Abstract
Osx plays essential roles in regulating osteoblast and chondrocyte differentiation, and bone formation during mouse skeletal development. However, many questions remain regarding the requirement for Osx in different cell lineages. In this study, we asked whether Osx is required for craniofacial bone formation derived from cranial neural crest (CNC) cells. The Osx gene was conditionally inactivated in CNC-derived cells using a Wnt1-Cre recombination system. Neural crest-specific inactivation of Osx resulted in the complete absence of intramembranous skeletal elements derived from the CNC, and CNC-derived endochondral skeletal elements were also affected by Osx inactivation. Interestingly, Osx inactivated CNC-derived cells, which were recapitulated by lacZ expression, occupied the same regions of craniofacial skeletal elements as observed for controls. However, cells lost their osteogenic ability to differentiate into functional osteoblasts by Osx inactivation. These results suggest that Osx is important for craniofacial bone formation by CNC-derived cells. This finding provides novel insights of the regulation of craniofacial development by the gene network and transcription factors, and the understanding of human diseases caused by neural crest developmental abnormalities.
Keywords: Osterix, Wnt1-Cre, Cranial neural crest cells, Craniofacial
1. Introduction
Neural crest cells are transient, pluripotent cells that develop into diverse cell lineages [1]. During craniofacial development, cranial neural crest (CNC) cells, derived from the lateral ridges of the neural plate, have a central role in the formation of the craniofacial mesenchyme that differentiates into craniofacial cartilage and bones [2–5]. CNC cells also contribute to the morphogenesis of thymus, mandibles, and teeth. CNC cells are known to migrate along defined pathways to form mesenchymal structures and to differentiate into a variety of cell types, which include chondrocytes, osteoblasts, and odontoblasts in the head and neck [6,7]. Most craniofacial skeletal elements originate from the neural crest and form via intramembranous or endochondral bone formation. The former process involves the direct differentiation of condensed mesenchymal cells into functional osteoblasts, whereas the latter involves the condensation of mesenchymal cells to form a cartilaginous template, which is then replaced by osteoblastic cells with infiltrating blood vesselsand converted into bone [8]. CNC cells contribute to both of these processes during craniofacial development. Neural crest development is regulated by gene networks that include transcription factors. However, because of a lack of marker genes, our understanding of the molecular mechanisms underlying CNC cell lineages and neural crest formation, and the craniofacial developmental process remains unclear.
The transcription factor Osterix (Osx) plays an essential role in bone formation [9]. It is first expressed in differentiating chondrocytes and continuously expressed in osteoblast progenitor cells and in all developing bones [9–11]. In Osx homozygous null mutants, no bone formation occurs in both intramembranous and endochondral ossification due to an arrest of osteoblast differentiation. In osteoblast-specific Osx conditional knockout mice, impaired intramembranous and endochondral ossification was observed to delay osteoblast maturation and cause an accumulation of immature osteoblasts [10]. Chondrocyte-specific Osx deficiency resulted in the impairment of chondrocyte differentiation and endochondral ossification [12]. In the present study, we conditionally inactivated the Osx gene specifically in neural crest cells using Wnt1-Cre, which contains an active Cre recombinase in a CNC-derived sub-population [2,5]. Osx conditional null mutants revealed obvious craniofacial bone developmental failure. In particular, these mutants completely lacked intramembranous skeletal elements, including mandibles, and exhibited the impaired endochondral skeletal elements, suggesting that Osx is a marker gene required for craniofacial bone formation by CNC-derived cells.
2. Materials and methods
2.1. Animals and genotyping
To cause neural crest-specific inactivation of Osx, conditional Osx mice harboring a floxed allele (Osxflox/+) [10], Osx heterozygous mice Osx+/− with a LacZ knock-in in the Osx locus (Osx+/−) [9], and Wnt1-Cre transgenic mice [2] were used. During the first cross, Wnt1-Cre transgenic mice were mated with Osx+/− to generate Osx+/−; Wnt1-Cre. Osx+/−; Wnt1-Cre male mice were then crossed with Osxflox/+ female mice to obtain Osxflox/+, Osxflox/−, Osxflox/+; Wnt1-Cre, and Osxflox/−; Wnt1-Cre. Genotypes of animals were determined by PCR using genomic DNA from yolk sacs or skin. The primer sets used and the PCR genotyping reactions were previously described [10]. All animal experiments were conducted after obtaining approval from Kyungpook National University.
