Abstract
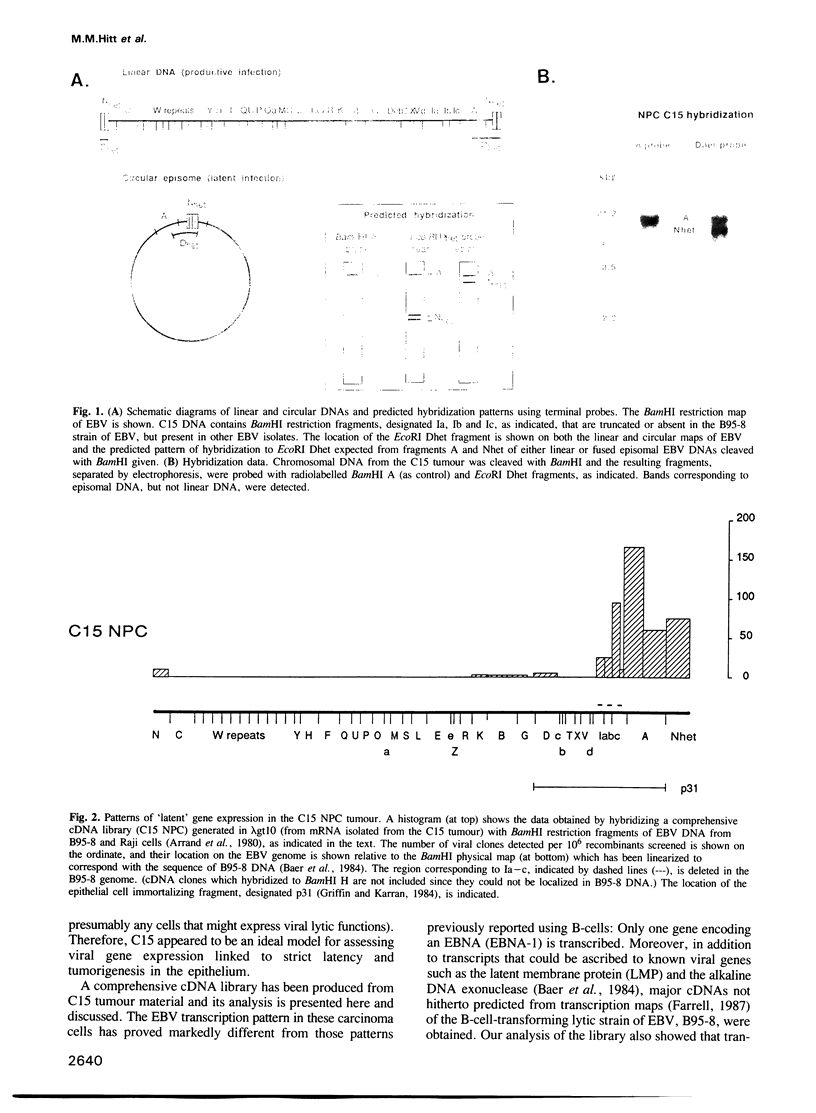
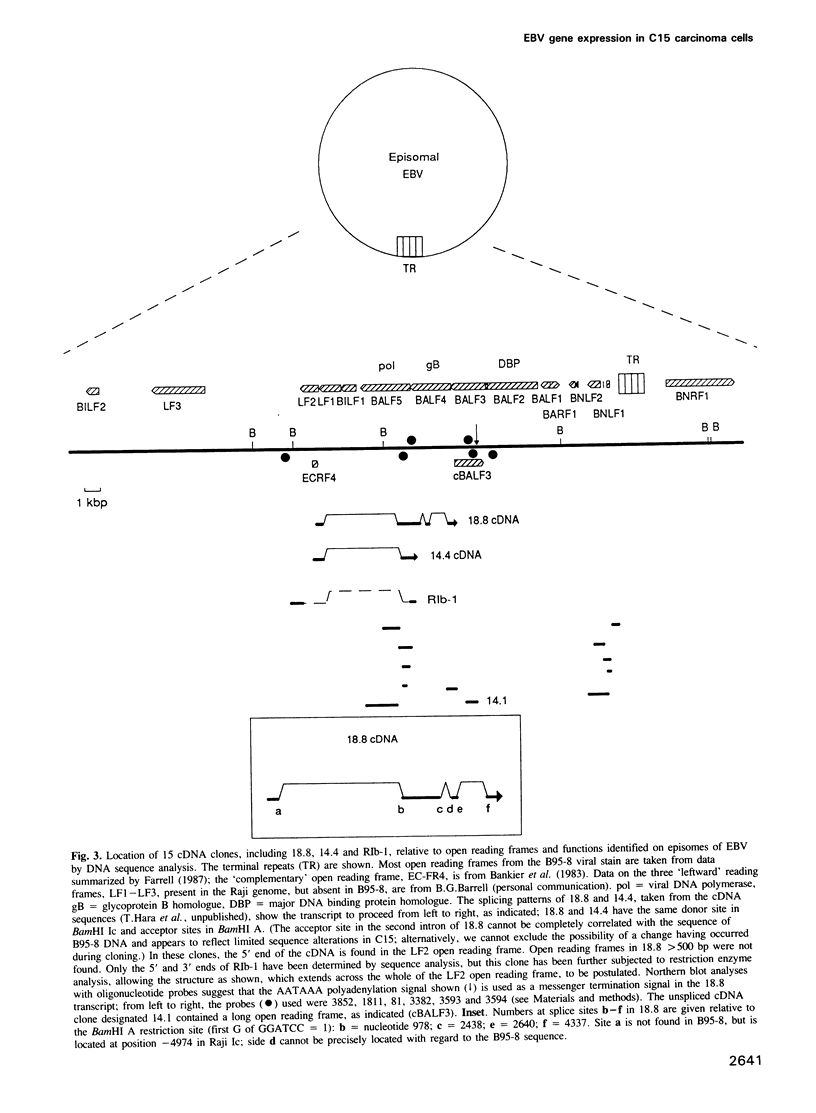
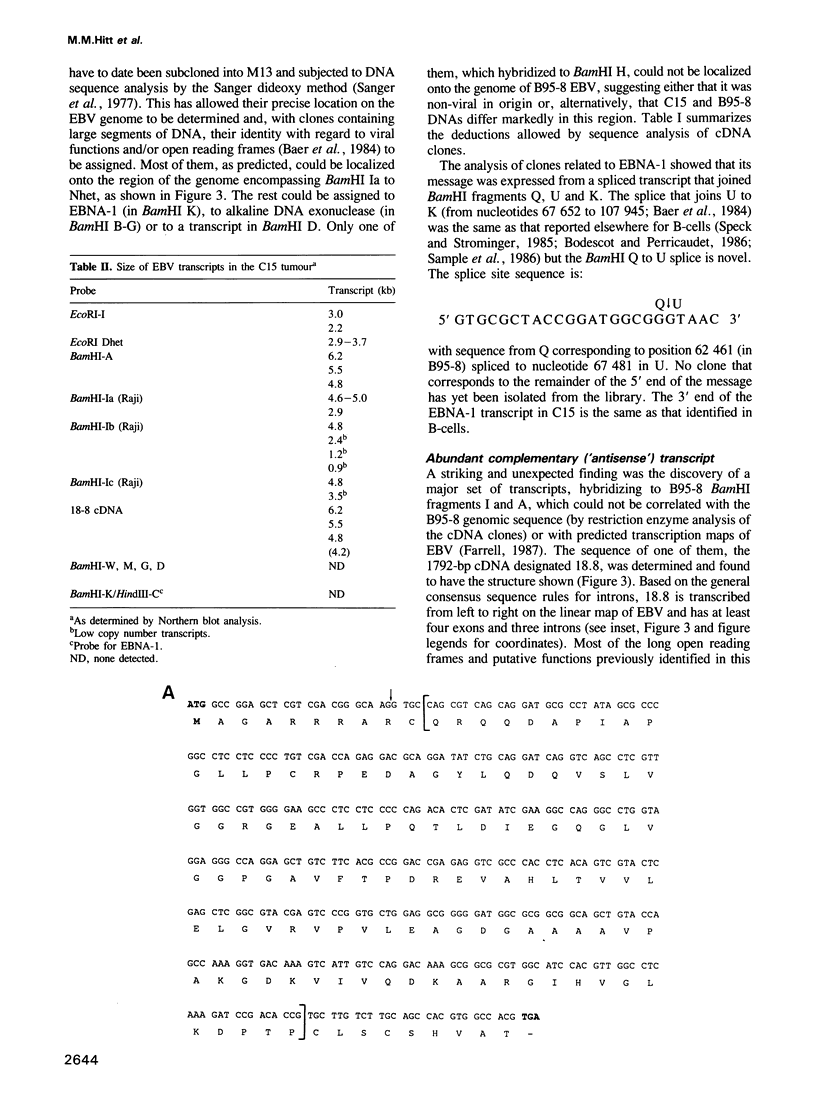
A nasopharyngeal carcinoma tumour (designated C15) propagated in nude mice has been used to generate a large cDNA library that we have analysed for Epstein-Barr virus (EBV) gene expression. No gross alterations exist in viral DNA from C15 relative to other human isolates and the large deletion present in the B95-8 'prototype' viral strain established in marmoset cells is not found; C15 contains no linear virion DNA. In the cDNA library, of the six EBV nuclear antigens (EBNAs) expressed in latently infected B-lymphocytes, only clones for EBNA-1 are found. These data are confirmed by immunoblotting. Sequence analysis shows the EBNA-1 mRNA splicing pattern in the carcinoma to differ from that observed in B-lymphocytes. Further, contrary to observations with B-cell lines, most viral transcription in the tumour is localized onto the 'rightmost' region of the conventional EBV physical map. Transcripts identified corresponding to known genes include those for the latent membrane protein (LMP), the alkaline DNA exonuclease and probably the terminal protein; major transcripts are also derived from the BamHI D fragment and the region deleted in B95-8 EBV DNA. Novel transcripts have also been identified that proceed in an anti-sense direction to genes encoding functions associated with replication, such as the viral DNA polymerase. They contain a large, hitherto unidentified, open reading frame in the viral genome that is complementary to the putative function known as BALF3 and a smaller open reading frame complementary to BALF5 (the DNA polymerase gene). From the present studies we can conclude that: (i) EBV transcription patterns in the epithelial cells vary markedly from those identified previously in B-cells, reflecting differential use of promoters or splicing patterns. (ii) Transcription is tightly regulated and restricted in the C15 tumour with many latent genes, notably EBNAs 2-6, being 'switched off.' (iii) A family of cytoplasmic RNAs are transcribed in an antisense direction to a number of existing open reading frames in the EBV genome. (iv) There are a number of mutations in C15 transcripts relative to the B95-8 genome, some of which could result in amino acid alterations in proteins.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allday M. J., Crawford D. H., Griffin B. E. Prediction and demonstration of a novel Epstein-Barr virus nuclear antigen. Nucleic Acids Res. 1988 May 25;16(10):4353–4367. doi: 10.1093/nar/16.