Abstract
Aims
Complications after cardiac implantable electronic device (CIED) treatment, including permanent pacemakers (PMs), cardiac resynchronization therapy devices with defibrillators (CRT-Ds) or without (CRT-Ps), and implantable cardioverter defibrillators (ICDs), are associated with increased patient morbidity, healthcare costs, and possibly increased mortality.
Methods and results
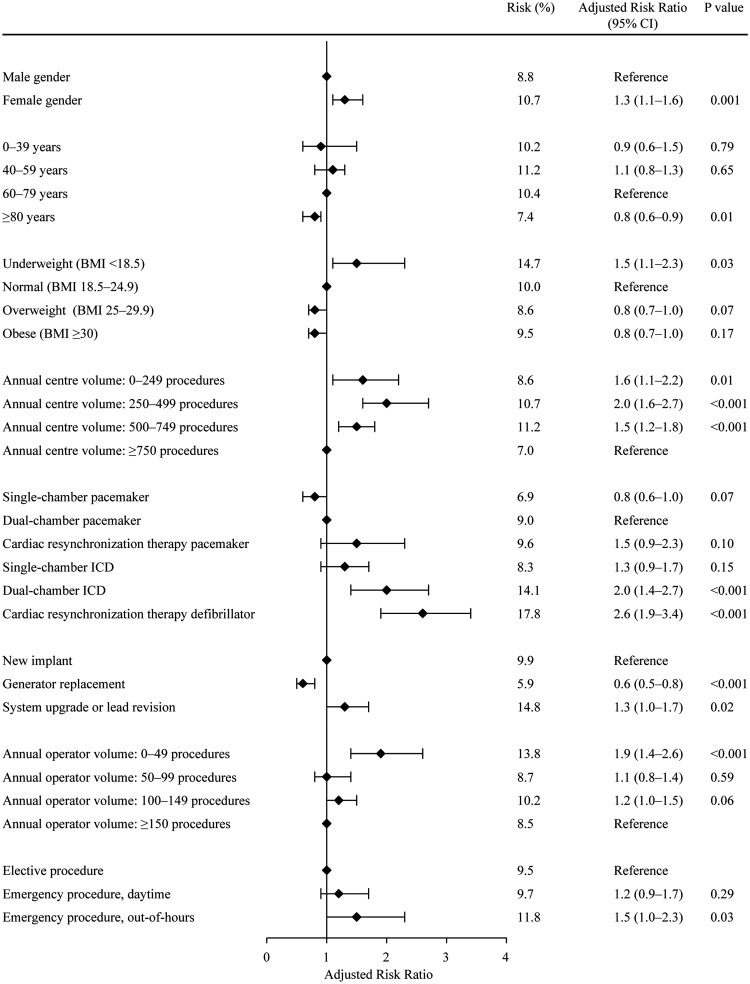
Population-based cohort study in all Danish patients who underwent a CIED procedure from May 2010 to April 2011. Data on complications were gathered on review of all patient charts while baseline data were obtained from the Danish Pacemaker and ICD Register. Adjusted risk ratios (aRRs) with 95% confidence intervals were estimated using binary regression. The study population consisted of 5918 consecutive patients. A total of 562 patients (9.5%) experienced at least one complication. The risk of any complication was higher if the patient was a female (aRR 1.3; 1.1–1.6), underweight (aRR 1.5; 1.1–2.3), implanted in a centre with an annual volume <750 procedures (0–249 procedures: aRR 1.6; 1.1–2.2, 250–499: aRR 2.0; 1.6–2.7, 500–749: aRR 1.5; 1.2–1.8), received a dual-chamber ICD (aRR 2.0; 1.4–2.7) or CRT-D (aRR 2.6; 1.9–3.4), underwent system upgrade or lead revision (aRR 1.3; 1.0–1.7), had an operator with an annual volume <50 procedures (aRR 1.9; 1.4–2.6), or underwent an emergency, out-of-hours procedure (aRR 1.5; 1.0–2.3).
Conclusion
CIED complications are more frequent than generally acknowledged. Both patient- and procedure-related predictors may identify patients with a particularly high risk of complications. This information should be taken into account both in individual patient treatment and in the planning of future organization of CIED treatment.
Keywords: Complication, Predictors, Cardiac resynchronization therapy, Implantable cardioverter defibrillator, Pacemaker
See page 1167 for the editorial comment on this article (doi:10.1093/eurheartj/eht568)
Introduction
Cardiac implantable electronic devices (CIEDs), including permanent pacemakers (PMs), cardiac resynchronization therapy devices with defibrillators (CRT-Ds) or without (CRT-Ps), and implantable cardioverter defibrillators (ICDs), are implanted worldwide in increasing numbers.1,2 Post-procedural complications are associated with increased patient morbidity, healthcare costs, and even mortality.3–6 Published data on these complications are based primarily on secondary analyses of strictly controlled randomized trials,7,8 observational single-centre studies,9,10 or registry-based studies.6,11–13 Unselected, real-life population-based complications data to evaluate the quality of routine CIED treatment and to identify high-risk patients are lacking.
We aimed (i) to provide complete and validated data on complications within the first 6 months after a CIED procedure and (ii) to identify predictors for CIED complications in a nationwide cohort of consecutive CIED patients.
Methods
Study design and study population
A population-based cohort study was performed in all Danish patients who underwent a CIED procedure from May 2010 to April 2011. Eligible patients and their baseline characteristics were identified in the Danish Pacemaker and ICD Register (DPIR). Data on complications were gathered on review of all patient charts. Patients with epicardial systems were excluded.
Centre structure in Denmark
In Denmark, CIED implantation and follow-up are centralized to 14 centres covering a total population of 5.6 million. All transvenous procedures are performed by electrophysiologists or cardiologists, and epicardial procedures are performed by thoracic surgeons. All centres perform PM implants, with five university centres performing, in addition, ICD and CRT implants.
