery significantly silenced the non-pump resistance mechanism, increasing the efficacy of DOX 132 times compared to free DOX (Figure 1b)11. Another recent approach to load siRNA and drug was achieved by using a PEI coating to load P-glycoprotein siRNA on the surface of the MSNP and phosphonate coated pores to load water soluble DOX. The presence of DOX did not affect the loading and release of siRNA and vice versa. The MSNP exhibited a transfection efficiency higher than that of Lipofectamine 2000, and was able to reduce the expression of multiple drug resistance protein 1 (MDR-1) by 90%. Additionally, by silencing P-glycoprotein, the intracellular concentration of DOX was significantly increased due to fewer drugs being pumped out. This was also demonstrated through decreased IC50 value of the siRNA-PEI-DOX-MSNP in an additive manner. The electrostatic binding of DOX ensured a pH-sensitive release in this case, which is in contrast to the intracellularly triggered release in the previous case.
Figure 8.
A schematic of mesoporous silica nanoparticles containing a hydrophilic drug and DNA/siRNA in the pores (top left), a hydrophobic drug and a hydrophilic drug in the pores (top right), and a hydrophobic drug in the pores with DNA/siRNA complexed to the surface (bottom).
Enzyme triggered release has also been explored using hollow mesoporous particles. As a proof of concept fluorescein was loaded within the particles. The particles were coated with positively charged PLL and negatively charged CpG ODN using Layer by layer method. Figure 2aiii shows the release of CpG-ODN and fluorescein in the presence and absence of α-chymotrypsin. There was negligible release in the absence of the enzyme however in the presence of the enzyme 45% of the CpG-ODN was released within 24 hrs. Fluorescein was also released albeit at a much slower rate initially22.
When taken together, these examples demonstrate the versatility of silica nanoparticles as carriers capable of modulating release properties12. The same group also recently performed extensive screening assays using PEI-PEG coated MSNPs to optimize the siRNA/drug combination to overcome multidrug resistance in MCF-7/MDR cells. Among the various siRNA targets tested, the PgP-targeting siRNA combined with DOX showed superior synergism in vitro. This synergism was also demonstrated in vivo using xenografts from MCF-7/MDR cells in nude mice118. Finally, another group also successfully loaded a gene/siRNA with chloroquine to enhance the transfection efficiency of the system113.
Conventional nanocarriers such as liposomes are inherently unstable in physiological conditions and tend to undergo premature release. However, MSNPs are capable of zero release and stimuli-responsive release21, 117. Both release profiles were achieved in a system that used MSNPs to deliver cAMP and gluconic modified insulin (G-Ins). G-Ins was immobilized on the surface and also acted as a cap to prevent premature release of the drug. cAMP was loaded in the pores and was delivered to activate the existing cells to secrete insulin because the glucose-responsive delivery system usually experiences a decrease of insulin with repeated cycles. In the absence of glucose, less than 10% of cAMP was released. However, when triggered with 50mM glucose, more than 80% of the drug was released in less than 22 hours, demonstrating the glucose-triggered nature of the system (Figure 2aii). Since cAMP cannot permeate the membrane surface, endocytosis of MSNPs allows for cAMP to be delivered internally into the cells to exert its effect21.
Conventional polymeric drug delivery systems typically have to rely on chemical conjugation to co-deliver a hydrophobic and a hydrophilic drug, increasing the complexity of formulation. Recently, a group developed a simple sequential loading method using different solvents to load a hydrophobic and hydrophilic drug (DOX-paclitaxel and DOX-rapamycin) within the pores of an MSNP through molecular interaction between the hydroxyl groups of silica and the drug molecules. In addition to obtaining a high loading of the hydrophobic and hydrophilic drug, they were also able to manipulate the ratio of the two drugs loaded by varying the feed ratio116. The next generation of MSNP carriers is likely to be a multifunctional system endowed with targeting, stimuli-responsive, multiple-release, and theranostic capabilities.
Janus nanoparticles
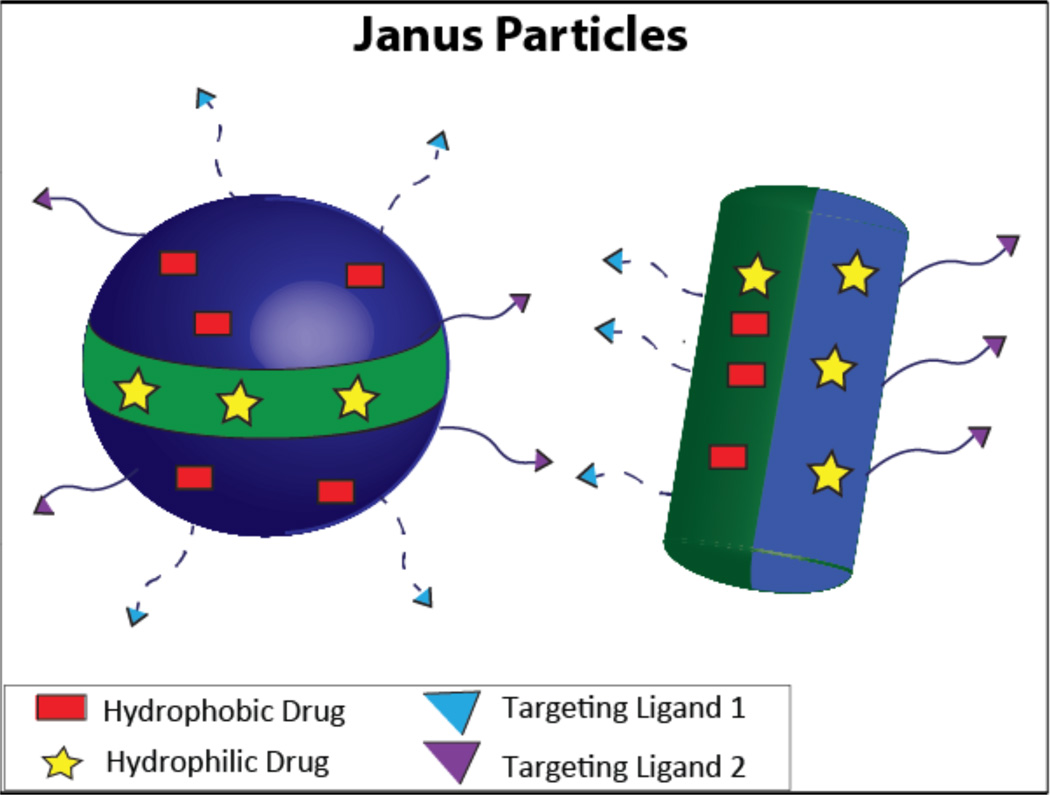
Janus particles have two or more distinct segments with different properties, which could include regions differing in chemistry, polarity, and functionalization (Figure 9). Due to their unique nature, they have great potential as a co-delivery carrier. The multiple segments allow for loading multiple drugs with a wide disparity in their properties; the architecture of Janus particles ensures that there is an absence of any interaction between these drugs, thereby generating diverse release profiles for the individual drugs119. In addition, a wide variety of features can be included in the same particle, such as pH responsiveness120, NIR responsiveness121, and multiple targeting ligands122.
