Abstract
Rheumatoid arthritis is an autoimmune disease that primarily affects the limbs, but the pathogenic mechanism remains unclear. γδ T cells, a T-cell subpopulation, are characterized by multiple biological functions and associated with a variety of diseases. This study investigated the antigen-presenting effects of γδ T cells and their relationship with rheumatoid arthritis development. We found that Vγ9Vδ2 T cells (the predominant subtype of γδ T cells in peripheral blood) were activated by isopentenyl pyrophosphate to continuously proliferate and differentiate into effector memory cells. The effector memory Vγ9Vδ2 T cells exhibited phenotypic characteristics of specific antigen-presenting cells, including high HLA-DR and CD80/86 expression. These Vγ9Vδ2 T cells could present soluble antigens and synthetic peptides to CD4+ T cells. Vγ9Vδ2 T cells with different phenotypes showed different cytokine secretion patterns. Effector memory Vγ9Vδ2 T cells simultaneously secreted not only interferon (IFN)-γ but also IL-17. The peripheral blood and joint synovial fluid from RA patients contained numerous heterogeneous γδ T cells that were predominantly effector memory Vγ9Vδ2 T cells with the ability to secrete inflammatory factors. We also found that γδ T cells had a similar antigen-presenting capability to B cells. These results suggest that during the development of rheumatoid arthritis, γδ T cells can aggravate immune dysfunction and produce abnormal immune damage by secreting cytokines and inducing inflammatory cells to participate in synergistic inflammatory responses. Furthermore, γδ T cells can behave similarly to B cells to present viral peptides and autoantigen peptides to CD4+ T cells, thus sustaining CD4+ T-cell activation.
Keywords: antigen-presenting function, rheumatoid arthritis, γδ T cell, TEM Vγ9Vδ2 T cell
Introduction
Rheumatoid arthritis (RA) is a common and systemic autoimmune disease predominantly affecting the limbs. The prevalence of RA worldwide is 0.5%–1%, with 4 000 000 new cases reported every year. The development of RA is mediated by immune system dysfunction, although the exact pathogenic mechanisms remain unclear. In particular, the hypothesis that latent Epstein–Barr virus (EBV) in susceptible individuals with particular genetic backgrounds (e.g., HLA-DRB1*01 or HLA-DRB1*04) is repeatedly activated and leads to RA has been intensively studied.1 Previous studies showed that there are close correlations between the HLA-DR4-positive genetic background and the occurrence and development of RA.2, 3, 4 Additionally, studies on ‘molecular mimicry' suggested that EBVgp110 has a common motif with HLA-DR4 and thus can induce and activate autoreactive T cells and cause a series of autoimmune responses and pathologic damage.5 Although the roles of abnormal T/B cells in RA have been extensively investigated by a large number of researchers,6, 7, 8, 9, 10 a number of papers since the 1990s have revealed that γδ T cells with abnormal functions exist in the joints and that these cells characteristically express HLA-DR, suggesting that these γδ T cells may play unknown biological roles in RA.11, 12 Recent studies also suggested that γδ T cells are an important source of IL-17 and are involved in multiple diseases via their secretion of IL-17.13, 14, 15
γδ T cells, a small subpopulation of T cells, can be divided into several subtypes according to different combinations of the T-cell receptor (TCR) γ- and δ-chains, and they exhibit different biological characteristics because of the heterogeneity among the subtypes.16, 17, 18, 19 The small number of γδ T cells in peripheral blood makes them difficult to investigate. We have extensively studied a variety of ways to amplify γδ T cells to study their differentiation and immunological functions. γδ T cells are known to play important roles in infection, immunity, autoimmunity and tumor immunity20, 21, 22, 23, 24 and therefore, to some extent, can be regarded as pluripotent cells. These cells are involved in various human diseases and have substantial clinical implications. Our study investigated the latent antigen-presenting function of γδ T cells and their association with RA pathogenesis.
Materials and methods
Healthy donors and RA patients
Peripheral blood samples were taken from healthy donors 20–25 years of age that met the following criteria: no flu symptoms (e.g., fever or cough); negative blood, urine and fecal examinations; no chronic diseases such as hypertension, diabetes, kidney disease, heart disease or liver diseases; and no medical history or family history of autoimmune diseases. RA patients were recruited from Guanghua Rheumatoid Arthritis Specialized Hospital (Shanghai, China) with the approval from the Independent Ethics Committee and signed the informed consent forms themselves. The selection criteria are listed in Table 1.
Table 1. RA patients screening standards.
| A. >18 years, meeting the American College of Rheumatology (ACR) diagnostic criteria | |
| B. Has developed RA for >6 months but <8 months per year | |
| C. Any three of four | At least eight joints with tenderness |
| Scoring by clinician: 2–4 (full score: 5) | |
| Self-scoring patient: 2–4 (full score: 5) | |
| Serum C-reactive protein >15 mg/dl | |
| D. Laboratory examination | Hemoglobin (HB) ≥5.5 mmol/l |
| White blood cells (WBC) ≥3.5×109/l | |
| Platelets ≥100×109/l | |
| Serum bilirubin, aspartate amino transferase (AST), alanine amino transferase (ALT) and alkaline phosphatase (AKP), <1.5× the normal upper limit; serum creatinine ≤150 µmol/l | |
Cell isolation and culture
Density gradient centrifugation was used to isolate peripheral blood mononuclear cells (PBMCs) and synovial fluid mononuclear cells (SFMCs). Magnetic-activated cell sorting was performed according to the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany) to isolate CD4+ T cells and B cells from the PBMCs. Fluorescence-activated cell sorting (FACS) was used to isolate the Vγ9Vδ2 T cells. After the isolation of CD4+ T and B cells from the PBMCs of healthy donors, the remaining PBMCs or other cell populations were diluted to 1×106 cells/ml with RPMI-1640 containing 10% fetal bovine serum (FBS), isopentenylpyrophosphate (IPP, 2 µg/ml) and low concentrations (5 U/ml) of IL-2, IL-7, IL-15 and/or IL-23. The cells were then cultured in 24-well sterile plates (1 ml/well) in a saturated humid environment containing 5% CO2 at 37 °C. Every 3 days, the cells were collected and labeled to detect their phenotypes, and half of the culture medium was exchanged. Vγ9Vδ2 T cells were sorted at appropriate time points.
