Abstract
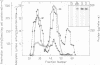
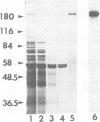
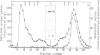
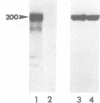
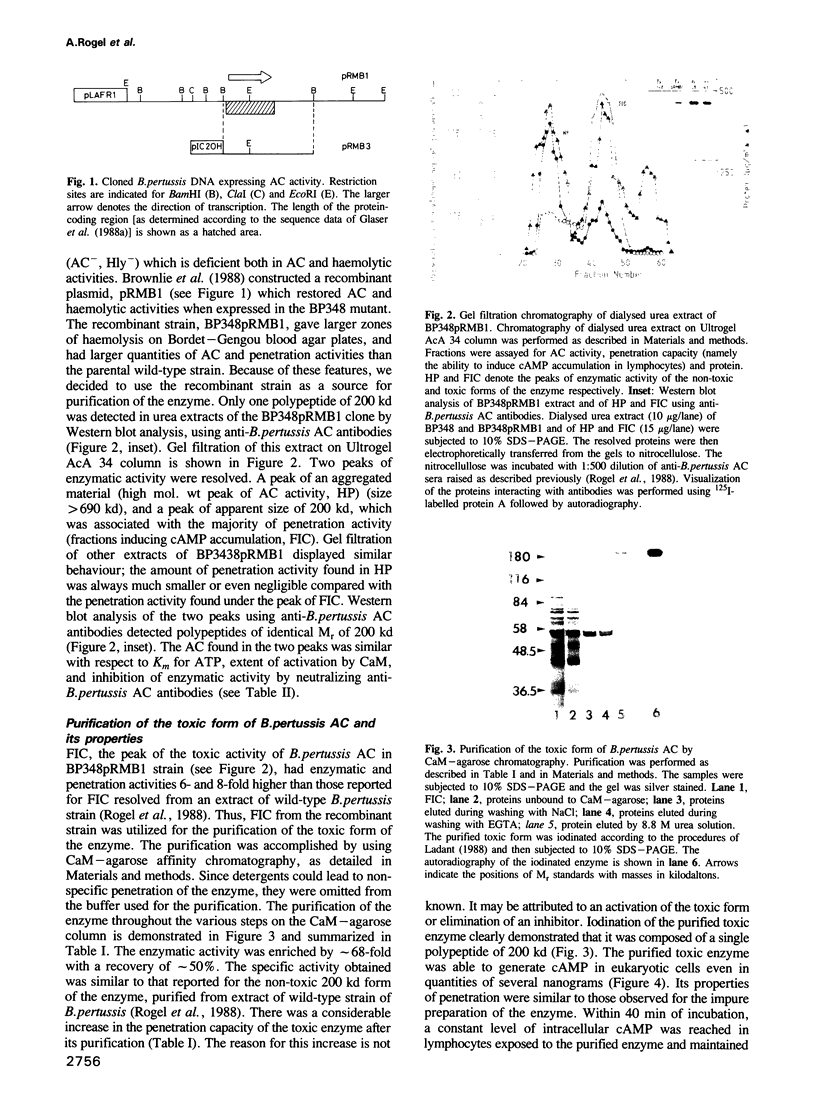
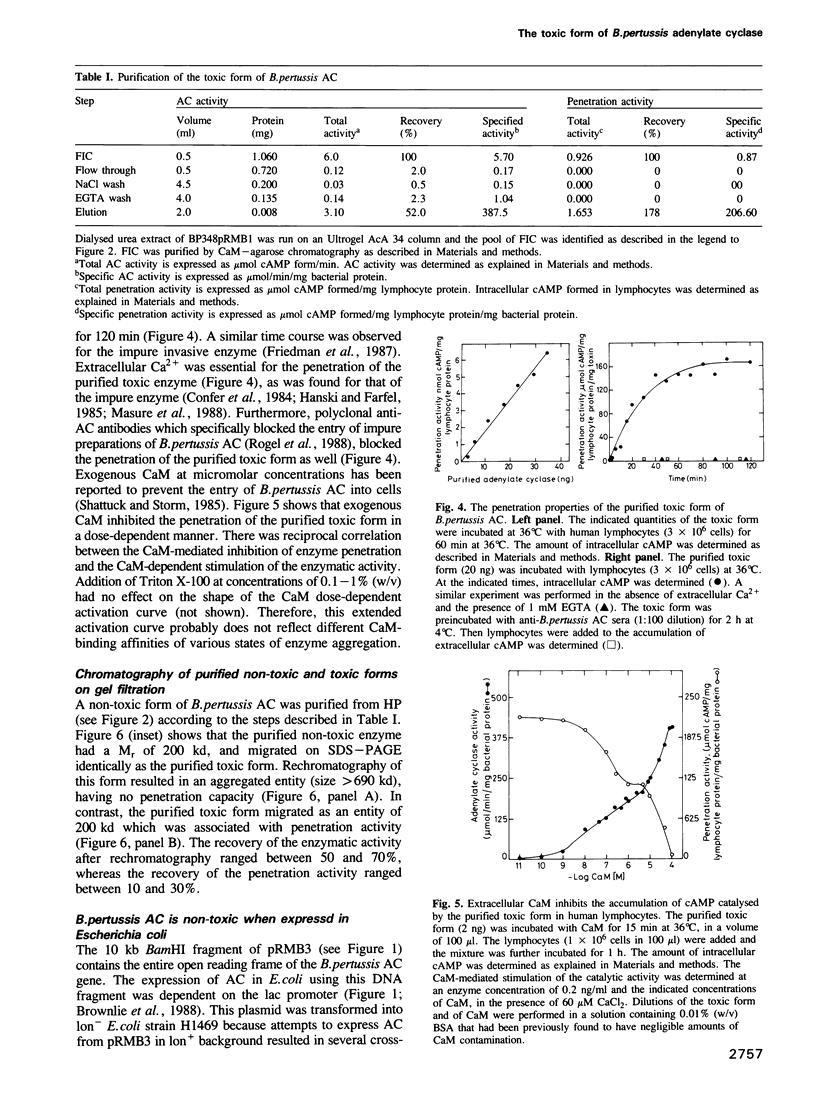
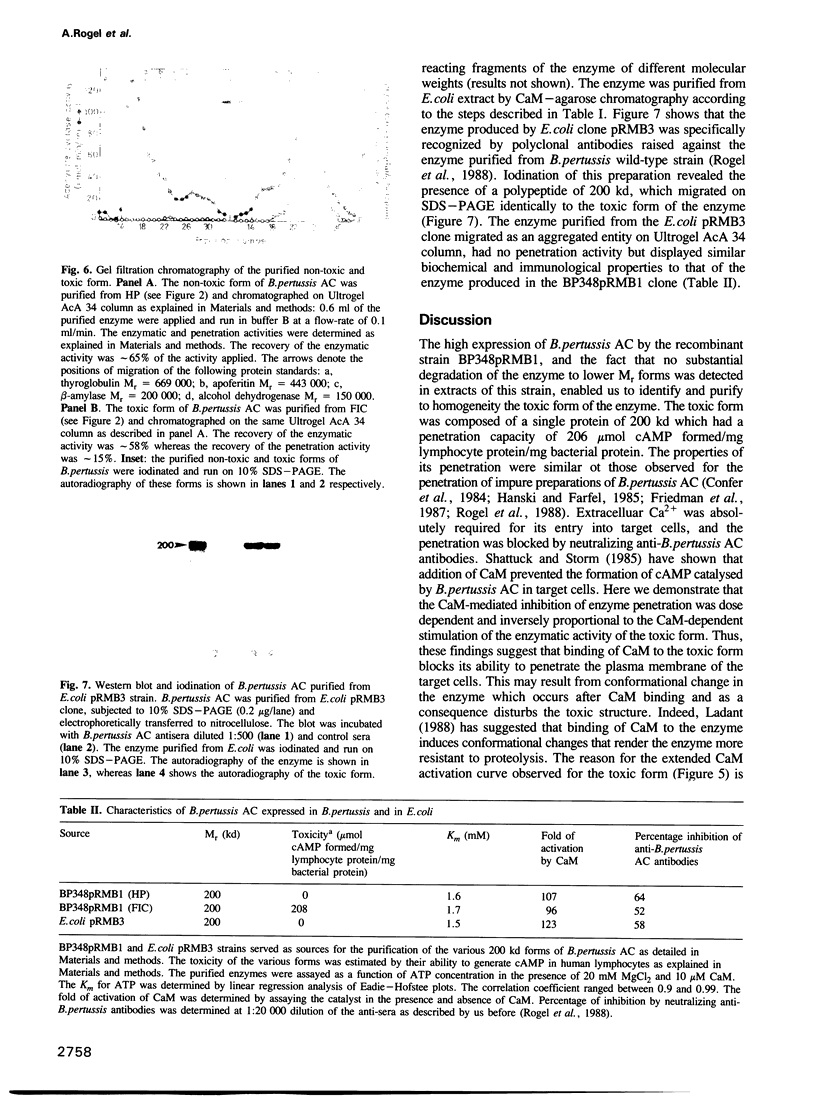
Bordetella pertussis produces a calmodulin-sensitive adenylate cyclase (AC) which is an essential virulence factor in mammalian pertussis. Here we report the purification and characterization of the toxic form of the enzyme, which penetrates eukaryotic cells and generates high levels of intracellular cAMP. This form was purified from an extract of B.pertussis strain carrying a recombinant plasmid which over-produced both enzymatic and toxic activities of the enzyme. Western blot analysis of the extract using anti-B.pertussis AC antibodies detected only one protein of 200 kd. However, gel filtration of the extract resolved two peaks of enzymatic activity. The first peak of aggregated material contained greater than 70% of the total enzymatic activity, and the second peak contained the majority of the toxic activity. Purification of the enzyme from both peaks yielded proteins of 200 kd, with similar biochemical and immunological properties. Yet only the enzyme purified from the second peak could penetrate human lymphocyte and catalyse the formation of intracellular cAMP. B.pertussis AC gene expressed in Escherichia coli produced a calmodulin-dependent enzyme of 200 kd, which lacked lymphocyte penetration capacity. It is proposed that a post-translational modification that occurs in B.pertussis but not in E.coli confers upon the 200 kd protein of B.pertussis AC the toxic properties.