Data sources
The Danish pacemaker and ICD register
The DPIR is a national clinical database into which implanting physicians have entered clinical and technical details of every CIED procedure performed since 1982, including implants, generator replacements, system upgrades/downgrades, and revisions.
Study outcome
Detailed information on complications after CIED procedures was collected by systematic review of all patient charts, also holding information regarding out-patient visits. The review was conducted by one investigator (REK).
Complications were categorized into major and minor complications according to severity. All re-interventions were categorized as major complications due to their inherently higher risk of infections.14,15 Major complications therefore included lead-related re-interventions, local infections requiring re-intervention, CIED-related systemic infections or endocarditis, pneumothorax requiring drainage, cardiac perforation, pocket revisions because of pain, generator-lead interface problems requiring re-intervention, haematomas requiring re-intervention, deep venous thrombosis, Twiddler's syndrome, wound revisions, stroke, myocardial infarctions, and procedure-related deaths. For patients who died before their first outpatient visit, cause of death was established by review of patient charts. Minor complications included haematomas resulting in a prolonged hospital stay, hospital re-admissions, or additional out-patient visits, wound infections treated with antibiotics, pneumothorax conservatively treated, and lead dislodgements without re-intervention.
Predictors
Patient- and procedure-related variables included gender, age, body mass index (BMI), centre volume, CIED type, procedure type, operator volume, and procedure priority. Age was divided into four groups: <39 years, 40–59 years, 60–79 years, and ≥80 years. BMI was categorized into four groups:16 <18.5 kg/m2 (underweight), 18.5–24.9 kg/m2 (normal weight), 25–29.9 kg/m2 (overweight), and ≥30 kg/m2 (obese). Centre volume was categorized according to procedure number during the study period: <249 procedures (five non-university centres), 250–499 (four non-university centres), 500–749 (three university centres), and ≥750 (two university centres). CIED type was categorized as a single-chamber PM, dual-chamber PM, CRT-P, single-chamber ICD, dual-chamber ICD, and CRT-D. Procedure type consisted of three groups: first implant, generator replacement, and surgical change of pacing mode (system upgrade), or lead revision. Operator volume was defined as the average annual procedure number of each operator for the period ranging from one year prior to the beginning of the study to the end of the study and was divided into four groups: <50 (low volume operators), 50–99, 100–149, and ≥150 (high volume operators). Procedure priority (elective, emergency daytime, and emergency out-of-hours) was recorded. Categorization of predictors was prespecified.
Statistical analysis
Differences between groups were evaluated with the χ2 test. Cumulative incidence proportions of complications six months after the procedure were estimated with 95% confidence intervals (CIs). Binary regression was used to estimate risk ratios (RRs) and 95% CIs for association between selected predictors and any complication, any major complication, or any minor complication. In adjusted analyses, we included a priori selected confounders (gender, age, BMI, centre volume, CIED type, procedure type, operator volume, and procedure priority). A sub-group analysis was performed using binary regression to estimate the RR for association between CIED type (PM/CRT-P vs. ICD/CRT-D) and right ventricular lead-related re-intervention. Additional binary regression analysis was performed with 6-month mortality as outcome. A P-value (two-sided) <0.05 was considered statistically significant. STATA software (STATA IC for Windows, version 11.2) was used for statistical analyses.
The Danish Data Protection Board and the DPIR steering committee approved the study.
Results
Study population
A total of 5942 patients underwent a CIED procedure during the study period. Patients with epicardial systems were excluded (n = 24). The final study population consisted of 5918 consecutive patients.
Patient and procedural characteristics
The majority of patients underwent new CIED implants (Table 1, see Supplementary material online, Table S1). Median age at implantation was 74 years (interquartile range: 65–83).
Table 1.
Patient and procedure characteristics
| Total (n = 5918) | No complication (n = 5356) | Complication (n = 562) | |
|---|---|---|---|
| Gender | |||
| Male | 3707 (63) | 3382 (63) | 325 (58) |
| Female | 2211 (37) | 1974 (37) | 237 (42) |
| Age group, years | |||
| 0–39 | 166 (3) | 149 (3) | 17 (3) |
| 40–59 | 713 (12) | 633 (12) | 80 (14) |
| 60–79 | 3096 (52) | 2775 (52) | 321 (57) |
| ≥80 | 1943 (33) | 1799 (34) | 144 (26) |
| Body mass index, kg/m2 | |||
| Underweight (<18.5) | 163 (3) | 139 (3) | 24 (4) |
| Normal (18.5–24.9) | 2483 (42) | 2236 (42) | 247 (44) |
| Overweight (25–29.9) | 2136 (36) | 1952 (37) | 184 (33) |
| Obese (≥30) | 1126 (19) | 1019 (19) | 107 (19) |
| Centre volume | |||
| 0–249 | 702 (12) | 642 (12) | 60 (11) |
| 250–499 | 1517 (26) | 1355 (25) | 162 (29) |
| 500–749 | 1912 (32) | 1697 (32) | 215 (38) |
| ≥750 | 1787 (30) | 1662 (31) | 125 (22) |
| CIED type | |||
| Single-chamber PM | 1160 (20) | 1080 (20) | 80 (14) |
| Dual-chamber PM | 3029 (51) | 2758 (52) | 271 (48) |
| CRT-P | 209 (4) | 189 (4) | 20 (4) |
| Single-chamber ICD | 684 (12) | 627 (12) | 57 (10) |
| Dual-chamber ICD | 391 (7) | 336 (6) | 55 (10) |
| CRT-D | 445 (8) | 366 (7) | 79 (14) |
| Procedure type | |||
| New implant | 4355 (74) | 3923 (73) | 432 (77) |
| Generator replacement | 1136 (19) | 1069 (20) | 67 (12) |
| System upgrade or lead revision | 427 (7) | 364 (7) | 63 (11) |
| Operator volume | |||
| 0–49 | 349 (6) | 301 (6) | 48 (9) |
| 50–99 | 1436 (24) | 1309 (24) | 125 (22) |
| 100–149 | 2257 (38) | 2027 (38) | 230 (41) |
| ≥150 | 1876 (32) | 1717 (32) | 159 (28) |
| Procedure priority | |||
| Elective | 5267 (89) | 4773 (89) | 498 (89) |
| Emergency, daytime | 340 (6) | 308 (6) | 33 (6) |
| Emergency, out-of-hours | 221 (4) | 195 (4) | 26 (5) |
| Procedure duration, median | 40 (30–56) | 40 (30–55) | 47 (36–65) |
Continuous variables are reported as median with 25th and 75th percentiles. Categorical variables are reported as absolute frequencies and percentages.
