Abstract
Tumor-associated antigen 90K is implicated in cell–cell and cell-extracellular matrix adhesion through its interaction with galectin-3 and integrin-β1 and is highly expressed in malignant tissues, making it a novel target for the development of new immunotherapies. We investigated a potential immunotherapy treatment for colon cancer using 90K-specific cytotoxic T lymphocytes induced by autologous dendritic cells and pulsed with 90K peptides. We selected three peptides (90K351, 90K5 and 90K523) that bind to HLA-A*0201 molecules on the basis of their binding affinity, as determined by a peptide-T2 binding assay. Dendritic cells pulsed with 90K peptides resulted in the efficient generation of mature dendritic cells and exhibited enhanced T-cell stimulation and polarization of naive T cells toward Th1. Dendritic cells pulsed with 90K peptides generated potent cytotoxic T-lymphocytes that lysed T2 cells loaded with each 90K peptide, and 90K+/HLA-A2+ colon cancer cell lines, including HCT116 and SW480, in a dose-dependent and HLA-A*0201-restricted manner. No killing was observed in 90K+/HLA-A2− DLD1 or 90K−/HLA-A2− K562 cells. Therefore, we believe that cytotoxic T-lymphocytes stimulated by 90K peptide-pulsed dendritic cells naturally recognize the 90K peptide presented by colon cancer cells in the context of HLA-A2, and kill 90K-positive tumor cells. Dendritic cells pulsed with 90K peptides led to the induction of granzyme B and perforin positive CD8+ T cells against HCT116 and SW480 cells, but not DLD1 cells. In conclusion, 90K-specific cytotoxic T lymphocytes, generated by stimulating T cells with 90K peptide-pulsed dendritic cells, could be useful effector cells for the immunotherapy treatment of colon cancer.
Keywords: 90K, colon cancer, dendritic cells, immunotherapy, peptide
Introduction
Although significant advances in colon cancer treatment have been achieved with the introduction of new chemotherapeutical and biological agents, advanced colon cancer remains difficult to treat; therefore, improved treatment is urgently needed. Immunological approaches to colon cancer therapy have evolved substantially over the past several years.1,2,3 Numerous tumor-associated antigens have been described in colon cancer, which display spontaneous T-cell responses to tumor antigens in patients without prior immunotherapy, suggesting immunogenicity of colon cancer.4,5,6
The 90K glycoprotein is composed of 90 kDa subunits, and it has been identified in culture fluid of human breast cancer cells.7 It was formerly named Mac-2-binding protein, because it is a ligand of galectin-3, formerly Mac-2.8,9 Numerous cell types, including hematopoietic cells and epithelial cells, synthesize and secrete 90K; thus, it is normally present in the serum and other biologic fluids.7,8,10 Although the functions of 90K are not well-defined, 90K has been implicated in cell–cell and cell–extracellular matrix adhesion through interaction with galectin-3 and integrin-β1.
Recently, we have shown that 90K suppresses Wnt/β-catenin signaling by targeting β-catenin in colon cancer.11 However, this activity of 90K can be masked through binding to extracellular galectins, and weakened in concert with downregulation of its binding partner, CD9/82. In contrast, 90K interacts with galectin on the tumor cell surface resulting in the formation of cell aggregates.12,13 It has been proposed that the interaction between these proteins may enhance the growth and biological behavior of cancer cells.
In cancerous tissues, 90K is expressed at high levels14,15,16 and is associated with poor prognosis.16,17,18 Immunohistochemical analyses of 90K expression have revealed weak or no expression in most normal tissues and strong upregulation in tumor cells of human neoplastic tissues,19 suggesting that 90K can be used as a target antigen in cancer immunotherapy. Ulmer et al.20 demonstrated that immunohistochemical analyses of colon tumors showed elevated expression of 90K in all tumor samples compared to normal colon samples. It is anticipated that 90K will be a novel target molecule for immunotherapy treatment of colon cancer, in addition to other 90K-positive cancers.
Recently, we determined that the 90K protein could be used for dendritic cell (DC)-based immunotherapy for colon cancer and showed that 90K protein-loaded DCs induced a strong antigen-specific cytotoxic T-lymphocyte (CTL) response against colon cancer cells.21 Human leukocyte antigen (HLA)-A*0201 is highly frequent in Northern Asia and North America, and is also the most popular type of HLA in Korea (∼32%). In this study, we selected three peptides derived from 90K with an HLA-A*0201-binding epitope recognized by CTLs, and investigated the possibility of immunotherapy for colon cancer using 90K-specific CTLs generated by stimulating autologous T cells with 90K peptide-pulsed DCs.
Materials and methods
Target cell lines
The human colon cancer cell lines used as target cells were as follows: HCT116, SW480, DKO, DLD1 and HCT8. All cell lines were purchased from the American Type Culture Collection and maintained in Dulbecco's Modified Eagle Medium (Hyclone, South Logan, Utah, USA) supplemented with 10% fetal bovine serum. K562 cells were obtained from the Korea Cell Line Bank and used as natural killer cell-sensitive targets.