Figure 9.
A schematic of Janus nanoparticles carrying both a hydrophobic drug and a hydrophilic drug, demonstrating the flexibility available for differences in spatial arrangement of drugs as well as surface functionalization.
The current methods of synthesizing Janus particles are masking, phase separation, self-assembly, and electrohydrodynamic co-jetting123. However, microfluidic-based synthesis has shown the most potential in creating uniform Janus particles124. One of the major disadvantages in loading drugs of varied properties is that there is no universal method for encapsulating all the drugs. Using a microfluidic system, one group created Janus particles comprised of PLGA that encapsulated hydrophilic doxorubicin hydrochloride (DOX) and hydrophobic paclitaxel (PTX) by nanoprecipitation and emulsion, respectively. This was accomplished when two different inlet streams, one containing PTX/PLGA in acetonitrile and the other containing a w/o emulsion of DOX/PLGA, came together to generate Janus droplets that subsequently underwent solvent evaporation to produce Janus particles. The drug loading efficiency was almost equivalent to that of monophasic particles. However, PTX from Janus particles had a larger burst release compared to monophasic particles, probably due to the fact that PTX is encapsulated differently in Janus particles. Encouragingly, the release of DOX was similar to that of monophasic particles.125
As has been discussed, the synergistic effect is greatly reliant on the ratio of the drugs incorporated in the carrier and the release rate of these drugs. A Janus PEG-based dendritic polymer synthesized via a convergent method allowed for exact control of the loading concentration and ratio of the drug. This was achieved by varying the dendron generation, which varied the number of drug loading sites. In addition, the release of the drugs was controlled by varying the linkers with different chemical stability (Figure 2bi)23.
Janus particles allow for loading of drugs with varied properties, as well as exact control of drug ratio, offering a promising platform for co-delivery of therapeutics in the future. Future applications might include immunization using both protein antigen and DNA antigen to stimulate the Th1 and Th2 pathways simultaneously, as well as co delivery of immunoadjuvants to the same immune cell for a more comprehensive immune response124.
Although Janus particles have great potential as a co-delivery carrier, poor structural control, difficult scale-up manufacturing, and low throughput act as barriers to translation of this technology. Better synthetic techniques must be developed before Janus particles can be widely applied for co-delivery applications.
DNA gels
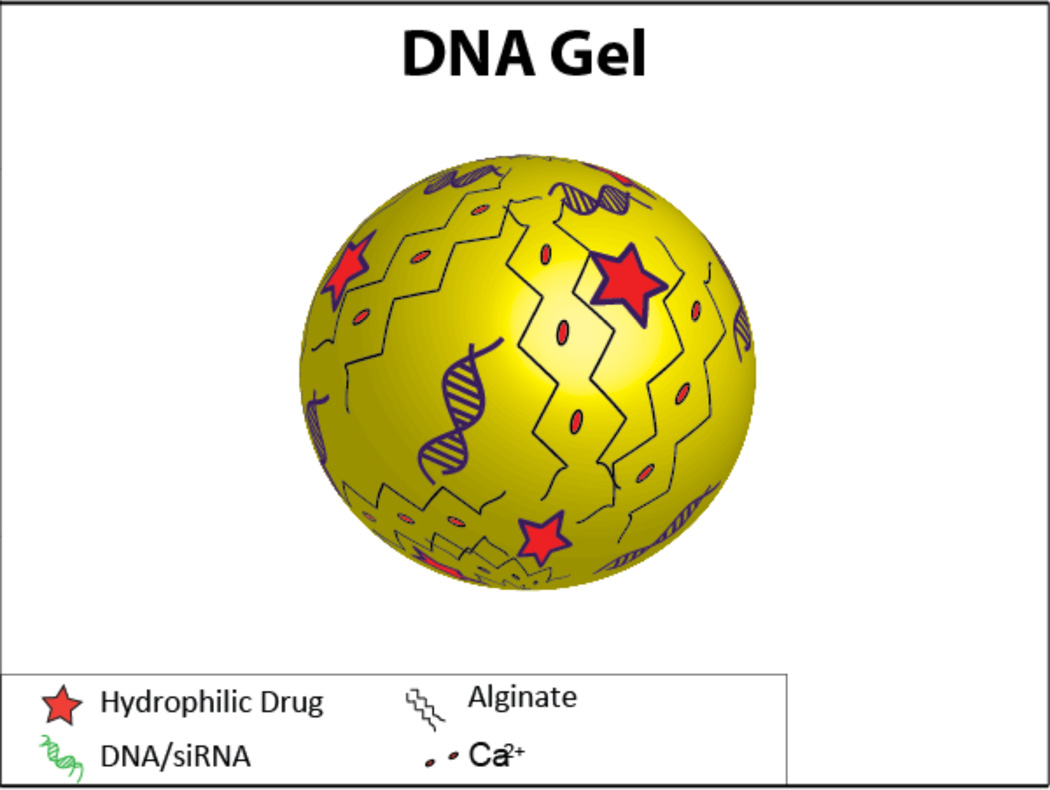
Gels are also candidates to be carriers of multiple therapeutics, especially in light of their biocompatibility and potential for modification to create a controlled release system. Thus far, they have been used to co-deliver plasmid DNA along with some type of small molecule (protein, drug, etc.)17, 126, 127 (Figure 10).