2.2. Skeletal preparation and histological analysis
Skeletal preparations were performed on skeletons from newborns. Briefly, skin and organs were removed, and skeletons were fixed in 95% ethanol and stained with alcian blue for cartilage and alizarin red S for bone. The stained skeletons were then cleared in 1% KOH containing 20% glycerol and observed under a stereomicroscope (Leica, Germany). For histological analysis, craniofacial bones of 15.5 days post coitum (dpc) and newborns were fixed in 4% paraformaldehyde (PFA), embedded in paraffin, sectioned at 6 μm, and stained with hematoxylin and eosin (H and E), and alcian blue. Von Kossa staining was performed to detect calcified tissues.
2.3. X-gal staining
X-gal staining of whole-mount craniofacial samples of newborn mice was performed as described previously [13]. Briefly, craniofacial samples were fixed for 1 h in a fixation solution containing 0.1 mol/L phosphate buffer (pH 7.5), 0.2% glutaraldehyde, 0.8% formaldehyde, 2 mM MgCl2, and 5 mM EGTA (pH 8.0). Then, the samples were washed with 0.1 mol/L phosphate buffer (pH 7.3) containing 2 mmol/L MgCl2, 0.2% Nonidet P-40, and 0.1% sodium deoxycholate. They were reacted with X-gal staining solution containing 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 1 mg/ml X-gal overnight in the dark, washed in 1×phosphate-buffered saline (PBS, pH 7.8) containing 10 mmol/L EDTA (pH 8.0), and refixed in 4% formaldehyde/1× PBS (pH 7.8) in the dark at 4 °C. For histological analysis, X-gal-stained samples were dehydrated, embedded in paraffin, sectioned at 10 μm, and counterstained with nuclear fast red.
3. Results
3.1. Conditional inactivation of the Osx gene by Wnt1-Cre
To inactivate Osx in neural crest cells, conditional Osx floxed mice and Wnt1-Cre transgenic mice were used in the mating scheme described in Section 2. Osxflox/+, Osxflox/−, and Osxflox/+; Wnt1-Cre littermates were also generated and used as controls for Osxflox/−; Wnt1-Cre. All control animals revealed the same phenotype (data not shown). In a previous study, Cre recombinase in Wnt1-Cre was active from embryonic day 8.5, and its activation was limited to migrating neural crest cells [2]. Wnt1-Cre transgenic mice do not exhibit any specific phenotypic alteration [2]. Newborn Osxflox/−; Wnt1-Cre were born at the expected Mendelian frequency, but they were not viable due to breathing difficulties and an inability to suckle.
3.2. Alterations of craniofacial bones in conditional Osx mutants by Wnt1-Cre
In newborn Osxflox/−; Wnt1-Cre mutants, obvious craniofacial deformities were observed by gross appearance and skeletons which were stained by alcian blue for cartilage and alizarin red for bone (Fig. 1). Whereas Osxflox/− controls revealed normal craniofacial bones, Osxflox/−; Wnt1-Cre exhibited the complete absence of cranial neural crest (CNC)-derived intramembranous bones, such as, frontal and nasal bones, zygomatic bone, tympanic ring, premaxilla, and maxilla (Fig. 2). Furthermore, parietal and interparietal bones were undersized in Osxflox/−; Wnt1-Cre versus Osxflox/− controls (Fig. 2). In particular, mandibles were tiny and rudimentary in Osxflox/−; Wnt1-Cre, though Meckel’s cartilage was normal (Fig. 2). A hypoplastic hyoid bone with a delay in size and mineralization was observed in Osxflox/−; Wnt1-Cre. Analysis of the cranial base in Osxflox/−; Wnt1-Cre revealed that CNC-derived endochondral bones in the prechordal region, such as, the basisphenoid and presphenoid, were smaller than in Osxflox/−, and that pterygoid processes of sphenoid bone were missing (Fig. 2). The caudal portion of the skull base, including the supraoccipital, exooccipital, and basioccipital bones, which do not originate from the neural crest, was conserved in Osxflox/−; Wnt1-Cre (Fig. 2). Moreover, although somewhat small, all cartilages, including the nasal and otic capsules, were unaffected in skeletal preparations of Osxflox/−; Wnt1-Cre (Fig. 2). No abnormalities were observed in the trunk or limbs of Osxflox/−; Wnt1-Cre (Fig. 1). These results show that Osxflox/−; Wnt1-Cre exhibited severe morphologic defects of CNC-derived craniofacial skeletal elements.
