Abstract
Women are at a twofold risk of developing late onset Alzheimer’s disease (LOAD) (onset ≥65 years of age) compared to men. During perimenopausal years, women undergo hormonal changes that are accompanied by metabolic, cardiovascular and inflammatory changes. These all together have been suggested as risk factors for LOAD. However, not all perimenopausal women develop AD; we hypothesize that certain genetic factors might underlie the increased susceptibility for developing AD in postmenopausal women. We investigated the androgen receptor (AR) gene in a clinical cohort of male and female AD patients and normal controls by sequencing all coding exons and evaluating the length and distribution of the CAG repeat in exon 1. We could not establish a correlation between the repeat length, gender and the disease status, nor did we identify possible pathogenic variants. AR is located on the X chromosome; in order to assess its role in AD, X-inactivation patterns will need to be studied to directly correlate the actual expressed repeat length to a possible sex specific phenotypic effect.
Keywords: Alzheimer’s disease, androgen receptor, menopause, X-inactivation, epigenetics
Introduction
Age is a major risk factor for Alzheimer’s disease (AD) (Rocca, Amaducci, & Schoenberg, 1986). Gender differences (Henderson, 1997) as well as hormonal changes (Pike, Rosario, & Nguyen, 2006; E. R. Rosario & Pike, 2008) have also been reported as possible risk factors leading to the development of AD.
Women are at a twofold risk of developing late onset Alzheimer’s disease (LOAD) (onset ≥65 years of age) compared to men (Alzheimer’s, 2012). Yet, while it is known that women have a longer life expectancy this difference in risk cannot be solely attributed to survival rate (Miniño & Murphy, 2011; Vina & Lloret, 2010).
Menopause in women is characterized by dramatic hormonal changes, primarily, an increase in the androgen/estrogen ratio due to the sudden drop in ovarian hormones, i.e. estrogen and progesterone. This near complete depletion of primary sex hormones is accompanied by metabolic, cardiovascular and inflammatory changes in women. Aging men also experience a decrease in their primary sex hormones, although in a much more gradual manner than women (Morley et al., 1997).
Estrogen and progesterone are synthesized in ovaries, testis and the adrenal cortex and play a neuroprotective role in the brain health of both women and men; it has been shown that estrogens may prevent or reduce the formation of amyloid deposits in the brain (Chang, Kwan, & Timiras, 1997; Singh & Su, 2012; Thomas & Rhodin, 2000). As it has been suggested that hormonal changes may influence increased susceptibility for developing AD (Pike et al., 2006; E. R. Rosario & Pike, 2008), one may argue that imbalanced levels of estrogens might strongly contribute to the higher incidence of AD in women. However, estrogen and progesterone depletion alone does not appear to exert a uniform effect across all postmenopausal women since not all women over the age of 65 years are affected by AD. Then, what is the cause of sex divergence/bias in the pathogenesis of AD? Why are not all perimenopausal women, who undergo hormonal changes, affected in a similar way cognitively?
We hypothesized that there must be other factors, specifically genetic differences, that confer susceptibility and increase the risk of developing AD in women.
In our ongoing longitudinal study we developed a targeted multiplex genotyping assay using the illumina Veracode technology to identify gender specific genetic entities that could be involved in the differential pathogenesis of AD in perimenopausal women. Statistical analysis revealed the Androgen Receptor gene (AR) to be associated with the AD status in women in our cohort (unpublished data).
Human androgen receptor gene (hAR, MIM313700) is a steroid hormone ligand-activated transcription factor located on the X chromosome. Exon 1 of AR encodes for a highly polymorphic polyglutamine repeat stretch located in the N-terminal domain of the AR protein. The range of the CAG repeat length in the unaffected population is reported to be 8–33 units with the mean of 21, while in individuals with disorders such as spinal bulbar muscular atrophy or Kennedy disease the repeat countreaches 44 triplets (Greenland, Beilin, Castro, Varghese, & Zajac, 2004; Rajender, Singh, & Thangaraj, 2007). It has been suggested that the activity of AR is inversely correlated to the length of CAG repeat (Chamberlain, Driver, & Miesfeld, 1994). Recent studies have shown that CAG repeat in AR modulates body fat and concentration of leptin and insulin in men implying to the role of androgen receptor in cardiovascular diseases (Zitzmann, Gromoll, von Eckardstein, & Nieschlag, 2003). However, similar results have not been found in women (Rexrode et al., 2008). Reduced AR CAG repeat has also been associated with violent criminal behavior in men (Rajender et al., 2008). The association of AR CAG repeat with the serum androgen level was previously examined in two independent studies: in a Swedish cohort study, premenopausal women with lower CAG repeat had higher serum androgen levels and in the other study where the sum of both alleles was counted (biallelic CAG count), postmenopausal women with lower repeat numbers also had higher serum androgen levels (Brum et al., 2005; Westberg et al., 2001).
In this study we sought to examine the role of AR gene in the pathogenesis of AD in women by screening the CAG repeat in exon 1 and sequencing exons 2–8 in a cohort of AD patients and neurologically normal controls from the Texas Alzheimer’s Research and Care Consortium (TARCC).