Flow cytometry
Extracellular staining
Flow cytometry was used to analyze the purity of the target cells after sorting by magnetic-activated cell sorting, the ratios of γδ T cells in the peripheral blood and synovial fluid of healthy donors and RA patients, the distribution and subtypes of Vγ9Vδ2 T cells and the phenotypic changes of Vγ9Vδ2 T cells after IPP induction. After rinsing with phosphate-buffered saline (PBS), the cells (1×106) were resuspended in PBS supplemented with 1% FBS that contained the following fluorescently labeled monoclonal antibodies or their isotype control antibodies: γδ-TCR, δ2-TCR, γ9-TCR, CD3, CD8, CD27, CD45RA, CD45RO, CD69, CD80, CD86 and/or HLADR. After incubation at 4 °C in the dark for 30 min, the cells were rinsed twice with PBS, fixed with 300 µl of PBS plus 1% paraformaldehyde and detected using a flow cytometer (BD FACS Calibur; BD Biosciences, Bedford, MA, USA). The results were analyzed with the BD Cellquest software (BD Biosciences).
Intracellular staining
The cytokine secretion patterns of the Vγ9Vδ2 T cells were analyzed by flow cytometry. Cells were harvested (1×106) before and after culturing and were rinsed with RPMI-1640. The cells were resuspended with 1 ml of RPMI-1640 culture medium containing 100 ng/ml phorbol 12-myristate 13-acetate, 0.75 µg/ml ionomycin and 1.5 µl/ml GolgiStop (BD Biosciences) and were cultured for 4 h. The cells were then rinsed with PBS plus 1% FBS, resuspended with 100 µl of PBS plus 1% FBS plus fluorescently labeled monoclonal antibodies against δ2-TCR and γ9-TCR or isotype control antibodies and stained extracellularly at 4 °C in the dark for 30 min. After two rinses with PBS plus 1% FBS, 250 µl of the fixative/membrane-rupturing buffer solution was added and mixed, and the cells were incubated at 4 °C in the dark for 20 min. After two more rinses with PBS plus 1% FBS, the cells were resuspended with 100 µl of PBS plus 1% FBS plus either fluorescently labeled monoclonal antibodies against IL-17 and interferon (IFN)-γ or isotype control antibodies and incubated at 4 °C in the dark for 30 min. Finally, the cells were rinsed twice with PBS plus 1% FBS, fixed with PBS plus 1% paraformaldehyde and detected by the BD FACS Calibur. The results were analyzed with BD Cellquest software.
Peptides synthesis
We commissioned the GL Biochem Ltd (Shanghai, China) to synthesize the three following peptides:
tetanus toxoid peptide (TTp) sequences: QYIKANSKFIGITE;
EBVgp110 sequences: CEQNQEQKRAAQRAA;
HLA-DR4 sequences: CKDLLEQKRAAVDTY.
Cellular proliferation
The 3H-TdR method was used to test the antigen-presenting efficacies of the different subtypes of the Vγ9Vδ2 T cells from the peripheral blood of healthy donors to purified protein derivative (PPD) and tetanus toxin (TT), the effector memory Vγ9Vδ2 T cells to synthetic peptides and the Vγ9Vδ2 T cells from RA patients to viral peptides and autoantigen peptides. B cells were used as controls.
Cytokine assays
The IL-17 and IFN-γ levels in the supernatant of the γδ T cell culture at different stages and in the serum and joint synovial fluid from RA patients were detected using a Quantikine ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
Statistical analysis
The paired Student's t-test (two-tailed) was used for the comparison of group values. The data were presented as mean±s.d. The significance was analyzed using Prism (GraphPad Software), and the values of P<0.05 were considered significant and are indicated in the corresponding figures (*, 0.01<P<0.05; **, 0.001<P<0.01; ***, P<0.001).
Results
Induction effect of IPP on γδ T cells
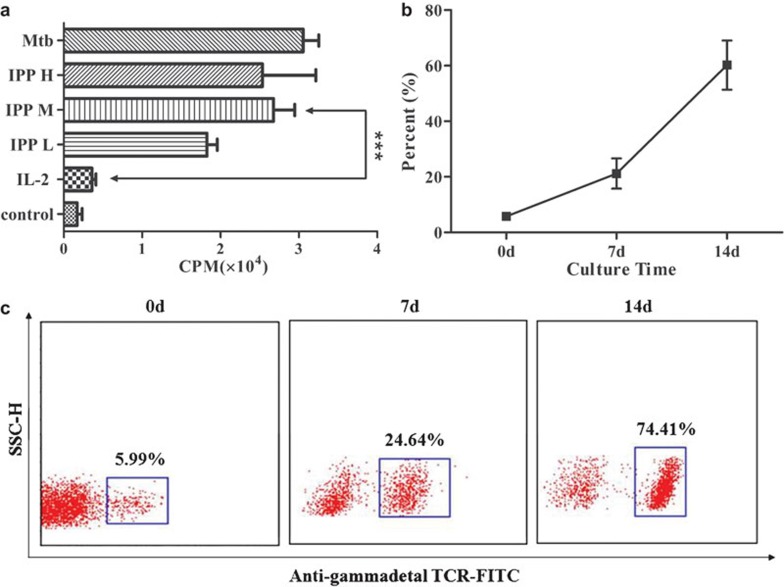
Previous studies have reported that γδ T cells can directly recognize some irregular antigen ligands such as the phosphorylated metabolites of microbiological organisms and lipid antigens (e.g., IPP, HMBPP and BrHPP) via TCRs,21, 25, 26 and could be activated directly without the assistance of antigen-presenting cells. Our results indicated that IPP was able to stimulate γδ T cells to proliferate at a certain concentration range. Furthermore, IL-2 can stimulate the proliferation of γδ T cells either alone or synergistically with IPP; the highest proliferation was seen when the γδ T cells were cultured with 2 µg/ml IPP and 5 U/ml IL-2. Under the synergic effect of IPP and IL-2, γδ T cells (primarily Vγ9Vδ2 T cells) can be selectively activated and stimulated to proliferate, resulting in an increased ratio of T lymphocytes to total PBMCs (Figure 1). Additionally, we also used IL-7, IL-15, and IL-23 to culture the γδ T cells but did not find a significant influence of these cytokines to affect the proliferation and differentiation potential of γδ T cells.
