Abstract
Immune system activation occurs not only due to foreign stimuli, but also due to endogenous molecules. As such, endogenous molecules that are released into the circulation due to cell death and/or injury alarm the immune system that something has disturbed homeostasis and a response is needed. Collectively, these molecules are known as damage-associated molecular patterns (DAMPs). Mitochondrial DAMPs (mtDAMPs) are potent immunological activators due to the bacterial ancestry of mitochondria. Mitochondrial DAMPs are recognized by specific pattern recognition receptors of the innate immune system, some of which are expressed in the cardiovascular system. Cell death leads to release of mtDAMPs that may induce vascular changes by mechanisms that are currently not well understood. This review will focus on recently published evidence linking mtDAMPs and immune system activation to vascular dysfunction and cardiovascular disease.
Keywords: Mitochondria, Damage-associated molecular patterns, Immune system, Vascular function, Cardiovascular disease
Introduction
The endosymbiotic theory suggests that mitochondria and other organelles represent formerly free-living bacteria. Dr Lynn Margulis1 first substantiated this theory with microbiological evidence in 1967. Margulis's theory gained support in the 1980s after research showed that the genetic material of mitochondria differed from that of nuclear DNA.2 Nowadays, the endosymbiotic theory of mitochondria embraces the knowledge that prokaryotic organisms (bacteria) entered into the eukaryotic cell and evolutionarily converted to an indispensible organelle.1,2
The endosymbiotic theory has raised new questions regarding the role of mitochondria, including their potential to contribute to unintended immune system activation, sterile inflammation, and disease pathogenesis, because of their bacterial origin. In an attempt to answer several questions that had remained unresolved by the traditional ‘self–non-self model of immunity’, in 1994 Dr Polly Matzinger3 proposed the ‘danger theory’ of immunological response centred on the idea that the immune system is more concerned by damage than by foreignness. The danger theory proposes that the activation of the immune system is trigged by danger signals from damaged tissues, rather than by the recognition of non-self. Such danger signals are provided by not only pathogens, but also by endogenous molecules released into the circulation or tissues due to cell death and/or injury. Normally, these endogenous molecules are shielded from immune system components by cellular membranes and compartmentalization within the cell; however, unintended cell injury and/or death trigger their release. Collectively, these released endogenous molecules are referred to as damage-associated molecular patterns (DAMPs). Damage-associated molecular patterns are derived from many sources within the cell, including the plasma membrane, nucleus, cytosol, endoplasmic reticulum (ER), and mitochondria.4 Mitochondrial DAMPs (MtDAMPs) express at least two unique molecular signatures that are evolutionarily conserved from their bacterial origin, N-formyl peptides, and mitochondrial DNA (mtDNA). N-Formyl peptides and mtDNA released inadvertently from damaged mitochondria and cells are recognized by specific pattern recognition receptors (PPRs) of the innate immune system, formyl peptide receptor (FPR), and Toll-like receptor (TLR) 9, respectively.5,6
Cell death leading to the release of DAMPs and immune system activation has been shown to have pathological implications in the cardiovascular system. As such, circulating DAMPs may be a common feature of the dysfunction seen in many vascular diseases. The bacterial ancestry of the mitochondria distinguishes it as novel DAMP with a rationale for its inflammatogenic properties. However, the mechanisms by which mtDAMPs may change vascular function are currently unclear. This review will focus on the recently published evidence linking mtDAMPs and immune system activation to vascular dysfunction and cardiovascular disease.
An overview of mitochondrial function
Mitochondria are essential organelles of all cells, their functions ranging from energy production and metabolic regulation to reactive oxygen species (ROS) signalling, calcium homeostasis, and apoptosis.