Material and Methods
Cohort
The cohort included 696 individuals subdivided into 241 female AD patients, 164 male AD patients, 198 female neurologically normal controls and 93 male neurologically normal controls enrolled by TARCC; in addition, 131 DNA samples of neurologically normal control subjects (n=68 females and n=63 males), obtained from Coriell Institute [NDP096 (http://ccr.coriell.org/Sections/Search/Panel_Detail.aspx?Ref=NDPT096&PgId=202) and NDP098 (http://ccr.coriell.org/Sections/Search/Panel_Detail.aspx?Ref=NDPT098&PgId=202)] were screened. The methodology for recruitment has been described in detail elsewhere (Waring SC, 2008). TARCC participants underwent a standardized annual examination at the respective sites that included a medical evaluation, neuropsychological testing, and interview. Each participant also provided blood for storage in the TARCC biobank. Diagnosis of AD status was based on National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria and control subjects performed within normal limits on psychometric assessment. Institutional Review Board approval was obtained at each site and written informed consent was obtained for all participants.
DNA sequencing and CAG repeat genotyping
The polymerase chain reaction (PCR) primers for AR exons 2–8 were designed using Primer 3 version 0.4.0 (frodo.wi.mit.edu/primer3). The primer sequences are available upon request. The PCR fragments were amplified using Roche FastStart PCR Mastermix (Roche Diagnostic, Corp., Indianapolis, IN, USA). Sequencing of purified PCR amplicons was carried out from one direction using the Big Dye Terminator kit (ABI, Foster City, CA, USA) using the manufacturer’s recommended protocol, run on a 3730 DNA analyzer (ABI) and analyzed using the Sequencher 4.9 software (Gene Codes Corporation, Ann Arbor, MI, USA). The effect of each SNP or novel variant in the protein structure was examined using PolyPhen-2 software (genetics.bwh.harvard.edu/pph2). The amplimer containing CAG repeat in the exon 1 was amplified as described in Ferlin et al. 2004. The amplimers were sequenced using the forward PCR primer. The sequences were analyzed using the Sequencher 4.9 software (Gene Codes Corporation, Ann Arbor, MI, USA).
Allelic distribution profile and statistical analysis
We divided our cohort into 4 groups consisting of: female AD patients (F-AD), female normal controls (F-NC), male AD patients (M-AD) and male normal controls (M-NC). In this study we compared the CAG allele distribution profile of women who have two alleles and men who have one allele as follows: female patients vs. female controls within one group and male patients vs. male controls within another group. We, therefore, first analyzed the allelic distribution of female and male participants separately. Subsequently we analyzed five modes of allelic presentations: 1- comparing the male alleles: AD vs. NC (Figure 1); 2- comparing the average allelic length for females: AD vs. NC (Figure 2); 3- comparing the long allelic length for females: AD vs. NC; 4-comparing the short allelic length for females: AD vs. NC; 5- comparing the long and short allelic length for female AD and NC (Figure 3). Modes 3, 4, and 5 were combined into a two-factor ANOVA with one between factor (diagnosis) and one within factor (allelic length). For modes 1 and 2, independent samples t-tests were performed. The assumptions for all statistical tests were satisfied.
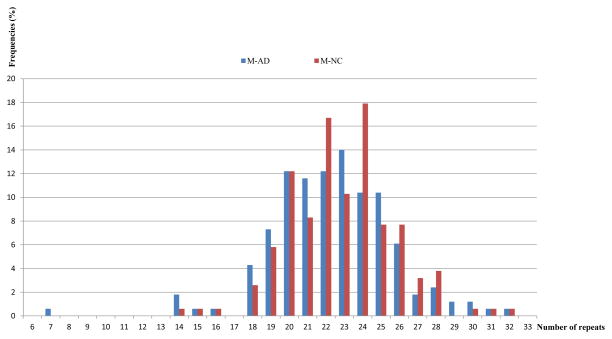
Figure 1.
The bar graph shows the frequencies of each allele of male patients compared to each allele of male controls for the CAG repeat polymorphism.
Abbreviations: M-AD = male Alzheimer’s disease patient; M-NC = male normal control.
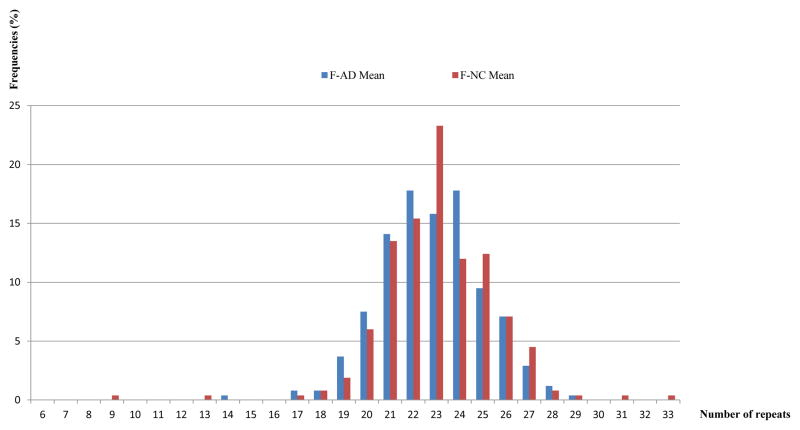
Figure 2.