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allday M. J., Jones M. D. Rapid processing of nitrocellulose filter lifts of bacteriophage lambda libraries. Nucleic Acids Res. 1987 Dec 23;15(24):10592–10592. doi: 10.1093/nar/15.24.10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrand J. R., Rymo L., Walsh J. E., Björck E., Lindahl T., Griffin B. E. Molecular cloning of the complete Epstein-Barr virus genome as a set of overlapping restriction endonuclease fragments. Nucleic Acids Res. 1981 Jul 10;9(13):2999–3014. doi: 10.1093/nar/9.13.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bankier A. T., Deininger P. L., Farrell P. J., Barrell B. G. Sequence analysis of the 17,166 base-pair EcoRI fragment C of B95-8 Epstein-Barr virus. Mol Biol Med. 1983 Jul;1(1):21–45. [PubMed] [Google Scholar]
- Bodescot M., Brison O., Perricaudet M. An Epstein-Barr virus transcription unit is at least 84 kilobases long. Nucleic Acids Res. 1986 Mar 25;14(6):2611–2620. doi: 10.1093/nar/14.6.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodescot M., Chambraud B., Farrell P., Perricaudet M. Spliced RNA from the IR1-U2 region of Epstein-Barr virus: presence of an open reading frame for a repetitive polypeptide. EMBO J. 1984 Aug;3(8):1913–1917. doi: 10.1002/j.1460-2075.1984.tb02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodescot M., Perricaudet M. Clustered alternative splice sites in Epstein-Barr virus RNAs. Nucleic Acids Res. 1987 Jul 24;15(14):5887–5887. doi: 10.1093/nar/15.14.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodescot M., Perricaudet M. Epstein-Barr virus mRNAs produced by alternative splicing. Nucleic Acids Res. 1986 Sep 11;14(17):7103–7114. doi: 10.1093/nar/14.17.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodescot M., Perricaudet M., Farrell P. J. A promoter for the highly spliced EBNA family of RNAs of Epstein-Barr virus. J Virol. 1987 Nov;61(11):3424–3430. doi: 10.1128/jvi.61.11.3424-3430.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkamm G. W., von Knebel-Doeberitz M., Lenoir G. M. No evidence for differences in the Epstein-Barr virus genome carried in Burkitt lymphoma cells and nonmalignant lymphoblastoid cells from the same patients. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4930–4934. doi: 10.1073/pnas.81.15.