The endosymbiotic theory helps explain both the energy-generating function of the mitochondria and their unique double-membrane structure. The outer mitochondrial membrane (OMM) resembles the plasma membrane and is significantly smaller in surface size than the bacterial-like inner mitochondrial membrane (IMM), containing the phospholipid cardiolipin. The IMM, crucial for the mitochondrial oxidative phosphorylation machinery, is enlarged by deep folds called cristae. The intermembrane space is similar in small molecule composition to the cytoplasm, due to porins in the OMM, but with different protein content, characteristically including cytochrome c, a component of the electron transport chain. Enclosed by the IMM is the matrix, where high-energy nutrients such as pyruvate from glycolysis or fatty acids are transformed into acetyl-CoA, the primary substrate of the Krebs cycle.7 Matrix enzymes complete the Krebs cycle that produces NADH and FADH2, which are used as a source of high energy electrons by respiratory complexes (NADH dehydrogenase, cytochrome b-c1, and cytochrome oxidase, or complexes I–III) that form the electron transfer chain in the IMM. These complexes ultimately use molecular oxygen as an electron acceptor, producing protons along the way, which after being pumped to the intermembrane space, form an electrochemical proton gradient. In most cells, during the last step of this extremely efficient system of oxidative phosphorlyation, adenosine triphosphate (ATP) synthase, also located in the IMM, uses the re-entry of protons in the matrix as a source of energy for ADP phosphorylation and ATP production. In some cell types however, such as brown adipocytes, mitochondrial respiration is uncoupled from ATP synthesis by proteins like uncoupling protein-1, and electron transfer results in heat generation.7
A certain amount of electrons escape the electron transfer chain, particularly at complex I and III sites, and produce the highly reactive superoxide anion via one electron reduction of molecular oxygen. In physiological conditions, this small amount of ROS contributes to normal cell signalling. Excessive amounts of mitochondrial ROS, produced via excessive NADH levels, low ATP production with high protonmotive force, or from other mitochondrial ROS sources, may overwhelm the powerful antioxidant systems present inside the matrix, such as manganese superoxide dismutase and glutathione peroxidase. Oxidative damage to mtDNA may subsequently result in mtDNA mutations, as well as autophagy and apoptosis.8 The free radical theory of ageing is based on strong evidence linking ageing with mitochondrial ROS production and accumulation of mtDNA. Mitochondrial ROS are produced in a controlled way through specialized enzymes, including p66Shc, a genetic determinant of life span in mammals.9 Mitochondrial p66Shc utilizes reducing equivalents of the mitochondrial electron transfer chain through the oxidation of cytochrome c to generate ROS and induces mitochondrial disruption.9
Mitochondria are also at the centre of the intrinsic apoptosis pathway induced by stimuli such as oxidative stress, DNA damage, ER stress, high calcium levels, or hypoxia. In this pathway, activation of pro-apoptotic Bax or Bak leads to OMM permeabilization and leakage of cytochrome c into the cytoplasm, a step considered to be ‘the point of no return’ of intrinsic apoptosis. Cytochrome c then binds apoptosis protease activating factor-1 (APAF-1) to form the apoptosome, structure that in turn activates caspase 9 in a cascade culminating in apoptosis.10
In addition to these functions, and important for vascular biology, mitochondria contribute to the maintenance of calcium homeostasis. Mitochondria and the ER physically associate in the so-called mitochondria-associated ER membrane (MAM), specialized domains that allow not only for transport of calcium, but also for cholesterol, ceramide, and phospholipids transfer.11 The latter is important for the frequent dynamic changes in mitochondria morphology resulting from constant fusion and fission. Rapid mitochondrial uptake of calcium is made possible in MAMs by voltage-dependent anion channels in the OMM and by the mitochondrial calcium uniporter in the IMM. Additional regulation of mitochondrial calcium levels is provided on the ER side of MAMs by IP3R and SERCA, as well as by calcium efflux via Na+/Ca2+ transporters in the IMM and the permeability transition pore in the OMM.12
Finally, mitochondria have been recently implicated in the regulation of danger signalling and immune system activation. The details of this novel role of mitochondria are provided below.
Immune system activation by mitochondria damage-associated molecular patterns
Immune defence involves two components: the innate immune system and the adaptive immune system. The innate immune system is the body's early warning system that rapidly detects and reacts to potentially dangerous antigens. This subsequently allows time for the adaptive immune system to mount an antigen-specific response.
Inflammation is one of the initial responses of the innate immune system, and this inflammation is the stimulus that subsequently signals for the adaptive immune system to elicit a more robust defence. Inflammation is described as dolour (pain), calor (heat), rubor (redness), tumour (swelling), and functio laesa (loss of function).13 While this definition of inflammation has maintained clinical relevance since its first description by Aulus Cornelius Celsus (De medicina, c. A.D. 25), inflammation nowadays can be explained more precisely as pro-inflammatory cytokine induction that is important for guiding the adaptive immune response. In addition to pro-inflammatory cytokines, inflammation can be characterized by the expression of chemokines (chemotactic cytokines) and cell adhesion molecules that direct the immune cell migration and diapedesis, respectively.14 Although the acute expression of these pro-inflammatory mediators is an important defence against short-term perturbations (e.g. pathogen invasion), chronic and/or excessive expression can contribute to a variety of pathologies, including cardiovascular disease.15
The traditional theory of immunological response and tolerance was based on the discrimination between self and non-self, where the immune system was activated by exogenous stimuli (e.g. bacteria, viruses, and fungus). Although this paradigm was acceptable for describing a situation in which the stimulus was a pathogen, many questions remained unresolved under this theoretical framework. For example, what happens when the host undergoes a transformation and subsequently appears different or foreign to the immune system? How do puberty, pregnancy, and ageing occur without eliciting a reaction to changing tissues? Why are tumours not rejected even though they express new and/or mutated proteins? Why are a few individuals susceptible to autoimmune diseases even though the majority of the population accommodates and tolerates autoreactive lymphocytes?16
As a result of these inconsistencies in the self–non-self model, a new theory of immune system activation was introduced, known as the danger model of immunity.3 This model focuses on the notion that the immune system is activated in response to stimuli that are dangerous and potentially damaging to the host. Therefore, immune system activation occurs not only due to foreign stimuli, such as pathogen-associated molecular pattern (PAMP), but also due to endogenous ‘alarmins’, if not properly shielded or compartmentalized away from immune system components. As such, endogenous molecules that are released into the circulation due to cell death and/or injury alarm the immune system that something has disturbed homeostasis and a response is needed. Collectively, these molecules are known as DAMPs. Potential DAMPs are varied and numerous, and by no means have been fully described yet, nor has their clinical efficacy as a therapeutic target been examined. Endogenous molecules (i.e. DAMPs) that arise from injured and dying cells and activate PPRs of the innate immune system include extracellular matrix components, plasma membrane, nuclear, and cytosolic proteins, and elements of damaged/fragmented organelles. Moreover, genetic abnormalities (e.g. polymorphisms and mutations) could turn a normally functioning protein into a DAMP.