The bar graph shows the frequencies of the mean values of two alleles of female patients compared to the mean values of two alleles of female controls for the CAG repeat polymorphism.
Abbreviations: F-AD = female Alzheimer’s disease patient; F-NC = female normal control.
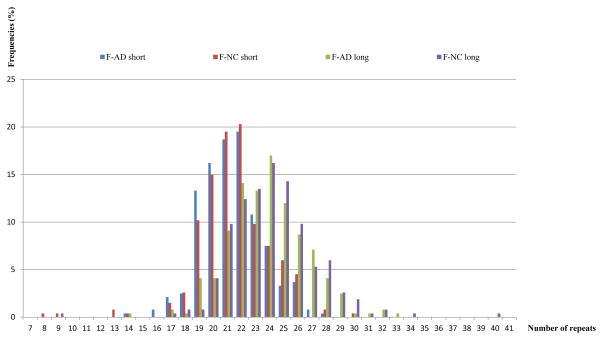
Figure 3.
The bar graph shows the frequencies of both (short and long) alleles of female patients compared to both (short and long) alleles of female controls for the CAG repeat polymorphism.
Abbreviations: F-AD = female Alzheimer’s disease patient; F-NC = female normal control.
IBM SPSS Statistics V20 was used to analyses these data. Significance was set to p < 0.05 and all tests were two tailed.
Results
CAG repeat allelic distribution statistical analysis
Androgen receptor gene AR CAG repeats in our cohort ranged from 7 to 40. These numbers exceed the highest range of CAG repeats reported in the literature, 8–31, in the normal population, while the reported disease ranges were at least 38 repeats for Kennedy disease, and 43–65 repeats for X-linked spinal and bulbar muscular atrophy (Beitel, Scanlon, Gottlieb, & Trifiro, 2005; Choong, Kemppainen, Zhou, & Wilson, 1996; Mariani et al., 2012). The range of CAG allelic distribution, mean, the median, standard deviation, percentile and number of alleles in each group of our cohort are summarized in Tables 1a–c.
Table 1a.
Descriptive Statistics – F-AD
| Allele | N | Mean | Standard Deviation | Minimum | Maximum | Percentiles | ||
|---|---|---|---|---|---|---|---|---|
| 25th | 50th (Median) | 75th | ||||||
| Short | 241 | 21.38 | 2.19 | 14 | 28 | 20 | 21 | 23 |
| Long | 241 | 23.77 | 2.79 | 14 | 33 | 22 | 24 | 25 |
Table 1c.
Descriptive Statistics – M-AD and M-NC
| Allele | N | Mean | Standard Deviation | Minimum | Maximum | Percentiles | ||
|---|---|---|---|---|---|---|---|---|
| 25th | 50th (Median) | 75th | ||||||
| M-AD | 164 | 22.37 | 3.35 | 7 | 32 | 20 | 22 | 24 |
| M-NC | 156 | 22.83 | 2.89 | 14 | 32 | 21 | 23 | 24 |
As it can be seen in Table 1a, the number of AR CAG repeats in female AD ranged from 14 to 33 with a median length of 21 for the short allele and 24 for the long allele. The number of CAG repeats among the female NC group (Table 1b) ranges from 8 to 40 with the median of 21 for the short allele and 24 for the long allele. The male group (Table 1c) shows allele sizes that range from 7 to 32 in the AD group and 14 to 32 in the NC group.
Table 1b.
Descriptive Statistics – F-NC
| Allele | N | Mean | Standard Deviation | Minimum | Maximum | Percentiles | ||
|---|---|---|---|---|---|---|---|---|
| 25th | 50th (Median) | 75th | ||||||
| Short | 266 | 21.50 | 2.59 | 8 | 30 | 20 | 21 | 23 |
| Long | 266 | 24.16 | 3.00 | 9 | 40 | 22 | 24 | 26 |
Table 2 provides the comparison of the allelic lengths for AD vs. NC males (Figure 1). The difference between normal controls and AD patients was non-significant (t=1.30, df=318, p=0.195).
Table 2.
Comparing the short allelic length for males: AD vs. NC
| DX Group | N | Mean | Standard Deviation | |
|---|---|---|---|---|
| Short Allele | NC | 156 | 22.83 | 2.89 |
| AD | 164 | 22.37 | 3.35 | |
Figure 2 presents the mean value of two alleles of female patients compared to the mean value of two alleles of female controls, in order to have a single presentation for each individual and to take into consideration that X chromosomes in women are subjected to random X-inactivation; as we did not have X-inactivation data on each person, instead, we calculated and considered the mean value. Additionally, in the study of Alzheimer’s disease, the conventional X-inactivation analysis assay, performed with DNA extracted from peripheral blood would not be informative as this is a disease of the central nervous system (CNS). To understand the X-inactivation or in other words, the methylation pattern of the DNA, one would need the areas of the brain which are affected by Alzheimer’s disease, for example the hippocampus, to determine the role of AR CAG repeat expression in the pathogenesis of the disease. As our cohort is clinical and our subjects are still alive, we could not investigate that aspect.
When comparing NC and AD groups using the average of the long and short alleles in females (Figure 2), the average allelic length was not significant (t=1.26, df=505, p=0.208) (Table 3).
Table 3.
