Abstract
People with autism spectrum disorders (ASD) have atypical visual perception of global and local information. Previous neuroimaging studies have examined the functional anatomy of locally-directed attention during visual processing in ASD, but few have examined differences in both globally-and locally-directed attention. We performed functional magnetic resonance imaging (fMRI) in 17 adults with ASD and 16 typically developing (TD) subjects to examine the neurobiology of both global- and local- level information processing in ASD using an abstract hierarchical design task. TD subjects showed no regions of increased brain activation relative to subjects with ASD using whole brain analysis. Subjects with ASD exhibited greater activation in right superior frontal gyrus during locally directed attention. During globally directed attention, the ASD group showed greater right lateral occipital activation. Additionally, subjects with ASD showed less deactivation in medial prefrontal cortex (part of the default mode network) in the globally directed attention condition. Our findings help elucidate networks of brain activation related to atyipcal global and local feature processing in ASD.
Keywords: (from Index Medicus): Autistic disorder, Magnetic resonance imaging, Child developmental disorders, pervasive, Asperger syndrome, Attention
1. Introduction
Autism spectrum disorders (ASD) are characterized by impaired social interaction and communication accompanied by repetitive behaviors and restricted interests (American Psychiatric Association, 2000). Despite the characterization of the disorder as a constellation of deficits, there may also be relative advantages. One such advantage is an enhanced ability to focus on details, exemplified by superior performance on visual search tasks such as the Embedded Figures Task (EFT) (Shah and Frith, 1983; Jolliffe and Baron-Cohen, 1997; Frith, 2003). The EFT involves searching for a particular shape within a larger, more complex figure (Briskman et al., 2001; Happé and Frith, 2006). At least two major theories attempt to explain how this constellation of symptoms and advantages might arise. One theory, known as weak central coherence (WCC), proposes that people with ASD exhibit a preference for local details compared with global perception whereas most people show a strong bias for global, holistic perception (Happé and Frith, 2006). The WCC theory emphasizes a relative primacy of local processing and a deficit in global processing (although recent revisions have altered this to ambivalence regarding impairment in global processing). A second theory known as enhanced perceptual functioning (EPF) posits that the default setting of autistic perception is more locally oriented than that of typically developing persons, without deficits in the processing of global aspects of information (Mottron et al., 2006; Wang et al., 2007). A major difference between the theories is that the revised WCC emphasizes enhanced local processing while remaining undecided regarding inferior global processing; whereas, EPF emphasizes that persons with ASD can process globally when required. Although several neuroimaging studies have attempted to provide a functional neuroanatomic understanding of these concepts, continued exploration is needed to provide a robust explanation of pathways underlying altered visual processing in ASD.
Individuals with ASD perform atypically on a range of tasks involving integration of parts and wholes (Happe and Booth, 2008). In the visual domain, subjects with ASD have shown preserved or superior performance of the Block Design subtest of the Wechsler Intelligence Scales and the EFT (Briskman et al., 2001; Happé and Frith, 2006). Other studies have shown performance on the EFT equivalent to that of typically developing (TD) subjects (Brian and Bryson, 1996; Ring et al., 1999). It is noteworthy that in the EFT, attention to the global form confers no advantage on target identification. Presumably, ASD participants perform better than intelligence quotient (IQ)-matched controls because they show a reduced global attention, enhanced local attention, or both. Other studies have also demonstrated the relative advantage in locally directed attention in ASD (Plaisted et al., 1998; O’Riordan et al., 2001). Conversely, people with ASD may have difficulty with tasks that load heavily on global perception (Happé and Frith, 2006). This was previously exemplified by studies showing people with ASD exhibit lower performance on tasks involving the recognition of faces—representing reduced configural processing (Behrmann et al., 2006). However, recent evidence has shown that the deficit in face recognition is related to face memory and eye recognition specifically, and not face recognition as a whole (Weigelt et al., 2012). These differences exemplify atypical global and local processing increasingly thought to contribute to the ASD endophenotype.
At least three lines of research highlight the value of functional neuroanatomic exploration of this aspect of the neurocognitive phenotype of autism. First, one recent study found that individuals with macrocepahly (measured by greater head circumference—an index of brain overgrowth that is a known neurodevelopmental correlate of autism) showed evidence of atypical local processing (White et al., 2009). Second, some broad autism phenotype studies have identified altered global processing among parents of individuals with ASD (Baron-Cohen and Hammer, 1997; Briskman et al., 2001). Third, in a Navon-type task participants with ASD were demonstrated increased local-to-global interference in naming time and accuracy (Navon, 1977; Wang et al., 2007). These findings demonstrate the need for continued exploration of the neuroanatomic basis of altered global and local processing in ASD.
Neuroanatomical studies have examined global and local visual processing differences in healthy subjects using functional magnetic resonance imaging (fMRI). Studies have shown preferential processing of global information in the right hemisphere (RH) and local information in the left hemisphere (LH), specifically in the right and left occipital cortex, respectively, and in anterior cingulate and parietal areas during local recognition (Fink et al., 1996; Martinez et al., 1997; Lux et al., 2004;). A more recent study found at least two components produce hemispheric asymmetries of global and local visual processing (Weissman and Woldorff, 2005). Structures that appear in task-relevant contrasts for healthy individuals include areas such as the cuneus, middle frontal gyrus, inferior frontal gyrus, middle occipital gyrus, superior occipital gyrus, inferior and posterior parietal regions and superior temporal gyrus (Ring et al., 1999; Weissman and Woldorff, 2005; Lee et al., 2007; Manjaly et al., 2007).