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brownlie R. M., Coote J. G., Parton R., Schultz J. E., Rogel A., Hanski E. Cloning of the adenylate cyclase genetic determinant of Bordetella pertussis and its expression in Escherichia coli and B. pertussis. Microb Pathog. 1988 May;4(5):335–344. doi: 10.1016/0882-4010(88)90061-7. [DOI] [PubMed] [Google Scholar]
- Confer D. L., Eaton J. W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- Confer D. L., Slungaard A. S., Graf E., Panter S. S., Eaton J. W. Bordetella adenylate cyclase toxin: entry of bacterial adenylate cyclase into mammalian cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:183–187. [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Friedman E., Farfel Z., Hanski E. The invasive adenylate cyclase of Bordetella pertussis. Properties and penetration kinetics. Biochem J. 1987 Apr 1;243(1):145–151. doi: 10.1042/bj2430145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L. Pertussis: the disease and new diagnostic methods. Clin Microbiol Rev. 1988 Oct;1(4):365–376. doi: 10.1128/cmr.1.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Ladant D., Sezer O., Pichot F., Ullmann A., Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):19–30. [PubMed] [Google Scholar]
- Glaser P., Sakamoto H., Bellalou J., Ullmann A., Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988 Dec 1;7(12):3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E., Farfel Z. Bordetella pertussis invasive adenylate cyclase. Partial resolution and properties of its cellular penetration. J Biol Chem. 1985 May 10;260(9):5526–5532. [PubMed] [Google Scholar]