Comparing the average allelic length for females: AD vs. NC
| Female Dx Group | N | Mean | Std. Deviation | |
|---|---|---|---|---|
| Average | NC | 266 | 22.83 | 2.41 |
| AD | 241 | 22.57 | 2.17 | |
To understand the paired allelic sizes in female patients and controls, we performed an ANOVA comparing allelic length (within factor) and diagnostic groups (between factor) for females (Tables 4a–c;). Table 4a shows that the comparison between the average short vs. long allelic length in females was significant (F=446.89, df=1/505, p<0.0001). This, however, has no biological significance as we have no data on which allele is inactivated and which is expressed in each patient. Table 4b presents the non-significant result of the average allelic length for females in the AD vs. NC groups (F=1.59, df=1/505, p=0.208). Table 4c presents the results of the interaction comparing the long and short allelic length for female AD and NC (Figure 3). The interaction of allelic length (long vs. short) and disease status (AD vs. NC) was not significant (F=1.23, df=1/505, p=0.269).
Table 4a.
Within (repeated) Factor: Long vs. Short Allele
| Allele Length | Mean | Standard Deviation | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Short | 21.44 | 2.41 | 21.23 | 21.65 |
| Long | 23.96 | 2.90 | 23.71 | 24.22 |
Table 4c.
Interaction: allelic length (short vs. long) by disease (AD vs. NC)
| DX Group | Length | Mean | Standard Deviation | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| NC | Short | 21.50 | 2.41 | 21.21 | 21.79 |
| Long | 24.16 | 2.90 | 23.81 | 24.51 | |
| AD | Short | 21.38 | 2.41 | 21.07 | 21.68 |
| Long | 23.77 | 2.90 | 23.40 | 24.14 | |
Table 4b.
Between Factor: AD vs. NC
| DX Group | Mean | Standard Deviation | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| NC | 22.83 | 2.30 | 22.55 | 23.11 |
| AD | 22.57 | 2.30 | 22.28 | 22.86 |
Sequence analysis
Sequencing of exons 2–8 in all the TARCC participants resulted in the identification of 3 variants of which one (IVS1-22 C>T) was novel and the other two had previously been reported in the SNP database and in Gottlieb et al. 2012 (Table 5) (Gottlieb, Beitel, Nadarajah, Paliouras, & Trifiro, 2012). The synonymous variant K558K was identified in two AD patient with no report on pathogenicity. The missense variant S598G, which was analyzed through PolyPhen2 software (Adzhubei et al., 2010) and was predicted, in silico, to be probably pathogenic, was detected in one AD patient and in one normal control in our cohort. This variant had been already isolated in a patient with Partial Androgen Insensitivity Syndrome (PAIS) and one normal control (Gottlieb et al., 2012). Interestingly, all variants were isolated in female individuals.
Table 5.
| DNA change | Exon | Predicted protein | Phenotype in current study | Phenotype reported in Gottlieb et al. | |
|---|---|---|---|---|---|
| 1 | IVS1-22 C>T | Intron 1 | - | Two female AD patients | - |
| 2 | c.1674 G>A | 2 | K558K | Two female AD patients | - |
| 3 | c. 1792 A>G | 3 | S598G | One female AD patient and one female normal control | PAIS and normal |
PAIS: Partial Androgen Insensitivity Syndrome
Discussion
The aim of this study was to examine the association of AR gene with the Alzheimer’s disease status in our cohort consisting of female and male AD patients and neurologically normal controls. The sequencing of AR exons 2–8 did not result in identification of any variants that could be associated with the disease status. Also, we could not establish significant correlation between the CAG repeat length located in AR exon 1 and the disease status or gender.
The only study of AR CAG repeats in AD, arbitrarily, divided the alleles into two groups, considering the short alleles in the range of ≤20 and the long allele in the range of >20 and reported an association of shorter allele with male AD patients (Lehmann et al., 2003). In our study we did not set an a priori threshold, instead, we interrogated the repeat distribution in a hypothesis free manner. CAG repeat length of androgen receptor gene is a functional polymorphism within the transcription activating domain of AR protein that affects the potential of AR to bind to the promoters of target genes. Studies have shown that plasma testosterone levels are higher in postmenopausal women with shorter CAG tracts (Brum et al., 2005). At this point, we could not establish a direct link between genetic variability in AR gene and sex specific phenotypic effects in our cohort.
In the study of LOAD, with specific focus on the sub-population of female patients, it is difficult to define a simple genotype-phenotype effect. Genetics of LOAD has proven to be complex and is thought to be the result of a multitude of genetic factors with small effects (Cruchaga et al., 2012; Singleton & Hardy, 2011; Tanzi, 2012). The recent genome wide association studies (GWAS) and whole exome/genome sequencing efforts have identified many factors which seem to exert small but definite incremental effects on the pathogenesis of LOAD (Bettens, Sleegers, & Van Broeckhoven, 2013; Guerreiro et al., 2013). In order to identify these factors, a study requires subject numbers in the magnitude of few thousands, as ascertained in GWAS. Our study and focus on AR however, was based on the results of a preliminary association study in our cohort (unpublished data). The association of the AR gene implies to the involvement of AR in the pathogenesis of AD. The genetic association could be the result of: 1- variations in the gene, which can be identified through Sanger sequencing. Point mutations in AR have been previously linked to PAIS, but we did not identify any pathogenic variations associated with the disease; 2- the repeat length variations such as AR CAG repeat or hexanucleotide repeat in C9ORF72 (Majounie et al., 2012) could be the reason for the genetic association. Even if not directly the cause of the disease, they could be modifiers of the phenotype or confer susceptibility to the carriers of such variants; 3- the expression of AR gene as a transcription factor may affect expression of downstream genes without any detectable variability in structure of AR gene.