Generation of DCs
Monocyte-derived mature DCs were generated from peripheral blood mononuclear cells of HLA-A*0201+ healthy donors and patients with colon cancer using an αDC1-polarizing cocktail composed of IL-1β (25 ng/ml), tumor-necrosis factor (TNF)-α (50 ng/ml), interferon (IFN)-α (3000 units/ml), IFN-γ (1000 units/ml), and polyinosinic∶polycytidylic acid (poly[I∶C] 20 µg/ml).22,23
Fluorescence-activated cell sorting analysis (FACS)
Analysis by FACS was used to examine the major histocompatibility complex (MHC) class I level of colon cancer cells. Colon cancer cells were stained with a fluorescein isothiocyanate (FITC)-conjugated anti-human HLA class ABC monoclonal antibody (mAb) (BD Bioscience) for 15 min on ice, after which the cells were washed three times. To determine the immunophenotype of DCs, mature DCs were stained with phycoerythrin (PE)-conjugated mAbs against CD86. In addition, DCs were stained with FITC-conjugated mAbs against CD80, CD83, HLA-ABC and CCR7. Flow cytometric analysis for FITC and PE fluorescence was performed using a FACSAria (Becton Dickinson), and data were analyzed with FACS Diva (Becton Dickinson) and Win MDI version 2.9 (Bio-Soft Net) software.
Measurement of cytokines
A sandwich ELISA was used for detecting IL-10 and IL-12p70 in culture supernatants. Assays of cytokines in culture medium were performed as recommended by the manufacturers (BD Biosciences). The levels of cytokines released into the culture medium were determined by measuring absorbance at 450 nm with a microplate reader. Cytokine concentrations in the samples were calculated using standard curves generated from recombinant cytokines, and the results were expressed in picograms per milliliter.
Peptide-T2 cell-binding assay
All peptides were synthesized by PEPTRON (Daejeon, Korea). Peptides binding to HLA-A*0201 molecules were measured using the T2 cell line according to a previously described protocol.24 T2 cells were incubated overnight with 3 µg/ml β2-microglobulin and peptides, followed by washing and incubation with the FITC-labeled anti-HLA-A*0201 mAb, BB7.2 (BD Pharmingen). After washing, cells were analyzed for levels of HLA-A*0201 expression by flow cytometry. The binding ability of the peptides to HLA-A*0201 was evaluated by mean fluorescent intensity of stained T2 cells that were pulsed with peptides.
Enzyme-linked immunosorbent spot assay (ELISPOT)
The ELISPOT assay for the enumeration of antigen-specific, IFN-γ-secreting cells was followed according to the manufacturer's protocol (BD Pharmingen). The number of IFN-γ spots was enumerated by Immuno Spot Analyzers (Cellular Technology Ltd). Data were expressed as the mean number of IFN-γ-secreting cells/104 T cells.
Carboxyfluorescein diacetate succinimidyl ester labeling assay
T cells were seeded into 96-well U-bottomed tissue culture plates in T cell medium and labeled with 5(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; 5 µM; Invitrogen) for 10 min at 37 °C. After washing, labeled T cells (2×105 cells/ml) were seeded into 96-well U-bottomed plates and incubated with autologous DCs (1×105 cells/ml) for 5 days. The proliferation of T cells was evaluated by gradual CFSE dilution in dividing T cells by flow cytometry.
Co-cultivation of DCs with J558/CD40L cells
Peptide-pulsed DCs were harvested and co-cultured with engineered J558/CD40L cells that express the CD40 ligand. DCs were mixed (1∶1 ratio) with J558/CD40L cells, which were irradiated before culture with DCs. At 24 h after cocultivation, the supernatant from the DCs was harvested and used for ELISA assay.
Intracellular granzyme B and perforin detection
Peptide-specific T cells were cocultured for 4 h with various tumor cells and stained with a PE-conjugated anti-CD8. Then cocultured cells were treated with a Fixation/Permeabilization solution for 20 min and subsequently labeled with an optimized concentration of FITC-conjugated mouse anti-human granzyme B Ab, perforin Ab or control FITC-conjugated murine IgG2 (Caltag Laboratories) for an additional 50 min on ice. Cells were then washed, suspended in balanced salt solution with 2% heat-inactivated FCS, and analyzed using a FACSAria cell sorter (Becton Dickinson), and the data analysis was performed with WIN MDI version 2.9 (Bio-Soft Net) software.
51Cr release assay
The standard 4-h 51Cr release assay was performed to measure the cytolytic activity of T cells with target cells. Target cells were incubated with 100 µCi 51Cr sodium chromate for 1 h, washed extensively, seeded (104 cells/well) into 96-well U-bottomed plates in T-cell medium, and cocultured for 4 h with various numbers of T cells. All assays were performed in triplicate. Results are expressed as the mean percentage of 51Cr release calculated as follows: [(experimental release−spontaneous release)/(maximum release−spontaneous release)]×100%. Spontaneous release was less than 20% of the maximum 51Cr uptake.