Figure 10.
A schematic of a DNA gel containing both a hydrophilic drug and DNA/siRNA.
One group used the plasmid DNA itself as the crosslinked gel component; they dissolved the plasmid DNA in water, chemically crosslinked it, and incorporated solutes by soaking the gel in a solution containing the desired solutes. The gels were crosslinked with ethylene diglycidyl ether (EGDE), which enables photochemical degradation of the gel upon exposure to UV light, allowing for controlled release of the gel components in response to the light energy and exposure127. Another group developed temperature-sensitive nanogels capable of loading larger-than-normal amounts of cargo by using a heating and cooling cycle that resulted in the collapse and subsequent re-swelling of the gel. The nanogels had a core-shell structure with a crosslinked hydrophobic core that could be loaded with proteins; the shell contained carbohydrate residues that allowed for the complexation of DNA. Once inside the cell, the pH-sensitive nanogel would degrade, releasing its contents126.
Alginate hydrogels have been formed to co-deliver the p53 antitumor gene along with encapsulated DOX for the treatment of cancer. In this case, inorganic ions co-precipitated calcium and the plasmid DNA; the presence of alginate in the gel retarded the growth of these co-precipitates without negatively impacting the encapsulation efficiency. These nanoparticles containing DOX alone were able to inhibit cells at a significantly higher rate than free DOX, and when delivered to HeLa cells, the co-delivery of p53 and DOX further potentiated the inhibitory effect compared to encapsulated-DOX alone, completely inhibiting the proliferation of the HeLa cells.17.
However, work must still be done to increase the effectiveness of these gels as a DNA carrier and to develop strategies for increasing drug encapsulation efficiencies. Although at a less-developed state than other carriers such as liposomes, DNA nanogels represent a promising avenue for future development; potential for controlled release coupled with the biocompatibility of hydrogels makes them an appealing co-delivery system.
Conclusion
Although co-delivery has shown great potential in improving the way we treat diseases, many challenges remain, some of which are similar to those confronting single-drug delivery but some are unique to co-delivery. Challenges experienced across the drug delivery field include short circulation times, poor bioavailability, undesirable release profiles and inability to cross various tissue and intracellular barriers. Challenges specific to co-delivery include firstly the judicious selection of drug combination that can elicit the maximum synergistic effect. Secondly, co-delivery should achieve an optimal ratio of the co-delivered drugs at the target site. The ratio of the drugs can direct the system to be synergistic, additive or antagonistic. Hence, extensive in vitro studies need to be done to optimize this parameter. This must be followed by in vitro-in vivo correlation. Finally, the drugs are in close proximity to each other, requiring that extensive characterization be done to ensure that the activity of one drug is not affected by the other. In addition to activity, the individual release profiles that can be potentially affected by drug-drug interactions must be addressed. This field of co-delivery is still in its infancy. Many of the studies have only demonstrated the proof-of-concept with model compounds and not active therapeutics, indicating that much remains to be done in terms of development and optimization.
Currently, most of the research regarding combination drug therapy is focused on selecting drug combinations to improve the efficacy of a particular therapy. This is understandable as the field is still in its initial stages. Given that drug development and drug delivery should go hand-in-hand, it would be wise to consider the design of carriers that can satisfy the demands of a particular combination of therapeutics early in the process. Most importantly, clinical translatability is the ultimate objective and regulatory hurdles should be taken in consideration as early as possible. The regulatory requirements are likely to increase with the complexity of a co-delivery system, which reinforces the idea that the therapeutic application must be judiciously selected.
The design and synthesis of carriers for co-delivery requires extra effort, as they need to cater to wide differences in the properties of the drugs loaded. Because the drugs may need to be released either simultaneously or sequentially, special formulation or manufacturing procedures may be required. Microfluidics-based production of controlled release particles is a fruitful direction that may address the manufacturing issue128. Many of the current single-drug delivery systems are moving towards multifunctional nanocarriers, which are equipped with imaging, targeting and controlled release abilities. Co-delivery system will also similarly be greatly benefited by such multifunctionality, but this will add another layer of complexity and further increase the burden of gaining regulatory approval. The field of co-delivery is facing a delicate balancing act between practicality and complex functionality as it moves toward clinical translation.
Table 2.