Mitochondrial DNA contains mostly unmethylated cytosine-phosphodiester-guanine (CpG) dinucleotides and all peptides are translated with an N-formyl group at the beginning.1,4,17 Therefore, when injured mitochondria are released into the circulation due to plasma membrane rupturing or improperly degraded within the cell, specific mitochondrial components are recognized by PRRs of the innate immune system as a PAMP, driving an inflammatory response. In fact, it was elegantly demonstrated by Zhang et al.18 that mtDAMPs released due to trauma were able to induce systemic inflammatory response syndrome (SIRS), in the absence of any bacterial contamination. Moreover, pressure-overload released mtDNA that escapes autophagic degradation leads to TLR9-mediated inflammatory responses in cardiomyocytes, and is capable of inducing myocarditis and dilated cardiomyopathy.19 As a result of this ability of mtDAMPs to induce systemic inflammation in non-infective conditions such as trauma and heart failure, it is our belief that this deleterious function of mtDAMPs can be extrapolated to other sterile inflammatory conditions (e.g. vascular diseases).
Mitochondrial DNA
Mitochondria contain their own genetic material known as mtDNA, which represents <1% of total cellular DNA. MtDNA is organized as a circular, double-stranded DNA and each mtDNA molecule consists of 15 000–17 000 bp. The nucleotide content of the two strands of mtDNA differs as the heavy (or H) strand is rich in guanine, whereas the light (or L) strand is rich in cytosine. The H strand encodes 28 genes, whereas the L strand encodes 9 genes, with a total of 37 genes encoded by the mitochondrial genome. Of these, 13 genes are for polypeptides, 22 are for transfer RNA (tRNA), and 2 are for the small and large subunits of ribosomal RNA.20 Mitochondrial DNA is packed into aggregates called nucleoids and the most common component of nucleoids is the transcription factor A (TFAM), which has similar actions with those of histones (e.g. compacting and packing DNA).21
The term mitochondrial dysfunction encompasses events such as decreased ATP production, increased generation of ROS, calcium dysregulation, and mtDNA damage.22 Mitochondria have significant DNA repair capacity, can increase their biogenesis, and harbour mechanisms such as mitophagy and the ubiquitin–proteasome system that are able to remove poorly performing mitochondria.23 Nevertheless, mtDNA is susceptible to oxidative stress due to its proximity with the respiratory chain and the lack of protection by histones and chromatin.22 Therefore, mitochondrial dysfunction and mtDNA damage are often involved in diseases associated with oxidative stress, such as diabetes, hypertension, and atherosclerosis. It has been proposed that mtDNA codes for mutated polypeptides and these mutations are responsible for loss of the integrity of the respiratory chain, which leads to increases in ROS and further nuclear and mtDNA damage.22 As a result, mitochondria-induced cellular dysfunction promotes apoptosis, cell cycle arrest, cell senescence, altered lipid processing, and inflammation, which are all characteristics of the development of atherosclerosis as well as other vascular diseases.24,25
As described above, mtDNA has emerged as a DAMP contributing to a systemic inflammatory response. Similar to bacterial DNA, mtDNA has unmethylated CpG DNA repeats, whereas nuclear DNA is modified by the addition of methyl groups on these motifs. This difference between bacterial and nuclear DNA allows immune cells to respond to invading pathogens by mounting an immune response. Bacterial DNA is recognized by the TLR9, a member of the highly conserved PRRs known as TLRs.26 Containing the inflammatogenic unmethylated CpG DNA motifs, mtDNA also acts as a ligand for TLR9.18 Activation of TLR9 induces the synthesis of pro-inflammatory cytokines and mitogen-activated protein kinase activation promoting an inflammatory response.26 TLR9 is expressed in immune,26 endothelial,27 and vascular smooth muscle cells28 and all these cells are involved in the development