Figure 1.
The IPP induced proliferation of γδ T cells. (a) IPP (IPP H, 5 µg/ml; IPP M, 2 µg/ml; IPP L, 0.8 µg/ml and in combination with 5 U/ml IL-2, IL-7, IL-15, IL-23) induced a massive proliferation of γδ T cells and had the equivalent effect with mycobacterium tuberculosis polypeptide antigen (Mtb)27 without a statistical difference. (b) Flow cytometry results indicated a gradual increase of γδ T cells relative to the total number of T cells. (c) A typical case of γδ T cell proliferation is shown (gated on CD3+ T cells). IPP, isopentenylpyrophosphate; TCR, T-cell receptor.
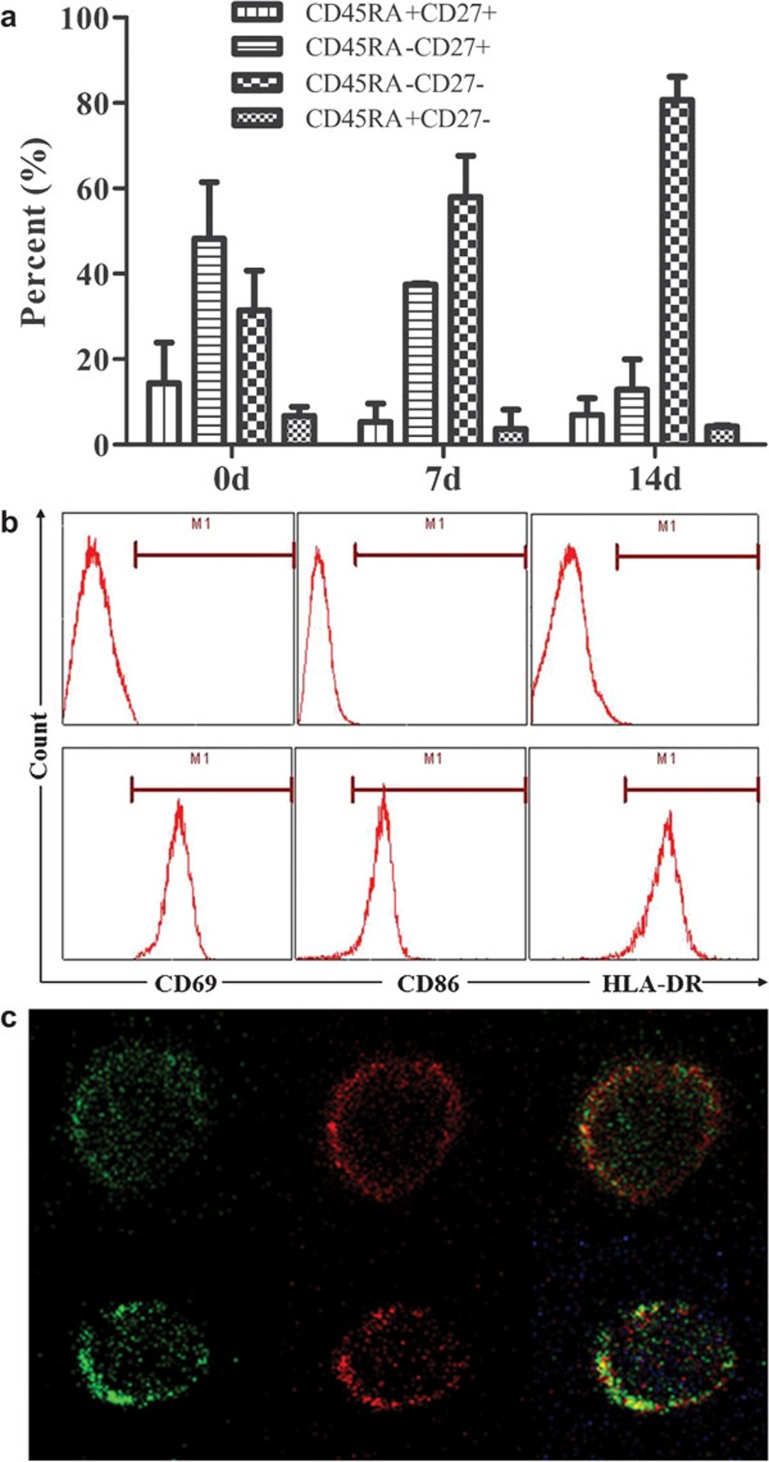
While the Vγ9Vδ2 T cells from the peripheral blood continuously proliferated in the presence of IPP, most of the Vγ9Vδ2 T cells were activated and transformed into effector memory cells (TEM, CD45RA−CD27−) (Figure 2a). During the differentiation of Vγ9Vδ2 T cells to TEM, CD69 (a marker of activated cells) began to be expressed, and then HLA-DR and CD86 (molecules related to the professional antigen-presenting function) appeared on the cells surface (Figure 2b and c). After induction and activation, Vγ9Vδ2 T cells exhibited the phenotypes of antigen-presenting cells (APCs) and acquired the potential to present antigens.