Data were incomplete for the following parameters: body mass index (n = 5908), procedure priority (n = 5828), and procedure duration (n = 5828).
In the two groups with centre volume <500 procedures (non-university centres), only PM procedures were performed. In the third group (500–749 procedures), 53% of procedures were CRT-P, ICD, or CRT-D procedures, and in the highest volume centres (>750 procedures), 40% were CRT-P, ICD, or CRT-D procedures. During the study period, 68 physicians performed CIED procedures. Emergency procedures involved new implant of single or dual-chamber PMs.
Complication risk
A total of 9.5% of all patients experienced at least one complication (Table 2), while 33 patients (0.6%) had more than one. Lead-related re-intervention was the single most common complication (2.4%). System upgrades or lead revisions had higher overall complication risk primarily because of infection (P = 0.001), and pocket revision due to pain (P < 0.001). The risk of infection was higher in generator replacement procedures compared with first implants (P = 0.001).
Table 2.
Cumulative incidence of complications at six monthsa
| All (n = 5918) | New implant (n = 4355) | Generator replacement (n = 1136) | Upgrade/ lead revision (n = 427) | |
|---|---|---|---|---|
| Any complication | 562 (9.5; 8.7–10.2) | 432 (9.9; 9.0–10.8) | 67 (5.9; 4.5–7.3) | 63 (14.8; 11.4–18.1) |
| Any major complication | 329 (5.6; 5.0–6.1) | 253 (5.8; 5.1–6.5) | 40 (3.5; 2.4–4.6) | 36 (8.4; 5.8–11.1) |
| Any minor complication | 250 (4.2; 3.7–4.7) | 189 (4.3; 3.7–4.9) | 30 (2.6; 1.7–3.6) | 31 (7.3; 4.8–9.7) |
| Major complications | ||||
| Lead related re-intervention | 143 (2.4; 2.0–2.8) | 120 (2.8; 2.3–3.2) | 10 (0.9; 0.3–1.4) | 13 (3.0; 1.4–4.7) |
| Infection | 49 (0.8; 0.6–1.1) | 24 (0.6; 0.3–0.8) | 17 (1.5; 0.8–2.2) | 8 (1.9; 0.6–3.2) |
| Local infection | 22 (0.4; 0.2–0.5) | 10 (0.2; 0.1–0.4) | 8 (0.7; 0.2–1.1) | 4 (1.0; 0.0–1.9) |
| Systemic infection/endocarditis | 27 (0.5; 0.3–0.6) | 14 (0.3; 0.2–0.5) | 9 (0.8; 0.3–1.3) | 4 (0.9; 0.0–1.9) |
| Pneumothorax requiring drainage | 51 (0.9; 0.6–1.1) | 45 (1.0; 0.7–1.3) | 0 | 6 (1.4; 0.3–2.5) |
| Cardiac perforation | 38 (0.6; 0.4–0.8) | 35 (0.8; 0.5–1.1) | 0 | 3 (0.7; 0.0–1.5) |
| No intervention | 21 (0.4; 0.2–0.5) | 18 (0.4; 0.2–0.6) | 0 | 3 (0.7; 0.0–1.5) |
| Interventionb | 17 (0.3; 0.2–0.4) | 17 (0.4; 0.2–0.6) | 0 | 0 |
| Pocket revision because of pain | 25 (0.4; 0.3–0.6) | 10 (0.2; 0.1–0.4) | 9 (0.8; 0.3–1.3) | 6 (1.4; 0.3–2.5) |
| Generator-lead interface problem with re-intervention | 7 (0.1; 0.0–0.2) | 3 (0.1; 0.0–0.1) | 4 (0.4; 0.0–0.7) | 0 |
| Haematoma requiring re-intervention | 10 (0.2; 0.1–0.3) | 9 (0.2; 0.1–0.3) | 1 (0.1; 0.0–0.3) | 0 |
| Otherc | 16 (0.3; 0.1–0.4) | 16 (0.4; 0.2–0.5) | 0 | 0 |
| Minor complications | ||||
| Haematomad | 138 (2.3; 1.9–2.7) | 104 (2.4; 1.9–2.8) | 20 (1.8; 1.0–2.5) | 14 (3.3; 1.6–5.0) |
| Wound infection treated with antibiotics | 69 (1.2; 0.9–1.4) | 47 (1.1; 0.8–1.4) | 12 (1.0; 0.5–1.7) | 10 (2.3; 0.9–3.8) |
| Pneumothorax conservatively treated | 39 (0.7; 0.5–0.9) | 32 (0.7; 0.5–1.0) | 0 | 7 (1.6; 0.4–2.8) |
| Lead dislodgement without re-intervention | 10 (0.2; 0.1–0.3) | 9 (0.2; 0.1–0.3) | 0 | 1 (0.2; 0.0–0.7) |
aReported as absolute frequencies and percentages with 95% CIs in parenthesis.
bLead revision, pericardiocentesis, or both.
cDeep venous thrombosis (n = 8), Twiddler's syndrome (n = 3), wound revision (n = 3), stroke (n = 1), myocardial infarction (n = 1)
dResulting in prolonged hospital stay, hospital re-admission, or additional out-patient visit.