Statistical analysis
All statistical analyses were performed with SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA). The Mann–Whitney U test was performed to analyze the statistical significance of non-parametric differences between groups. P<0.05 was considered to be statistically significant.
Results
Expression of 90K protein and MHC class I in colon cancer cell lines
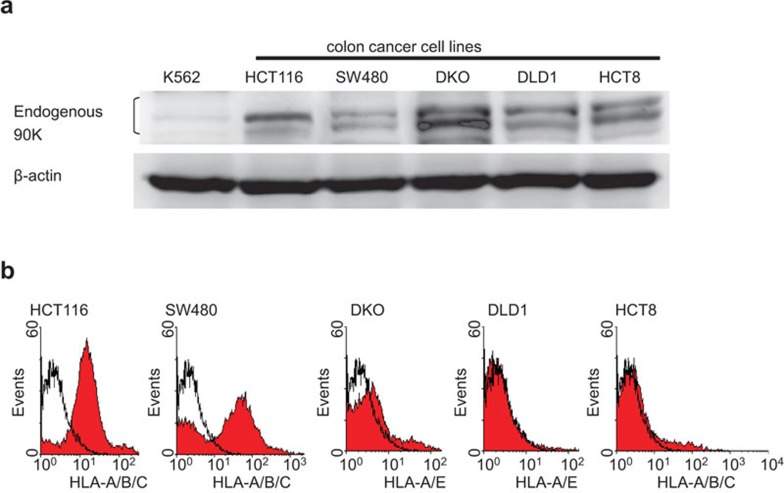
Western blot analysis was used to examine the expression of 90K in several colon cancer cell lines, including HCT116, SW480, DKO, DLD1, and HCT8. As shown in Figure 1a, 90K was expressed in all colon cancer cell lines. This suggests that 90K is a candidate for a colon cancer-associated tumor antigen. The expression of MHC class I in human colon cancer cells were also examined by flow cytometry. The results are shown in Figure 1b. Both HCT116 and SW480 cells strongly expressed MHC class I on the cell surface. In contrast, DKO, DLD1 and HCT8 cells were deficient in MHC class I expression on the cell surface. Therefore, we used HCT116 and SW480 cell lines as 90K+/HLA-A2+ targets and the DLD1 cell line as a 90K+/HLA-A2− target for further analysis. HLA-A2−/90K− K562 cells were used as natural killer cell-sensitive targets.
Figure 1.
Expression of 90K protein and MHC class I in colon cancer cell lines. (a) Western blot showing 90K protein expression in several colon cancer cell lines, including HCT116, SW480, DKO, DLD1, and HCT8. The K562 cell line was used as a negative control. (b) FACS analysis showing MHC class I expression in colon cancer cell lines. FACS, fluorescence-activated cell sorting analysis; MHC, major histocompatibility complex.
Selection of 90K peptides
The sequence of 90K was evaluated for peptides that could potentially bind to HLA-A*0201 using a peptide binding database (www.bimas.cit.nih.gov/molbio/hla_bind/). After comparing the predictive binding scores, we identified and selected three peptides (90K351, 90K5, 90K523) with high scores that could potentially bind to HLA-A*0201 molecules (Table 1). We measured and confirmed the binding affinity of the three peptides towards the restricting HLA-A*0201 allele using T2 cells, because HLA-A2 expression is stabilized and maintained on the surfaces of T2 cells when the peptides bind in the binding grooves of HLA-A2 molecules to form complexes. A peptide from HIV type 1 reverse transcriptase (HIV-pol)25 was used as a control.
Table 1. Binding affinity of 90K peptides and control peptide for HLA-A*0201 molecules.
| Peptide | Sequence | Predictive binding scorea | Mean fluorescent intensityb |
|---|---|---|---|
| No peptide | (−) | (−) | 11.80 |
| HIV-pol | ILKEPVHGV | 39.025 | 33.52 |
| 90K351 | MLPEELFEL | 1986.272 | 41.61 |
| 90K5 | RLFWVWLLV | 437.482 | 45.62 |
| 90K523 | LMLCEGLFV | 1737.776 | 53.47 |
Estimate of half-time for dissociation and calculated score in arbitrary units.
Mean fluorescent intensity of stained T2 cells that were pulsed with peptides.