However, the most plausible hypothetical mechanism which could explain higher prevalence of AD in women compared to men could be X-inactivation and mosaicism in women. It is well known that in the early stages of embryogenesis in females, one of the X chromosomes is inactivated, creating colonies of cells with either maternal or paternal active X chromosome. If the majority of CNS cells in a female individual express an X chromosome harboring mainly neuroprotective genes, in the areas susceptible to AD, the outcome would be healthy brain aging compared to those who harbor mainly risk factors on their active X chromosome. In these women, although both X chromosomes are present, the X-inactivation selection determines the mono-allelic expression of the genes in cells.
Female X-chromosome mosaicism has been suggested to be a survival mechanism (Aviv, 2007). If skewed gene inactivation leads to the expression of some X-chromosomal genes, disadvantageous for cognition, it might be one of the mechanisms underlying the higher prevalence of AD in women, Also, women who are not affected at the perimenopausal years may have a majority of cells in the CNS that express both copies of protective alleles. If X-chromosome carries multiple risk factors with variable degrees of penetrance and pathogenicity, the potential inactivation, partial or complete, has an immense effect on the expression of these variants and the possible pathogenesis of late onset Alzheimer’s disease. This mechanism can potentially explain the variability of phenotype in families with LOAD.
An alternative approach to study the involvement of AR gene in the gender specific pathogenesis of LOAD in women is to consider non-genetic or epigenetic mechanisms of action. Evidence suggests that aging may change the X-inactivation pattern in women (Cotton et al. 2011). This may impact the X-inactivation pattern of many genes on the X chromosome, including AR, with the possible consequence of changes in the androgen sensitivity. X-inactivation may explain sex differences in longevity, aging processes and prevalence of Alzheimer’s disease in the female population.
Comorbidities and epigenetics
The end of reproductive years at menopause coincides with comorbidities such as metabolic, cardiovascular and inflammatory changes. These conditions could represent a consequence of estrogen/progesterone depletion and androgen/testosterone increase in postmenopausal women.
Sex differences in longevity and disease have been attributed to hormones, genetic differences and behavior. The higher prevalence and incidence of diseases such as Alzheimer’s disease, systemic lupus erythematosus and autoimmune disease in women is a well-documented fact that cannot be only attributed to the environmental, behavioral and cultural factors (W. H. Brooks, 2010; Weckerle & Niewold, 2011). The genes that may grant health and resilience to women at the premenopausal stages of life, as they might be favorable in the context of coping with torrent of oxidative stress and other types of insults caused by cardiovascular, inflammatory and metabolic changes throughout life, may also confer disadvantage such as loss of cognitive ability and neurodegeneration in later life.
Female physiology is a life-long interplay of anti- and pro-inflammatory processes which are in balance throughout the normal production of female hormones until the end of productive years. Female hormones and their receptors are involved in many signaling and regulatory pathways. Estrogen receptors α and β are transcription factors that are activated through binding to estrogen and initiate transcription of downstream genes. It is conceivable that substantial changes in estrogen levels can influence expression of many genes, leading to diseases such as Alzheimer’s and autoimmune disease (Candore et al., 2010; Long, He, Shen, & Li, 2012; Simpkins, Singh, Brock, & Etgen, 2012). Androgens also play a neuroprotective role against Alzheimer’s disease, whereas, depletion thereof can lead to AD pathology in the brain (Azcoitia et al., 2001). Testosterone-mediated neuroprotective anti-apoptotic pathway, triggered by a decrease in serum androgen, is initiated by AR activation mechanism (B. P. Brooks et al., 1998; Hammond et al., 2001). Studies have shown that circulating levels of testosterone negatively correlate with A-β in the brains of aged men and decreased testosterone is correlated with accumulation of A-β deposits (Gandy & Petanceska, 2001; E.R. Rosario, Chang, Head, Stanczyk, & Pike, 2011).
Furthermore, high serum testosterone levels are associated with high levels of low density lipoprotein (LDL) which is known to be a risk factor for cardiovascular diseases, suggesting a mechanism for higher incidence of heart attack and stroke in men (Weidemann & Hanke, 2002). Female hormones, on the other hand, have a protective effect on the cardiovascular system; they are also neuroprotective and have anti-inflammatory effects on the female body. During menopause women become susceptible to cardiovascular diseases, auto-immune diseases and dementias because of the decrease in estrogens and progesterone and the comparative increase in levels of testosterone/estradiol (Czlonkowska et al., 2006; Mendelsohn & Karas, 1999; Straub, 2007).