Figure 2.
The TEM Vγ9Vδ2 T cells exhibited the phenotype of antigen-presenting cells. (a) The Vγ9Vδ2 T cells in the peripheral blood were activated by IPP and proliferated to TEM. After 14 days, the percentage of the TEM Vγ9Vδ2 T cells reached 80%. (b) Flow cytometry results indicated that after activation by IPP, Vγ9Vδ2 T cells expressed high levels of CD69, HLA-DR and CD86. (c) The immunofluorescence picture of the TEM Vγ9Vδ2 T cells (green) also showed that there are high levels of HLA-DR (red, upper) and CD86 (red, lower) expressed on the surface. IPP, isopentenylpyrophosphate.
Antigen-presenting function of TEM Vγ9Vδ2 T cells
Purified TEM Vγ9Vδ2 T cells with high levels of HLA-DR and CD86 and Vγ9Vδ2 T cells that were freshly isolated from the peripheral blood (non-effector memory type, non-TEM) were both treated with TT (10 ng/ml) for 24 h. The cells were exposed to γ-rays (4000 rad) and were then cocultured with purified autogenous CD4+ T cells (105 cells per well) at the best ratio. Non-TEM and TEM Vγ9Vδ2 T cells before and after irradiation were cultured alone and served as controls. The TEM Vγ9Vδ2 T cells had a stronger stimulating effect on the proliferation of the CD4+ T cells, and this stimulation could be blocked by an anti-HLA-DR antibody (Figure 3a), suggesting that the TEM Vγ9Vδ2 T cells may activate CD4+ T cells and stimulate their proliferation through the exogenous antigen-presenting pathway. In the culture system with PPD (10 µg/ml), TEM Vγ9Vδ2 T cells showed a similar efficacy (Figure 3b). Because both TT and PPD are soluble antigens, we used B cells as the positive control. After 7 days of coculture with B cells or TEM Vγ9Vδ2 T cells, the proliferation of CD4+ T cells was detected using the 3H-TdR method. We unexpectedly found that the TEM Vγ9Vδ2 T cells were more effective than the autogenous B cells to present the TT and PPD antigens (Figure 3c and d).
Figure 3.
The analysis of the TEM Vγ9Vδ2 T cells presenting TT and PPD. The cpm values were expressed as the mean±s.d. from five wells. (a) The TEM Vγ9Vδ2 T cells were effective at promoting CD4+ T-cell proliferation, which can be blocked by HLA-DR antibody. (b) The TEM Vγ9Vδ2 T cells could also promote CD4+ T cell proliferation in medium containing PPD as antigen. (c, d) The TEM Vγ9Vδ2 T cells were more effective than autogenous B cells at presenting TT and PPD. cpm, counts per million; PPD, purified protein derivative; TT, tetanus toxin.
Cytokine secretion patterns of TEM Vγ9Vδ2 T cells
The cell's function is not only related to its surface molecules but also to the cytokines secreted to the extracellular space. After activation by IPP, Vγ9Vδ2 T cells underwent proliferation and had changes in their subtype. We also measured the patterns of cytokines in the Vγ9Vδ2 T cell culture suspensions at different time points by ELISA. The concentration of IL-17 increased with the increase in the percentage of Vγ9Vδ2 T cells, while IFN-γ did not show any obvious changes (Figure 4a).
Figure 4.
The secretion patterns of the Vγ9Vδ2 T cells at various differentiation states. (a) IFN-γ in the suspension is at a higher level. (b) IL-17 in the suspension increased following Vγ9Vδ2 T-cell proliferation and differentiation. (c) Non-TEM (non-activated) and TEM (activated) Vγ9Vδ2 T cells from the PB of healthy donors were sorted by FACS. (d, e) The cytokine levels were assayed by ELISA 4 h after the Vγ9Vδ2 T cells were stimulated by PMA and ionomycin. The non-TEM Vγ9Vδ2 T cells mainly secreted IFN-γ, while the TEM Vγ9Vδ2 T cells secreted high levels of both IFN-γ and IL-17. (f) Flow cytometry data showed consistent results with ELISA. The non-TEM Vγ9Vδ2 T cells primarily secreted IFN-γ, while the TEM Vγ9Vδ2 T cells mainly secreted IL-17 (gated on Vγ9Vδ2 T cells). FACS, fluorescence-activated cell sorting; IFN, interferon; PB, peripheral blood; PMA, phorbol 12-myristate 13-acetate; TCR, T-cell receptor.
To understand whether the changes in IL-17 were directly related to the proliferation and differentiation of the Vγ9Vδ2 T cells, we measured the levels of IL-17 and IFN-γ in the supernatant of the media used to culture the purified non-TEM and TEM Vγ9Vδ2 T cells from healthy donors (both stimulated with phorbol 12-myristate 13-acetate and ionomycin). The results indicated that during the IPP-stimulation of the Vγ9Vδ2 T cells, IFN-γ was maintained at a high level (Figure 4a), suggesting that all of the subtypes of Vγ9Vδ2 T cells could secrete IFN-γ. However, IL-17 increased with the differentiation of Vγ9Vδ2 T cells (Figure 4b), suggesting that TEM Vγ9Vδ2 T cells have a stronger capacity to secrete IL-17. We then sorted pure non-TEM and TEM Vγ9Vδ2 T cells by FACS (Figure 4c). The non-TEM Vγ9Vδ2 T cells mainly secreted IFN-γ (Figure 4d), and only a portion of them secreted IL-17 (Figure 4e). The secretion pattern of the TEM Vγ9Vδ2 T cells changed substantially; they secreted not only IFN-γ but also high levels of IL-17 (Figure 4d and e). Intracellular staining of Vγ9Vδ2 T cells revealed that both the non-TEM and TEM Vγ9Vδ2 T cells were able to secret IFN-γ, but only the TEM cells secreted high levels of IL-17 (Figure 4f). Therefore, the Vγ9Vδ2 T cells that had different phenotypic characteristics and secretion patterns were likely to possess various immune functions. The research on the Vγ9Vδ2 T cells phenotypes and functions has provided a basis for further studies on the relationship between γδ T cells and diseases such as RA and pulmonary tuberculosis.