Properties of different co-delivery systems carrying similar cargo. Drug loading is provided as a weight percent, which is the mass of the drug loaded into the final formulation divided by the mass of the carrier material (i.e. polymer). In some cases where drug loading was not provided as a weight percent, the encapsulation efficiency, the percentage of the drug present during formulation that was actually encapsulated within the carrier, is used as an alternative measurement.
| Carrier | Specific Therapeutic | Approximate Size (nm) |
Drug Loading | Ref | |
|---|---|---|---|---|---|
| Drug/Gene | |||||
| Liposome | Paclitaxel and pEGFP-hTRAIL gene | 74.8 | 2.2 wt% | 16 | |
| Polymeric Micelle | DOX and reporter gene | 280 | 23.3 wt% | 80 | |
| Dendrimer | DOX and pORF-hTRAIL gene | 200 | 33.3 wt% | 14 | |
| Linear Conjugate | PC-DOX and p53 gene | 220–400 | 5 wt% | 61 | |
| Mesoporous Silica | Chloroquine and reporter gene | 130 | 32 wt% | 113 | |
| DNA gel | DOX and p53 gene | 145 | 1 wt% | 17 | |
| Drug/siRNA | |||||
| Liposome | DOX and siRNA targeting MRP-1 and Bcl-2 | 100–140 | 70% encapsulation efficiency | 34 | |
| Polymeric Micelle | DOX and Bcl-2 siRNA | 190 | 6.56 wt% | 73 | |
| DOX and P-gp siRNA | 93–108 | 6 wt% | 82 | ||
| Dendrimer | DOX and GAPDH siRNA | 56 | 65% encapsulation efficiency | 104 | |
| Mesoporous Silica | DOX and P-gp siRNA | 100–120 | 8.4 wt% | 12 | |
| Drug/Drug hydrophobic/hydrophobic | |||||
| Polymeric Micelle | DOX and Wortmannin | < 100 | 22.9–26.7 wt% of each drug | 72 | |
| Dendrimer | DOX and 10-Hydroxycamptothecin | 89 | 41.2 wt% DOX 19.2 wt% HCPT |
111 | |
| Linear Conjugate | DOX and Dexamethasone | 8.5 | 8.4 wt% DOX 6.6 wt% Dex |
59 | |
| hydrophobic/hydrophobic | |||||
| Polymeric Micelle | Paclitaxel and Gemcitabine hydrochloride | 70 | 1.6 wt% Ptxl 1.6 wt% Gemcitabine |
71 | |
| Dendrimer | Paclitaxel and Alendronate | 190 | 4.7 wt% Ptxl 11 wt% ALN |
105 | |
| Mesoporous Silica | DOX and Paclitaxel | 50 | 24.2 wt% DOX 15.6 wt% Ptxl |
116 | |
| Janus Particle | DOX and Paclitaxel | 305 | 0.6 wt% DOX 1.15 wt% Ptxl |
125 | |
| PLGA Nanoparticle | DOX and Paclitaxel | 243 | 9.67 wt% total drug | 10 | |
References
- 1.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Nat. Rev. Drug Discovery. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 2.Barth A, Morton DL. Cancer. 1995;75:726–734. doi: 10.1002/1097-0142(19950115)75:2+<726::aid-cncr2820751417>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.Miller MJ, Foy KC, Kaumaya PT. Discov Med. 2013;15:166–176. [PubMed] [Google Scholar]
- 4.Dai W, Jin W, Zhang J, Wang X, Wang J, Zhang X, Wan Y, Zhang Q. Pharm. Res. 2012;29:2902–2911. doi: 10.1007/s11095-012-0797-2. [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Lu Y, Lee A, Pan X, Yang X, Zhao X, Lee RJ. Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Société canadienne des sciences pharmaceutiques. 2007;10:350–357. [PubMed] [Google Scholar]
- 6.Kim J-y, Shim G, Choi H-w, Park J, Chung SW, Kim S, Kim K, Kwon IC, Kim C-W, Kim SY, Yang VC, Oh Y-K, Byun Y. Biomaterials. 2012;33:4424–4430. doi: 10.1016/j.biomaterials.2012.02.066. [DOI] [PubMed] [Google Scholar]
- 7.Riviere K, Kieler-Ferguson HM, Jerger K, Szoka FC. J. Controlled Release. 2011;153:288–296. doi: 10.1016/j.jconrel.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishida T, Shiraga E, Kiwada H. Journal of Controlled Release. 2009;134:194–200. doi: 10.1016/j.jconrel.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Zucker D, Barenholz Y. J. Controlled Release. 2010;146:326–333. doi: 10.1016/j.jconrel.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Zhao Y, Wu Y, Hu Y-l, Nan K, Nie G, Chen H. Biomaterials. 2011;32:8281–8290. doi: 10.1016/j.biomaterials.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Chen AM, Zhang M, Wei D, Stueber D, Taratula O, Minko T, He H. Small. 2009;5:2673–2677. doi: 10.1002/smll.200900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng H, Liong M, Xia T, Li Z, Ji Z, Zink JI, Nel AE. ACS Nano. 2010;4:4539–4550. doi: 10.1021/nn100690m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun T-M, Du J-Z, Yao Y-D, Mao C-Q, Dou S, Huang S-Y, Zhang P-Z, Leong KW, Song E-W, Wang J. ACS Nano. 2011;5:1483–1494. doi: 10.1021/nn103349h. [DOI] [PubMed] [Google Scholar]
- 14.Han L, Huang R, Li J, Liu S, Huang S, Jiang C. Biomaterials. 2011;32:1242–1252. doi: 10.1016/j.biomaterials.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 15.Xiao W, Chen X, Yang L, Mao Y, Wei Y, Chen L. Int. J. Pharm. 2010;393:119–126. doi: 10.1016/j.ijpharm.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Sun X, Pang Z, Ye H, Qiu B, Guo L, Li J, Ren J, Qian Y, Zhang Q, Chen J, Jiang X. Biomaterials. 2012;33:916–924. doi: 10.1016/j.biomaterials.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 17.Zhao D, Liu C-J, Zhuo R-X, Cheng S-X. Mol. Pharmaceutics. 2012;9:2887–2893. doi: 10.1021/mp3002123. [DOI] [PubMed] [Google Scholar]