Women had higher risk of pneumothorax (2.2 vs. 1.1%, P = 0.02), and cardiac perforation (1.1 vs. 0.4%, P < 0.001). Risk of pneumothorax increased with decreasing BMI from 0.8% in overweight or obese, 2.3% in normal weight, to 5.5% in underweight patients (P < 0.001). Furthermore, minor haematomas were more frequent in underweight than in normal weight patients (4.9 vs. 2.3%, P = 0.001). Patients older than 80 years had lower risk of any lead-related re-intervention (1.0 vs. 3.1%, P = 0.001) compared with patients who were 60–79 years of age. Centres with <750 annual procedures had higher complication risks with no predisposition to any specific complication. In dual-chamber ICD and CRT-D procedures, higher complication risks were observed compared with dual-chamber PM procedures, primarily lead-related re-interventions (dual-chamber ICD: 3.6 vs. 2.3%, P = 0.001; CRT-D: 4.7 vs. 2.3%, P = 0.001). Low volume operators (<50 annual procedures) had higher complication risks overall. Particularly, their risks of cardiac perforation (1.4 vs. 0.5%, P = 0.04), infection (1.7 vs. 0.5%, P = 0.02), and minor haematoma (4.3 vs. 1.9%, P = 0.005) were higher compared with higher volume operators. Emergency, out-of-hours procedures had higher risk of cardiac perforation (2.3 vs. 0.6%, P = 0.003).
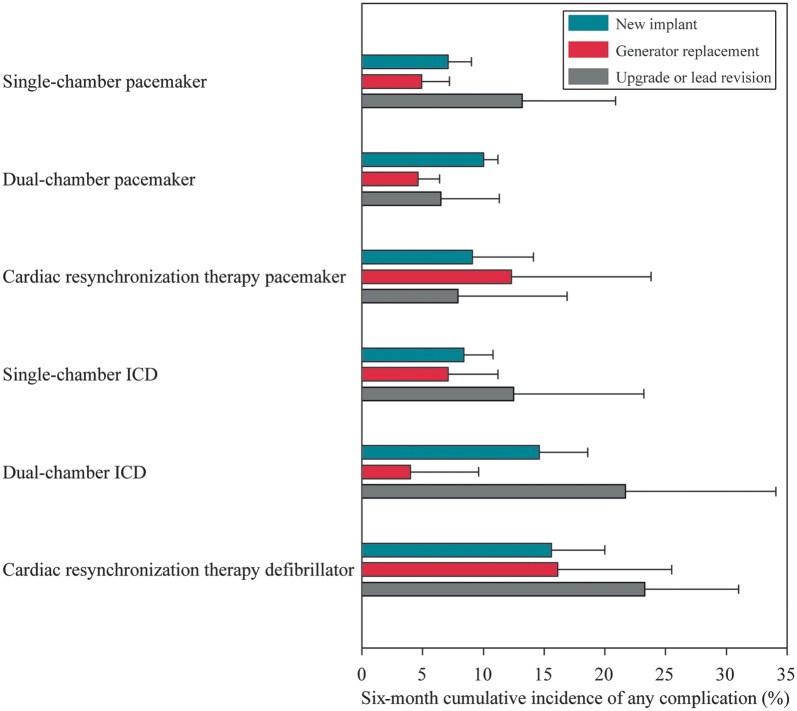
Large differences in risk of any complication were observed between device and procedure types (Figure 1).
Figure 1.
Risk of any complication according to procedure and CIED type (with 95% CI).
The risk of right ventricular lead complications resulting in re-intervention was 1.2% after PM and CRT-P procedures, and 2.4% after ICD and CRT-D procedures. The risk of atrial lead complications was 1.2% (PM/CRT-P), and 1.3% (ICD/CRT-D), and the risk of left ventricular lead complications was 2.9% (CRT-P), and 1.8% (CRT-D).
Predictors
In multivariate analyses, increased risk of any complication was seen if the patient was a female, underweight, implanted in a centre with an annual volume <750 procedures, had a dual-chamber ICD or CRT-D implanted, underwent a system upgrade or lead revision, had an operator with an annual volume <50 procedures, or underwent an emergency, out-of-hours procedure (Figure 2). Decreased risk was present in patients older than 80 years, or receiving a generator replacement. These trends in predictor associations were also observed for the occurrence of any major or minor complication, although the strength of associations varied (Table 3).
Figure 2.
Predictors of any complication.
Table 3.