Characterization of DCs pulsed with 90K peptides
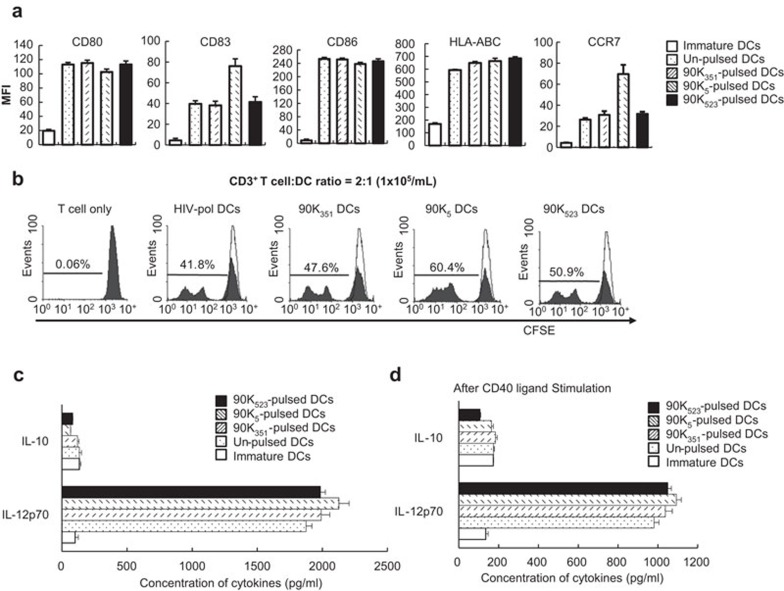
Based on FACS analysis, which compared the phenotypes of peptide-pulsed mature DCs and un-pulsed mature DCs, both DCs exhibited high expression of CD80, CD83, CD86, HLA-ABC and CCR7, indicating the typical phenotype of mature DCs (Figure 2a). Thus, DCs pulsed with 90K peptides resulted in efficient generation of mature DCs without altering the phenotype of the mature DCs. To evaluate the T cell-proliferating capacity of DCs, autologous CD3+ T cells obtained by HLA-A*0201+ healthy donors were stimulated with various DCs, and the CFSE-labeling assay was performed for three independent experiments (Figure 2b). The divided cells made up 47.6%, 60.4% and 50.9% of the T cells in response to DCs pulsed with 90K351, 90K5 and 90523, respectively, suggesting that 90K peptides were desirable epitopes of T cell stimulation by DCs presented with antigen.
Figure 2.
Characteristics of DCs pulsed with the 90K peptide. (a) FACS was performed to analyze cell surface markers (CD80, CD83, CD86, HLA-ABC and CCR7) of DCs pulsed with or without 90K peptides. Data are expressed as the mean fluorescence intensity increase over the isotype control±s.d. from three independent experiments. (b) Cell proliferation, as measured by CFSE dilution, of CD3+ T cells from a HLA-A*0201+ blood donor in response to unpulsed DCs or DCs pulsed with 90K peptides or the control HIV-pol peptide. Data are expressed as percentages of the mean frequency of dividing CD3+ T cells in three independent experiments. (c, d) Cytokines secreted into the culture supernatants were measured by ELISA. Production of IL-12p70 and IL-10 during maturation of DCs (c) and after stimulation with CD40 ligand-transfected J558 cells (d). The results are illustrated as the mean (pg/ml)±s.d. of triplicate cultures from two independent experiments. CFSE, 5(6)-carboxyfluorescein diacetate succinimidyl ester; DC, dendritic cell; FACS, fluorescence-activated cell sorting analysis; IL, interleukin.
One of the best ways of determining DC function is to examine cytokine secretion. It has been reported that the level of IL-12 production by DCs is a major factor driving the development of Th1 cells. In contrast, IL-10 is a pleiotropic cytokine known to have inhibitory effects on the accessory functions of DCs. Therefore, we measured IL-12p70 and IL-10 production by immature DCs and by mature DCs pulsed with or without 90K peptides (Figure 2c). Mature DCs showed high IL-12p70 production and this enhancement of IL-12p70 production was not suppressed by loading the cells with 90K peptides. Furthermore, the mature DCs pulsed with or without 90K peptides showed further production of IL-12p70 after subsequent stimulation with CD40L-transfected J558 cells (Figure 2d). By contrast, production of the inhibitory cytokine IL-10 by mature DCs was not increased even after CD40L stimulation.