- 18.Greco F, Vicent MJ, Gee S, Jones AT, Gee J, Nicholson RI, Duncan R. J. Controlled Release. 2007;117:28–39. doi: 10.1016/j.jconrel.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Lehar J, Krueger AS, Avery W, Heilbut AM, Johansen LM, Price ER, Rickles RJ, Short GF, 3rd, Staunton JE, Jin X, Lee MS, Zimmermann GR, Borisy AA. Nat. Biotechnol. 2009;27:659–666. doi: 10.1038/nbt.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao H, Li W, Qi R, Yan L, Wang R, Liu S, Zheng Y, Xie Z, Huang Y, Jing X. J. Controlled Release. 2012;163:304–314. doi: 10.1016/j.jconrel.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Trewyn BG, Slowing II, Lin VS-Y. J. Am. Chem. Soc. 2009;131:8398–8400. doi: 10.1021/ja901831u. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Y, Meng W, Gao H, Hanagata N. The Journal of Physical Chemistry C. 2011;115:13630–13636. [Google Scholar]
- 23.Acton AL, Fante C, Flatley B, Burattini S, Hamley IW, Wang Z, Greco F, Hayes W. Biomacromolecules. 2013;14:564–574. doi: 10.1021/bm301881h. [DOI] [PubMed] [Google Scholar]
- 24.Yang H, Tyagi P, Kadam RS, Holden CA, Kompella UB. ACS Nano. 2012;6:7595–7606. doi: 10.1021/nn301873v. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Radovic-Moreno AF, Alexis F, Gu FX, Basto PA, Bagalkot V, Jon S, Langer RS, Farokhzad OC. ChemMedChem. 2007;2:1268–1271. doi: 10.1002/cmdc.200700121. [DOI] [PubMed] [Google Scholar]
- 26.Sengupta S, Eavarone D, Capila I, Zhao G, Watson N, Kiziltepe T, Sasisekharan R. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 27.Tardi PG, Gallagher RC, Johnstone S, Harasym N, Webb M, Bally MB, Mayer LD. Biochimica et biophysica acta. 2007;1768:678–687. doi: 10.1016/j.bbamem.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Feldman EJ, Kolitz JE, Trang JM, Liboiron BD, Swenson CE, Chiarella MT, Mayer LD, Louie AC, Lancet JE. Leukemia research. 2012;36:1283–1289. doi: 10.1016/j.leukres.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K. Nanoscale Research Letters. 2013;8:102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zucker D, Andriyanov AV, Steiner A, Raviv U, Barenholz Y. J. Controlled Release. 2012;160:281–289. doi: 10.1016/j.jconrel.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Shim G, Han S-E, Yu Y-H, Lee S, Lee HY, Kim K, Kwon IC, Park TG, Kim YB, Choi YS, Kim C-W, Oh Y-K. J. Controlled Release. 2011;155:60–66. doi: 10.1016/j.jconrel.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Wu JJ, Huang L. Mol. Ther. 2010;18:828–834. doi: 10.1038/mt.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang SH, Cho H-J, Shim G, Lee S, Kim S-H, Choi H-G, Kim C-W, Oh Y-K. Pharm. Res. 2011;28:3069–3078. doi: 10.1007/s11095-011-0569-4. [DOI] [PubMed] [Google Scholar]
- 34.Saad M, Garbuzenko OB, Minko T. Nanomedicine (London, UK) 2008;3:761–776. doi: 10.2217/17435889.3.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong Z, Wan Y, Shi S, Han J, Zhang Z, Sun X. Pharm. Res. 2012;29:145–157. doi: 10.1007/s11095-011-0521-7. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y-f, Wang J-c, Bian D-y, Zhang X, Zhang Q. European Journal of Pharmaceutics and Biopharmaceutics. 2010;74:467–473. doi: 10.1016/j.ejpb.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Mendonça LS, Moreira JN, de Lima MCP, Simões S. Biotechnol. Bioeng. 2010;107:884–893. doi: 10.1002/bit.22858. [DOI] [PubMed] [Google Scholar]
- 38.Tardi P, Johnstone S, Harasym N, Xie S, Harasym T, Zisman N, Harvie P, Bermudes D, Mayer L. Leukemia research. 2009;33:129–139. doi: 10.1016/j.leukres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 39.Dicko A, Kwak S, Frazier AA, Mayer LD, Liboiron BD. J. Pharm. Sci. 2010;391:2540–2548. doi: 10.1016/j.ijpharm.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Hourigan CS, Karp JE. Current opinion in investigational drugs (London, England : 2000) 2010;11:669–677. [PMC free article] [PubMed] [Google Scholar]
- 41.Lim W-S, Tardi PG, Dos Santos N, Xie X, Fan M, Liboiron BD, Huang X, Harasym TO, Bermudes D, Mayer LD. Leukemia research. 2010;34:1214–1223. doi: 10.1016/j.leukres.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 42.Lim W-S, Tardi PG, Xie X, Fan M, Huang R, Ciofani T, Harasym TO, Mayer LD. Leukemia & lymphoma. 2010;51:1536–1542. doi: 10.3109/10428194.2010.490312. [DOI] [PubMed] [Google Scholar]