The aging process is always accompanied by many comorbidities and onset of diseases of old age. Women endure many diseases as they enter menopause and experience the comorbidities that coincide with termination of reproductive life. As women experience metabolic, inflammatory and cardiovascular changes many common diseases such as dementias, diabetes, metabolic disorder and heart disease are manifested in women. If these comorbidities epidemiologically coincide with the diseases of old age such as dementias or cancer, it is quite possible that hormonal changes and the consequent cardiovascular, inflammatory and metabolic changes act as epigenetic forces on the genome to change the pattern of gene expression. Hence, the cause of the late onset diseases could be found in the epigenetic mechanisms rather than in the sequences of the genome alone. A probable reason for the limited success in the quest for finding the late onset Alzheimer’s disease gene may have been the conventional approach. We may therefore, from now on, look at the epigenetic mechanisms that affect gene expression.
A change in X-inactivation patterns could be among the epigenetic effects of aging, altering even a number of regions that escape inactivation including coding regions and regions of long non-coding RNAs (lnc-RNAs). Through such changes, a female individual not only faces a tremendous imbalance of gene dosage but also a sex bias that can lead to a sex specific phenotypic effect.
Role of AR
The brain is one of the androgen responsive tissues of the body. Given dense localization of androgen receptors in the hippocampus, androgen receptors, together with estrogen receptors, support the notion of a strong role of sex hormones in brain development and neuronal protection, specifically, in the areas affected by Alzheimer’s disease (Kerr, Allore, Beck, & Handa, 1995; Simerly, Chang, Muramatsu, & Swanson, 1990; Tohgi, Utsugisawa, Yamagata, & Yoshimura, 1995). If the AR gene exerts its effect in the pathogenesis of AD through modulating plasma androgen levels, the genetic effect of CAG repeat length could be possibly examined and verified by investigating the X-inactivation patterns in these areas of the brain. Studies have indicated that blood cells X-inactivation pattern, are not in concordance with the pattern of X-inactivation in other tissues (Cotton et al., 2011; Sharp, Robinson, & Jacobs, 2000). Our approach interrogated the number of AR CAG repeats in each patient in a clinical cohort. Our findings, however, did not support this hypothesis. As it was a clinical cohort, we could not verify which X chromosome, harboring a certain CAG repeat (long or short), has been inactivated in the brains of the female subjects. This is the crucial point in determining what allele is associated with the disease status.
In the future, the correlation between the AR repeat length and its expression needs to be verified in the brain samples of male and female AD patients and controls, to identify the role of AR and X chromosome in the pathogenesis of AD in women.
Supplementary Material
Acknowledgments
The authors thank the Investigators from the Texas Alzheimer’s Research Consortium: Baylor College of Medicine: Susan Rountree, Eveleen Darby, Aline Hittle, Aisha Khaleeg; Texas Tech University Health Science Center (TTUHSC): Benjamin Williams, Larry Hill; University of North Texas Health Science Center: Janice Knebl, Lisa Alvarez, Douglas Mains, Thomas Fairchild, James Hall; University of Texas Southwestern Medical Center: Perrie Adams, Roger Rosenberg, Ryan Huebinger, Janet Smith, Mechelle Murray, Tomequa Sears. Authors also thank Abu Minhajuddin and Julia Kozlitina for preliminary association analysis on illumina Veracode genotyping data. The molecular genetic study in Dr. Momeni’s laboratory and this work was supported by a grant from the CH Foundation, Laura W Bush Institute for Women’s Health (LWBIWH), the financial and administrative support from the Office of the Dean and Department of Internal Medicine at TTUHSC. The collection of samples and the corresponding clinical data for this study was made possible by the Texas Alzheimer’s Research and Care Consortium (TARCC) funded by the state of Texas through the Texas Council on Alzheimer’s Disease and Related Disorders.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s, A. 2012 Alzheimer’s Disease Facts and Figures. 2012 http://www.alz.org/downloads/facts_figures_2012.pdf.