Predictors for complications
| Any major complication |
Any minor complication |
|||||
|---|---|---|---|---|---|---|
| Risk (%) | aRRb (95% CI) | P-value | Risk (%) | aRRb (95% CI) | P-value | |
| Gender | ||||||
| Malea | 5.0 | – | – | 4.0 | – | – |
| Female | 6.5 | 1.4 (1.2–1.8) | 0.001 | 4.6 | 1.2 (0.9–1.5) | 0.22 |
| Age group, years | ||||||
| 0–39 | 7.8 | 1.3 (0.7–2.2) | 0.36 | 3.0 | 0.5 (0.2–1.5) | 0.23 |
| 40–59 | 7.2 | 1.1 (0.8–1.5) | 0.38 | 4.8 | 1.0 (0.7–1.5) | 0.94 |
| 60–79a | 6.3 | – | – | 4.4 | – | – |
| ≥80 | 3.7 | 0.6 (0.5–0.8) | 0.001 | 3.9 | 1.0 (0.7–1.3) | 0.81 |
| Body mass index, kg/m2 | ||||||
| Underweight (<18.5) | 8.0 | 1.5 (0.8–2.5) | 0.17 | 6.8 | 1.5 (0.8–2.8) | 0.21 |
| Normal (18.5–24.9)a | 5.6 | – | – | 4.4 | – | – |
| Overweight (25–29.9) | 5.3 | 0.9 (0.7–1.2) | 0.41 | 3.7 | 0.8 (0.6–1.1) | 0.15 |
| Obese (≥30) | 5.2 | 0.8 (0.6–1.1) | 0.13 | 4.6 | 0.9 (0.7–1.3) | 0.70 |
| Centre volume | ||||||
| 0–249 | 5.7 | 1.4 (0.9–2.0) | 0.13 | 2.9 | 1.7 (0.9–3.1) | 0.09 |
| 250–499 | 5.3 | 1.4 (1.0–2.0) | 0.054 | 5.7 | 3.5 (2.2–5.4) | <0.001 |
| 500–749 | 6.4 | 1.2 (0.9–1.6) | 0.19 | 5.2 | 2.1 (1.4–3.0) | <0.001 |
| ≥750a | 5.0 | – | – | 2.4 | – | – |
| CIED type | ||||||
| Single-lead PM | 3.3 | 0.7 (0.5–1.0) | 0.03 | 3.7 | 0.9 (0.6–1.3) | 0.66 |
| Dual-chamber PMa | 5.5 | – | – | 3.8 | – | – |
| CRT-P | 6.7 | 1.6 (0.9–2.8) | 0.11 | 3.8 | 1.5 (0.7–3.1) | 0.30 |
| Single-chamber ICD | 5.4 | 1.2 (0.8–1.8) | 0.39 | 3.2 | 1.3 (0.8–2.3) | 0.52 |
| Dual-chamber ICD | 6.7 | 1.4 (0.9–2.2) | 0.15 | 7.7 | 2.8 (1.7–4.5) | <0.001 |
| CRT-D | 11.0 | 2.4 (1.6–3.5) | <0.001 | 7.4 | 2.8 (1.7–4.4) | <0.001 |
| Procedure type | ||||||
| New implanta | 5.8 | – | – | 4.3 | – | – |
| Generator replacement | 3.5 | 0.6 (0.5–0.9) | 0.01 | 2.6 | 0.6 (0.4–0.9) | 0.02 |
| Upgrade/lead revision | 8.4 | 1.3 (0.9–1.8) | 0.18 | 7.3 | 1.5 (1.0–2.3) | 0.03 |
| Operator volume | ||||||
| 0–49 | 7.7 | 2.0 (1.3–3.1) | 0.002 | 6.6 | 1.9 (1.2–3.1) | 0.01 |
| 50–99 | 5.7 | 1.3 (0.9–1.8) | 0.11 | 3.2 | 0.8 (0.5–1.2) | 0.24 |
| 100–149 | 5.8 | 1.4 (1.0–1.8) | 0.03 | 4.8 | 1.1 (0.8–1.5) | 0.71 |
| ≥150a | 4.9 | – | – | 3.9 | – | – |
| Procedure priority | ||||||
| Electivea | 5.5 | – | – | 4.3 | – | – |
| Emergency, daytime | 6.5 | 1.3 (0.8–2.0) | 0.24 | 3.5 | 1.1 (0.6–2.0) | 0.76 |
| Emergency, out-of-hours | 7.2 | 1.6 (1.0–2.7) | 0.07 | 4.5 | 1.4 (0.7–2.7) | 0.32 |
aReference group.
bAdjusted for gender, age, body mass index, centre volume, CIED type, procedure type, procedure priority, and operator volume.
The risk of re-intervention due to right ventricular lead complications was higher in ICD and CRT-D procedures (i.e. high-voltage leads) compared with pacing leads, aRR 3.2; 95% CI 1.7–5.8, P < 0.001.
Mortality
A total of 327 patients (5.5%) died within the first 6 months. One death was possibly procedure-related; a patient, who had severe chronic obstructive pulmonary disease, was discharged from hospital with an unrecognized minor pneumothorax, and died few days later because of an unknown cause. There was no indication that any other patients died from procedure-related complications. Ninety-day mortality was 3.2% (n = 187). Thirty-day mortality was 1.4% (n = 81). In-hospital mortality was 0.1% (n = 7).
In multivariate analysis, a higher 6-month mortality was observed in patients older than 80 years (aRR 2.2), underweight (aRR 2.3), or receiving a single-chamber ventricular PM (aRR 2.4).
Discussion
The present study provides detailed and complete data on the risks and predictors of CIED complications in a nationwide cohort of consecutive CIED patients.
Complication risk
The almost 10% overall risk of any complication is higher than expected from previous studies, as is the 6% risk of major complications.
Most studies report risks of 5–6% for any complication7,13,17,18 and 3–4% for major complications after PM implantations.9,19 Complication risks after ICD and CRT-D procedures are reported to be between 3 and 8%, although comparisons are impeded by varying follow-up periods and definition of complications.6,10–12,20,21 More consistent with our findings, however, are reported in-hospital complication risks of 11–16% after ICD and CRT-D procedures,3,4,22 from studies using administrative data from Medicare. Similarly, the FOLLOWPACE trial reported a complication risk of 12.4% within the first two months after PM implant.23
We found higher risks of any complication (5.9%) after generator replacement compared with a report from the Ontario ICD database.24 Similarly, we found higher or equal complication risks for system upgrades and lead revisions compared with those reported by the REPLACE Registry, which specifically studied complication risks of re-intervention procedures.25 Taken together with these previous findings, our results confirm that any type of re-intervention carries higher complication risk compared with new CIED implants. This emphasizes the importance of careful consideration of CIED prescription at new implant in order to avoid the need for future re-intervention.