Characteristics of γδ T cells in RA patients
Several studies11, 12 reported that γδ T cells with abnormal functions existed in the joints of RA patients, although the immunopathological mechanism of RA is unclear. Therefore, we analyzed the phenotypes of γδ T cells in the peripheral blood and synovial fluid of RA patients. We found that γδ T cells in the synovial fluid were different from those in the peripheral blood because the ratio of γδ T cells in the synovial fluid was significantly higher (about 20%) (Figure 5a). Vγ9Vδ2 T cells were the predominant subpopulation of γδ T cells in both the peripheral blood and also the synovial fluid from RA patients, and flow cytometry analyses indicated that the TEM Vγ9Vδ2 T cells were more predominant in the synovial fluid (Figure 5b). Furthermore, many of the Vγ9Vδ2 T cells in the synovial fluid expressed HLA-DR (85.75±0.96%) and CD86 (42.85±5.9%) (Figure 5c and d). These results suggested that a large number of the Vγ9Vδ2 T cells with potential antigen-presenting capability existed in the synovial fluid. The function of these cells may be similar to the function of APCs that can continuously activate CD4+ T cells, which then leads to an intense immunoreaction in the joint and aggravates the immune injury.
Figure 5.
The phenotypic characteristics of γδ T cells in RA patients' peripheral blood and synovial fluid. (a) There was a similar percentage of γδ T cell in the peripheral blood of RA patients and healthy donors, but the ratio of γδ T cells in the RA patients' synovial fluid was about 20% and was significantly higher than that in the peripheral blood. (b) γδ T cells in RA patients' peripheral blood (mainly Vγ9Vδ2 T cells) were mostly central memory and effector memory T cells, while the TEM Vγ9Vδ2 T cells in the synovial fluid had a higher percentage of effector memory cells. (c, d) The Vγ9Vδ2 T cells from RA patients expressed HLA-DR and CD86 molecules on the surface, and the Vγ9Vδ2 T cells in the synovial fluid had a higher surface expression of HLA-DR and CD86 molecules (HLA-DR: 85.75%±0.96%, CD86: 42.85%±5.9%). PB, peripheral blood; RA, rheumatoid arthritis; SF, synovial fluid.
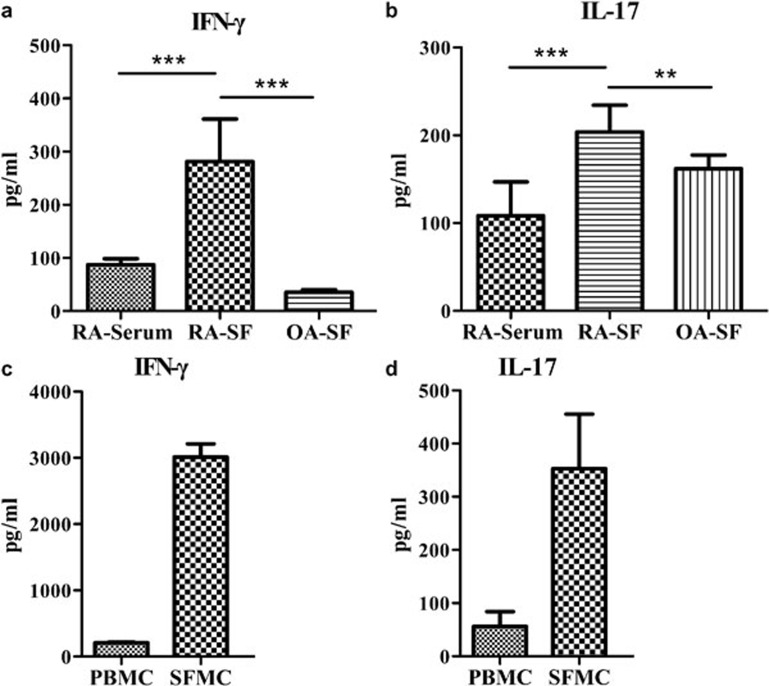
Analyses of the IFN-γ and IL-17 levels in the serum and synovial fluid of RA patients revealed that the levels of both cytokines were higher in the synovial fluid than in the serum (Figure 6a and b). Moreover, compared with the synovial fluid from osteoarthritis patients, the synovial fluid from RA patients had higher titers of both IFN-γ and IL-17, suggesting a more severe inflammatory reaction in the joints of the RA patients. Higher levels of IFN-γ and IL-17 were detected in the SFMCs than in the PBMCs, which was probably because of the presence of a large number of TEM Vγ9Vδ2 T cells (Figure 6c and d). These results indicated that γδ T cells were an important source of inflammatory factors in the RA joints and that the γδ T cells (particularly the Vγ9Vδ2 T cells) could secrete many cytokines that aggravated inflammation and immune disorder.
Figure 6.
γδ T cells were an important source of IFN-γ and IL-17 in the joints of the RA patients. (a) The levels of IFN-γ and IL-17 in the RA synovial fluid were significantly higher than the levels in the serum and the OA synovial fluid. (b) SFMCs have a stronger capacity to produce IFN-γ and IL-17 than PBMCs, and high levels of IFN-γ and IL-17 were detected in the SFMCs culture suspension. IFN, interferon; OA, osteoarthritis; PBMC, peripheral blood mononuclear cell; RA, rheumatoid arthritis; SFMC, synovial fluid mononuclear cell.