of vascular dysfunction associated with hypertension, ischaemia, and atherosclerosis. In unstimulated cells, TLR9 is located in the ER. Upon stimulation by CpG DNA, TLR9 translocates to the membranes of endosomes.29 The expression of TLR9 in the ER contrasts sharply with the expression pattern of other TLRs such as TLR2 and TLR4, which both enter the secretory pathway and travel to the plasma membrane, where they recognize their ligands and initiate cellular activation.29 Interestingly, previous studies have shown that circulating mtDNA is increased in individuals with hypertension30 and in pregnant women with preeclampsia, a hypertensive disorder of pregnancy.31 More recently, our laboratory reported that the in vivo activation of TLR9 by synthetic CpG oligonucleotides induces preeclampsia-like symptoms in pregnant rats6 and increases vascular responses to contractile stimuli (unpublished observations). Collectively, these data suggest that mtDNA either in the circulation or in the cytoplasm has the ability of inducing an inflammatory response via activation of the immune system. The presence of TLR9 in vascular and immune cells suggests a potentially functional role of the mtDNA/TLR9 axis in the vasculature in pathologic conditions.
Mitochondrial N-formyl peptides
As described earlier, mitochondria carry hallmarks of their bacterial ancestry.17 One of these hallmarks is that this organelle still uses an N-formyl-methionyl-tRNA as an initiator of protein synthesis.17 Mitochondria synthesize only 13 proteins which all contain an N-formyl group at the beginning of the amino acid sequence. These proteins are found in the IMM, where they participate in electron transport for oxidation phosphorylation.17
It is known that the N-formyl peptide sequence, such as N-formyl-methionyl-leucyl-phenylalanine from gram-negative bacteria, is a potent chemoattractant32 with a high-affinity binding site for FPR.32 The FPR family, a subfamily of G protein-coupled receptors, is represented by at least, three isoforms FPR 1, 2, and 3. Formyl peptide receptors are expressed at high levels on neutrophils and monocytes, and when activated by binding of FMLP, they induce NADPH oxidase activation, ROS generation, and cell chemotaxis.5,32 Interestingly, Hauser et al.33 showed that mtDAMPs from human femoral reaming lead to pulmonary inflammation via activation of FPR on neutrophils.
Besides being expressed in neutrophils, FPR family is expressed on a range of somatic cells and tissues, including the endothelium, epithelium, spleen, lung, liver, skeletal, and smooth muscle.5,32 Nevertheless, its biological function in other tissues is not fully understood. As such, FPR activation in the cardiovascular system has been shown to have functional implications, such as modulation of vascular tone, even in the absence of leucocytes. Specifically, Bode et al.34 demonstrated that FMLP activates cells of an unknown identity in the adventitia or media of human coronary arteries and produced vasoconstrictor cyclooxygenase-derived products. In addition, Keitoku et al.35 reported that FMLP produced biphasic responses (contraction with subsequent relaxation) in human coronary arteries, mainly via generation of thromboxane A2 and prostacyclin. On the other hand, Laplante et al.36 demonstrated that FMLP has a relaxant effect on rabbit vascular strips (portal veins and pulmonary arteries). This relaxant response was decreased by indomethacin in pulmonary arteries. Recently, we reported that FMLP and N-formyl peptides derived from mitochondria (F-MIT) have a powerful relaxant effect in resistance arteries, and that cyclosporine H (FPR antagonist) inhibits this response, suggesting that FPR has an important role in vascular function.5 It is noteworthy that we performed our experiments with formylated peptide corresponding to the N-terminus of mitochondria NADPH dehydrogenase subunit 6. This peptide is equally potent to activate the FPR in human promyelocytic leukaemia cells.37 However, the FPR signalling downstream that induces relaxation is currently under investigation.