In ASD, functional imaging studies have focused mostly on tasks that demand attention to local detail, and in which the global percept confers no advantage, such as the EFT. One recent study was the first, in our knowledge, to examine global-level interference during local processing in ASD, using a functional connectivity analysis (Liu et al., 2011). In this study subjects had to count colored lines associated with a three-dimensional (3D) object. The study found subjects with ASD to have a lower level of activation of executive brain regions and synchronization between executive and posterior visuospatial regions, and concluced that subject with ASD were less or not at all affected by the presence of a 3D figure, whereas control subjects needed to suppress automatic processing of global information. In fMRI studies emphasizing attention to detail, such as those using the EFT or Hidden Figures Task (HFT), subjects with ASD have generally exhibited increased task-related activations in posterior regions including the right cuneus, right occipital gyri and right inferior parietal areas in adults (Ring et al., 1999), adolescents (Manjaly et al., 2007), and children (Lee et al., 2007; Malisza et al., 2010) with ASD. Additionally, in a visual matrix reasoning task including Raven’s Standard Progressive Matrices, subjects with ASD demonstrated greater occipital activation combined with lesser prefrontal activation compared with typically developing subjects (Soulieres et al., 2009). A meta-analysis using Activation Likelihood Estimation (ALE) of fMRI studies of visual processing in ASD demonstrated atypical allocation of activity in visual regions in ASD for object processing (Samson et al., 2012). Objects included in that meta-analysis included nameable objects. We are not aware, however, of studies to date examining whole brain differences in global and local processing in ASD using an abstract hierarchical figure (as opposed to namable letters such as in traditional Navon-type tasks). Using a hierarchical abstract figure reduces confounding cognitive processes from mental identification of letters, numbers, or other nameable objects, thus focusing on visual perception alone. Therefore, neural processes known to be involved with reading are avoided and the emphasis is placed on processes related to early visual perception and attention.
The goal of the present study was to examine whole brain differences in both local- and global-level attention in ASD using a hierarchical, abstract shape recognition task (Martinez et al., 1997). We hypothesized greater activation of primary visual cortex and other visual areas in subjects with ASD in both global- and local- conditions. Additionally, we predicted that the ASD group would exhibit decreased activity in the right hemisphere during the global condition, and increased left hemispheric activity in the local condition.
2. Methods
2. 1. Participants
The study included 33 adult participants −17 individuals with ASD (high functioning autistic disorder or Asperger syndrome) (mean age 32, range 18–55, of which 3 were female), and 16 typically developing (TD) subjects (mean age 33, range 18–55, of which 3 were female). Subjects were matched for age, gender, intelligence quotient (IQ; using the Wechsler Abreviated Scale of Intelligence or WASI), and socioeconomic status. See Table 1 for demographic information. Diagnoses were confirmed with the Autism Diagnostic Observation Schedule -Generic (ADOS-G) and DSM-IV checklist completed by an experienced clinician (SH). Nine subjects in the ASD group met clinical criteria criteria (via the DSM-IV checklist) for Autistic Disorder and 8 for Asperger syndrome. All met criteria for Autistic Disorder on the ADOS-G. Full scale IQ for all subjects was greater than 70. Exclusion criteria were any known seizure disorder or single-gene genetic association with autism such as fragile X, tuberous sclerosis, etc. In addition, typically developing subjects were screened for any personal or family history of a developmental disorder, Axis I illness or neurological disorder. All subjects gave written consent to participate in the research consistent with the Declaration of Helsinki and the local guidelines of the Colorado Multiple Institution Review Board.
Table 1.
Demographic information.
| ASD | TD | t Value | P Value | ||
|---|---|---|---|---|---|
| Age (years) | Mean +/−SD | 31.8 +/− 12.0 | 34.8+/−11.4 | 0.49 | NS |
| Handedness | Right: Left | 13:3 | 14:1 | NA | NA |
| Gender | Male: Female | 13:3 | 12:3 | NA | NA |
| VIQ | Mean +/−SD | 108.3+/−15.7 | 115.9+/−16.8 | 0.38 | NS |
| PIQ | Mean +/−SD | 109.5+/−12.5 | 116.8+/−18.0 | 0.29 | NS |
| FSIQ | Mean +/−SD | 109.6+/−12.1 | 116.7+/−18.1 | 0.24 | NS |
2. 2. fMRI Task Design and Stimuli
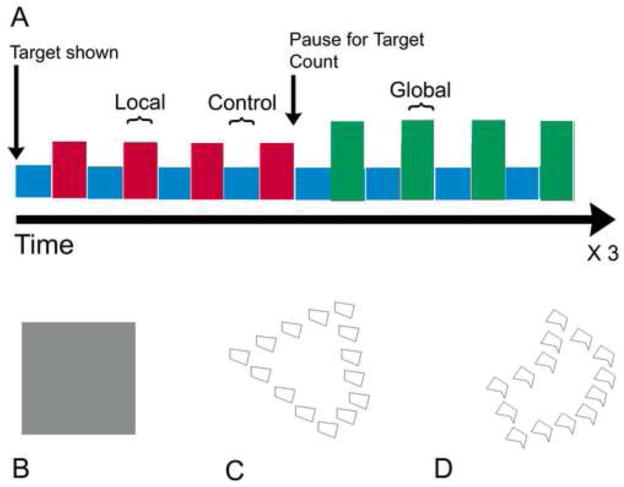
During fMRI sessions, subjects were engaged in a hierarchical shape recognition task conducted in a blocked fashion (Martinez et al., 1997). There were 3 task conditions: 1) attend to the global pattern level, 2) attend to the local pattern, or 3) watch a control stimulus (see Figure I). The stimuli were abstract shapes comprised of smaller abstract shapes. The control condition consisted of passive viewing of grey squares and was alternated between blocks of attending to local or global patterns. Prior to entering the magnet, subjects were presented with a practice module using E-Prime to verify understanding of the task. Subjects were asked the following: “Silently count the number of figures that match the global (or local) target figure; you will be asked to state how many matching figures you counted”. The target figure appeared in each block 11 to 13 percent (only at the attended level). Each global- and local- block lasted for 20 seconds and was repeated 12 times per condition. Control blocks also lasted 20 seconds and were repeated 24 times. Block order was counterbalanced by subject, with every other subject starting with the global block, the remaining with the local block. The total experiment comprised of 24 blocks of attention to shape (12 at each level) and 24 interspersed control blocks, for a total task duration of 16 min (20 seconds per block * 48 blocks = 960 sec or 16 min). Stimuli were presented to subjects in the magnet using MR-compatible goggles (Resonance Technology, Inc.) using E-Prime.
