Abstract
We have analysed homeotic gene expression in the embryonic visceral mesoderm of segmentation mutants by antibody staining against Ultrabithorax, Antennapedia and Sex combs reduced protein. We found that even-skipped (eve) function is crucially required for homeotic gene expression, whereas most other segmentation mutations have only minor effects on position and/or width of the homeotic expression domains in this germ layer. Analysis of pair-rule double mutants indicates that complete loss of homeotic gene activity in the visceral mesoderm, as observed in amorphic eve mutants, correlates with loss of engrailed (en) expression in the epidermis and loss of segmentation. We suggest that the establishment of parasegment borders, a consequence of eve expression and witnessed by subsequent en expression, is a necessary precondition for homeotic gene expression in the visceral mesoderm.
Full text
PDF

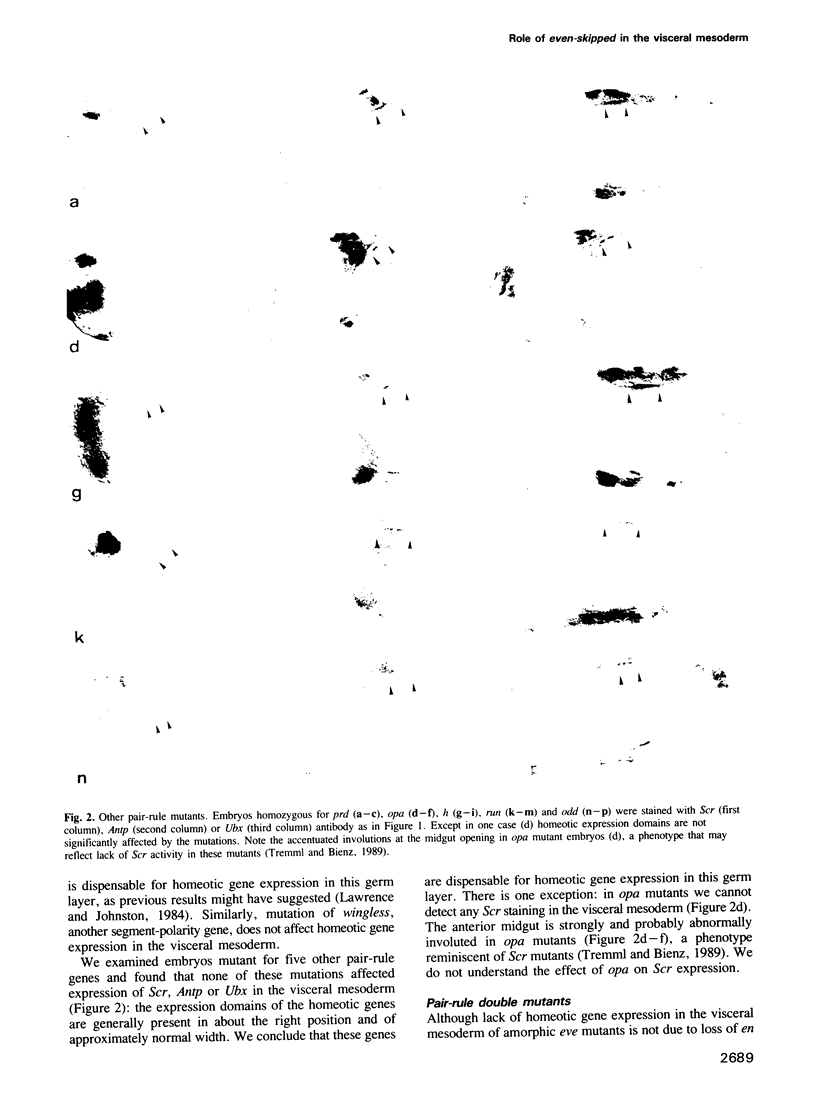




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. E., Martinez-Arias A. The distribution of Ultrabithorax transcripts in Drosophila embryos. EMBO J. 1985 Jul;4(7):1689–1700. doi: 10.1002/j.1460-2075.1985.tb03838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987 Sep;101(1):1–22. [PubMed] [Google Scholar]
- Baker N. E. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J. 1987 Jun;6(6):1765–1773. doi: 10.1002/j.1460-2075.1987.tb02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M., Saari G., Tremml G., Müller J., Züst B., Lawrence P. A. Differential regulation of Ultrabithorax in two germ layers of Drosophila. Cell. 1988 May 20;53(4):567–576. doi: 10.1016/0092-8674(88)90573-9. [DOI] [PubMed] [Google Scholar]
- Bienz M., Tremml G. Domain of Ultrabithorax expression in Drosophila visceral mesoderm from autoregulation and exclusion. Nature. 1988 Jun 9;333(6173):576–578. doi: 10.1038/333576a0. [DOI] [PubMed] [Google Scholar]
- Carroll S. B., DiNardo S., O'Farrell P. H., White R. A., Scott M. P. Temporal and spatial relationships between segmentation and homeotic gene expression in Drosophila embryos: distributions of the fushi tarazu, engrailed, Sex combs reduced, Antennapedia, and Ultrabithorax proteins. Genes Dev. 1988 Mar;2(3):350–360. doi: 10.1101/gad.2.3.350. [DOI] [PubMed] [Google Scholar]
- Carroll S. B., Laughon A., Thalley B. S. Expression, function, and regulation of the hairy segmentation protein in the Drosophila embryo. Genes Dev. 1988 Jul;2(7):883–890. doi: 10.1101/gad.2.7.883. [DOI] [PubMed] [Google Scholar]
- Carroll S. B., Laymon R. A., McCutcheon M. A., Riley P. D., Scott M. P. The localization and regulation of Antennapedia protein expression in Drosophila embryos. Cell. 1986 Oct 10;47(1):113–122. doi: 10.1016/0092-8674(86)90372-7. [DOI] [PubMed] [Google Scholar]
- Carroll S. B., Scott M. P. Localization of the fushi tarazu protein during Drosophila embryogenesis. Cell. 1985 Nov;43(1):47–57. doi: 10.1016/0092-8674(85)90011-x. [DOI] [PubMed] [Google Scholar]