Our finding of higher overall complication risk can be attributed to the comprehensiveness of our study design, which consisted of a systematic review of all patient charts in a consecutive cohort. The majority of our knowledge on complications derives from randomized trials, which typically observe fewer complications than in a real-life setting due to strict patient selection criteria, and selection of more experienced operators. In recent years, large, registry-based studies on complications have emerged with more robust estimates of complications; however, data on complications are typically self-reported by CIED centres such that underreporting is likely to occur. Furthermore, many registries are restricted to in-hospital follow-up, leaving a large proportion of longer-term CIED complications unaccounted for.6
Predictors
In centres with an annual volume <750 procedures, we demonstrated 50–100% higher risks of any complication after CIED procedures compared with the highest volume centres, in accord with previous studies.20,26,27 A likely explanation is more experienced operators and ancillary personnel in the highest volume centres. Similar to previous reports, we showed that low volume operators had a 90% increased complication risk compared with high volume operators,4,22,28 with a critical threshold of approximately 50 procedures per year.9,24 Despite relative centralization of CIED treatment in Denmark compared with most Western countries,1,4 we were still able to demonstrate marked variation of complication risks between both centres and operators. This variation may be more marked in countries where CIED treatment is decentralized.
Implantation of high-voltage leads (i.e. ICD-leads) had an increased risk of re-interventions compared with implantation of RV pacing leads. Very few data exist on this topic, however, a recently published study reports similar findings.29 This can be attributed to the more complex structure, larger calibre, and increased rigidity of high-voltage leads, which in addition, require more stringent implant and follow-up lead parameters. This higher complication risk should be taken into account when planning the implantation of ICD and CRT-D systems.
Women had a 30% higher risk of any complication, mainly due to pneumothorax, and cardiac perforation. This gender difference in CIED complication risk is consistent with other reports,6,11,13,21 and underlying explanations may include differences in body composition, and hormonal differences.
In contrast to previous reports,5,19,30,31 age >80 years was associated with a 20% reduction in complication risk, particularly fewer lead-related re-interventions were seen. The reason for this is unknown, although possibly related to a higher tendency to accept suboptimal lead function, a higher proportion of simpler CIED types, or less physical activity with lower strain on the CIED in this patient group.
Reduced BMI was associated with increased risk of complications after CIED procedures; in particular, the risks of pneumothorax and haematoma were higher, consistent with findings from recent studies.8,23,32 Haematomas may be more easily recognized in underweight patients, and a closer proximity of the pleural space to the venous access point in these patients may explain the higher risk of pneumothorax.
System upgrades and lead revisions increased the risk of complications by 30%, mainly because of elevated risk of infections, as reported previously.14,25 Generator replacements had lower risk of any complication, but were associated with increased infection risk as anticipated.14,15
Emergency, out-of-hours procedures were associated with increased complication risk, likely due to more urgent indications for CIED implantation (e.g. third degree atrioventricular block and haemodynamic instability).
Mortality
Mortality after CIED procedures is inconsistently reported and meaningful comparisons are difficult. It is, however, apparent that procedure-related mortality is low;24 we observed only one potentially procedure-related death in our cohort. Previous studies reported all-cause in-hospital mortality between 0.4 and 1.3%,3,11,13 highest in PM populations13 and in registry-based studies.4,5 We found a somewhat lower in-hospital mortality of 0.1%. Compared with results from the MOST trial,7 our 30-day mortality rate was twice as high, likely reflecting the differences in prognosis between a consecutive cohort and patients qualifying for inclusion in a randomized trial. Al-Khatib et al.4 reported a 90-day mortality higher than ours, most likely explained by their study cohort being older than 65 years.
Patients older than 80 years, patients with single-chamber ventricular PM procedures, and underweight patients had higher 6-month mortality. This likely reflects that single-chamber ventricular PMs are often selected for patients with high burden of comorbidity.
We report acceptably low in-hospital, 30-day, 90-day, and 6-month all-cause mortality rates, not significantly related to centre or operator volumes.
Study limitations
Only complications documented in the patient charts were identified. However, in our opinion, this was the most accurate and comprehensive way of identifying complications.
Our complication risk figures are only applicable to healthcare systems where CIED procedures are performed by cardiologists and electrophysiologists working within a similar system to ours, and should be interpreted with care in countries where general internists and thoracic surgeons also perform these procedures.28,33
Our results may have been confounded by other factors that may affect complications, such as anti-thrombotic and antiplatelet treatment, and the use of steroids, for which data were not collected. Similarly, we were unable to account for procedures where lead extraction occurred concurrently with the CIED procedure being examined. These procedures will undoubtedly carry higher risks of complications. Because of the non-randomized nature of the study, the difference in complication risk between CIED types may in part be explained by residual confounding.
We did not study long-term complication risks. However, our aim was to investigate short-term complications after CIED procedures, and the large majority of these occur within a 6 month period.23,34 Nevertheless, prospective studies examining long-term outcome after CIED procedures and CIED complications are needed to further investigate the quality of CIED treatment. Longer follow-up is especially important for CIED-related infections.
Conclusions
Complications following CIED treatment are more frequent than generally acknowledged. Both patient- and procedure-related predictors may identify patients with particularly high risk of complications. This information should be taken into account in individual patient treatment, and when planning the implantation of more complex CIED types. In order to minimize later need for system upgrade, carefully considered CIED therapy prescription is essential. Low volume centres and operators had higher risk of complications, and minimum operator volume of 50 procedures per year seems advisable.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by unrestricted research grants from the Institute of Clinical Medicine, Aarhus University; Biotronik Denmark; Medtronic Denmark A/S; St. Jude Medical; ViCare Medical A/S; Gangstedfonden; the Foundation of Sophus Jacobsen and Astrid Jacobsen; the Central Denmark Region Research Foundation; Department of Cardiology, Odense University Hospital; and the Danish Heart Foundation (12-04-R90-A3850-22712). Funding to pay the Open Access publication charges for this article was provided by The Danish Pacemaker and ICD Register.
Conflict of interest: J.B.J.: speakers fee (<10 K$) from Biotronik, and Boston-Scientific within latest 12 months. J.C.N.: research Grant from Biosense Webster (>100 K$) for MANTRA-PAF trial, speakers fee (<10 K$) from Biotronik and Biosense Webster within latest 12 months.