Figure 1. Abstract Shape Recognition Task.
A: Control condition and global- or local- condition blocks were repeated in an alternating manner for a total of four blocks of each control and global- or local- condition, prior to a pause to report target count. At the end of each block subjects were asked to report the number of figures that matched the target for that block. Each global- and local- block lasted for 20 seconds and was repeated 12 times per condition. Control blocks also lasted 20 seconds and were repeated 24 times. Block order was counterbalanced by subject, with every other subject starting with the global block, the remaining with the local block. The total experiment comprised of 24 blocks of attention to shape (12 at each level) and 24 interspersed control blocks (Total experiment time was 20 seconds per block * 48 blocks = 960 sec or 16 min in the scanner).
B: During the control condition, subjects attend to a simple gray square occupying the visual field.
C, D: Two examples of stimuli (hierarchical shapes) used during the fMRI task. During the global condition, subjects are presented with a target shape and match the larger shape to matching larger shapes that flash every 500ms, ignoring the smaller shapes. During the local condition, subjects are presented with a target shape and match the smaller shape to matching smaller shapes that flash every 500ms, ignoring the larger shape.
2.3. Image Acquisition
T2* images were acquired on a 3T GE Signa using an 8-channel coil. A high-resolution T1-weighted anatomical scan was acquired for each subject for coregistration to functional data (inversion recovery spoiled gradient-recall acquisition [IR-SPGR], repetition time=9 msec, echo time=1.9 msec, inversion time=500 msec, flip angle=10 degrees, matrix=256×256, field of view=220 mm2, 124 coronal slices 1.7 mm thick). Functional images were acquired with a gradient-echo T2* blood-oxygen-level-dependent (BOLD) contrast technique (repetition time=2000 msec, echo time=30 msec, field of view=220 mm2, 64×64 matrix, 31 slices 4 mm thick, no gap, angled parallel to the planum sphenoidale). A total of 480 EPI volumes were acquired, plus 4 additional “dummy” scans to achieve a T1 relaxation steady-state. Additionally, one inversion recovery-echo planar imaging (IR-EPI) volume (inversion time=505 msec) was acquired to improve co-registration between the functional and anatomical scans. Head motion was minimized with a VacFix head-conforming vacuum cushion (Par Scientific A/S, Odense, Denmark).
2.4. fMRI Data Analysis
Data were analyzed using SPM8 (Wellcome Department of Imaging Neuroscience, London). After discarding the first four scans from each run for saturation effects, data from each participant were realigned to the first volume, and normalized to the Montreal Neurological Institute (MNI) template using a gray-matter-segmented IR-EPI as an intermediate and smoothed with a 6-mm full width at half maximum Gaussian kernel. Data were evaluated using the GLM in a random effects analysis. Contrasts of interest were generated for each subject at the first level to account for the variability of the data on a voxel by voxel basis, using an HRF-convolved boxcar function. A 128 s high pass filter was applied to remove low frequency fluctuation in the BOLD signal and a first-order autocorrelation model, AR(1), was used to address temporal autocorrelations. Six motion parameters (x, y and z translation and rotation) were included at the first level to model and remove the effects of subject motion. Subjects with head motion exceeding one voxel (two subjects from the control group and one subject with ASD) were excluded from analysis. The total number of subjects (N=33) reported in our analysis does not include these three subjects.
At the first-level (within-subject), contrasts were created for global- and local-conditions versus control condition for each group (ASD and TD). Additional contrasts were formed for the global condition compared to the local condition for group-wise analyses at the second level.
Second level, group-wise analysis was performed to incorporate both within subject and between subject variance, using one-sample t-tests to compare the first level contrasts (global > control and local > control) within each group and two-sample t tests to compare contrasts (global > control, local > control, global < local and global > local) between groups. Additionally, to improve statistical sensitivity, results were masked with a gray-matter mask consisting of the average gray matter from their unified segmentation from their T1 scans. To control for multiple comparisons, the AlphaSim program from Analysis of Functional Neuroimages (AFNI) was used with 10,000 Monte Carlo simulations to yield a combined height threshold of p < 0.01 and 34 voxels for a whole brain FWE of p < 0.05.
Additionally, in order to examine lateralization of brain activation for expected activations in the occipital and temporal regions, predefined 6 mm spherical regions of interest (ROI) were examined, centered on the MNI coordinates +/− 43.33x, −56.44y and −5.74z (lateral-occipital) and +/− 14.96x, −68.02y and 36.82z (occipito-temporal). The MNI coordinates were converted from Talairach-space to MNI space using the tal2icbm_spm function from GingerAle (Eickhoff et al., 2009). These coordinates were taken from the prior published work from which we adapted this task (Martinez et al., 1997), for the ROI exhibiting the greatest activations in the attend-global or attend-local conditions. Within each ROI, weighted means were calculated using the Lateralization Index (LI) toolbox for SPM8 (Wilke and Lidzba, 2007). The LI measure ranges from 1 (entirely left lateralized) to −1 (entirely right lateralized). A 2 × 2 × 2 (group by condition by region) mixed design ANOVA, with group as a between subjects factor, condition (global or local) and region (occipital or temporal) as within subjects factors, was evaluated in SPSS, version 19.