Based on these findings, we hypothesized that in conditions of sterile inflammation (e.g. tissue damage/trauma), FPR activation and associated signalling leads to exacerbated relaxation. This novel concept has clinical relevance in the context of SIRS, as FPR agonists released from damaged tissue may induce exacerbated vasodilatation and uncontrolled systemic and local inflammation, leading to hypotension and shock.5
Other mitochondrial-derived damage-associated molecular patterns
The mitochondrion is not only the key site for lipid biosynthesis and oxidation within the cells, but also houses its own unique lipid environment. An important mitochondrial phospholipid is cardiolipin, which acts to anchor cytochrome c to the inner membrane and provides structural and functional support to proteins necessary to carry out oxidative phosphorylation. Disturbances in mitochondrial function disrupt the phospholipid environment leading to the release of cardiolipin, which undergoes rapid oxidation due to its high content of unsaturated fatty acid chains thus turning it into a DAMP. The presence of oxidized cardiolipin in apoptotic cells of atherosclerotic lesions has implicated it in the pathological immune response associated with atherosclerosis.38 Additionally, elevated natural antibodies to cardiolipin have been associated with higher carotid intima-media thickness in patients with rheumatoid arthritis.39
Another biologically active molecule that can act as DAMP is ATP.40 Mitochondria are the major source of ATP production within the cell and intracellular concentrations range from 1 to 10 mM. Once released, ATP binds to the plasmatic membrane family receptors, P2X (inotropic ligand-gated non-selective cation channels) and P2Y (G protein coupled receptors). Released ATP activates P2X7 receptors to trigger potassium efflux, procaspase-1 cleavage, and conversion of pro-IL-1β into mature IL-1β.41 In endothelial cells, shear stress can lead to the release of ATP and intravascular release of ATP contributes to tissue damage by induction of an inflammatory microenvironment and recruitment of circulating neutrophils.42 It is believed that ATP acts as a second signal following TLR induction of pro-IL-1β to drive this inflammatory response through activation of the inflammasome. The combined effects of these two signalling cascades lead to the formation of active caspase-1 and cleavage of pro-IL-1β to active and secreted forms.43 Furthermore, high concentrations of extracellular ATP induce a proinflammatory response in human microvascular endothelial cells leading to the release of IL-6, IL-8, and MCP-1 and increase ICAM-1 expression.44 Our lab has shown that lipopolysaccharide-induced vascular hyporeactivity is amplified following P2X7 receptor activation.45 This brings to light a new response of the P2X and P2Y receptor family members in not only mediating changes in vascular reactivity but also playing a role in vascular inflammation.
Mitochondrial TFAM, a highly abundant mitochondrial protein that is functionally and structurally homologous to high mobility group box protein 1, is a cytosolic and nuclear protein that can act like a DAMP when released from necrotic cells. Like many other high mobility group family proteins, TFAM has the ability to bind to DNA in a sequence-independent manner.46,47 Accordingly, Julian et al.46 reported that TFAM synergizes with mtDNA. Upon release from cell injury, mtDNA remained in association with TFAM to promote endosomal signal transduction via PI3K/Akt and ERK in plasmacytoid dendritic cells (pDC) and that exposure to TFAM alone was insufficient to elicit pDC activation. However, TLR9-mediated immune responses to mtDNA were significantly amplified by TFAM.46 Additionally, Chaung et al.47 demonstrated that TFAM was released into the circulation in rats after haemorrhagic shock. Extracellular TFAM stimulated pro-inflammatory cytokine release in primary peritoneal macrophages and intravenously administration provoked inflammatory responses and led to organ injury in healthy animals. These authors conclude that TFAM can act as an mtDAMP,47 however if this mtDAMP may modify vascular function remains unknown.
Cellular stress leads to the release of cytochrome c from the mitochondria into the cytosol where it can act as an intracellular danger signal. Once in the cytosol, cytochrome c activates caspase family members through a complex with Apaf-1. Serum concentrations of cytochrome c are elevated in patients with SIRS compared with control subjects.48 Moreover, the concentration of cytochrome c in non-survivors was significantly elevated compared with survivors of SIRS identifying cytochrome c as a survival prognostic. Paradoxically, beneficial effects of cytochrome c were observed when cytochrome c loaded nanoparticles targeted to newly formed blood vessels feeding adipocytes were administered in a mouse model of diet induced obesity and the increase in body weight was prevented.49 This gives evidence to the efficacy of using this DAMP in a directly targeted therapeutic approach to the treatment of diseases with unwanted to abnormal angiogenesis.
Are microRNAs mitochondrial-derived damage-associated molecular patterns?
MicroRNAs (miRNA) are class of gene regulators that repress mRNAs post-transcriptionally by binding to partially complementary sites.50 However, recent studies suggest that miRNAs have more functions than initially demonstrated.51 Thus, miRNAs are able to mediate intercellular communication or regulate their own expression.51 Recent studies have shown that miRNAs are ligands for TLRs, a function that is independent of their role in post-transcriptional gene regulation.51 Fabbri et al.52 have shown that miR-21 and -29a secreted by tumour cells in exosomes activate TLR8 and 7 receptors in immune cells, leading to NF-κB activation and inflammatory cytokines production. Interestingly, in 2011 Dr Gidrol and colleagues53 have discovered pre-miRNA and miRNA in human mitochondria from skeletal muscle cells.