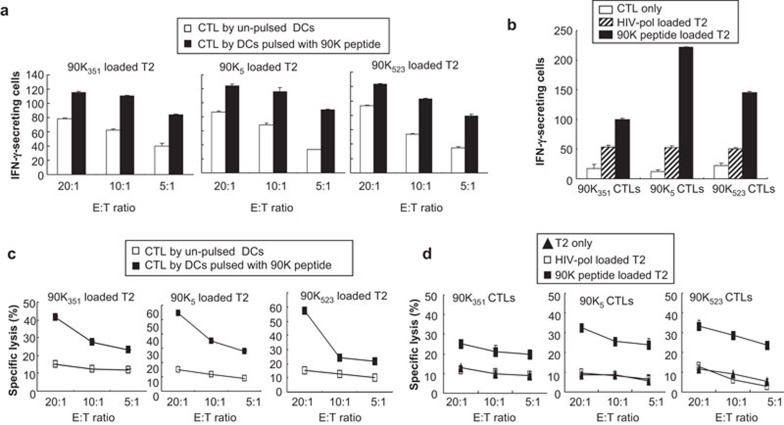
Characterization of 90K peptide-specific CTLs against T2 cells
To generate 90K peptide-specific CTLs from HLA-A*0201+ healthy donors, autologous DCs pulsed with 90K peptides were used as antigen-presenting cells (APCs). Specific responses of the CTLs to 90K peptides were examined when co-cultured with T2 cells (HLA-A2+/90K−) using an ELISPOT assay. CTLs that were stimulated by 90K peptide-pulsed DCs displayed a greater number of IFN-γ-secreting cells against T2 cells loaded with each 90K peptide compared to those stimulated by un-pulsed DCs (P<0.05) (Figure 3a). These results indicate that the 90K peptides induced a strong peptide-specific CTL response. CTLs stimulated by 90K peptides also produced a greater number of IFN-γ-secreting cells against T2 cells loaded with each 90K peptide compared to T2 cells loaded with the control peptide (HIV-pol), indicating the specificity of the CTLs (Figure 3b). Next, the cytotoxic activity of the CTLs against T2 cells was assessed using a standard 4-h 51Cr release assay. As shown in Figure 3c, CTLs stimulated by 90K peptide-pulsed DCs displayed cytotoxic activity in a dose-dependent manner that was more potent than CTLs stimulated by unpulsed DCs. CTLs stimulated by DCs pulsed with 90K peptides also lysed more T2 cells loaded with the same 90K peptides than T2 cells loaded with the control peptide, or unloaded T2 cells in a dose-dependent manner (Figure 3d), confirming the specificity of the CTLs.
Figure 3.
Characterization of 90K peptide-specific CTL responses against T2 cells. (a, b) ELISPOT assay showing the number of IFN-γ-secreting cells per 104 CD8+ T cells. (a) Comparison of IFN-γ-secreting cells from CTLs stimulated by un-pulsed and 90K peptide-pulsed DCs against 90K peptide-loaded T2 cells. (b) Comparison of IFN-γ-secreting cells from 90K-specific CTLs stimulated by 90K peptide-pulsed DCs against 90K peptide-loaded T2 cells and control (HIV-pol) peptide-loaded T2 cells. Unprimed CD8+ T cells (CTL only) were used as a negative control. (c, d) Cytotoxicity of CTLs against T2 cells. Lytic activity of CTLs generated by unpulsed DCs or 90K peptide-pulsed DCs against 90K peptide-loaded T2 cells (c) and lytic activity of CTLs generated by 90K peptide-pulsed DC against T2 cells only or HIV-pol loaded T2 cells or 90K peptide-loaded T2 cells (d). CTLs were incubated with radiolabeled target cells at effector/target ratios of 20∶1, 10∶1 and 5∶1. The amount of 51Cr release from the lysed target cells was measured. The results are illustrated as the mean values (±s.d.) of triplicate cultures from three independent experiments. CTL, cytotoxic T-lymphocyte; DC, dendritic cell; IFN, interferon.
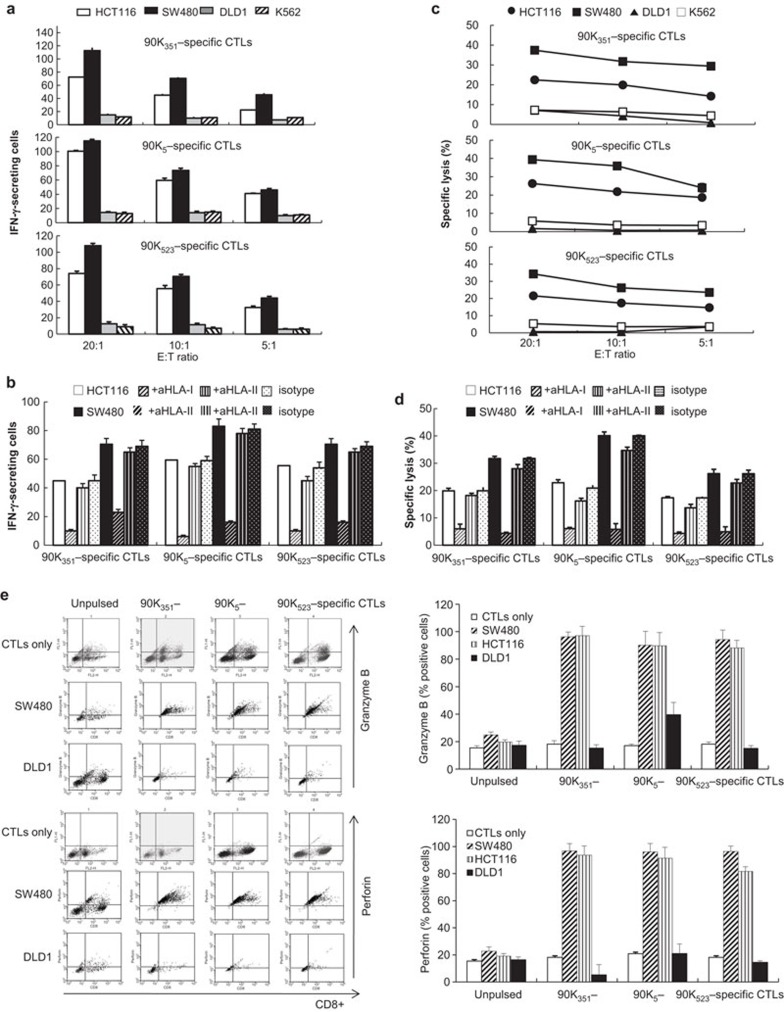
Characterization of 90K peptide-specific CTLs against colon cancer cells