3. Results
3.1 Within-group task results
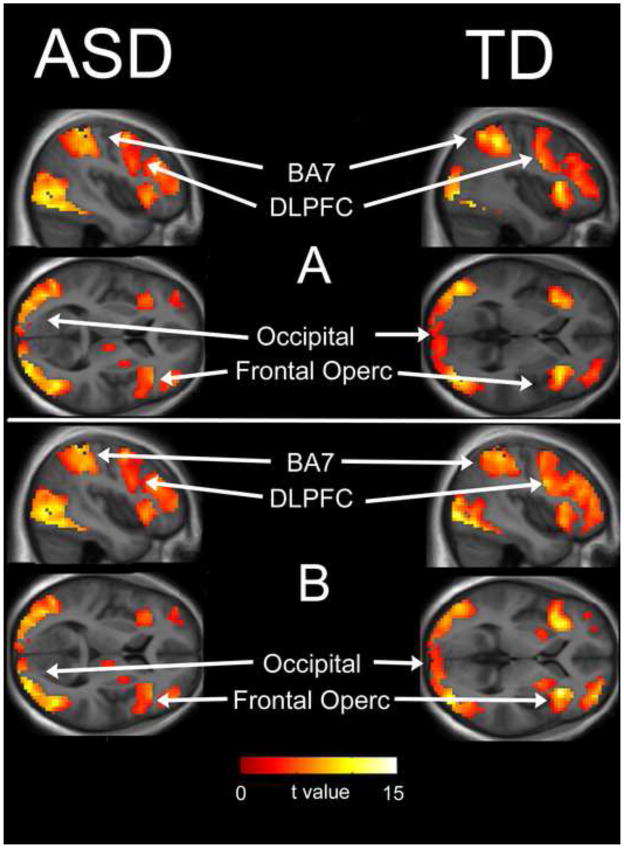
The within-group pattern of activation for both groups and both attention conditions (local > control and global > control) was similar for both ASD and typically developing subjects and included activation of attention and cognitive control networks (bilateral parietal BA7, frontal operculum, dorsolateral prefrontal cortex and pre-supplemental motor area/cingulate gyrus), as well as early activation of ventral visual stream regions including the lateral occipital cortex. (Table 2, Figure 2).
Table 2.
One-sample results
| Condition | Brain Region | ke | t | x | y | z |
|---|---|---|---|---|---|---|
| R lateral occipital | 3436 | 12.2 | 42 | −70 | 7 | |
| L lateral occipital | 3436 | 10.56 | −42 | −82 | −5 | |
| R DLPFC | 1754 | 6.49 | 39 | 2 | 55 | |
| L DLPFC | 345 | 4.5 | −42 | 11 | 28 | |
| R frontal operculum | 1754 | 6.19 | 33 | 20 | −5 | |
| L frontal operculum | 189 | 4.49 | −33 | 20 | 7 | |
| L middle frontal gyrus | 44 | 3.29 | −33 | 44 | 13 | |
| ASD: Global > Control | ||||||
|
| ||||||
| ASD: Local > Control | R lateral occipital | 2187 | 12.22 | 48 | −64 | 1 |
| R lateral occipital | 2187 | 9.62 | 30 | −91 | −2 | |
| L inferior occipital | 1626 | 10.16 | −36 | −85 | −5 | |
| L lateral occipital | 1626 | 8.13 | −39 | −79 | 4 | |
| R DLPFC | 1993 | 9.46 | 30 | −1 | 61 | |
| L DLPFC | 483 | 5.63 | −33 | −7 | 58 | |
| L middle frontal gyrus | 109 | 3.95 | −42 | 50 | 4 | |
| L Inferior frontal gyrus | 109 | 3.5 | −35 | 38 | 16 | |
| L frontal operculum | 162 | 5.15 | −39 | 23 | 4 | |
| R caudate nucleus | 72 | 4.31 | 15 | −4 | 16 | |
| R thalamus | 72 | 3.61 | 12 | −16 | 10 | |
|
| ||||||
| ASD: Global > Local | R superior occipital | 45 | 3.31 | 27 | −91 | 25 |
| R cuneus | 45 | 3.83 | 15 | −91 | 16 | |
|
| ||||||
| ASD: Local > Global | R DLPFC | 39 | 4.78 | 45 | −10 | 58 |
| L superior parietal | 66 | 3.14 | −51 | −61 | 52 | |
| L superior parietal | 66 | 3.14 | −39 | −13 | 49 | |
|
| ||||||
| TD: Global > Control | L lateral occipital | 3697 | 11.02 | −33 | −85 | −8 |
| R superior parietal | 3697 | 13.97 | 27 | −58 | 52 | |
| R frontal operculum | 1560 | 10.56 | 39 | 20 | 4 | |
| L frontal operculum | 255 | 8.69 | −33 | 17 | 7 | |
| L DLPFC | 549 | 6.42 | −54 | 8 | 40 | |
| R DLPFC | 1560 | 7.41 | 48 | 11 | 25 | |
| L middle cingulate gyrus | 442 | 7.41 | −3 | 11 | 46 | |
| R medial frontal | 442 | 7.32 | 6 | 5 | 64 | |
|
| ||||||
| TD: Local > Control | R lateral occipital | 3584 | 10.67 | 42 | −82 | −2 |
| R superior parietal | 3584 | 12.57 | 39 | −55 | 55 | |
| R frontal operculum | 2659 | 10.71 | 33 | 23 | 1 | |
| L frontal operculum | 1160 | 9.6 | −33 | 20 | 1 | |
| L inferior frontal gyrus | 1160 | 9.97 | −48 | 11 | 25 | |
| R DLFPC | 2659 | 8.35 | 39 | 38 | 25 | |
| L DLPFC | 1160 | 7.34 | −48 | 2 | 43 | |
| L DLPFC | 125 | 6.27 | −30 | 47 | 25 | |
| R thalamus | 125 | 4.44 | 9 | −19 | 16 | |
| R caudate nucleus | 125 | 6.1 | 18 | 5 | 16 | |
|
| ||||||
| TD: Global > Local | None | |||||
|
| ||||||
| TD: Local > Global | R lateral occipital | 45 | 3.87 | 30 | −91 | −2 |
| L inferior frontal gyrus | 338 | 5.16 | −51 | 20 | 25 | |
| R angular gyrus | 185 | 5.12 | 45 | −61 | 25 | |
| R middle cingulate gyrus | 81 | 4.21 | 6 | −28 | 52 | |
| L middle cingulate gyrus | 81 | 3.16 | −3 | −28 | 52 | |
| L caudate nucleus | 81 | 3.5 | −18 | 11 | 10 | |
| L DLPFC | 154 | 5.28 | −51 | 26 | 7 | |
| R medial frontal | 121 | 3.7 | 6 | 50 | 43 | |
| L medial frontal | 121 | 3.33 | −3 | 41 | 34 | |
Figure 2. One-Sample T-test Results.