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busson P., Braham K., Ganem G., Thomas F., Grausz D., Lipinski M., Wakasugi H., Tursz T. Epstein-Barr virus-containing epithelial cells from nasopharyngeal carcinoma produce interleukin 1 alpha. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6262–6266. doi: 10.1073/pnas.84.17.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busson P., Ganem G., Flores P., Mugneret F., Clausse B., Caillou B., Braham K., Wakasugi H., Lipinski M., Tursz T. Establishment and characterization of three transplantable EBV-containing nasopharyngeal carcinomas. Int J Cancer. 1988 Oct 15;42(4):599–606. doi: 10.1002/ijc.2910420422. [DOI] [PubMed] [Google Scholar]
- Chen J. Y., Chen C. J., Liu M. Y., Cho S. M., Hsu M. M., Lynn T. C., Shieh T., Tu S. M., Beasley R. P., Hwang L. Y. Antibody to Epstein-Barr virus-specific DNase as a marker for field survey of patients with nasopharyngeal carcinoma in Taiwan. J Med Virol. 1989 Apr;27(4):269–273. doi: 10.1002/jmv.1890270403. [DOI] [PubMed] [Google Scholar]
- Croen K. D., Ostrove J. M., Dragovic L. J., Smialek J. E., Straus S. E. Latent herpes simplex virus in human trigeminal ganglia. Detection of an immediate early gene "anti-sense" transcript by in situ hybridization. N Engl J Med. 1987 Dec 3;317(23):1427–1432. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- Dillner J., Kallin B. The Epstein-Barr virus proteins. Adv Cancer Res. 1988;50:95–158. doi: 10.1016/s0065-230x(08)60436-4. [DOI] [PubMed] [Google Scholar]
- Fennewald S., van Santen V., Kieff E. Nucleotide sequence of an mRNA transcribed in latent growth-transforming virus infection indicates that it may encode a membrane protein. J Virol. 1984 Aug;51(2):411–419. doi: 10.1128/jvi.51.2.411-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fåhraeus R., Fu H. L., Ernberg I., Finke J., Rowe M., Klein G., Falk K., Nilsson E., Yadav M., Busson P. Expression of Epstein-Barr virus-encoded proteins in nasopharyngeal carcinoma. Int J Cancer. 1988 Sep 15;42(3):329–338. doi: 10.1002/ijc.2910420305. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Karran L. Immortalization of monkey epithelial cells by specific fragments of Epstein-Barr virus DNA. Nature. 1984 May 3;309(5963):78–82. doi: 10.1038/309078a0. [DOI] [PubMed] [Google Scholar]