- Aviv A. Cardiovascular Diseases, Aging and the Gender Gap in the Human Longevity. J Am Soc Hypertens. 2007;1(3):185–188. doi: 10.1016/j.jash.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM. Brain aromatase is neuroprotective. J Neurobiol. 2001;47(4):318–329. doi: 10.1002/neu.1038. [DOI] [PubMed] [Google Scholar]
- Beitel LK, Scanlon T, Gottlieb B, Trifiro MA. Progress in Spinobulbar muscular atrophy research: insights into neuronal dysfunction caused by the polyglutamine-expanded androgen receptor. Neurotox Res. 2005;7(3):219–230. doi: 10.1007/BF03036451. [DOI] [PubMed] [Google Scholar]
- Bettens K, Sleegers K, Van Broeckhoven C. Genetic insights in Alzheimer’s disease. Lancet Neurol. 2013;12(1):92–104. doi: 10.1016/s1474-4422(12)70259-4. [DOI] [PubMed] [Google Scholar]
- Brooks BP, Merry DE, Paulson HL, Lieberman AP, Kolson DL, Fischbeck KH. A cell culture model for androgen effects in motor neurons. J Neurochem. 1998;70(3):1054–1060. doi: 10.1046/j.1471-4159.1998.70031054.x. [DOI] [PubMed] [Google Scholar]
- Brooks WH. X chromosome inactivation and autoimmunity. Clin Rev Allergy Immunol. 2010;39(1):20–29. doi: 10.1007/s12016-009-8167-5. [DOI] [PubMed] [Google Scholar]
- Brum IS, Spritzer PM, Paris F, Maturana MA, Audran F, Sultan C. Association between androgen receptor gene CAG repeat polymorphism and plasma testosterone levels in postmenopausal women. J Soc Gynecol Investig. 2005;12(2):135–141. doi: 10.1016/j.jsgi.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Candore G, Balistreri CR, Colonna-Romano G, Lio D, Listi F, Vasto S, Caruso C. Gender-related immune-inflammatory factors, age-related diseases, and longevity. Rejuvenation Res. 2010;13(2–3):292–297. doi: 10.1089/rej.2009.0942. [DOI] [PubMed] [Google Scholar]
- Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994;22(15):3181–3186. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D, Kwan J, Timiras PS. Estrogens influence growth, maturation, and amyloid beta-peptide production in neuroblastoma cells and in a beta-APP transfected kidney 293 cell line. Adv Exp Med Biol. 1997;429:261–271. doi: 10.1007/978-1-4757-9551-6_19. [DOI] [PubMed] [Google Scholar]
- Cholerton B, Gleason CE, Baker LD, Asthana S. Estrogen and Alzheimer’s disease: the story so far. Drugs Aging. 2002;19(6):405–427. doi: 10.2165/00002512-200219060-00002. [DOI] [PubMed] [Google Scholar]
- Choong CS, Kemppainen JA, Zhou ZX, Wilson EM. Reduced androgen receptor gene expression with first exon CAG repeat expansion. Mol Endocrinol. 1996;10(12):1527–1535. doi: 10.1210/mend.10.12.8961263. [DOI] [PubMed] [Google Scholar]
- Cotton AM, Lam L, Affleck JG, Wilson IM, Penaherrera MS, McFadden DE, Brown CJ. Chromosome-wide DNA methylation analysis predicts human tissue-specific X inactivation. Hum Genet. 2011;130(2):187–201. doi: 10.1007/s00439-011-1007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruchaga C, Haller G, Chakraverty S, Mayo K, Vallania FL, Mitra RD, Goate AM. Rare variants in APP, PSEN1 and PSEN2 increase risk for AD in late-onset Alzheimer’s disease families. PLoS One. 2012;7(2):e31039. doi: 10.1371/journal.pone.0031039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czlonkowska A, Ciesielska A, Gromadzka G, Kurkowska-Jastrzebska I. Gender differences in neurological disease: role of estrogens and cytokines. Endocrine. 2006;29(2):243–256. doi: 10.1385/endo:29:2:243. [DOI] [PubMed] [Google Scholar]
- Ferlin A, Bartoloni L, Rizzo G, Roverato A, Garolla A, Foresta C. Androgen receptor gene CAG and GGC repeat lengths in idiopathic male infertility. Mol Hum Reprod. 2004;10(6):417–421. doi: 10.1093/molehr/gah054. [DOI] [PubMed] [Google Scholar]
- Gandy S, Petanceska S. Regulation of alzheimer beta-amyloid precursor trafficking and metabolism. Adv Exp Med Biol. 2001;487:85–100. doi: 10.1007/978-1-4615-1249-3_7. [DOI] [PubMed] [Google Scholar]
- Gottlieb B, Beitel LK, Nadarajah A, Paliouras M, Trifiro M. The androgen receptor gene mutations database: 2012 update. Hum Mutat. 2012;33(5):887–894. doi: 10.1002/humu.22046. [DOI] [PubMed] [Google Scholar]
- Greenland KJ, Beilin J, Castro J, Varghese PN, Zajac JD. Polymorphic CAG repeat length in the androgen receptor gene and association with neurodegeneration in a heterozygous female carrier of Kennedy’s disease. J Neurol. 2004;251(1):35–41. doi: 10.1007/s00415-004-0266-x. [DOI] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Hardy J. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368(2):117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77(5):1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Henderson VW. The epidemiology of estrogen replacement therapy and Alzheimer’s disease. Neurology. 1997;48(5 Suppl 7):S27–35. doi: 10.1212/wnl.48.5_suppl_7.27s. [DOI] [PubMed] [Google Scholar]
- Henderson VW. Estrogen-containing hormone therapy and Alzheimer’s disease risk: understanding discrepant inferences from observational and experimental research. Neuroscience. 2006;138(3):1031–1039. doi: 10.1016/j.neuroscience.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for Alzheimer’s disease. Exp Gerontol. 2004;39(11–12):1633–1639. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136(8):3213–3221. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- Lehmann DJ, Butler HT, Warden DR, Combrinck M, King E, Nicoll JA, Smith AD. Association of the androgen receptor CAG repeat polymorphism with Alzheimer’s disease in men. Neurosci Lett. 2003;340(2):87–90. doi: 10.1016/s0304-3940(03)00069-7. [DOI] [PubMed] [Google Scholar]