Whole-brain analysis of areas of activation for globally-directed attention condition (A) and locally-directed attention condition (B) for ASD and TD groups. Areas of activation included bilateral attention and cognitive control areas and visual areas including: dorsolateral prefrontal cortex, occipital cortex, and frontal operculum (see Table 2).
3.2. Comparisons between TD and ASD group
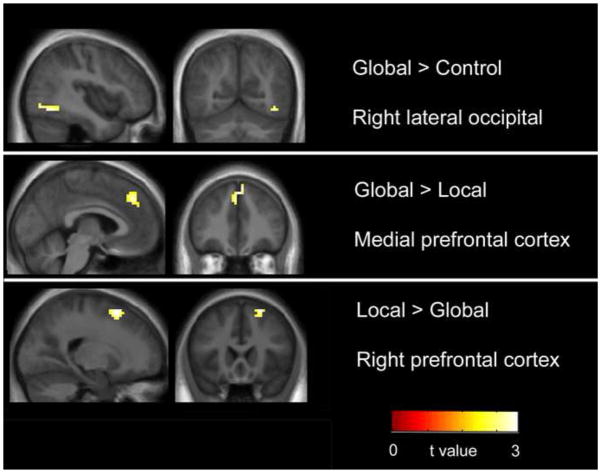
No areas were found in which typically developing subjects showed greater activation than subjects with ASD, in either the global- or local- conditions. The ASD group showed greater activation than TD subjects in right prefrontal cortex for local > control and right lateral occipital cortex for global > control (Table 3, Figure 3). The ASD group showed less deactivation than the TD group in medial prefrontal cortex in both clusters (calculated using percent signal change, see Figure 4) in the global > local contrast. Participants in both groups were all at the performance ceiling for the task and there were no significant differences between groups (i.e., no variance to evaluate).
Table 3. Two-sample results.
Whole-brain FWE (p < 0.05) corrected results for regions showing more activity in ASD (N=17) subjects relative to TD subjects (N=16).
| Brain region | Cluster Size | t | x | y | z |
|---|---|---|---|---|---|
| ASD > TD | |||||
| Global > Control | |||||
| Right lateral occipital | 32 | 3.29 | 48 | −73 | −8 |
| 3.03 | 42 | −64 | −11 | ||
| Local > Control | |||||
| Right superior frontal gyrus | 42 | 3.61 | 21 | 14 | 55 |
| Global > Local | 40 | 3.61 | 3 | 38 | 46 |
| Medial frontal gyrus | 3.30 | −3 | 38 | 37 | |
Note. All labels derived from visual inspection (see text). Peak voxels reported within clusters of the same size belong to clusters that are contiguous.
Figure 3. Group Comparisons.
Whole-brain comparison for ASD > TD (see Table 3). No regions of increased activation were found for TD> ASD. In the Global > Control comparison right lateral occipital area showed greater activation. In the Global > Local comparison medial prefrontal cortex was found to show increased deactivation calculated by percent signal change (see figure 4). Finally in the Local > Global comparison right prefrontal cortex showed increased activation in the ASD group compared to TD.
Figure 4. Medial Prefrontal Cortex Deactivation.
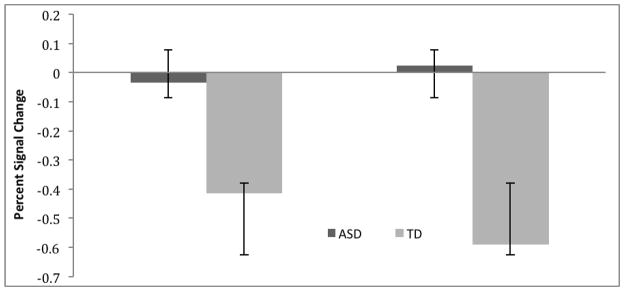
BOLD percent signal change (mean +/− SD), showing deactivation greater in the TD group compared with the ASD group in medial prefrontal cortex in the group comparison (6mm spheres created at two clusters centering on MNI coordinates −3, 38, 46 (left); and 3, 38, 37 (right)).