- 43.Feldman EJ, Lancet JE, Kolitz JE, Ritchie EK, Roboz GJ, List AF, Allen SL, Asatiani E, Mayer LD, Swenson C, Louie AC. Journal of Clinical Oncology. 2011;29:979–985. doi: 10.1200/JCO.2010.30.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HP, Gerhard B, Harasym TO, Mayer LD, Hogge DE. Experimental hematology. 2011;39:741–750. doi: 10.1016/j.exphem.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Lancet JCJ, Kovacsovics T, Hogge D, Kolitz JE, Tallman MS, Chiarella M, Louie A, Feldman E. American Society of Clinical Oncology (ASCO) Annual Meeting. 2011 [Google Scholar]
- 46.Batist G, Gelmon KA, Chi KN, Miller WH, Chia SKL, Mayer LD, Swenson CE, Janoff AS, Louie AC. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:692–700. doi: 10.1158/1078-0432.CCR-08-0515. [DOI] [PubMed] [Google Scholar]
- 47.Mayer LD, Harasym TO, Tardi PG, Harasym NL. Mol. Cancer Ther. 2006;5:1854–1863. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- 48.Harasym TO, Tardi PG, Harasym NL, P H, A JS, D ML. Oncology Research. 2007;16:361–374. doi: 10.3727/000000006783980937. [DOI] [PubMed] [Google Scholar]
- 49.Batist G, Sawyer M, Gabrail N, Christiansen N. Journal of Clinical Oncology (Meeting Abstracts) 2008;26:4108. [Google Scholar]
- 50.Li WM, Dragowska WH, Bally MB, Schutze-Redelmeier M-P. Vaccine. 2003;21:3319–3329. doi: 10.1016/s0264-410x(03)00172-5. [DOI] [PubMed] [Google Scholar]
- 51.Jiao X, Wang RY-H, Qiu Q, Alter HJ, Shih JW-K. J. Gen. Virol. 2004;85:1545–1553. doi: 10.1099/vir.0.79896-0. [DOI] [PubMed] [Google Scholar]
- 52.Badiee A, Jaafari MR, Samiei A, Soroush D, Khamesipour A. Clin. Vaccine Immunol. 2008;15:668–674. doi: 10.1128/CVI.00413-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tafaghodi M, Jaafari M, Sajaditabassi S. European Journal of Pharmaceutics and Biopharmaceutics. 2006;64:138–145. doi: 10.1016/j.ejpb.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Bu J, Song Y, Rompato G, Burgess D, Garmendia A. Comparative Immunology, Microbiology and Infectious Diseases. 2003;26:175–187. doi: 10.1016/s0147-9571(02)00050-4. [DOI] [PubMed] [Google Scholar]
- 55.Andrews CD, Provoda CJ, Ott G, Lee K-D. Bioconjugate Chem. 2011;22:1279–1286. doi: 10.1021/bc100436y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halwani M, Yebio B, Suntres ZE, Alipour M, Azghani AO, Omri A. J. Antimicrob. Chemother. 2008;62:1291–1297. doi: 10.1093/jac/dkn422. [DOI] [PubMed] [Google Scholar]
- 57.Halwani M, Hebert S, Suntres ZE, Lafrenie RM, Azghani AO, Omri A. Int. J. Pharm. 2009;373:141–146. doi: 10.1016/j.ijpharm.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Alipour M, Suntres ZE, Lafrenie RM, Omri A. J. Antimicrob. Chemother. 2010;65:684–693. doi: 10.1093/jac/dkq036. [DOI] [PubMed] [Google Scholar]
- 59.Krakovičová H, Etrych T, Ulbrich K. Eur. J. Pharm. Sci. 2009;37:405–412. doi: 10.1016/j.ejps.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 60.Lammers T, Subr V, Ulbrich K, Peschke P, Huber PE, Hennink WE, Storm G. Biomaterials. 2009;30:3466–3475. doi: 10.1016/j.biomaterials.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 61.Lu X, Wang Q-Q, Xu F-J, Tang G-P, Yang W-T. Biomaterials. 2011;32:4849–4856. doi: 10.1016/j.biomaterials.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 62.Miller K, Eldar-Boock A, Polyak D, Segal E, Benayoun L, Shaked Y, Satchi-Fainaro R. Mol. Pharmaceutics. 2011;8:1052–1062. doi: 10.1021/mp200083n. [DOI] [PubMed] [Google Scholar]
- 63.Segal E, Pan H, Benayoun L, Kopeckova P, Shaked Y, Kopecek J, Satchi-Fainaro R. Biomaterials. 2011;32:4450–4463. doi: 10.1016/j.biomaterials.2011.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Segal E, Pan H, Ofek P, Udagawa T, Kopeckova P, Kopecek J, Satchi-Fainaro R. PLoS One. 2009;4:e5233. doi: 10.1371/journal.pone.0005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vicent MJ, Greco F, Nicholson RI, Paul A, Griffiths PC, Duncan R. Angew. Chem., Int. Ed. 2005;44:4061–4066. doi: 10.1002/anie.200462960. [DOI] [PubMed] [Google Scholar]
- 66.Lammers T. Adv. Drug Delivery Rev. 2010;62:203–230. doi: 10.1016/j.addr.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 67.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. J. Controlled Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 68.Greco F, Vicent MJ. Adv. Drug Delivery Rev. 2009;61:1203–1213. doi: 10.1016/j.addr.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 69.Aryal S, Hu C-MJ, Zhang L. Mol. Pharm. 2011;8:1401–1407. doi: 10.1021/mp200243k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Gao S, Ye W-H, Yoon HS, Yang Y-Y. Nat. Mater. 2006;5:791–796. doi: 10.1038/nmat1737. [DOI] [PubMed] [Google Scholar]