- Carroll S. B., Scott M. P. Zygotically active genes that affect the spatial expression of the fushi tarazu segmentation gene during early Drosophila embryogenesis. Cell. 1986 Apr 11;45(1):113–126. doi: 10.1016/0092-8674(86)90543-x. [DOI] [PubMed] [Google Scholar]
- Carroll S. B., Winslow G. M., Schüpbach T., Scott M. P. Maternal control of Drosophila segmentation gene expression. Nature. 1986 Sep 18;323(6085):278–280. doi: 10.1038/323278a0. [DOI] [PubMed] [Google Scholar]
- DiNardo S., O'Farrell P. H. Establishment and refinement of segmental pattern in the Drosophila embryo: spatial control of engrailed expression by pair-rule genes. Genes Dev. 1987 Dec;1(10):1212–1225. doi: 10.1101/gad.1.10.1212. [DOI] [PubMed] [Google Scholar]
- Driever W., Nüsslein-Volhard C. A gradient of bicoid protein in Drosophila embryos. Cell. 1988 Jul 1;54(1):83–93. doi: 10.1016/0092-8674(88)90182-1. [DOI] [PubMed] [Google Scholar]
- Duncan I. Control of bithorax complex functions by the segmentation gene fushi tarazu of D. melanogaster. Cell. 1986 Oct 24;47(2):297–309. doi: 10.1016/0092-8674(86)90452-6. [DOI] [PubMed] [Google Scholar]
- Frasch M., Hoey T., Rushlow C., Doyle H., Levine M. Characterization and localization of the even-skipped protein of Drosophila. EMBO J. 1987 Mar;6(3):749–759. doi: 10.1002/j.1460-2075.1987.tb04817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch M., Levine M. Complementary patterns of even-skipped and fushi tarazu expression involve their differential regulation by a common set of segmentation genes in Drosophila. Genes Dev. 1987 Nov;1(9):981–995. doi: 10.1101/gad.1.9.981. [DOI] [PubMed] [Google Scholar]
- Frasch M., Warrior R., Tugwood J., Levine M. Molecular analysis of even-skipped mutants in Drosophila development. Genes Dev. 1988 Dec;2(12B):1824–1838. doi: 10.1101/gad.2.12b.1824. [DOI] [PubMed] [Google Scholar]
- Gaul U., Seifert E., Schuh R., Jäckle H. Analysis of Krüppel protein distribution during early Drosophila development reveals posttranscriptional regulation. Cell. 1987 Aug 14;50(4):639–647. doi: 10.1016/0092-8674(87)90037-7. [DOI] [PubMed] [Google Scholar]
- Gergen J. P., Butler B. A. Isolation of the Drosophila segmentation gene runt and analysis of its expression during embryogenesis. Genes Dev. 1988 Sep;2(9):1179–1193. doi: 10.1101/gad.2.9.1179. [DOI] [PubMed] [Google Scholar]
- Hafen E., Kuroiwa A., Gehring W. J. Spatial distribution of transcripts from the segmentation gene fushi tarazu during Drosophila embryonic development. Cell. 1984 Jul;37(3):833–841. doi: 10.1016/0092-8674(84)90418-5. [DOI] [PubMed] [Google Scholar]
- Harding K., Levine M. Gap genes define the limits of antennapedia and bithorax gene expression during early development in Drosophila. EMBO J. 1988 Jan;7(1):205–214. doi: 10.1002/j.1460-2075.1988.tb02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding K., Rushlow C., Doyle H. J., Hoey T., Levine M. Cross-regulatory interactions among pair-rule genes in Drosophila. Science. 1986 Aug 29;233(4767):953–959. doi: 10.1126/science.3755551. [DOI] [PubMed] [Google Scholar]