- Joab I., Rowe D. T., Bodescot M., Nicolas J. C., Farrell P. J., Perricaudet M. Mapping of the gene coding for Epstein-Barr virus-determined nuclear antigen EBNA3 and its transient overexpression in a human cell line by using an adenovirus expression vector. J Virol. 1987 Oct;61(10):3340–3344. doi: 10.1128/jvi.61.10.3340-3344.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. H., Hayward S. D., Rawlins D. R. Interaction of the lymphocyte-derived Epstein-Barr virus nuclear antigen EBNA-1 with its DNA-binding sites. J Virol. 1989 Jan;63(1):101–110. doi: 10.1128/jvi.63.1.101-110.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W., Thomas-Powell A. L., Raab-Traub N., Hawke M., Kieff E. Epstein-Barr virus RNA. V. Viral RNA in a restringently infected, growth-transformed cell line. J Virol. 1980 Nov;36(2):506–518. doi: 10.1128/jvi.36.2.506-518.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Giovanella B. C., Lindahl T., Fialkow P. J., Singh S., Stehlin J. S. Direct evidence for the presence of Epstein-Barr virus DNA and nuclear antigen in malignant epithelial cells from patients with poorly differentiated carcinoma of the nasopharynx. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4737–4741. doi: 10.1073/pnas.71.12.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause P. R., Croen K. D., Straus S. E., Ostrove J. M. Detection and preliminary characterization of herpes simplex virus type 1 transcripts in latently infected human trigeminal ganglia. J Virol. 1988 Dec;62(12):4819–4823. doi: 10.1128/jvi.62.12.4819-4823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux G., Freese U. K., Fischer R., Polack A., Kofler E., Bornkamm G. W. TPA-inducible Epstein-Barr virus genes in Raji cells and their regulation. Virology. 1988 Feb;162(2):503–507. doi: 10.1016/0042-6822(88)90496-5. [DOI] [PubMed] [Google Scholar]
- Laux G., Perricaudet M., Farrell P. J. A spliced Epstein-Barr virus gene expressed in immortalized lymphocytes is created by circularization of the linear viral genome. EMBO J. 1988 Mar;7(3):769–774. doi: 10.1002/j.1460-2075.1988.tb02874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung M. L., Chang R. S., Jones J. H. Genetic polymorphism of natural Epstein-Barr virus isolates from infectious mononucleosis patients and healthy carriers. J Virol. 1988 Oct;62(10):3862–3866. doi: 10.1128/jvi.62.10.3862-3866.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfitzner A. J., Strominger J. L., Speck S. H. Characterization of a cDNA clone corresponding to a transcript from the Epstein-Barr virus BamHI M fragment: evidence for overlapping mRNAs. J Virol. 1987 Sep;61(9):2943–2946. doi: 10.1128/jvi.61.9.2943-2946.