Fig. 1.
Conditional inactivation of the Osx gene by Wnt1-Cre. Gross appearance (upper) and skeletal preparation (lower) of newborn mice. Skeletons were stained with alcian blue for cartilage followed by alizarin red for bone.
Fig. 2.
Higher magnified lateral, dorsal, and basal images of the skeletons of Fig. 1. In Osxflox/−; Wnt1-Cre mutants, the morphology of overall cartilage was conserved, but frontal and nasal bones, premaxilla, maxilla, and mandible were missing. Osxflox/−; Wnt1-Cre mutants showed a progressively decreased size and mineralization in hyoid bone and rudimentary mandibles. n, nasal; f, frontal; p, parietal; ip, interparietal; s, supraoccipital; e, exooccipital; px, premaxilla; x, maxilla; md, mandible; tr, tympanic ring; z, zygomatic; bo, basioccipital; bs, basisphenoid; ps, presphenoid.
3.3. LacZ gene expression in the craniofacial bones of conditional Osx mutant
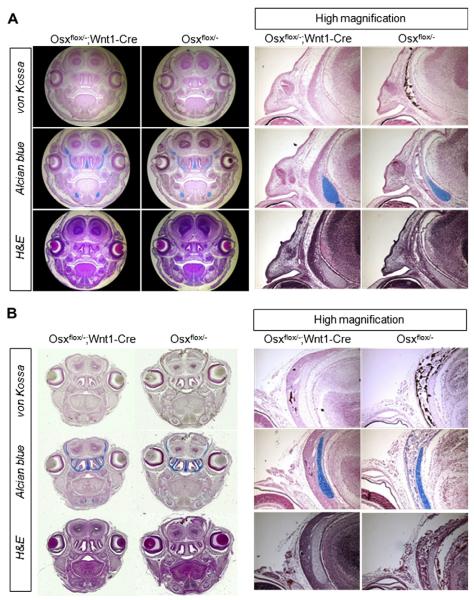
In Osx heterozygotes, the lacZ gene was inserted in the Osx locus to recapitalize the expression of Osx after Osx gene knockout [9]. Thus, the lacZ expression enabled us to visualize patterns of endogenous Osx gene expression by X-gal staining [9]. In order to observe the Osx gene expression in CNC cells, we examined the localization of lacZ expressing cells in Osxflox/− and Osxflox/−; Wnt1-Cre. In Osxflox/− controls and Osxflox/−; Wnt1-Cre mutants, which were examined in dorsal view, lacZ expression was observed in all skull bones, such as, frontal and nasal bones, parietal and interparietal bones (Fig. 3A, upper panel). Surprisingly, the strong expression of lacZ gene was observed in intramembranous bones of Osxflox/−; Wnt1-Cre mutants, such as, the frontal and nasal bones, which were totally absent in skeletal preparations shown in Fig. 2. LacZ gene expression was also observed in zygomatic bones and the mandibles of Osxflox/−; Wnt1-Cre (data not shown). Consequently, the overall pattern of lacZ expression in Osx-null mutant cells was identical to that observed for Osx heterozygous cells, even in terms of missing craniofacial skeletal elements. Next, the mineral bone deposition, originally detected by von Kossa staining, was histologically examined in whole mount X-gal stained skull. In Osxflox/− controls, the mineral bone deposition was observed within matrix associated with lacZ expressing cells, but in lacZ expressing cells of Osxflox/−; Wnt1-Cre, von Kossa stained regions showed no mineral bone deposition (Fig. 3A, lower panel and Fig. 3B). This complete lack of mineral bone deposition was attributable to the failure of CNC-derived cells not expressing Osx to differentiate into functional osteoblasts. Our results show that no functional osteoblasts were present in Osxflox/−; Wnt1-Cre mutants in regions where craniofacial bone formation derived from CNC cells occurred in Osxflox/− controls.
Fig. 3.

Localization of lacZ expressing cells in Osxflox/−; Wnt1-Cre mutants. LacZ expressing cells were stained blue by whole mount X-gal staining (A, upper) and mineral deposition was detected by von Kossa staining in X-gal stained sections (A, lower and B). In Osxflox/− controls, mineral deposition occurred within matrix in association with lacZ expressing cells. However, mineral deposition was totally lacking in Osxflox/−; Wnt1-Cre mutants.