Analysis of the antigen-presenting capacity of γδ T cells of RA patients
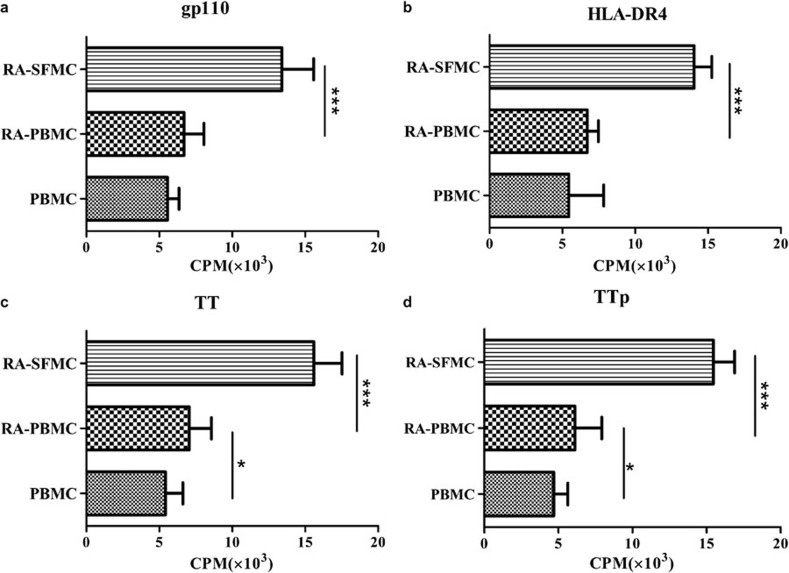
It is known that HLA DRB1*04 is the major genotype in Chinese RA patients (47.37%) and that the EBVgp110 protein shares a common motif with the HLA DR4 subtype.4 These facts suggest that EBV infection may be associated with the development of RA. We showed above that γδ T cells can present both PPD (a large protein) and also TT (a small antigen). Thus, we synthesized three peptides: TTp (TT purified from natural resources has cellular toxicity), EBVgp110 and HLA-DR4. We explored the optimal reactive concentration of these antigens (data not shown) and then measured the immune response of PBMCs and SFMCs from RA patients to the established optimal concentration of TT, TTp, EBVgp110 and HLA-DR4. We used PBMCs from healthy donors as the controls. The PBMCs from the RA patients showed a stronger response to the antigens than the PBMCs from healthy donors. Moreover, the SFMCs responded more strongly to the viral antigen (EBVgp110) and the autoantigen (HLA-DR4) (Figure 7). These differences may be attributed to two factors. First, in the RA synovial fluid, the αβ T cells were more active and there were more specific autoreactive CD4+ T cells that had been previously activated, and thus, a secondary response could be quickly generated after these CD4+ T cells were exposed to a viral antigen or autoantigen. Second, there were more γδ T cells with the potential to present antigen in the synovial fluid, and they could recognize and present antigenic peptide to either the initial CD4+ T cells or the autoreactive CD4+ T cells more effectively and activate these cells to cause excessive immune responses.
Figure 7.
The responses of the PBMCs and SFMCs from RA patients to viral peptides and autoantigen peptides. Compared to the PBMCs from healthy donors, EBVgp110 and HLA-DR4 induced a more significant proliferation of PBMCs and SFMCs from RA patients. SFMCs had the strongest reactive efficacy (all experiments were repeated three times). cpm, counts per million; EBV, Epstein–Barr virus; PBMC, peripheral blood mononuclear cell; RA, rheumatoid arthritis; SFMC, synovial fluid mononuclear cell; TT, tetanus toxin; TTp, tetanus toxoid peptide.
We also studied the optimal concentration of EBVgp110 and HLA-DR4 (data not shown) and the optimal ratio of γδ T cells and CD4+ T cells. At a constant CD4+ T cell number, the CD4+ T cells had the strongest proliferation at a 10∶1 ratio of CD4+ T cells to γδ T cells. When purified γδ T cells and B cells from RA patients were separately cocultured with CD4+ T cells at various ratio (1∶2.5, 1∶5, 1∶10, 1∶20 and 1∶40), the results indicated that the γδ T cells from the RA patients can effectively present EBVgp110 and HLA-DR4, and the efficacy of presentation was significantly higher than that of B cells (Figure 8). Therefore, we concluded that the TEM Vγ9Vδ2 T cells have a role as professional APCs in the pathogenicity of RA.
Figure 8.
The antigen-presenting efficacy of γδ T cells from RA patients. Purified γδ T cells and B cells from RA patients were pre-treated with EBVgp110, HLA-DR4, TT and TTp, irradiated by γ-rays (4000 rad), and cocultured with CD4+ T cells (each well had 105 cells) at a ratio of 1∶2.5, 1∶5, 1∶10, 1∶20 or 1∶40. Seven days later, the proliferation was measured with the 3H-TdR method. The data were expressed as mean±s.d. APC, antigen-presenting cell; cpm, counts per million; EBV, Epstein–Barr virus; RA, rheumatoid arthritis; TT, tetanus toxin; TTp, tetanus toxoid peptide.
Discussion
Epidemiological studies have revealed that 90% of people have a history of EBV infection and that EBV IgG-related antibodies can be detected, albeit at low levels, in the peripheral blood of most people. EBV can lurk in the human body for a long time after infection without producing any symptoms and can be activated to form a recurrent infection when the host immune function is compromised. We detected a high level of EBV-related antibodies and Dw4 antibodies (data not shown here) in the serum and synovial fluid of RA patients, suggesting a relationship between EBV infection and RA occurrence; the mechanisms of the immune disorder in RA patients are complex and involve many factors.