3.3. Lateralization of Brain Activation
There were no significant effects of group or condition on the LI measure. There was, however, a significant effect of region, F(1, 31)=35.86, p < 0.001, indicating that the lateral-occipital ROI was more left lateralized (0.29 +/− 0.07) while the occipito-temporal ROI was more right lateralized (−0.20 +/− 0.08). The group by condition was also significant, F(1,31)=5.03, p < 0.04. The group by region interaction was non-significant. Post-hoc testing revealed that for TD subjects only, there was change in lateralization between conditions (greater in the global condition) in both ROI, but in opposing directions for each ROI (i.e., the lateral-occipital was more left lateralized in the global condition than in the local, and the occipito-temporal ROI was more right lateralized in the global condition than in the local, both p < 0.02). Subjects with ASD exhibited no significant differences in lateralization between conditions.
4. Discussion
The present study used a hierarchical abstract shape recognition task to examine both global- and local- level visual processing differences in subjects with ASD. Compared with previous studies that have employed the EFT, HFT, or used functional connectivity analysis, our study separately examines both local- and global- level processing, using a whole brain comparison. In general, both groups showed a similar pattern of activation of early visual areas including lateral occipital region, and attention and cognitive control networks including dorsolateral prefrontal areas, superior parietal and frontal operculum. In the comparison between TD and ASD groups, the following areas were found to be differentially activated in the group with ASD: 1) increased activation of right lateral occipital areas in the global condition, 2) increased activation of right prefrontal cortex in the local condition; and 3) decreased deactivation of medial prefrontal cortex in the global condition. We next discuss each of these findings in turn and consider the possible relevance to the autism phenotype.
4.1 Early Visual Processing
As expected, both ASD and TD groups were found to activate bilateral ventral visual processing stream including bilateral middle and lateral occipital areas during both local and global conditions. In the group comparison, subjects with ASD showed greater right lateral occipital activation than TD subjects in the global condition. In a recent meta-analysis including evaluation of “object” processing using fMRI ALE analysis, Samson and colleagues demonstrated increased activation in occipital areas in ASD, consistent with findings in our study. These findings also correspond to those in previous studies examining only local-level attention using the EFT (Ring et al., 1999; Lee et al., 2007; Manjaly et al., 2007).
Both the lateral-occipital ROI and the occipito-parietal ROI exhibited significant lateralization effects. The lateral-occipital ROI was left lateralized and the occipito-parietal ROI was right lateralized. Only control subjects exhibited changes in lateralization due to attention focus during the task, showing increases in lateralization in the global condition for both ROI. For the occipito-parietal ROI, this increase in right lateralization during attention to global form is consistent with the previous work of Martinez et al. (1997). Increased left-lateralization of lateral-occipital regions was not observed in the Martinez et al. (1997) study, however. For TD subjects, an asymmetry for global processing emerges at the latter site (occipito-temporal) as would be predicted. This emergence of asymmetry was not found for subjects with ASD. Diminished lateralization has recently been demonstrated as a finding frequently found in fMRI studies of ASD (Philip et al., 2012; Samson et al., 2012).
4.2 Executive Function/Cognitive Control/Attention
Both groups showed activation of attention and cognitive control networks including bilateral dorsolateral prefrontal cortex; bilateral frontal operculum; and superior parietal areas, which corresponds with findings in previous studies (Ring et al., 1999; Lee et al., 2007; Manjaly et al., 2007; Malisza et al., 2010). The ASD group showed increased activation of right prefrontal cortex compared to the TD group in the local condition compared with the control condition. Right prefrontal cortex has been associated with nonverbal working memory (Smith and Jonides, 1999) and with visual memory, specifically memory related to non-nameable objects similar to those used in our study (Buckner et al., 1999). In ASD, studies have shown increased right-sided prefrontal cortex activation in tasks involving visual memory (Koshino et al., 2005). Thus, in our study, the finding of increased right-sided prefrontal cortex activation during the local condition is consistent with the broader literature.
Given that the demand characteristics of the EFT involve locating embedded features that have no meaningful relationship to the global form, it makes sense that reduced top down processing and enhanced bottom up processing would yield superior performance. Indeed, this argument has been advanced for superior performance in visual search (Joseph et al., 2009). In a behavioral visual search paradigm, Joseph and colleagues performed a number of task manipulations in order to eliminate possible arguments favoring top down control (e.g., superior deployment of attention), and concluded that superior performance was associated with enhanced perception of featural information.
Of course, visual perception tasks involving hierarchical designs differ from the EFT in important ways. In the present paradigm, participants were directed to attend to either the global- or local-level in alternate blocks. Thus, the task does not involve locating a local element within a global context. Rather, successful performance requires the deployment of attention to one level or the other and the resistance of conflict from shapes at the irrelevant level—an attentional demand not clearly present in visual search or in the EFT. Extensive evidence has demonstrated that typically developing individuals experience greater global attention (Kimchi, 1992). However recent evidence supports the existence of local bias among some individuals within the normal population. Billington et al. (2008) investigated the relationship between “systemizing”—a characteristic of the cognitive phenotype of autism — in typically developing individuals. During a hierarchical Navon type task, subjects were scanned using fMRI in a global and local block (Billington et al., 2008). They examined whether autistic symptomatology in a typically developing sample relates to neural mechanisms of global-local processing. As in ASD, systemizing in normally developing individuals was correlated with relatively greater attention to local level information and, importantly, among high systemizers, conflict was associated with relatively stronger activation of lateral prefrontal cortex—a pattern that has been associated with maintaining attentional set, particularly under conditions involving interference (Banich et al., 2000; Weissman et al., 2002; Fan et al., 2003)—including that in our study.