- Howard K., Ingham P. Regulatory interactions between the segmentation genes fushi tarazu, hairy, and engrailed in the Drosophila blastoderm. Cell. 1986 Mar 28;44(6):949–957. doi: 10.1016/0092-8674(86)90018-8. [DOI] [PubMed] [Google Scholar]
- Ingham P. W., Ish-Horowicz D., Howard K. R. Correlative changes in homoeotic and segmentation gene expression in Krüppel mutant embryos of Drosophila. EMBO J. 1986 Jul;5(7):1659–1665. doi: 10.1002/j.1460-2075.1986.tb04409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham P. W., Martinez-Arias A. The correct activation of Antennapedia and bithorax complex genes requires the fushi tarazu gene. Nature. 1986 Dec 11;324(6097):592–597. doi: 10.1038/324592a0. [DOI] [PubMed] [Google Scholar]
- Ingham P. W. The molecular genetics of embryonic pattern formation in Drosophila. Nature. 1988 Sep 1;335(6185):25–34. doi: 10.1038/335025a0. [DOI] [PubMed] [Google Scholar]
- Knipple D. C., Seifert E., Rosenberg U. B., Preiss A., Jäckle H. Spatial and temporal patterns of Krüppel gene expression in early Drosophila embryos. Nature. 1985 Sep 5;317(6032):40–44. doi: 10.1038/317040a0. [DOI] [PubMed] [Google Scholar]
- Kuroiwa A., Kloter U., Baumgartner P., Gehring W. J. Cloning of the homeotic Sex combs reduced gene in Drosophila and in situ localization of its transcripts. EMBO J. 1985 Dec 30;4(13B):3757–3764. doi: 10.1002/j.1460-2075.1985.tb04145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. A., Johnston P., Macdonald P., Struhl G. Borders of parasegments in Drosophila embryos are delimited by the fushi tarazu and even-skipped genes. 1987 Jul 30-Aug 5Nature. 328(6129):440–442. doi: 10.1038/328440a0. [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Johnston P. On the role of the engrailed+ gene in the internal organs of Drosophila. EMBO J. 1984 Dec 1;3(12):2839–2844. doi: 10.1002/j.1460-2075.1984.tb02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. A., Johnston P. Pattern formation in the Drosophila embryo: allocation of cells to parasegments by even-skipped and fushi tarazu. Development. 1989 Apr;105(4):761–767. doi: 10.1242/dev.105.4.761. [DOI] [PubMed] [Google Scholar]
- Lehmann R., Nüsslein-Volhard C. hunchback, a gene required for segmentation of an anterior and posterior region of the Drosophila embryo. Dev Biol. 1987 Feb;119(2):402–417. doi: 10.1016/0012-1606(87)90045-5. [DOI] [PubMed] [Google Scholar]
- Leiss D., Hinz U., Gasch A., Mertz R., Renkawitz-Pohl R. Beta 3 tubulin expression characterizes the differentiating mesodermal germ layer during Drosophila embryogenesis. Development. 1988 Dec;104(4):525–531. doi: 10.1242/dev.104.4.525. [DOI] [PubMed] [Google Scholar]
- Lewis E. B. A gene complex controlling segmentation in Drosophila. Nature. 1978 Dec 7;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Macdonald P. M., Ingham P., Struhl G. Isolation, structure, and expression of even-skipped: a second pair-rule gene of Drosophila containing a homeo box. Cell. 1986 Dec 5;47(5):721–734. doi: 10.1016/0092-8674(86)90515-5. [DOI] [PubMed] [Google Scholar]