It is well known that individuals carrying particular MHC antigenic determinants are susceptible to specific diseases. For example, individuals carrying HLA-B27 are susceptible to ankylosing spondylitis, and those carrying HLA-DQ6 are susceptible to hypnolepsy. Hypothetically, this selective susceptibility is attributed to the high affinity between the MHC molecules and the pathogenic viral antigens (foreign or lurking in vivo), which results in recognition by autoreactive T cells in patients with these diseases. It was reported that 17.3% of the general population in China carry the HLA-DR4 gene compared to 43.8% of Chinese RA patients. As a subtype of HLA-II, HLA-Dw4 has a homologous sequence to EBVgp110;4 therefore, the fact that RA patients with a HLA-DR4 genetic background had repetitive EBV infection history suggests a relationship between EBV infection and RA. Our study demonstrates that both EBVgp110 and HLA-DR4 peptide segments can induce the proliferation of T cells from the peripheral blood and synovial fluid of RA patients and that SFMCs have a stronger response to viral and autoantigen peptides. Furthermore, these findings explain that the occurrence of RA is focal in nature and is related to the specific genetic background and autoimmune disorders resulting from a viral infection.
More importantly, we found that the γδ T cells in the peripheral blood from RA patients have a higher capacity to present EBVgp110 and HLA-DR4 than the B cells. According to the characteristics of γδ T cells in the synovial fluid of RA patients, this higher capacity may be attributed to the large number of TEM Vγ9Vδ2 T cells (CD45RA−CD27−) that could continuously activate CD4+ T cells by presenting antigens to cause a sustained chronic inflammatory reaction in the joint. Furthermore, the TEM Vγ9Vδ2 T cells secrete various inflammatory factors (e.g., IFN-γ and IL-17) and thus aggravate the inflammatory reaction.
Different results have been reported showing that the γδ T cells were stimulated by inflammation independently of antigen at the affected joints. γδ T cells have also been shown to produce enhanced IL-17, which exacerbated the arthritis in mice with collagen-induced arthritis but not in SKG mice, which are genetically prone to develop autoimmune arthritis, or in patients with RA.28 Also, it was observed that in the arthritic joints, the collagen II-specific Th17 cells rather than the IL-17+ γδ T cells are the main effectors that trigger osteoclast-mediated joint destruction in an IL-23-dependent manner;29 neither of these studies characterized the IL-17A-and Il-22-producting Vγ2Vδ2 T cells in detail nor examined the cytokine requirements for IL-17A production by human γδ T cells. Thus, the potential role of γδ T cells as sources of IL-17 and Il-22 in human immune responses is unclear.30
However, γδ T cells are often the major source of IL-17; their response can be very rapid during bacterial infections, and the response has been shown to be protective. However, IL-17-producing γδ T cells have been found to exacerbate collagen-induced arthritis. Interestingly, some of the γδ T cells produce IL-17 in response to IL-23 alone, even in naive animals, suggesting that the γδ T cells are already differentiated and may develop differently than the CD4+ αβ Th17 cells.13 Using IL-23R reporter mice, it has been shown that γδ T cells were the first cells to respond to IL-23 during experimental autoimmune encephalomyelitis. Thus, IL-23, which by itself has no direct effect on regulatory T cells, is able to disarm regulatory T cell responses and promote antigen-specific effector T-cell responses via the activation of γδ T cells.31 This year, Silva-Santos B also showed that human Vγ9Vδ2 T cells can secrete the proinflammatory cytokine IL-17.32
Additionally, γδ T cells can affect the response of the B cells by direct cell-to-cell mediation.33 Autoreactive B cells can maintain the activation of fibroblasts and also produce LT-β to participate in the formation of lymphoid tissues in the synovial tissue,34 thus playing an important role in the development and progression of RA. Previous studies reported that γδ T cells can be detected in the follicular germinal centers of B cells.33 Recently, a new type of T cell (follicular helper cells, termed TFH) that can help the activity of B cells has been identified. The TFH cells can effectively support the responses of B cells, including their proliferation, differentiation and production of antibodies.35 Despite this progress, direct evidence of γδ T cell-assisted B-cell activities during RA remains to be found.
In summary, γδ T cells may have different biological functions in the development of RA through multiple pathways at various stages of the disorder. The exact mechanism requires more investigation.
Acknowledgments
This work was supported by the grants from the following: National Natural Science Foundation of China (no. 30471593, 30872304 and 81072470), Shanghai Commission of Science and Technology (no. 10JC14 08500 and 10ZR1426100), Shanghai Leading Academic Discipline-Surgery (no. S30204-K01), Shanghai Municipal education Commission (no. J50207 and 09YZ102), Shanghai Institute of Immunology (no. 08-A04), Clinical Medicine Technology Development Foundation of Jiangsu University (no. JLY2010091) and Foundation of Shanghai Xuhui Central Hospital (no. 2011XHCH07).