4.3 Medial Prefrontal Cortex
Medial prefrontal cortex (MPFC) is a key hub of the default mode network (DMN) (Buckner et al., 2008). This structure—part of a baseline network of structures—is thought to be activated in the mind’s resting state, and deactivated during task-related functional activity (Raichle and Snyder, 2007). In numerous studies of healthy control subjects, MPFC is consistently deactivated during task positive processing in fMRI and positron emission topography (PET) studies (Buckner et al., 2008). In the current study, the group with ASD exhibited less deactivation of MPFC compared with TD subjects in the globally directed attention condition, in contrast to the locally directed condition. This finding may imply less attention devoted to global-level condition than local condition in the ASD group. Failure to suppress task-related activation of medial prefrontal cortex has been linked to distractibility in attention deficit hyperactivity disorder (ADHD) (Mason et al., 2007; Fassbender et al., 2009). Because ADHD and autism are highly comorbid (Simonoff et al., 2008), this may indicate a particular difficulty with distraction during the global condition. In this view, difficulty modulating between task negative DMN and task positive attention during the global condition in subjects with ASD may be related to the impairments in global feature processing (Assaf et al., 2001).
4.4 Conclusions and Limitations
Given the wide range of tasks and natural settings in which atypical global and local processing have been observed, it seems reasonable to speculate that observations of WCC across a wide range of different cognitive domains and tasks may reflect a number of non-mutually exclusive mechanisms both at the cognitive level (e.g., increased local attention, reduced global attention) and the neural level (e.g., increased bottom up processing, decreased top down processing) (Happé and Booth, 2008). Our fMRI findings of increased activation in early visual areas in ASD are consistent with prior studies of local level processing (Ring et al., 1999; Manjaly et al., 2007; Malisza et al., 2010).
We acknowledge the lack of strong normative data available for this specific task in both neurotypical persons and in persons with ASD as a limitation. Hierarchical stimuli consisting of letters and shapes (i.e., nameable objects) have wider usage in global-local studies in normative developmental and autism studies. Such studies in ASD, however, are also limited in that there are well replicated deficits in verbal abilities in the spectrum, including deficits in phonological processing that may affect nameable Navon-stimulus perception (reviewed in Boucher, 2012). Non-letter object naming performance in autism may be more complicated still, depending on the type of non-letter objects employed. Both deficits and enhancements in processing stimuli such as faces, simple shapes (triangles, squares, etc.), complex nameable shapes (houses, cars, etc.) have been reported in previous research reports. Walenski et al. (2008) reported that picture naming was faster in ASD to pictures with low frequency word associations, but no different than controls when pictures were associated with higher frequency words, suggesting that word frequency effects that are commonly observed in healthy individuals may be heightened in ASD. Given the added cognitive dimensions and evidence for strengths and weaknesses in autism in those very dimensions, it seems prudent to employ stimuli that do not engage automatic lexical/semantic processes. Future studies could benefit from directly contrasting nameable to non-nameable stimuli in the context of local/global perceptual ability in ASD.
Acknowledgments
This work was supported by grants from the Public Health Service (R01 MH082820) and Autism Speaks. We gratefully thank all participants who took part in our study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 2000. text revision. [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, O’Boyle JG, Schultz RT, Pearlson GD. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2011;53:247–256. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang Z, Barad V, Gullett D, Shah C, Brown C. Prefrontal regions play a predominant role in imposing an attentional ‘set’: Evidence from fmri. Cognitive Brain Research. 2000;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Hammer J. Parents of children with asperger syndrome: What is the cognitive phenotype? Journal of Cognitive Neuroscience. 1997;9:548–554. doi: 10.1162/jocn.1997.9.4.548. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Thomas C, Humphreys K. Seeing it differently: Visual processing in autism. Trends Cognitive Science. 2006;10:258–264. doi: 10.1016/j.tics.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Billington J, Baron-Cohen S, Bor D. Systemizing influences attentional processes during the navon task: An fmri study. Neuropsychologia. 2008;46:511–520. doi: 10.1016/j.neuropsychologia.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Boucher J, Mayes A, Bigham S. Memory in autistic spectrum disorder. Psychological Bulletin. 2012;138:458–496. doi: 10.1037/a0026869. [DOI] [PubMed] [Google Scholar]
- Brian JA, Bryson SE. Disembedding performance and recognition memory in autism/pdd. Journal of Child Psychology and Psychiatry. 1996;37:865–872. doi: 10.1111/j.1469-7610.1996.tb01482.x. [DOI] [PubMed] [Google Scholar]
- Briskman J, Happe F, Frith U. Exploring the cognitive phenotype of autism: Weak “central coherence” in parents and siblings of children with autism: Real life skills and preferences. Journal of Child Psychology and Psychiatry. 2001;42:309–316. [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Kelley WM, Petersen SE. Frontal cortex contributes to human memory formation. Nature Neuroscience. 1999;2:311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. NeuroImage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. A lack of default network suppression is linked to increased distractibility in adhd. Brain Research. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Where in the brain does visual attention select the forest and the trees? Nature. 1996;382:626–628. doi: 10.1038/382626a0. [DOI] [PubMed] [Google Scholar]
- Frith U. Autism: Explaining the Enigma. Wiley; Malden: 2003. [Google Scholar]