- Hewlett E. L., Urban M. A., Manclark C. R., Wolff J. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1926–1930. doi: 10.1073/pnas.73.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessin R. H., Franke J. Secreted adenylate cyclase of Bordetella pertussis: calmodulin requirements and partial purification of two forms. J Bacteriol. 1986 Apr;166(1):290–296. doi: 10.1128/jb.166.1.290-296.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilhoffer M. C., Cook G. H., Wolff J. Calcium-independent activation of adenylate cyclase by calmodulin. Eur J Biochem. 1983 Jun 1;133(1):11–15. doi: 10.1111/j.1432-1033.1983.tb07423.x. [DOI] [PubMed] [Google Scholar]
- Ladant D., Brezin C., Alonso J. M., Crenon I., Guiso N. Bordetella pertussis adenylate cyclase. Purification, characterization, and radioimmunoassay. J Biol Chem. 1986 Dec 5;261(34):16264–16269. [PubMed] [Google Scholar]
- Ladant D. Interaction of Bordetella pertussis adenylate cyclase with calmodulin. Identification of two separated calmodulin-binding domains. J Biol Chem. 1988 Feb 25;263(6):2612–2618. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackman N., Nicaud J. M., Gray L., Holland I. B. Secretion of haemolysin by Escherichia coli. Curr Top Microbiol Immunol. 1986;125:159–181. doi: 10.1007/978-3-642-71251-7_10. [DOI] [PubMed] [Google Scholar]
- Masure H. R., Oldenburg D. J., Donovan M. G., Shattuck R. L., Storm D. R. The interaction of Ca2+ with the calmodulin-sensitive adenylate cyclase from Bordetella pertussis. J Biol Chem. 1988 May 15;263(14):6933–6940. [PubMed] [Google Scholar]