Next, we investigated whether the 90K-specific CTLs recognize 90K-derived peptides that are endogenously processed and presented in the context of HLA-A2 molecules in 90K-positive colon cancer cells. To generate 90K peptide-specific T cells from HLA-A*0201+ patients with colon cancer, autologous, mature DCs pulsed with peptides were used as APCs. In these experiments, HCT116 (HLA-A2+/90K+), SW480 (HLA-A2+/90K+), DLD1 (HLA-A2−/90K+), and K562 cells (HLA-A2−/90K−, natural killer cell-sensitive) were used as target cells. As shown in Figure 4a, CTLs stimulated by 90K peptides produced a greater number of IFN-γ-secreting cells against HCT116 or SW480 cells compared to DLD1 or K562 cells. This stimulation depended on the effector-to-target ratio. Blocking studies using MHC class I or II blocking antibodies showed specific responses of CTLs against HCT116 or SW480 cells that are MHC class I-restricted (Figure 4b). In addition, 90K peptide-specific CTLs specifically lysed HCT116 and SW480 cells, but not DLD1 or K562 cells (Figure 4c), further confirming the specificity of CTLs. The cytolysis of the CTLs from patients with colon cancer against 90K+/HLA-A2+ colon cancer cells was blocked by antibodies against HLA class I, but not by antibodies against HLA class II (Figure 4d). Therefore, we hypothesized that CD8+ CTLs stimulated by 90K peptide-pulsed DCs recognize the 90K peptide naturally presented by colon cancer cells in the context of HLA-A2, and are able to kill 90K-positive tumor cells.
Figure 4.
Characterization of 90K peptide-specific CTL responses against colon cancer cells. Production of IFN-γ-secreting cells (a, b) and cytolytic activity (c, d) of 90K-specific CTLs against HLA-A2+/90K+ colon cancer cell lines (HCT116, SW480), the HLA-A2−/90K+ colon cancer cell line (DLD1) and HLA-A2−/90K− K562 cells. To generate 90K-specific CD8+ T cells from HLA-A*0201+ patients with colon cancer, autologous, mature DCs pulsed with 90K peptides were used as APCs. (b, d) MHC restriction of the CTLs was analyzed using an MHC class I-specific mAb or an MHC class II-specific mAb. A nonspecific response was determined by irrelevant Ig blockade. The results are illustrated as the mean values (±s.d.) of triplicate cultures from two independent experiments (e) FACS was performed to analyze the expression of intracellular granzyme B and perforin in CD8+ T cells. Bar graphs indicate the percentage of CD8+ T cells expressing granzyme B or perforin. The results are illustrated as the mean±s.d. of three experiments. APC, antigen-presenting cell; CTL, cytotoxic T lymphocyte; DC, dendritic cell; FACS, fluorescence-activated cell sorting analysis; IFN, interferon; mAb, monoclonal antibody; MHC, major histocompatibility complex.
CTLs contribute to tumor cell clearance by releasing perforin and granzymes from cytoplasmic granules. We investigated whether 90K peptide-pulsed DCs led to the induction of granzyme B and perforin positive CD8+ T cells. Flow cytometry analysis was used to examine the expression of intracellular granzyme B and perforin. 90K-specific CD8+ T cells were led to the induction of granzyme B and perforin against HCT116 and SW480 cells, but not against DLD1 cells (Figure 4e). These data suggest that stimulation of DCs by 90K peptides is highly effective in priming the cytotoxic T cell response upon exposure to the specific 90K antigen in an MHC class-I restricted manner.