- Happé F, Frith U. The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2006;36:5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Happé FGE, Booth RDL. The power of the positive: Revisiting weak coherence in autism spectrum disorders. The Quarterly Journal of Experimental Psychology. 2008;61:50–63. doi: 10.1080/17470210701508731. [DOI] [PubMed] [Google Scholar]
- Jolliffe T, Baron-Cohen S. Are people with autism and asperger syndrome faster than normal on the embedded figures test? Journal of Child Psychology and Psychiatry. 1997;38:527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Keehn B, Connolly C, Wolfe JM, Horowitz TS. Why is visual search superior in autism spectrum disorder? Developmental Science. 2009;12:1083–1096. doi: 10.1111/j.1467-7687.2009.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi R. Primacy of wholistic processing and global/local paradigm: A critical review. Psychological Bulletin. 1992;112:24–38. doi: 10.1037/0033-2909.112.1.24. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fmri working memory task in high-functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Lee PS, Foss-Feig J, Henderson JG, Kenworthy LE, Gilotty L, Gaillard WD, Vaidya CJ. Atypical neural substrates of embedded figures task performance in children with autism spectrum disorder. Neuroimage. 2007;38:184–193. doi: 10.1016/j.neuroimage.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cherkassky VL, Minshew NJ, Just MA. Autonomy of lower-level perception from global processing in autism: Evidence from brain activation and functional connectivity. Neuropsychologia. 2011;49:2105–2111. doi: 10.1016/j.neuropsychologia.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S, Marshall JC, Ritzl A, Weiss PH, Pietrzyk U, Shah NJ, Zilles K, Fink GR. A functional magnetic resonance imaging study of local/global processing with stimulus presentation in the peripheral visual hemifields. Neuroscience. 2004;124:113–120. doi: 10.1016/j.neuroscience.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Clancy C, Shiloff D, Foreman D, Holden J, Jones C, Paulson K, Summers R, Yu CT, Chudley AE. Functional evaluation of hidden figures object analysis in children with autistic disorder. Journal of Autism and Developmental Disorders. 2010;41:13–22. doi: 10.1007/s10803-010-1013-z. [DOI] [PubMed] [Google Scholar]
- Manjaly ZM, Bruning N, Neufang S, Stephan KE, Brieber S, Marshall JC, Kamp-Becker I, Remschmidt H, Herpertz-Dahlmann B, Konrad K, Fink GR. Neurophysiological correlates of relatively enhanced local visual search in autistic adolescents. Neuroimage. 2007;35:283–291. doi: 10.1016/j.neuroimage.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Moses P, Frank L, Buxton R, Wong E, Stiles J. Hemispheric asymmetries in global and local processing: Evidence from fmri. Neuroreport. 1997;8:1685–1689. doi: 10.1097/00001756-199705060-00025. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Navon D. Forest before trees: The precedence of global features in visual perception. Cognitive Psychology. 1977;9:353–383. [Google Scholar]
- O’Riordan MA, Plaisted KC, Driver J, Baron Cohen S. Superior visual search in autism. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:719–730. doi: 10.1037//0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- Philip RC, Dauvermann MR, Whalley HC, Baynham K, Lawrie SM, Stanfield AC. A systematic review and meta-analysis of the fmri investigation of autism spectrum disorders. Neuroscience and Biobehavioral Reviews. 2012;36:901–942. doi: 10.1016/j.neubiorev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Plaisted K, O’Riordan M, Baron-Cohen S. Enhanced visual search for a conjunctive target in autism: A research note. Journal of Child Psychology and Psychiatry. 1998;39:777–783. [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1089. [DOI] [PubMed] [Google Scholar]
- Ring HA, Baron-Cohen S, Wheelwright S, Williams SC, Brammer M, Andrew C, Bullmore ET. Cerebral correlates of preserved cognitive skills in autism: A functional mri study of embedded figures task performance. Brain. 1999;122:1305–1315. doi: 10.1093/brain/122.7.1305. [DOI] [PubMed] [Google Scholar]
- Samson F, Mottron L, Soulieres I, Zeffiro TA. Enhanced visual functioning in autism: An ALE meta-analysis. Human Brain Mapping. 2012;33:1553–1581. doi: 10.1002/hbm.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Frith U. An islet of ability in autistic children: A research note. Journal of Child Psychology and Psychiatry. 1983;24:613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Soulieres I, Dawson M, Samson F, Barbeau EB, Sahyoun CP, Strangman GE, Zeffiro TA, Mottron L. Enhanced visual processing contributes to matrix reasoning in autism. Human Brain Mapping. 2009;30:4082–4107. doi: 10.1002/hbm.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenski M, Mostofsky SH, Gidley-Larson JC, Ullman MT. Brief report: Enhanced picture naming in autism. Journal of Autism and Developmental Disorders. 2008;38:1395–1399. doi: 10.1007/s10803-007-0513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Mottron L, Peng D, Berthiaume C, Dawson M. Local bias and local-to-global interference without global deficit: A robust finding in autism under various conditions of attention, exposure time, and visual angle. Cognitive Neuropsychology. 2007;24:550–574. doi: 10.1080/13546800701417096. [DOI] [PubMed] [Google Scholar]
- Weigelt S, Koldewyn K, Kanwisher N. Face identity recognition in autism spectrum disorders: A review of behavioral studies. Neuroscience and Biobehavioral Reviews. 2012;36:1060–1084. doi: 10.1016/j.neubiorev.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Weissman D, Woldorff M. Hemispheric asymmetries for different components of global/local attention occur in distinct temporo-parietal loci. Cerebral Cortex. 2005;15:870–876. doi: 10.1093/cercor/bhh187. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Mangun GR, Woldorff MG. A role for top-down attentional orienting during interference between global and local aspects of hierarchical stimuli. NeuroImage. 2002;17:1266–1276. doi: 10.1006/nimg.2002.1284. [DOI] [PubMed] [Google Scholar]
- White S, O’Reilly H, Frith U. Big heads, small details and autism. Neuropsychologia. 2009;47:1274–1281. doi: 10.1016/j.neuropsychologia.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Wilke M, Lidzba K. Li-tool: A new toolbox to assess lateralization in functional mr-data. Journal of Neuroscience Methods. 2007;163:128–136. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]