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab-Traub N., Dambaugh T., Kieff E. DNA of Epstein-Barr virus VIII: B95-8, the previous prototype, is an unusual deletion derivative. Cell. 1980 Nov;22(1 Pt 1):257–267. doi: 10.1016/0092-8674(80)90173-7. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N., Flynn K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell. 1986 Dec 26;47(6):883–889. doi: 10.1016/0092-8674(86)90803-2. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N., Hood R., Yang C. S., Henry B., 2nd, Pagano J. S. Epstein-Barr virus transcription in nasopharyngeal carcinoma. J Virol. 1983 Dec;48(3):580–590. doi: 10.1128/jvi.48.3.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins D. R., Milman G., Hayward S. D., Hayward G. S. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell. 1985 Oct;42(3):859–868. doi: 10.1016/0092-8674(85)90282-x. [DOI] [PubMed] [Google Scholar]
- Rowe D. T., Rowe M., Evan G. I., Wallace L. E., Farrell P. J., Rickinson A. B. Restricted expression of EBV latent genes and T-lymphocyte-detected membrane antigen in Burkitt's lymphoma cells. EMBO J. 1986 Oct;5(10):2599–2607. doi: 10.1002/j.1460-2075.1986.tb04540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Rowe D. T., Gregory C. D., Young L. S., Farrell P. J., Rupani H., Rickinson A. B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 1987 Sep;6(9):2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymo L., Lindahl T., Adams A. Sites of sequence variability in Epstein-Barr virus DNA from different sources. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2794–2798. doi: 10.1073/pnas.76.6.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample J., Hummel M., Braun D., Birkenbach M., Kieff E. Nucleotide sequences of mRNAs encoding Epstein-Barr virus nuclear proteins: a probable transcriptional initiation site. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5096–5100. doi: 10.1073/pnas.83.14.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck S. H., Strominger J. L. Analysis of the transcript encoding the latent Epstein-Barr virus nuclear antigen I: a potentially polycistronic message generated by long-range splicing of several exons. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8305–8309. doi: 10.1073/pnas.82.24.8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987 Dec;61(12):3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I., Spivack J. G., Lirette R. P., Brown S. M., MacLean A. R., Subak-Sharpe J. H., Fraser N. W. Herpes simplex virus type 1 latency-associated transcripts are evidently not essential for latent infection. EMBO J. 1989 Feb;8(2):505–511. doi: 10.1002/j.1460-2075.1989.tb03404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Strauss M., Streuli C. H., Griffin B. E. Efficient oligodeoxyribonucleotide-directed deletion mutagenesis using pEMBL vectors: removal of early region introns from polyoma virus mutants. Gene. 1986;49(3):331–340. doi: 10.1016/0378-1119(86)90369-0. [DOI] [PubMed] [Google Scholar]
- Tugwood J. D., Lau W. H., O S. K., Tsao S. Y., Martin W. M., Shiu W., Desgranges C., Jones P. H., Arrand J. R. Epstein-Barr virus-specific transcription in normal and malignant nasopharyngeal biopsies and in lymphocytes from healthy donors and infectious mononucleosis patients. J Gen Virol. 1987 Apr;68(Pt 4):1081–1091. doi: 10.1099/0022-1317-68-4-1081. [DOI] [PubMed] [Google Scholar]
- Wagner E. K., Flanagan W. M., Devi-Rao G., Zhang Y. F., Hill J. M., Anderson K. P., Stevens J. G. The herpes simplex virus latency-associated transcript is spliced during the latent phase of infection. J Virol. 1988 Dec;62(12):4577–4585. doi: 10.1128/jvi.62.12.4577-4585.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Petti L., Braun D., Seung S., Kieff E. A bicistronic Epstein-Barr virus mRNA encodes two nuclear proteins in latently infected, growth-transformed lymphocytes. J Virol. 1987 Apr;61(4):945–954. doi: 10.1128/jvi.61.4.945-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel R., Miller G. Latent and viral replicative transcription in vivo from the BamHI K fragment of Epstein-Barr virus DNA. J Virol. 1985 May;54(2):501–508. doi: 10.1128/jvi.54.2.501-508.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Dawson C. W., Clark D., Rupani H., Busson P., Tursz T., Johnson A., Rickinson A. B. Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J Gen Virol. 1988 May;69(Pt 5):1051–1065. doi: 10.1099/0022-1317-69-5-1051. [DOI] [PubMed] [Google Scholar]
- Zhang C. X., Decaussin G., Daillie J., Ooka T. Altered expression of two Epstein-Barr virus early genes localized in BamHI-A in nonproducer Raji cells. J Virol. 1988 Jun;62(6):1862–1869. doi: 10.1128/jvi.62.6.1862-1869.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimber U., Adldinger H. K., Lenoir G. M., Vuillaume M., Knebel-Doeberitz M. V., Laux G., Desgranges C., Wittmann P., Freese U. K., Schneider U. Geographical prevalence of two types of Epstein-Barr virus. Virology. 1986 Oct 15;154(1):56–66. doi: 10.1016/0042-6822(86)90429-0. [DOI] [PubMed] [Google Scholar]