Discussion
DCs capture and process proteins and present peptides on the cell surface in the context of MHC I and MHC II molecules to induce antigen-specific T-cell immune responses.26,27 Tumor antigens coded by peptides, proteins, DNA and RNA have been used to sensitize DCs. Among these approaches, one of the most relevant methods for the development of tumor immunotherapy is peptide-based, cancer-specific immunotherapy using antigens that are expressed in tumor tissues but not in normal tissues. Recently, we showed that 90K protein-loaded DCs induce a strong antigen-specific CTL response against colon cancer cells and suggested that 90K has the potential to be a novel target molecule for immunotherapy treatment for colon cancer.11 In the present study, we identified T-cell epitopes from the 90K protein for the development of 90K peptide-based immunotherapy against colon cancer. Previously, Ozaki et al.28 identified 90K-derived CTL epitopes that may be useful target antigenic epitopes for clinical immunotherapy of lung cancer. CTLs were generated from peripheral blood lymphocytes by multiple in vitro stimulations with the 90K peptide. In this study, we investigated the possibility of immunotherapy for colon cancer using 90K-specific CTLs generated by 90K peptide-pulsed DCs. We identified and synthesized three 90K peptides (90K351, 90K5 and 90K523) for HLA-A*0201 molecules and generated 90K peptide-specific CTLs using 90K peptide-pulsed DCs as APCs. The CTLs showed strong cytolytic activity against 90K peptide-loaded T2 cells and 90K+/HLA-A2+ colon cancer cell lines, including HCT116 and SW480. Cell death was not observed in 90K+/HLA-A2− DLD1 cells or 90K−/HLA-A2− K562 cells. This suggests that CTLs stimulated by 90K peptide-pulsed DCs recognize the 90K peptide naturally presented by colon cancer cells in the context of HLA-A2, and kill 90K-positive tumor cells. In conclusion, 90K-specific CTLs generated by stimulating T cells with 90K peptide-pulsed DCs could be useful effector cells for immunotherapy of colon cancer.
Acknowledgments
This study was financially supported by a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family affairs, Republic of Korea (0720570) and the National Research Foundation of Korea (2012-0002351).
References
- Morse M, Langer L, Starodub A, Hobeika A, Clay T, Lyerly HK. Current immunotherapeutic strategies in colon cancer. Surg Oncol Clin N Am. 2007;16:873–900. doi: 10.1016/j.soc.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Ogino S, Galon J, Fuchs CS, Dranoff G. Cancer immunology-analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8:711–719. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Hu X, Zimmerman M, Waller JL, Wu P, Hayes-Jordan A, et al. TNFα cooperates with IFN-γ to repress Bcl-xL expression to sensitize metastatic colon carcinoma cells to TRAIL-mediated apoptosis. PLoS One. 2011;6:e16241. doi: 10.1371/journal.pone.0016241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother. 2005;54:187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagorsen D, Keilholz U, Rivoltini L, Schmittel A, Letsch A, Asemissen AM. Natural T-cell response against MHC class I epitopes of epithelial cell adhesion molecule, HER-2/neu, and carcinoembryonic antigen in patients with colorectal cancer. Cancer Res. 2000;60:4850–4854. [PubMed] [Google Scholar]
- Nagorsen D, Scheibenbogen C, Marincola FM, Letsch A, Keilholz U. Natural T cell immunity against cancer. Clin Cancer Res. 2003;9:4296–4303. [PubMed] [Google Scholar]
- Iacobelli S, Arnò E, D'Orazio A, Coletti G. Detection of antigens recognized by a novel monoclonal antibody in tissue and serum from patients with breast cancer. Cancer Res. 1986;46:3005–3010. [PubMed] [Google Scholar]
- Rosenberg I, Cherayil BJ, Isselbacher KJ, Pillai S. Mac-2-binding glycoproteins. Putative ligands for a cytosolic beta-galactosidelectin. J Biol Chem. 1991;266:18731–18736. [PubMed] [Google Scholar]
- Koths K, Taylor E, Halenbeck R, Casipit C, Wang A. Cloning and characterization of a human Mac-2-binding protein, a new member of the superfamily defined by the macrophage scavenger receptor cysteine-rich domain. J Biol Chem. 1993;268:14245–14249. [PubMed] [Google Scholar]
- Ullrich A, Sures I, D'Egidio M, Jallal B, Powell TJ, Herbst R, et al. The secreted tumor-associated antigen 90K is a potent immune stimulator. J Biol Chem. 1994;269:18401–18407. [PubMed] [Google Scholar]