- Martinez-Arias A., Ingham P. W., Scott M. P., Akam M. E. The spatial and temporal deployment of Dfd and Scr transcripts throughout development of Drosophila. Development. 1987 Aug;100(4):673–683. doi: 10.1242/dev.100.4.673. [DOI] [PubMed] [Google Scholar]
- Martinez-Arias A., Lawrence P. A. Parasegments and compartments in the Drosophila embryo. Nature. 1985 Feb 21;313(6004):639–642. doi: 10.1038/313639a0. [DOI] [PubMed] [Google Scholar]
- Martinez-Arias Alfonso. The Antennapedia gene is required and expressed in parasegments 4 and 5 of the Drosophila embryo. EMBO J. 1986 Jan;5(1):135–141. doi: 10.1002/j.1460-2075.1986.tb04187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt H. Hierarchical inductions of cell states: a model for segmentation in Drosophila. J Cell Sci Suppl. 1986;4:357–381. doi: 10.1242/jcs.1986.supplement_4.20. [DOI] [PubMed] [Google Scholar]
- Meinhardt H. Space-dependent cell determination under the control of morphogen gradient. J Theor Biol. 1978 Sep 21;74(2):307–321. doi: 10.1016/0022-5193(78)90078-4. [DOI] [PubMed] [Google Scholar]
- Muriel W. J., Cole J., Lehmann A. R. Molecular analysis of ouabain-resistant mutants of the mouse lymphoma cell line L5178Y. Mutagenesis. 1987 Sep;2(5):383–389. doi: 10.1093/mutage/2.5.383. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Frohnhöfer H. G., Lehmann R. Determination of anteroposterior polarity in Drosophila. Science. 1987 Dec 18;238(4834):1675–1681. doi: 10.1126/science.3686007. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Kluding H., Jürgens G. Genes affecting the segmental subdivision of the Drosophila embryo. Cold Spring Harb Symp Quant Biol. 1985;50:145–154. doi: 10.1101/sqb.1985.050.01.020. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980 Oct 30;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Tremml G., Bienz M. Homeotic gene expression in the visceral mesoderm of Drosophila embryos. EMBO J. 1989 Sep;8(9):2677–2685. doi: 10.1002/j.1460-2075.1989.tb08408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakimoto B. T., Turner F. R., Kaufman T. C. Defects in embryogenesis in mutants associated with the antennapedia gene complex of Drosophila melanogaster. Dev Biol. 1984 Mar;102(1):147–172. doi: 10.1016/0012-1606(84)90182-9. [DOI] [PubMed] [Google Scholar]
- White R. A., Lehmann R. A gap gene, hunchback, regulates the spatial expression of Ultrabithorax. Cell. 1986 Oct 24;47(2):311–321. doi: 10.1016/0092-8674(86)90453-8. [DOI] [PubMed] [Google Scholar]
- White R. A., Wilcox M. Distribution of Ultrabithorax proteins in Drosophila. EMBO J. 1985 Aug;4(8):2035–2043. doi: 10.1002/j.1460-2075.1985.tb03889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. A., Wilcox M. Protein products of the bithorax complex in Drosophila. Cell. 1984 Nov;39(1):163–171. doi: 10.1016/0092-8674(84)90202-2. [DOI] [PubMed] [Google Scholar]
- Wieschaus E., Nusslein-Volhard C., Kluding H. Krüppel, a gene whose activity is required early in the zygotic genome for normal embryonic segmentation. Dev Biol. 1984 Jul;104(1):172–186. doi: 10.1016/0012-1606(84)90046-0. [DOI] [PubMed] [Google Scholar]