Supplementary Material
Acknowledgements
We wish to thank the DPIR steering committee for supporting the data collection: Sam Riahi, Aalborg University Hospital; Søren Højberg, Bispebjerg Hospital; Elsebeth Friis, Esbjerg Hospital; Michael Vinther, Gentofte University Hospital; Lene Svendstrup, Haderslev Hospital; Jerzy Malczynski, Herning Hospital; Tommi Bo Lindhardt, Hillerød Hospital; Regitze Videbæk, Rigshospitalet; Thomas Melchior, Roskilde Hospital; Birger Engby, Vejle Hospital; and Per Dahl Christensen, Viborg Hospital.
References
- 1.Mond HG, Irwin M, Ector H, Proclemer A. The world survey of cardiac pacing and cardioverter-defibrillators: calendar year 2005 an International Cardiac Pacing and Electrophysiology Society (ICPES) project. Pacing Clin Electrophysiol. 2008;31:1202–1212. doi: 10.1111/j.1540-8159.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- 2.Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Kirchhof P, Blomstrom-Lundqvist C, Badano LP, Aliyev F, Bansch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC) Developed in collaboration with the European Heart Rhythm Association (EHRA) Eur Heart J. 2013;34:2281–2329. doi: 10.1093/eurheartj/eht150. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds MR, Cohen DJ, Kugelmass AD, Brown PP, Becker ER, Culler SD, Simon AW. The frequency and incremental cost of major complications among medicare beneficiaries receiving implantable cardioverter-defibrillators. J Am Coll Cardiol. 2006;47:2493–2497. doi: 10.1016/j.jacc.2006.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Khatib SM, Lucas FL, Jollis JG, Malenka DJ, Wennberg DE. The Relation Between Patients’ Outcomes and the Volume of Cardioverter-Defibrillator Implantation Procedures Performed by Physicians Treating Medicare Beneficiaries. J Am Coll Cardiol. 2005;46:1536–1540. doi: 10.1016/j.jacc.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 5.Swindle JP, Rich MW, McCann P, Burroughs TE, Hauptman PJ. Implantable cardiac device procedures in older patients: use and in-hospital outcomes. Arch Intern Med. 2010;170:631–637. doi: 10.1001/archinternmed.2010.30. [DOI] [PubMed] [Google Scholar]
- 6.Lee DS, Krahn AD, Healey JS, Birnie D, Crystal E, Dorian P, Simpson CS, Khaykin Y, Cameron D, Janmohamed A, Yee R, Austin PC, Chen Z, Hardy J, Tu JV. Evaluation of early complications related to De Novo cardioverter defibrillator implantation insights from the Ontario ICD database. J Am Coll Cardiol. 2010;55:774–782. doi: 10.1016/j.jacc.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Ellenbogen KA, Hellkamp AS, Wilkoff BL, Camunas JL, Love JC, Hadjis TA, Lee KL, Lamas GA. Complications arising after implantation of DDD pacemakers: the MOST experience. Am J Cardiol. 2003;92:740–741. doi: 10.1016/s0002-9149(03)00844-0. [DOI] [PubMed] [Google Scholar]
- 8.van Eck JW, van Hemel NM, Zuithof P, van Asseldonk JP, Voskuil TL, Grobbee DE, Moons KG. Incidence and predictors of in-hospital events after first implantation of pacemakers. Europace. 2007;9:884–889. doi: 10.1093/europace/eum113. [DOI] [PubMed] [Google Scholar]
- 9.Tobin K, Stewart J, Westveer D, Frumin H. Acute complications of permanent pacemaker implantation: their financial implication and relation to volume and operator experience. Am J Cardiol. 2000;85:774–776. doi: 10.1016/s0002-9149(99)00861-9. [DOI] [PubMed] [Google Scholar]
- 10.Duray GZ, Schmitt J, Cicek-Hartvig S, Hohnloser SH, Israel CW. Complications leading to surgical revision in implantable cardioverter defibrillator patients: comparison of patients with single-chamber, dual-chamber, and biventricular devices. Europace. 2009;11:297–302. doi: 10.1093/europace/eun322. [DOI] [PubMed] [Google Scholar]
- 11.Peterson PN, Daugherty SL, Wang Y, Vidaillet HJ, Heidenreich PA, Curtis JP, Masoudi FA. Gender differences in procedure-related adverse events in patients receiving implantable cardioverter-defibrillator therapy. Circulation. 2009;119:1078–1084. doi: 10.1161/CIRCULATIONAHA.108.793463. [DOI] [PubMed] [Google Scholar]
- 12.Dewland TA, Pellegrini CN, Wang Y, Marcus GM, Keung E, Varosy PD. Dual-chamber implantable cardioverter-defibrillator selection is associated with increased complication rates and mortality among patients enrolled in the NCDR implantable cardioverter-defibrillator registry. J Am Coll Cardiol. 2011;58:1007–1013. doi: 10.1016/j.jacc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 13.Nowak B, Misselwitz B, Erdogan A, Funck R, Irnich W, Israel CW, Olbrich HG, Schmidt H, Sperzel J, Zegelman M. Do gender differences exist in pacemaker implantation?—results of an obligatory external quality control program. Europace. 2010;12:210–215. doi: 10.1093/europace/eup312. [DOI] [PubMed] [Google Scholar]
- 14.Johansen JB, Jorgensen OD, Moller M, Arnsbo P, Mortensen PT, Nielsen JC. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J. 2011;32:991–998. doi: 10.1093/eurheartj/ehq497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM, Stoner SM, Baddour LM. Risk factor analysis of permanent pacemaker infection. Clin Infect Dis. 2007;45:166–173. doi: 10.1086/518889. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organisation Technical Report Services. Obesity: preventing and managing the global epidemic. 2000 Report of a WHO Consultation. [PubMed] [Google Scholar]
- 17.Annual Statistics Report. Swedish ICD and Pacemaker Register https://www.pacemakerregistret.se. Last accessed 13 February 2013.