- Lee JH, Bae JA, Seo YW, Kho DH, Sun EG, Lee SE, et al. Glycoprotein 90K, downregulated in advanced colorectal cancer tissues, interacts with CD9/CD82 and suppresses the Wnt/β-catenin signal via ISGylation of β-catenin. Gut. 2010;59:907–917. doi: 10.1136/gut.2009.194068. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Brakebusch C, Engel J, Timpl R. Mac-2 binding protein is a cell-adhesive protein of the extracellular matrix which self-assembles into ring-like structures and binds beta1 integrins, collagens and fibronectin. EMBO J. 1998;17:1606–1613. doi: 10.1093/emboj/17.6.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinari N, Kuwabara I, Huflejt ME, Shen PF, Iacobelli S, Liu FT. Glycoprotein 90K/MAC-2BP interacts with galectin-1 and mediates galectin-1-induced cell aggregation. Int J Cancer. 2001;91:167–172. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1022>3.3.co;2-q. [DOI] [PubMed] [Google Scholar]
- Fusco O, Querzoli P, Nenci I, Natoli C, Brakebush C, Ullrich A, et al. 90K (MAC-2 BP) gene expression in breast cancer and evidence for the production of 90K by peripheral-blood mononuclear cells. Int J Cancer. 1998;20; 79:23–26. doi: 10.1002/(sici)1097-0215(19980220)79:1<23::aid-ijc5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Park YP, Choi SC, Kim JH, Song EY, Kim JW, Yoon DY, et al. Upregulation of Mac-2 binding protein by hTERT in gastric cancer. Int J Cancer. 2007;120:813–820. doi: 10.1002/ijc.22369. [DOI] [PubMed] [Google Scholar]
- Ozaki Y, Kontani K, Hanaoka J, Chano T, Teramoto K, Tezuka N, et al. Expression and immunogenicity of a tumor-associated antigen, 90K/Mac-2 binding protein, in lung carcinoma. Cancer. 2002;95:1954–1962. doi: 10.1002/cncr.10899. [DOI] [PubMed] [Google Scholar]
- Marchetti A, Tinari N, Buttitta F, Chella A, Angeletti CA, Sacco R, et al. Expression of 90K (Mac-2 BP) correlates with distant metastasis and predicts survival in stage I non-small cell lung cancer patients. Cancer Res. 2002;62:2535–2539. [PubMed] [Google Scholar]
- Kim SJ, Lee SJ, Sung HJ, Choi IK, Choi CW, Kim BS, et al. Increased Serum 90K and Galectin-3 Expression Are Associated with Advanced Stage and a Worse Prognosis in Diffuse Large B-Cell Lymphomas. Acta Haematol. 2008;120:211–216. doi: 10.1159/000193223. [DOI] [PubMed] [Google Scholar]
- Becker R, Lenter MC, Vollkommer T, Boos AM, Pfaff D, Augustin HG, et al. Tumor stroma marker endosialin (Tem1) is a binding partner of metastasis-related protein Mac-2 BP/90K. Faseb J. 2008;22:3059–3067. doi: 10.1096/fj.07-101386. [DOI] [PubMed] [Google Scholar]
- Ulmer TA, Keeler V, Loh L, Chibbar R, Torlakovic E, Andre S, et al. Tumor-associated antigen 90K/Mac-2-binding protein: possible role in colon cancer. J Cell Biochem. 2006;98:1351–1366. doi: 10.1002/jcb.20784. [DOI] [PubMed] [Google Scholar]
- Lee JH, Park MS, Chung IJ. Induction of 90K-specific cytotoxic T lymphocytes for colon cancer immunotherapy. Immune Netw. 2010;10:206–211. doi: 10.4110/in.2010.10.6.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, et al. Alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Foon KA, Mailliard RB, Muthuswamy R, Kalinski P. Type 1-polarized dendritic cells loaded with autologous tumor are a potent immunogen against chronic lymphocytic leukemia. J Leukoc Biol. 2008;84:319–325. doi: 10.1189/jlb.1107737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijman HW, Houbiers JG, Vierboom MP, van der Burg SH, Drijfhout JW, D'Amaro J, et al. Identification of peptide sequences that potentially trigger HLA-A2.1-restricted cytotoxic T lymphocytes. Eur J Immunol. 1993;23:1215–1219. doi: 10.1002/eji.1830230603. [DOI] [PubMed] [Google Scholar]
- Tsomides TJ, Walker BD, Eisen HN. An optimal viral peptide recognized by CD8+ T cells binds very tightly to the restricting class I major histocompatibility complex protein on intact cells but not to the purified class I protein. Proc Natl Acad Sci U S A. 1991;88:11276–11280. doi: 10.1073/pnas.88.24.11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MJ. Antigen recognition. Class discrimination in the world of immunology. Nature. 1987;325:192–193. doi: 10.1038/325192b0. [DOI] [PubMed] [Google Scholar]
- Jondal R, Schirmbeck R, Reimann J. MHC class I-restricted CTL responses to exogenous antigens. Immunity. 1996;5:295–302. doi: 10.1016/s1074-7613(00)80255-1. [DOI] [PubMed] [Google Scholar]
- Ozaki Y, Kontani K, Teramoto K, Fujita T, Tezuka N, Sawai S, et al. Identification of antigenic epitopes recognized by Mac-2 binding protein-specific cytotoxic T lymphocytes for use in cancer immunotherapy. Biochem Biophys Res Commun. 2004;317:1089–1095. doi: 10.1016/j.bbrc.2004.03.155. [DOI] [PubMed] [Google Scholar]