3.4. Histological analysis of craniofacial bone
To examine alterations in craniofacial bones, histological analysis was performed by H and E, alcian blue and von Kossa staining at 15.5 dpc and in newborns (Fig. 4). Alcian blue and von Kossa staining enabled to assess cartilage matrix and mineral bone deposition, respectively. Cartilage matrix stained by alcian blue was not significantly altered in Osxflox/− controls and Osxflox/−; Wnt1-Cre mutants. However, no mineral bone deposition was observed in the craniofacial skeletal elements of Osxflox/−; Wnt1-Cre mutants, whereas normal calcification was present in Osxflox/− controls at both times.
Fig. 4.
Histological analysis of Osxflox/−; Wnt1-Cre mutants at 15.5 dpc (A) and in newborns (B). H and E, alcian blue, and von Kossa staining were performed in coronal sections of Osxflox/− controls and Osxflox/−; Wnt1-Cre mutants. Alcian blue and nuclear fast red staining showed no change in cartilage formation in either group. However, von Kossa staining showed the complete absence of mineral deposition in Osxflox/−; Wnt1-Cre mutants at 15.5 dpc and in newborns.
4. Discussion
The skull is a unique skeletal structure consisting of cranial vault bones formed by intramembranous ossification and cranial base bones formed by endochondral ossification. Unlike other parts of the skeleton, the skull is derived from the neural crest. Neural crest cells arise in the ectoderm at the margins of the neural tube, migrate in many different locations, and then differentiate into many cell types to contribute to neurons, teeth, head, face, and other cranial features [2,14]. CNC cells, which generate most of the skull, provide patterning information for craniofacial morphogenesis [15–17], and several genes have been suggested to play important regulatory roles in craniofacial patterning by CNC cells and CNC-derived cell lineages.
Transcription factor AP-2 is expressed in CNC cells, cranial and spinal sensory ganglia, and facial mesenchyme [18]. Furthermore, AP-2 knockout mice display severe dismorphogeneses of the face, skull, and cranial closure, indicating that AP-2 is necessary for craniofacial development [19]. Members of the Dlx homeobox gene family are expressed in craniofacial ectoderm and CNC and function in the patterning of bronchial arches [20]. In addition, Dlx5 is expressed in CNC for the development of craniofacial hard tissues, and Dlx5-deficient mice show defective craniofacial structures, frontonasal development, and maxillae and mandibles, which suggests that Dlx5 is required for craniofacial development and that it regulates the patterning of craniofacial morphology through the CNC [21]. Mutations in Pax6, a crucial regulator of craniofacial development, cause craniofacial skeletal defects that arise from the arrested migration of midbrain CNC-derived cells to the lateral frontonasal region [22,23]. During craniofacial development in mouse, Osx is expressed in condensed mesenchymes of the developing frontal bone, maxilla, and mandible [9]. However, the importance of Osx in CNC-derived cells for craniofacial bone formation has not been well investigated.
In the present study, to further understand the significance of Osx in CNC-derived cells, the Osx gene was specifically inactivated by Wnt1-Cre-mediated deletion. Wnt1 is required for the anterior–posterior patterning of the central nervous system (CNS), and notably, in the CNS, CNC cells in branchial arches are wholly derived from Wnt1-expressing cells [24,25]. Thus, in the Wnt1-Cre recombination system, Cre recombinase is specifically active in the neural crest and exhibits an expression pattern identical to that of the endogenous Wnt1 gene [2,24]. The Wnt1-Cre system also provides a good tool for the analysis of neural crest development, and by using this system, several group have elucidated the functions of specific genes and the molecular mechanisms underlying CNC-derived cell lineages and of neural crest formation. For instance, Sox9 inactivation in CNC cells resulted in a complete absence of CNC-derived endochondral bones and cartilages, but did not affect any intramembranous bone [26]. CNC-derived Sox9-null cells failed to differentiate into chondrocytes, and instead expressed osteoblast marker genes [26]. These results were due to a failure of CNC cells to differentiate into chondrocytes, and suggest that Sox9 is required for determining the cell fates of chondrocyte and osteoblast lineages during CNC-derived endochondral