- Masure H. R., Shattuck R. L., Storm D. R. Mechanisms of bacterial pathogenicity that involve production of calmodulin-sensitive adenylate cyclases. Microbiol Rev. 1987 Mar;51(1):60–65. doi: 10.1128/mr.51.1.60-65.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masure H. R., Storm D. R. Characterization of the bacterial cell associated calmodulin-sensitive adenylate cyclase from Bordetella pertussis. Biochemistry. 1989 Jan 24;28(2):438–442. doi: 10.1021/bi00428a005. [DOI] [PubMed] [Google Scholar]
- Nicaud J. M., Mackman N., Gray L., Holland I. B. Characterisation of HlyC and mechanism of activation and secretion of haemolysin from E. coli 2001. FEBS Lett. 1985 Aug 5;187(2):339–344. doi: 10.1016/0014-5793(85)81272-2. [DOI] [PubMed] [Google Scholar]
- Rogel A., Farfel Z., Goldschmidt S., Shiloach J., Hanski E. Bordetella pertussis adenylate cyclase. Identification of multiple forms of the enzyme by antibodies. J Biol Chem. 1988 Sep 15;263(26):13310–13316. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Shattuck R. L., Storm D. R. Calmodulin inhibits entry of Bordetella pertussis adenylate cyclase into animal cells. Biochemistry. 1985 Nov 5;24(23):6323–6328. doi: 10.1021/bi00344a001. [DOI] [PubMed] [Google Scholar]
- Wagner W., Kuhn M., Goebel W. Active and inactive forms of hemolysin (HlyA) from Escherichia coli. Biol Chem Hoppe Seyler. 1988 Jan;369(1):39–46. doi: 10.1515/bchm3.1988.369.1.39. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- Wolff J., Cook G. H., Goldhammer A. R., Berkowitz S. A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]