- 71.Aryal S, Hu CM, Zhang L. Small. 2010;6:1442–1448. doi: 10.1002/smll.201000631. [DOI] [PubMed] [Google Scholar]
- 72.Bae Y, Diezi TA, Zhao A, Kwon GS. J. Controlled Release. 2007;122:324–330. doi: 10.1016/j.jconrel.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 73.Cao N, Cheng D, Zou S, Ai H, Gao J, Shuai X. Biomaterials. 2011;32:2222–2232. doi: 10.1016/j.biomaterials.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 74.Duong HTT, Marquis CP, Whittaker M, Davis TP, Boyer C. Macromolecules. 2011;44:8008–8019. [Google Scholar]
- 75.Hu QD, Fan H, Ping Y, Liang WQ, Tang GP, Li J. Chem. Commun. (Cambridge, U. K.) 2011;47:5572–5574. doi: 10.1039/c1cc10721f. [DOI] [PubMed] [Google Scholar]
- 76.Kolishetti N, Dhar S, Valencia PM, Lin LQ, Karnik R, Lippard SJ, Langer R, Farokhzad OC. Proc. Natl. Acad. Sci. U. S. A. 2010;107:17939–17944. doi: 10.1073/pnas.1011368107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee AL, Wang Y, Cheng HY, Pervaiz S, Yang YY. Biomaterials. 2009;30:919–927. doi: 10.1016/j.biomaterials.2008.10.062. [DOI] [PubMed] [Google Scholar]
- 78.Men K, Gou ML, Guo QF, Wang XH, Shi S, Kan B, Huang MJ, Luo F, Chen LJ, Zhao X, Qian ZY, Liang SF, Wei YQ. J. Nanosci. Nanotechnol. 2010;10:7958–7964. doi: 10.1166/jnn.2010.2668. [DOI] [PubMed] [Google Scholar]
- 79.Pramod PS, Takamura K, Chaphekar S, Balasubramanian N, Jayakannan M. Biomacromolecules. 2012;13:3627–3640. doi: 10.1021/bm301583s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qiu LY, Bae YH. Biomaterials. 2007;28:4132–4142. doi: 10.1016/j.biomaterials.2007.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shin HC, Alani AW, Cho H, Bae Y, Kolesar JM, Kwon GS. Mol. Pharm. 2011;8:1257–1265. doi: 10.1021/mp2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiong XB, Lavasanifar A. ACS Nano. 2011;5:5202–5213. doi: 10.1021/nn2013707. [DOI] [PubMed] [Google Scholar]
- 83.Zhang J, Sun H, Ma PX. ACS Nano. 2010;4:1049–1059. doi: 10.1021/nn901213a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Capretto L, Mazzitelli S, Colombo G, Piva R, Penolazzi L, Vecchiatini R, Zhang X, Nastruzzi C. Int. J. Pharm. 2012 doi: 10.1016/j.ijpharm.2012.07.057. [DOI] [PubMed] [Google Scholar]
- 85.Shi S, Zhu X, Guo Q, Wang Y, Zuo T, Luo F, Qian Z. Int. J. Nanomedicine. 2012;7:1749–1759. doi: 10.2147/IJN.S28932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu C, Jung S, Luo S, Meng F, Zhu X, Park TG, Zhong Z. Biomaterials. 2010;31:2408–2416. doi: 10.1016/j.biomaterials.2009.11.077. [DOI] [PubMed] [Google Scholar]
- 87.Wang Z, Ho PC. Biomaterials. 2010;31:7115–7123. doi: 10.1016/j.biomaterials.2010.05.075. [DOI] [PubMed] [Google Scholar]
- 88.Gaucher G, Dufresne MH, Sant VP, Kang N, Maysinger D, Leroux JC. J. Controlled Release. 2005;109:169–188. doi: 10.1016/j.jconrel.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 89.Chen S, Cheng SX, Zhuo RX. Macromol. Biosci. 2011;11:576–589. doi: 10.1002/mabi.201000427. [DOI] [PubMed] [Google Scholar]
- 90.Miller T, van Colen G, Sander B, Golas MM, Uezguen S, Weigandt M, Goepferich A. Pharm. Res. 2013;30:584–595. doi: 10.1007/s11095-012-0903-5. [DOI] [PubMed] [Google Scholar]
- 91.Diwan M, Tafaghodi M, S J. J. Controlled Release. 2002;85:247–262. doi: 10.1016/s0168-3659(02)00275-4. [DOI] [PubMed] [Google Scholar]
- 92.Hamdy S, Molavi O, Ma Z, Haddadi A, Alshamsan A, Gobti Z, Elhasi S, Samuel J, Lavasanifar A. Vaccine. 2008;26:5046–5057. doi: 10.1016/j.vaccine.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 93.Jeon SY, Park JS, Yang HN, Woo DG, Park K-H. Biomaterials. 2012;33:4413–4423. doi: 10.1016/j.biomaterials.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 94.Jiang B, Zhang G, Brey EM. Acta Biomaterialia. 2012;9:4976–4984. doi: 10.1016/j.actbio.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 95.Molavi O, Ma Z, Hamdy S, Lavasanifar A, Samuel J. Immunopharmacology and Immunotoxicology. 2009;31:214–221. doi: 10.1080/08923970802380452. [DOI] [PubMed] [Google Scholar]
- 96.Patil YB, Swaminathan SK, Sadhukha T, Ma L, Panyam J. Biomaterials. 2010;31:358–365. doi: 10.1016/j.biomaterials.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.San Romáan B, Irache JM, Gómez S, Tsapis N, Gamazo C, Espuelas MS. European Journal of Pharmaceutics and Biopharmaceutics. 2008;70:98–108. doi: 10.1016/j.ejpb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 98.Singh A, Dilnawaz F, Mewar S, Sharma U, Jagannathan NR, Sahoo SK. ACS Appl. Mater. Interfaces. 2011;3:842–856. doi: 10.1021/am101196v. [DOI] [PubMed] [Google Scholar]