- 18. Jahresbericht 2010 des Deutschen herzschrittmacher- und defibrillator-registers http://www.pacemaker-register.de. Last accessed 13 February 2013.
- 19.Eberhardt F, Bode F, Bonnemeier H, Boguschewski F, Schlei M, Peters W, Wiegand UK. Long term complications in single and dual chamber pacing are influenced by surgical experience and patient morbidity. Heart. 2005;91:500–506. doi: 10.1136/hrt.2003.025411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman JV, Wang Y, Curtis JP, Heidenreich PA, Hlatky MA. The relation between hospital procedure volume and complications of cardioverter-defibrillator implantation from the implantable cardioverter-defibrillator registry. J Am Coll Cardiol. 2010;56:1133–1139. doi: 10.1016/j.jacc.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 21.MacFadden DR, Crystal E, Krahn AD, Mangat I, Healey JS, Dorian P, Birnie D, Simpson CS, Khaykin Y, Pinter A, Nanthakumar K, Calzavara AJ, Austin PC, Tu JV, Lee DS. Sex differences in implantable cardioverter-defibrillator outcomes: findings from a prospective defibrillator database. Ann Intern Med. 2012;156:195–203. doi: 10.7326/0003-4819-156-3-201202070-00007. [DOI] [PubMed] [Google Scholar]
- 22.Al-Khatib SM, Greiner MA, Peterson ED, Hernandez AF, Schulman KA, Curtis LH. Patient and implanting physician factors associated with mortality and complications after implantable cardioverter-defibrillator implantation, 2002–2005. Circ Arrhythm Electrophysiol. 2008;1:240–249. doi: 10.1161/CIRCEP.108.777888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Udo EO, Zuithoff NP, van Hemel NM, de Cock CC, Hendriks T, Doevendans PA, Moons KG. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm. 2012;9:728–735. doi: 10.1016/j.hrthm.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Krahn AD, Lee DS, Birnie D, Healey JS, Crystal E, Dorian P, Simpson CS, Khaykin Y, Cameron D, Janmohamed A, Yee R, Austin PC, Chen Z, Hardy J, Tu JV. Predictors of short-term complications after implantable cardioverter-defibrillator replacement: results from the Ontario ICD Database. Circ Arrhythm Electrophysiol. 2011;4:136–142. doi: 10.1161/CIRCEP.110.959791. [DOI] [PubMed] [Google Scholar]
- 25.Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge R, Gottipaty V, Shinn T, Dan D, Feldman LA, Seide H, Winston SA, Gallagher JJ, Langberg JJ, Mitchell K, Holcomb R. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122:1553–1561. doi: 10.1161/CIRCULATIONAHA.110.976076. [DOI] [PubMed] [Google Scholar]
- 26.Kirkfeldt RE, Johansen JB, Nohr EA, Moller M, Arnsbo P, Nielsen JC. Risk factors for lead complications in cardiac pacing: a population-based cohort study of 28,860 Danish patients. Heart Rhythm. 2011;8:1622–1628. doi: 10.1016/j.hrthm.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Kirkfeldt RE, Johansen JB, Nohr EA, Moller M, Arnsbo P, Nielsen JC. Pneumothorax in cardiac pacing: a population-based cohort study of 28 860 Danish patients. Europace. 2012;14:1132–1138. doi: 10.1093/europace/eus054. [DOI] [PubMed] [Google Scholar]
- 28.Freeman JV, Wang Y, Curtis JP, Heidenreich PA, Hlatky MA. Physician procedure volume and complications of cardioverter-defibrillator implantation. Circulation. 2012;125:57–64. doi: 10.1161/CIRCULATIONAHA.111.046995. [DOI] [PubMed] [Google Scholar]
- 29.Schuchert A, Muto C, Maounis T, Frank R, Boulogne E, Polauck A, Padeletti L. Lead complications, device infections, and clinical outcomes in the first year after implantation of cardiac resynchronization therapy-defibrillator and cardiac resynchronization therapy-pacemaker. Europace. 2013;15:71–76. doi: 10.1093/europace/eus247. [DOI] [PubMed] [Google Scholar]
- 30.Armaganijan LV, Toff WD, Nielsen JC, Andersen HR, Connolly SJ, Ellenbogen KA, Healey JS. Are elderly patients at increased risk of complications following pacemaker implantation? A meta-analysis of randomized trials. Pacing Clin Electrophysiol. 2012;35:131–134. doi: 10.1111/j.1540-8159.2011.03240.x. [DOI] [PubMed] [Google Scholar]
- 31.Tsai V, Goldstein MK, Hsia HH, Wang Y, Curtis J, Heidenreich PA. Influence of age on perioperative complications among patients undergoing implantable cardioverter-defibrillators for primary prevention in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:549–556. doi: 10.1161/CIRCOUTCOMES.110.959205. [DOI] [PubMed] [Google Scholar]
- 32.Hsu JC, Varosy PD, Bao H, Wang Y, Curtis JP, Marcus GM. Low body mass index but not obesity is associated with in-hospital adverse events and mortality among implantable cardioverter-defibrillator recipients: insights from the national cardiovascular data registry. J Am Heart Assoc. 2012;1:e003863. doi: 10.1161/JAHA.112.003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curtis JP, Luebbert JJ, Wang Y, Rathore SS, Chen J, Heidenreich PA, Hammill SC, Lampert RI, Krumholz HM. Association of physician certification and outcomes among patients receiving an implantable cardioverter-defibrillator. JAMA. 2009;301:1661–1670. doi: 10.1001/jama.2009.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kron J, Herre J, Renfroe EG, Rizo-Patron C, Raitt M, Halperin B, Gold M, Goldner B, Wathen M, Wilkoff B, Olarte A, Yao Q. Lead- and device-related complications in the antiarrhythmics versus implantable defibrillators trial. Am Heart J. 2001;141:92–98. doi: 10.1067/mhj.2001.111261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.