References
- Takeda T, Mizugaki Y, Matsubara L, Imai S, Koike T, Takada K. Lytic Epstein–Barr virus infection in the synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:1218–1225. doi: 10.1002/1529-0131(200006)43:6<1218::AID-ANR4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Zoschke D, Segall M. Dw subtypes of DR4 in rheumatoid arthritis: evidence for a preferential association with Dw4. Hum Immunol. 1986;15:118–124. doi: 10.1016/0198-8859(86)90322-8. [DOI] [PubMed] [Google Scholar]
- Nepom GT, Byers P, Seyfried C, Healey LA, Wilske KR, Stage D, et al. HLA genes associated with rheumatoid arthritis. Identification of susceptibility alleles using specific oligonucleotide probes. Arthritis Rheum. 1989;32:15–21. doi: 10.1002/anr.1780320104. [DOI] [PubMed] [Google Scholar]
- Roudier J, Petersen J, Rhodes GH, Luka J, Carson DA. Susceptibility to rheumatoid arthritis maps to a T-cell epitope shared by the HLA-Dw4 DR beta-1 chain and the Epstein–Barr virus glycoprotein gp110. Proc Natl Acad Sci USA. 1989;86:5104–5108. doi: 10.1073/pnas.86.13.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C, Mathis D. Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry. Nat Immunol. 2001;2:797–801. doi: 10.1038/ni0901-797. [DOI] [PubMed] [Google Scholar]
- Cope AP. T cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10:S1–S10. doi: 10.1186/ar2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope AP, Schulze-Koops H, Aringer M. The central role of T cells in rheumatoid arthritis. Clin Exp Rheumatol. 2007;25:S4–S11. [PubMed] [Google Scholar]
- Thomas R, Turner M, Cope AP. High avidity autoreactive T cells with a low signalling capacity through the T-cell receptor: central to rheumatoid arthritis pathogenesis. Arthritis Res Ther. 2008;10:210. doi: 10.1186/ar2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran CN, Lundy SK, Fox DA. Synovial biology and T cells in rheumatoid arthritis. Pathophysiology. 2005;12:183–189. doi: 10.1016/j.pathophys.2005.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayi GS. B cells: a fundamental role in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2005;44:ii3–ii7. doi: 10.1093/rheumatology/keh616. [DOI] [PubMed] [Google Scholar]
- Bodman-Smith MD, Anand A, Durand V, Youinou PY, Lydyard PM. Decreased expression of FcgammaRIII (CD16) by gammadelta T cells in patients with rheumatoid arthritis. Immunology. 2000;99:498–503. doi: 10.1046/j.1365-2567.2000.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J. Activation of gammadelta T cells by mycobacterial antigens in rheumatoid arthritis. Microbes Infect. 1999;1:197–202. doi: 10.1016/s1286-4579(99)80034-3. [DOI] [PubMed] [Google Scholar]
- Roark CL, Simonian PL, Fontenot AP, Born WK, O'Brien RL. gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura M, Kawabe T, Shudo K, Kidoya H, Fukui M, Asano M, et al. Involvement of IL-17 in Fas ligand-induced inflammation. Int Immunol. 2004;16:1099–1108. doi: 10.1093/intimm/dxh111. [DOI] [PubMed] [Google Scholar]
- Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette–Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- Fischer S, Scheffler A, Kabelitz D. Activation of human γδ T-cells by heat-treated mistletoe plant extracts. Immunol Lett. 1996;52:69–72. doi: 10.1016/0165-2478(96)02584-9. [DOI] [PubMed] [Google Scholar]
- Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, et al. Differentiation of effector/memory Vδ2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlazzo V, Sferrazza C, Caccamo N, Di Fede G, Di Lorenzo G, D'Asaro M, et al. In vitro effects of aminobisphosphonates on Vγ9Vδ2 T cell activation and differentiation. Int J Immunopathol Pharmacol. 2006;19:309–317. doi: 10.1177/039463200601900208. [DOI] [PubMed] [Google Scholar]
- Born WK, Reardon CL, O'Brien RL. The function of gammadelta T cells in innate immunity. Curr Opin Immunol. 2006;18:31–38. doi: 10.1016/j.coi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Beetz S, Wesch D, Marischen L, Welte S, Oberg HH, Kabelitz D. Innate immune functions of human gammadelta T cells. Immunobiology. 2008;213:173–182. doi: 10.1016/j.imbio.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Gryglewski A, Majcher P, Bryniarski K, Konturek S, Ptak M, Ptak W, et al. Mesenteric lymph node Tgammadelta cells induced by gastrectomy in mice suppress cell-mediated immune response in vitro via released TGF-beta. J Surg Res. 2007;142:66–71. doi: 10.1016/j.jss.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- Green AE, Lissina A, Hutchinson SL, Hewitt RE, Temple B, James D, et al. Recognition of nonpeptide antigens by human Vγ9Vδ2 T cells requires contact with cells of human origin. Clin Exp Immunol. 2004;136:472–482. doi: 10.1111/j.1365-2249.2004.02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelitz D, Glatzel A, Wesch D. Antigen recognition by human gammadelta T lymphocytes. Int Arch Allergy Immunol. 2000;122:1–7. doi: 10.1159/000024353. [DOI] [PubMed] [Google Scholar]
- Peng MY, Wang ZH, Yao CY, Jiang LN, Jin QL, Wang J, et al. Interleukin 17-producing gamma delta T cells increased in patients with active pulmonary tuberculosis. Cell Mol Immunol. 2008;5:203–208. doi: 10.1038/cmi.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lu H, Li B, Wang W, Zhang H. Stimulatory effect of purified mycobacterium tuberculosis peptide antigen on human γδT lymphocyte proliferation. Chin J Immunol. 2004;20:661–664. [Google Scholar]
- Ito Y, Usui T, Kobayashi S, Iguchi-Hashimoto M, Ito H, Yoshitomi H, et al. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum. 2009;60:2294–2303. doi: 10.1002/art.24687. [DOI] [PubMed] [Google Scholar]
- Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells. J Immunol. 2010;184:7268–7280. doi: 10.4049/jimmunol.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöllinger B, Junt T, Metzler B, Walker UA, Tyndall A, Allard C, et al. Th17 cells, not IL-17+ gamma/delta T cells, drive arthritic bone destruction in mice and humans. J Immunol. 2011;186:2602–2612. doi: 10.4049/jimmunol.1003370. [DOI] [PubMed] [Google Scholar]
- Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, et al. Gamma/delta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Santos B. gamma/delta cells making IL-17. Blood. 2011;118:3–5. doi: 10.1182/blood-2011-05-351726. [DOI] [PubMed] [Google Scholar]
- Moser B, Eberl M. gammadelta T cells: novel initiators of adaptive immunity. Immunol Rev. 2007;215:89–102. doi: 10.1111/j.1600-065X.2006.00472.x. [DOI] [PubMed] [Google Scholar]
- Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- Brandes M, Willimann K, Lang AB, Nam KH, Jin C, Brenner MB, et al. Flexible migration program regulates gamma delta T-cell involvement in humoral immunity. Blood. 2003;102:3693–3701. doi: 10.1182/blood-2003-04-1016. [DOI] [PubMed] [Google Scholar]