ossification. However, Sox9 is not needed for the osteoblast lineage during CNC-derived intramembranous ossification [26]. Alagille syndrome caused by mutations in the Jagged1 gene exhibits craniofacial anomalies [27]. The requirement for Jagged1 in CNC cell populations during craniofacial development was investigated using CNC cell lineage-specific Jagged1 conditional knockout models. Jagged1 inactivation in CNC cells caused the craniofacial abnormalities observed in Alagille syndrome, including midfacial hypoplasia and aberrant craniofacial growth [27]. This result demonstrates that the Jagged1 plays an important role in the CNC during craniofacial formation. The conditional inactivation of Tgfβr2 in the CNC resulted in severe skull defects, cleft palate, small mandibles and maxillae, and missing frontal and retarded parietal bones in mice [28,29]. Furthermore, in this conditional knockout model, FGF2, a downstream gene of TGFβ signaling, rescued impaired cell proliferation in frontal bone [30]. These results demonstrate that TGFβ signaling is essential for the regulation of the fate of CNC cells during craniofacial development. In the present study, conditional inactivation of Osx in CNC-derived cells was achieved using a Wnt1-Cre recombination system and conditional Osx inactivation in CNC cells caused severe defects in craniofacial bone development, including the complete absence of intramembranous skeletal elements and the impairment of endochondral skeletal elements. These results suggest that Osx is required for craniofacial bone formation by CNC-derived cells. Moreover, in conditional Osx null mutants in CNC cells, Osx inactivated CNC-derived cells occupied the same region as in the control. However, although they migrated to the correct locations, they failed to differentiate into functional osteoblasts to generate mineral bone matrix. This finding indicates that Osx inactivated CNC-derived cells do not differentiate into mature osteoblasts and cannot participate in the formation of craniofacial bones. Taken together, this study provides novel insights of the regulation of craniofacial development by network via the osteoblast-specific transcription factor Osx and enhances our understanding of human diseases caused by neural crest developmental abnormalities.
Acknowledgments
This work was supported by Basic Science Research Program through The National Research Foundation of Korea (NRF) funded by The Ministry of Education, Science and Technology (2012R1A1A2007161 and 2012R1A6A3A01017109).
References
- [1].Huang X, Saint-Jeannet JP. Induction of the neural crest and the opportunities of life on the edge. Dev. Biol. 2004;275:1–11. doi: 10.1016/j.ydbio.2004.07.033. [DOI] [PubMed] [Google Scholar]
- [2].Chai Y, Jiang X, Ito Y, Bringas P, Jr., Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- [3].Cerny R, Lwigale P, Ericsson R, Meulemans D, Epperlein HH, Bronner-Fraser M. Developmental origins and evolution of jaws: new interpretation of “maxillary” and “mandibular”. Dev. Biol. 2004;276:225–236. doi: 10.1016/j.ydbio.2004.08.046. [DOI] [PubMed] [Google Scholar]
- [4].Taneyhill LA. To adhere or not to adhere: the role of Cadherins in neural crest development. Cell Adhes. Migr. 2008;2:223–230. doi: 10.4161/cam.2.4.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jiang X, Sachiko I, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev. Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- [6].Bronner-Fraser M. Origins and developmental potential of the neural crest. Exp. Cell Res. 1995;218:405–417. doi: 10.1006/excr.1995.1173. [DOI] [PubMed] [Google Scholar]
- [7].Hall BK. The Neural Crest in Development and Evolution. Springer; New York: 1999. [Google Scholar]
- [8].Olsen BR, Reginato AM, Wang W. Bone development. Annu. Rev. Cell Dev. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- [9].Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng J, Behringer R, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- [10].Baek WY, Lee MA, Jung JW, Kim SY, Akiyama H, de Crombrugghe B, Kim JE. Positive regulation of adult bone formation by osteoblast-specific transcription factor Osterix. J. Bone Miner. Res. 2009;24:1055–1065. doi: 10.1359/jbmr.081248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baek WY, de Crombrugghe B, Kim JE. Postnatally induced inactivation of Osterix in osteoblasts results in the reduction of bone formation and maintenance. Bone. 2010;46:920–928. doi: 10.1016/j.bone.