With regards to the cardiovascular system, miRNAs have been associated to cardiovascular development, cardiac fibrosis, arrhythmia, and vascular diseases. Liu et al.54 observed that miRNAs are overexpressed in carotid arteries after angioplasty. Also, they demonstrated that in cultured vascular smooth muscle cells, miR-221 and miR-222 expression was increased by growth stimulators and the knockdown of these miRNAs decreased vascular smooth muscle cell proliferation.54 These new evidences suggest a potentially functional role of the miRNA in the vasculature in pathologic conditions.
Conclusion and perspective
Aberrant immune system activation and low-grade systemic inflammation may be a common feature of the dysfunction seen in many vascular diseases. The bacterial ancestry of the mitochondria distinguishes it as novel DAMP with a rationale for its inflammatogenic properties; however, the molecular mechanisms associated with vascular changes induced by mtDAMPs require further investigation (Figure 1). Future studies should examine the ability of mtDAMPs to induce systemic inflammation in non-infective conditions such as trauma, hypertension, and heart failure. The relationship between sterile inflammatory conditions (e.g. vascular diseases) and innate immune system may be important in view of new therapeutic strategies for cardiovascular diseases. These therapies should focus on targeting potent immunological activators, such as mtDAMPs, their release, activity, and/or clearance.
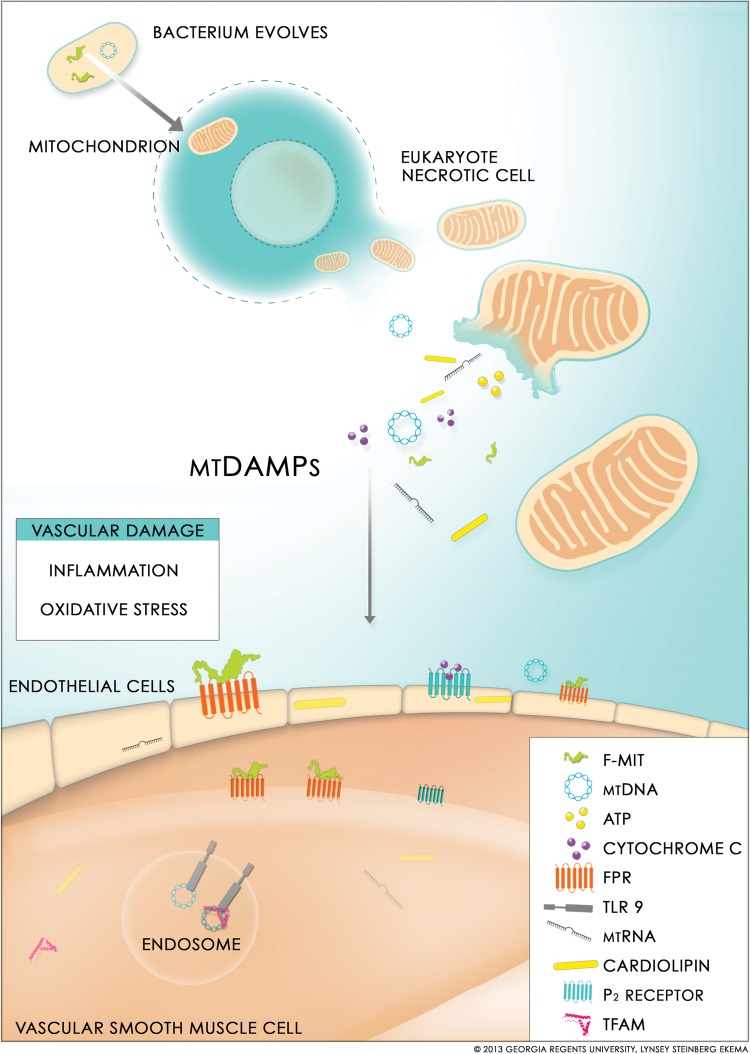
Figure 1.
Cell and/or tissue injury (e.g. necrosis) trigger mitochondria DAMPs (mtDAMPs) release. MtDAMPs are potent immunological activators due to the bacterial ancestry and once released from necrosis they can lead to vascular damage. F-MIT (mitochondria-derived formyl peptide); mtDNA (mitochondria DNA); TLR 9 (Toll like receptor 9); FPR (formyl peptide receptor); P2 (purinergic receptor); ATP (adenosine triphosphate); mtRNA (mitochondrial RNA and microRNA); TFAM (mitochondrial transcription factor A).
Funding
National Institute of Health; American Heart Association; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Conflict of interest: none declared.
Acknowledgements
We would like to thank Lynsey Ekema, MSMI, for her artistic contribution to our figure.