- 99.Song XR, Cai Z, Zheng Y, He G, Cui FY, Gong DQ, Hou SX, Xiong SJ, Lei XJ, Wei YQ. Eur. J. Pharm. Sci. 2009;37:300–305. doi: 10.1016/j.ejps.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 100.Wang H, Zhao P, Su W, Wang S, Liao Z, Niu R, Chang J. Biomaterials. 2010;31:8741–8748. doi: 10.1016/j.biomaterials.2010.07.082. [DOI] [PubMed] [Google Scholar]
- 101.Zhang X-Q, Dahle CE, Weiner GJ, Salem AK. J. Pharm. Sci. 2007;96:3283–3292. doi: 10.1002/jps.20978. [DOI] [PubMed] [Google Scholar]
- 102.Zhang L, Chan JM, Gu FX, Rhee JW, Wang AZ, Radovic-Moreno AF, Alexis F, Langer R, Farokhzad OC. ACS Nano. 2008;2:1696–1702. doi: 10.1021/nn800275r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wischke C, Schwendeman SP. Int. J. Pharm. 2008;364:298–327. doi: 10.1016/j.ijpharm.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 104.Biswas S, Deshpande PP, Navarro G, Dodwadkar NS, Torchilin VP. Biomaterials. 2013;34:1289–1301. doi: 10.1016/j.biomaterials.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Clementi C, Miller K, Mero A, Satchi-Fainaro R, Pasut G. Mol. Pharm. 2011;8:1063–1072. doi: 10.1021/mp2001445. [DOI] [PubMed] [Google Scholar]
- 106.Kaneshiro TL, Lu Z-R. Biomaterials. 2009;30:5660–5666. doi: 10.1016/j.biomaterials.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 107.Lee I-H, An S, Yu MK, Kwon H-K, Im S-H, Jon S. J. Controlled Release. 2011;155:435–441. doi: 10.1016/j.jconrel.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 108.Miller K, Clementi C, Polyak D, Eldar-Boock A, Benayoun L, Barshack I, Shaked Y, Pasut G, Satchi-Fainaro R. Biomaterials. 2013;34:3795–3806. doi: 10.1016/j.biomaterials.2013.01.052. [DOI] [PubMed] [Google Scholar]
- 109.Ren Y, Kang C-S, Yuan X-B, Zhou X, Xu P, Han L, Wang GX, Jia Z, Zhong Y, Yu S, Sheng J, Pu P-Y. J. Biomater. Sci., Polym. Ed. 2010;21:303–314. doi: 10.1163/156856209X415828. [DOI] [PubMed] [Google Scholar]
- 110.Tekade RK, Dutta T, Tyagi A, Bharti AC, Das BC, Jain NK. J. Drug Targeting. 2008;16:758–772. doi: 10.1080/10611860802473154. [DOI] [PubMed] [Google Scholar]
- 111.Zhang Y, Xiao C, Li M, Chen J, Ding J, He C, Zhuang X, Chen X. Macromol. Biosci. 2013 doi: 10.1002/mabi.201200441. [DOI] [PubMed] [Google Scholar]
- 112.Cheng Y, Zhao L, Li Y, Xu T. Chem. Soc. Rev. 2011;40:2673–2703. doi: 10.1039/c0cs00097c. [DOI] [PubMed] [Google Scholar]
- 113.Bhattarai SR, Muthuswamy E, Wani A, Brichacek M, Castañeda AL, Brock SL, Oupický D. Adv. Healthcare Mater. 2012;27:2556–2568. doi: 10.1007/s11095-010-0245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Choudhury NN, He H. Curr. Pharm. Biotechnol. 2012;13:1317–1331. doi: 10.2174/138920112800624418. [DOI] [PubMed] [Google Scholar]
- 115.He Q, Gao Y, Zhang L, Zhang Z, Gao F, Ji X, Li Y, Shi J. Biomaterials. 2011;32:7711–7720. doi: 10.1016/j.biomaterials.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 116.Liu Q, Zhang J, Sun W, Xie QR, Xia W, Gu H. Int. J. Nanomedicine. 2012;7:999–1013. doi: 10.2147/IJN.S28088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu Y, Meng W, Hanagata N. Dalton transactions (Cambridge, England : 2003) 2011;40:10203–10208. doi: 10.1039/c1dt11114k. [DOI] [PubMed] [Google Scholar]
- 118.Meng H, Mai WX, Zhang H, Xue M, Xia T, Lin S, Wang X, Zhao Y, Ji Z, Zink JI, Nel AE. ACS Nano. 2013;7:994–1005. doi: 10.1021/nn3044066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hwang S, Lahann J. Macromol. Rapid Commun. 2012;33:1178–1183. doi: 10.1002/marc.201200054. [DOI] [PubMed] [Google Scholar]
- 120.Isojima T, Lattuada M, Vander Sande JB, Hatton TA. ACS Nano. 2008;2:1799–1806. doi: 10.1021/nn800089z. [DOI] [PubMed] [Google Scholar]
- 121.Sun L, Ma X, Dong C-M, Zhu B, Zhu X. Biomacromolecules. 2012;13:3581–3591. doi: 10.1021/bm3010325. [DOI] [PubMed] [Google Scholar]
- 122.Wu LY, Ross BM, Hong S, Lee LP. Small. 2010;6:503–507. doi: 10.1002/smll.200901604. [DOI] [PubMed] [Google Scholar]
- 123.Hu J, Zhou S, Sun Y, Fang X, Wu L. Chem. Soc. Rev. 2012;41:4356–4378. doi: 10.1039/c2cs35032g. [DOI] [PubMed] [Google Scholar]
- 124.Yang S, Guo F, Kiraly B, Mao X, Lu M, Leong KW, Huang TJ. Lab Chip. 2012;12:2097. doi: 10.1039/c2lc90046g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xie H, She Z-G, Wang S, Sharma G, Smith JW. Langmuir. 2012;28:4459–4463. doi: 10.1021/la2042185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ahmed M, Narain R. Mol. Pharm. 2012;9:3160–3170. doi: 10.1021/mp300255p. [DOI] [PubMed] [Google Scholar]
- 127.Costa D, Valente AJM, Miguel MG, Queiroz J. Langmuir. 2011;27:13780–13789. doi: 10.1021/la2026285. [DOI] [PubMed] [Google Scholar]
- 128.Zhang Y, Chan HF, Leong KW. Adv. Drug Delivery Rev. 2012;65:104–120. doi: 10.1016/j.addr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]