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Oh JH, Park SY, de Crombrugghe B, Kim JE. Chondrocyte-specific ablation of Osterix leads to impaired endochondral ossification. Biochem. Biophys. Res. Commun. 2012;418:634–640. doi: 10.1016/j.bbrc.2012.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim JE, Nakashima K, de Crombrugghe B. Transgenic mice expressing a ligand-inducible cre recombinase in osteoblasts and odontoblasts: a new tool to examine physiology and disease of postnatal bone and tooth. Am. J. Pathol. 2004;165:1875–1882. doi: 10.1016/S0002-9440(10)63240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Trainor P, Krumlauf R. Plasticity in mouse neural crest cells reveals a new patterning role for cranial mesoderm. Nat. Cell Biol. 2000;2:96–102. doi: 10.1038/35000051. [DOI] [PubMed] [Google Scholar]
- [15].Le Douarin NM, Kalcheim C. The Neural Crest. second ed Cambridge University Press; Cambridge: 1982. [Google Scholar]
- [16].Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993;117:409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- [17].D’Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am. J. Anat. 1983;166:445–468. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- [18].Mitchell PJ, Timmons PM, Hébert JM, Rigby PW, Tjian R. Transcription factor AP-2 is expressed in neural crest cell lineages during mouse embryogenesis. Genes Dev. 1991;5:105–119. doi: 10.1101/gad.5.1.105. [DOI] [PubMed] [Google Scholar]
- [19].Schorle H, Meier P, Buchert M, Jaenisch R, Mitchell PJ. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature. 1996;381:235–238. doi: 10.1038/381235a0. [DOI] [PubMed] [Google Scholar]
- [20].Qiu M, Bulfone A, Ghattas I, Meneses JJ, Christensen L, Sharpe PT, Presley R, Pedersen RA, Rubenstein JLR. Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: mutations of Dlx-1, Dlx-2, and Dlx-1 and -2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Dev. Biol. 1997;185:165–184. doi: 10.1006/dbio.1997.8556. [DOI] [PubMed] [Google Scholar]
- [21].Depew MJ, Liu JK, Long JE, Presley R, Meneses JJ, Pedersen RA, Rubenstein JLR. Dlx5 regulates regional development of the branchial arches and sensory capsules. Development. 1999;126:3831–3846. doi: 10.1242/dev.126.17.3831. [DOI] [PubMed] [Google Scholar]
- [22].Depew MJ, Tucker AS, Sharpe PT. Craniofacial development. In: Rossant J, Tam PPL, editors. Mouse Development: Patterning, Morphogenesis, and Organogenesis. Academic Press; San Diego: 2002. pp. 421–498. [Google Scholar]
- [23].Compagnucci C, Fish JL, Schwark M, Tarabykin V, Depew MJ. Pax6 regulates craniofacial form through its control of an essential cephalic ectodermal patterning center. Genesis. 2011;49:307–325. doi: 10.1002/dvg.20724. [DOI] [PubMed] [Google Scholar]
- [24].McMahon AP, Joyner AL, Bradley A, McMahon JA. The midbrain–hindbrain phenotype of Wnt-1-/Wnt-1-mice results from stepwise deletion of engrailed-expressing cells by 9.5 days post-coitum. Cell. 1992;69:581–595. doi: 10.1016/0092-8674(92)90222-x. [DOI] [PubMed] [Google Scholar]
- [25].Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen inducible form of Cre recombinase. Curr. Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- [26].Mori-Akiyama Y, Akiyama H, Rowitch DH, de Crombrugghe B. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc. Natl. Acad. Sci. USA. 2003;100:9360–9365. doi: 10.1073/pnas.1631288100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Humphreys R, Zheng W, Prince LS, Qu X, Brown C, Loomes K, Huppert SS, Baldwin S, Goudy S. Cranial neural crest ablation of Jagged1 recapitulates the craniofacial phenotype of Alagille syndrome patients. Hum. Mol. Genet. 2012;15:1374–1383. doi: 10.1093/hmg/ddr575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ito Y, Yeo JY, Chytil A, Han J, Bringas P, Jr, Nakajima A, Shuler CF, Moses HL, Chai Y. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development. 2003;130:5269–5280. doi: 10.1242/dev.00708. [DOI] [PubMed] [Google Scholar]
- [29].Chai Y, Ito Y, Han J. TGF-β signaling and its functional significance in regulating the fate of cranial neural crest cells. Crit. Rev. Oral Biol. Med. 2003;14:78–88. doi: 10.1177/154411130301400202. [DOI] [PubMed] [Google Scholar]
- [30].Sasaki T, Ito Y, Bringas P, Jr., Chou S, Urata MM, Slavkin H, Chai Y. TGFβ-mediated FGF signaling is crucial for regulating cranial neural crest cell proliferation during frontal bone development. Development. 2005;133:371–381. doi: 10.1242/dev.02200. [DOI] [PubMed] [Google Scholar]