References
- 1.Sagan L. On the origin of mitosing cells. J Theor Biol. 1967;14:255–274. doi: 10.1016/0022-5193(67)90079-3. [DOI] [PubMed] [Google Scholar]
- 2.Margulis L, Bermudes D. Symbiosis as a mechanism of evolution: status of cell symbiosis theory. Symbiosis. 1985;1:101–124. [PubMed] [Google Scholar]
- 3.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 4.Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32:157–164. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Wenceslau CF, McCarthy CG, Goulopoulou S, Szasz T, NeSmith EG, Webb RC. Mitochondrial-derived N-formyl peptides: novel links between trauma, vascular collapse and sepsis. Med Hypotheses. 2013;81:532–535. doi: 10.1016/j.mehy.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goulopoulou S, Matsumoto T, Bomfim GF, Webb RC. Toll-like receptor 9 activation: a novel mechanism linking placenta-derived mitochondrial DNA and vascular dysfunction in pre-eclampsia. Clin Sci. 2012;123:429–435. doi: 10.1042/CS20120130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alberts B. Molecular Biology of the Cell. 5th edn. New York: Garland Science; 2008. [Google Scholar]
- 8.Addabbo F, Montagnani M, Goligorsky MS. Mitochondria and reactive oxygen species. Hypertension. 2009;53:885–892. doi: 10.1161/HYPERTENSIONAHA.109.130054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowland AA, Voeltz GK. Endoplasmic reticulum–mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol. 2012;13:607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 13.Rather LJ. Disturbance of function (functio laesa): the legendary fifth cardinal sign of inflammation, added by Galen to the four cardinal signs of Celsus. Bull N Y Acad Med. 1971;47:303–322. [PMC free article] [PubMed] [Google Scholar]
- 14.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 15.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133–1145. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 17.Taanman JW. The mitochondrial genome: structure, transcription, translation and replication. Biochim Biophys Acta. 1999;1410:103–123. doi: 10.1016/s0005-2728(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacobazzi V, Castegna A, Infantino V, Andria G. Mitochondrial DNA methylation as a next-generation biomarker and diagnostic tool. Mol Genet Metab. 2013;110:25–34. doi: 10.1016/j.ymgme.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman DBA, Durisic N, Mativetsky JM, Costantino S, Hancock MA, Grutter P, Shoubridge EA. The mitochondrial transcription factor TFAM coordinates the assembly of multiple DNA molecules into nucleoid-like structures. Mol Biol Cell. 2007;18:3225–3236. doi: 10.1091/mbc.E07-05-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu E, Mercer J, Bennett M. Mitochondria in vascular disease. Cardiovas Res. 2012;95:173–182. doi: 10.1093/cvr/cvs111. [DOI] [PubMed] [Google Scholar]
- 23.Chan NC, Chan DC. Parkin uses the UPS to ship off dysfunctional mitochondria. Autophagy. 2011;7:771–772. doi: 10.4161/auto.7.7.15453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 25.Kukat A, Edgar D, Bratic I, Maiti P, Trifunovic A. Random mtDNA mutations modulate proliferation capacity in mouse embryonic fibroblasts. Biochem Biophys Res Commun. 2011;409:394–399. doi: 10.1016/j.bbrc.2011.04.145. [DOI] [PubMed] [Google Scholar]
- 26.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 27.Ding Z, Liu S, Wang X, Khaidakov M, Dai Y, Mehta JL. Oxidant stress in mitochondrial DNA damage, autophagy and inflammation in atherosclerosis. Sci Rep. 2013;3:1077. doi: 10.1038/srep01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erridge C, Burdess A, Jackson AJ, Murray C, Riggio M, Lappin D, Milligan S, Spickett CM, Webb DJ. Vascular cell responsiveness to Toll-like receptor ligands in carotid atheroma. Eur J Clin Invest. 2008;38:713–720. doi: 10.1111/j.1365-2362.2008.02010.x. [DOI] [PubMed] [Google Scholar]
- 29.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 30.Veiko NN, Konorova IL, Neverova ME, Didelina OV, Mkrtumova NA, Ershova ES, Kon'kona MS, Postnov AY. The effect of CpG-rich DNA fragments on the development of hypertension on spontaneously hypertensive rats. Biomed Chem. 2010;4:269–278. doi: 10.18097/pbmc20105606686. [DOI] [PubMed] [Google Scholar]