- Long J, He P, Shen Y, Li R. New evidence of mitochondria dysfunction in the female Alzheimer’s disease brain: deficiency of estrogen receptor-beta. J Alzheimers Dis. 2012;30(3):545–558. doi: 10.3233/jad-2012-120283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majounie E, Renton AE, Mok K, Dopper EG, Waite A, Rollinson S, Traynor BJ. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol. 2012;11(4):323–330. doi: 10.1016/s1474-4422(12)70043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani S, Fiore D, Barbaro G, Basciani S, Saponara M, D’Arcangelo E, Gnessi L. Association of epicardial fat thickness with the severity of obstructive sleep apnea in obese patients. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340(23):1801–1811. doi: 10.1056/nejm199906103402306. [DOI] [PubMed] [Google Scholar]
- Miniño AM, Murphy SL. Death in the United States, 2010. 2011. [PubMed] [Google Scholar]
- Morley JE, Kaiser FE, Perry HM, 3rd, Patrick P, Morley PM, Stauber PM, Garry PJ. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46(4):410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Rosario ER, Nguyen TV. Androgens, aging, and Alzheimer’s disease. Endocrine. 2006;29(2):233–241. doi: 10.1385/endo:29:2:233. [DOI] [PubMed] [Google Scholar]
- Rajender S, Pandu G, Sharma JD, Gandhi KP, Singh L, Thangaraj K. Reduced CAG repeats length in androgen receptor gene is associated with violent criminal behavior. Int J Legal Med. 2008;122(5):367–372. doi: 10.1007/s00414-008-0225-7. [DOI] [PubMed] [Google Scholar]
- Rajender S, Singh L, Thangaraj K. Phenotypic heterogeneity of mutations in androgen receptor gene. Asian J Androl. 2007;9(2):147–179. doi: 10.1111/j.1745-7262.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- Rexrode KM, Ridker PM, Hegener HH, Buring JE, Manson JE, Zee RY. Genetic variation of the androgen receptor and risk of myocardial infarction and ischemic stroke in women. Stroke. 2008;39(5):1590–1592. doi: 10.1161/strokeaha.107.508218. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Amaducci LA, Schoenberg BS. Epidemiology of clinically diagnosed Alzheimer’s disease. Ann Neurol. 1986;19(5):415–424. doi: 10.1002/ana.410190502. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiology of aging. 2011;32(4):604–613. doi: 10.1016/j.neurobiolaging.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Pike CJ. Androgen regulation of beta-amyloid protein and the risk of Alzheimer’s disease. Brain Res Rev. 2008;57(2):444–453. doi: 10.1016/j.brainresrev.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp A, Robinson D, Jacobs P. Age- and tissue-specific variation of X chromosome inactivation ratios in normal women. Hum Genet. 2000;107(4):343–349. doi: 10.1007/s004390000382. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Singh M, Brock C, Etgen AM. Neuroprotection and estrogen receptors. Neuroendocrinology. 2012;96(2):119–130. doi: 10.1159/000338409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Su C. Progesterone, brain-derived neurotrophic factor and neuroprotection. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton A, Hardy J. A generalizable hypothesis for the genetic architecture of disease: pleomorphic risk loci. Hum Mol Genet. 2011;20(R2):R158–162. doi: 10.1093/hmg/ddr358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Tanzi RE. A Brief History of Alzheimer’s Disease Gene Discovery. J Alzheimers Dis. 2012 doi: 10.3233/jad-2012-129044. [DOI] [PubMed] [Google Scholar]
- Thomas T, Rhodin J. Vascular actions of estrogen and Alzheimer’s disease. Ann N Y Acad Sci. 2000;903:501–509. doi: 10.1111/j.1749-6632.2000.tb06406.x. [DOI] [PubMed] [Google Scholar]
- Tohgi H, Utsugisawa K, Yamagata M, Yoshimura M. Effects of age on messenger RNA expression of glucocorticoid, thyroid hormone, androgen, and estrogen receptors in postmortem human hippocampus. Brain Res. 1995;700(1–2):245–253. doi: 10.1016/0006-8993(95)00971-r. [DOI] [PubMed] [Google Scholar]
- Vina J, Lloret A. Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J Alzheimers Dis. 2010;20(Suppl 2):S527–533. doi: 10.3233/jad-2010-100501. [DOI] [PubMed] [Google Scholar]
- Waring SC, O’Bryant SE, Reisch J, Diaz-Arrastia R, Knebl J, Doody RS for the TARC. The Texas Alzheimer’s Research Consortium longitudinal research cohort:Study design and baseline characteristics. J Tex Pub Hlth Assn. 2008;(63):9–13. [Google Scholar]
- Weckerle CE, Niewold TB. The unexplained female predominance of systemic lupus erythematosus: clues from genetic and cytokine studies. Clin Rev Allergy Immunol. 2011;40(1):42–49. doi: 10.1007/s12016-009-8192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann W, Hanke H. Cardiovascular effects of androgens. Cardiovasc Drug Rev. 2002;20(3):175–198. doi: 10.1111/j.1527-3466.2002.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Westberg L, Baghaei F, Rosmond R, Hellstrand M, Landen M, Jansson M, Eriksson E. Polymorphisms of the androgen receptor gene and the estrogen receptor beta gene are associated with androgen levels in women. J Clin Endocrinol Metab. 2001;86(6):2562–2568. doi: 10.1210/jcem.86.6.7614. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Gromoll J, von Eckardstein A, Nieschlag E. The CAG repeat polymorphism in the androgen receptor gene modulates body fat mass and serum concentrations of leptin and insulin in men. Diabetologia. 2003;46(1):31–39. doi: 10.1007/s00125-002-0980-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.