- 31.Colleoni F, Lattuada D, Garretto A, Massari M, Mando C, Somigliana E, Cetin I. Maternal blood mitochondrial DNA content during normal and intrauterine growth restricted (IUGR) pregnancy. Am J Obstet Gynecol. 2010;203:365. doi: 10.1016/j.ajog.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 32.Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23:541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- 33.Hauser CJ, Sursal T, Rodriguez EK, Appleton PT, Zhang Q, Itagaki K. Mitochondrial DAMPs from femoral reamings activate neutrophils via formyl peptide receptors and P44/42 MAP kinase. J Orthop Trauma. 2010;24:534–538. doi: 10.1097/BOT.0b013e3181ec4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bode SM, Kuhn M, Förstermann U. Chemotactic peptide FMLP contracts human coronary arteries via cyclooxygenase products. Am J Physiol. 1990;258:H848–H853. doi: 10.1152/ajpheart.1990.258.3.H848. [DOI] [PubMed] [Google Scholar]
- 35.Keitoku M, Kohzuki M, Katoh H, Funakoshi M, Suzuki S, Takeuchi M, Karibe A, Horiguchi S, Watanabe J, Satoh S, Nose M, Abe K, Okayama H, Shirato K. FMLP actions and its binding sites in isolated human coronary arteries. J Mol Cell Cardiol. 1997;29:881–894. doi: 10.1006/jmcc.1996.0291. [DOI] [PubMed] [Google Scholar]
- 36.Laplante C, Tremblay B, Marceau F. Relaxant effect of N-formyl-methionyl-leucyl-phenylalanine on rabbit vascular strips. J Pharmacol Exp Ther. 1989;248:774–780. [PubMed] [Google Scholar]
- 37.Rabiet MJ, Huet E, Boulay F. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur J Immunol. 2005;35:2486–2495. doi: 10.1002/eji.200526338. [DOI] [PubMed] [Google Scholar]
- 38.Tuominen A, Miller YI, Hansen LF, Kesaniemi YA, Witztum JL, Horkko S. A natural antibody to oxidized cardiolipin binds to oxidized low-density lipoprotein, apoptotic cells, and atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2006;26:2096–2102. doi: 10.1161/01.ATV.0000233333.07991.4a. [DOI] [PubMed] [Google Scholar]
- 39.Sherer Y, Gerli R, Gilburd B, Bartoloni Bocci E, Vaudo G, Mannarino E, Shoenfeld Y. Thickened carotid artery intima-media in rheumatoid arthritis is associated with elevated anticardiolipin antibodies. Lupus. 2007;16:259–264. doi: 10.1177/0961203307076697. [DOI] [PubMed] [Google Scholar]
- 40.Gorini S, Gatta L, Pontecorvo L, Vitiello L, la Sala A. Regulation of innate immunity by extracellular nucleotides. Am J Blood Res. 2013;3:14–28. [PMC free article] [PubMed] [Google Scholar]
- 41.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 42.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 43.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 44.Seiffert K, Ding W, Wagner JA, Granstein RD. ATPgammaS enhances the production of inflammatory mediators by a human dermal endothelial cell line via purinergic receptor signaling. J Invest Dermatol. 2006;126:1017–1027. doi: 10.1038/sj.jid.5700135. [DOI] [PubMed] [Google Scholar]
- 45.Chiao CW, Tostes RC, Webb RC. P2X7 receptor activation amplifies lipopolysaccharide-induced vascular hyporeactivity via interleukin-1 beta release. J Pharmacol Exp Ther. 2008;326:864–870. doi: 10.1124/jpet.107.135350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Julian MW, Shao G, Bao S, Knoell DL, Papenfuss TL, VanGundy ZC, Crouser ED. Mitochondrial transcription factor A serves as a danger signal by augmenting plasmacytoid dendritic cell responses to DNA. J Immunol. 2012;189:433–443. doi: 10.4049/jimmunol.1101375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaung WW, Wu R, Ji Y, Dong W, Wang P. Mitochondrial transcription factor A is a proinflammatory mediator in hemorrhagic shock. Int J Mol Med. 2012;30:199–203. doi: 10.3892/ijmm.2012.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adachi N, Hirota M, Hamaguchi M, Okamoto K, Watanabe K, Endo F. Serum cytochrome c level as a prognostic indicator in patients with systemic inflammatory response syndrome. Clin Chim Acta. 2004;342:127–136. doi: 10.1016/j.cccn.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 49.Hossen N, Kajimoto K, Akita H, Hyodo M, Ishitsuka T, Harashima H. Therapeutic assessment of cytochrome c for the prevention of obesity through endothelial cell-targeted nanoparticulate system. Mol Ther J Am Soc Gene Ther. 2013;21:533–541. doi: 10.1038/mt.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8:93–103. doi: 10.1038/nrg1990. [DOI] [PubMed] [Google Scholar]
- 51.Chen X, Liang H, Zhang J, Zen K, Zhang CY. microRNAs are ligands of Toll-like receptors. RNA. 2013;19:737–739. doi: 10.1261/rna.036319.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrey E, Saint-Auret G, Bonnamy B, Damas D, Boyer O, Gidrol X. Pre-microRNA and mature microRNA in human mitochondria. PLoS ONE. 2011;6:e20220. doi: 10.1371/journal.pone.0020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]