Abstract
Humanized mouse models that have received human cells or tissue transplants are extremely useful in basic and applied human disease research. Highly immunodeficient mice, which do not reject xenografts and support cell and tissue differentiation and growth, are indispensable for generating additional appropriate models. Since the early 2000s, a series of immunodeficient mice appropriate for generating humanized mice has been successively developed by introducing the IL-2Rγnull gene (e.g., NOD/SCID/γcnull and Rag2nullγcnull mice). These strains show not only a high rate of human cell engraftment, but also generate well-differentiated multilineage human hematopoietic cells after human hematopoietic stem cell (HSC) transplantation. These humanized mice facilitate the analysis of human hematology and immunology in vivo. However, human hematopoietic cells developed from HSCs are not always phenotypically and functionally identical to those in humans. More recently, a new series of immunodeficient mice compensates for these disadvantages. These mice were generated by genetically introducing human cytokine genes into NOD/SCID/γcnull and Rag2nullγcnull mice. In this review, we describe the current knowledge of human hematopoietic cells developed in these mice. Various human disease mouse models using these humanized mice are summarized.
Keywords: animal model, immunodeficient mice, immunology, hematology, humanized mice
Introduction
‘Humanized' mouse models in which various types of human cells and tissues are engrafted and function, as they would in humans are considered extremely useful in basic and applied human disease research.1, 2, 3, 4 For this purpose, highly immunodeficient mice, which do not reject xenografts and support the differentiation and growth of cells and tissues, are indispensable. The support system that maintains human cells and tissues is also a key factor. In addition, technical modifications necessary for the generation of humanized mice are important.
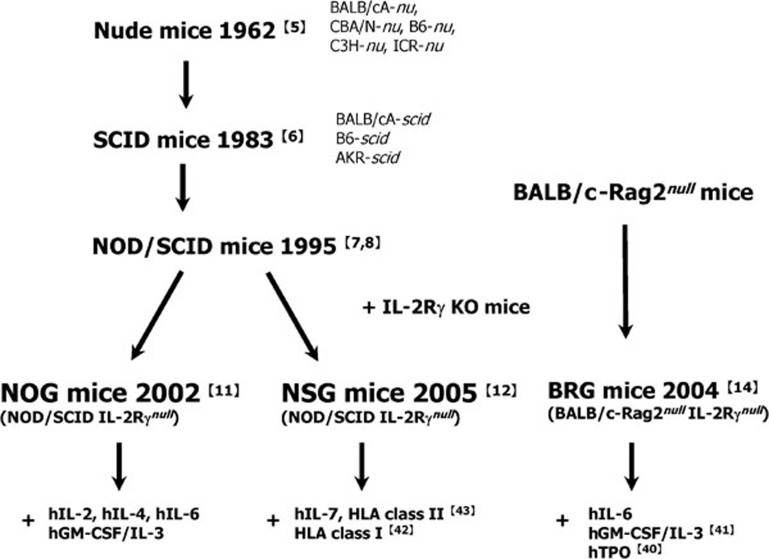
The discoveries of nude and severe combined immunodeficiency (SCID) mice were key advances in the development of immunodeficient mice for xenotransplantation.5, 6 The development of nonobese diabetic (NOD)/SCID mice via the introduction of the Prdkcscid gene into a NOD inbred strain also contributed to the generation of humanized mice.7, 8 NOD/SCID/β2mnull and NOD/Rag1nullPfpnull mice were subsequently derived from NOD/SCID and NOD/Rag1null mice.9, 10
Since the early 2000s, immunodeficient mice appropriate for generating humanized mice have been successively developed by introducing the mutant IL2rα gene into NOD/SCID and RAG1/2null mice by backcross mating, thus resulting in NOD/SCID/γcnull mice11, 12 and Rag1/2nullγcnull mice.13, 14, 15, 16 These mice show multiple immunodeficiencies, including defects in T, B and natural killer (NK) cells, and reduced macrophage (Mφ) and dendritic cell (DC) function.11 In these mice, extremely high human cell engraftment rates and increases in well-differentiated human multilineage hematopoietic cells from human hematopoietic stem cells (HSCs), as compared with parent immunodeficient mice, were observed.17, 18, 19 Humanized mice that retain various human immune cells are often termed human immune system mice3 or human hematolymphoid system mice.20 The production of humanized mice that are reconstructed with human cells would facilitate analysis of the underlying mechanisms of human disease pathogenesis. Indeed, various humanized models have been developed using these mice.
Various technical modifications have been used when generating humanized mice. These include modifications in the HSC injection route, age of mice, and HSC and irradiation sources, each of which may affect the efficacy of human cell engraftment. In terms of the injection route and age, intrahepatic or intravenous injection (through the facial vein) into newborn mice and intravenous injection (via the tail vein) into adult mice were generally used.11, 14, 21 Brehm et al.16 examined various parameters, including injection route, injection age and immunodeficient mouse strains. By comparing engraftment rates of human cells from HSCs, they concluded that intrahepatic injection of HSCs into newborn mice enhanced engraftment as compared with adult mice. With respect to the source of HSCs, CD34+ cells from cord blood or fetal liver were typically used. However, CD34+ cells from granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood (PB) or bone marrow (BM) served as additional sources. Lepus et al.22 reported that CD34+ cells from fetal liver were more efficient than those from cord blood or G-CSF-mobilized PB. In addition, Matsumura et al.23 reported that CD34+ cells from cord blood were more effective than those from G-CSF-mobilized PB and BM. Busulfan treatment can also be used in place of irradiation, and results in more effective differentiation of B cells from HSCs as compared with irradiation.24 The use of this drug would be beneficial for research, since special equipment is not required. The engraftment rate of human cells from HSCs varies between reports. A possible explanation for this difference is the quality of HSCs, which is influenced by different isolation techniques and the HSC source (i.e., cord blood or fetal liver).
Despite these efforts, some human cell lineages, such as erythrocytes and neutrophils, have not yet been developed in humanized mice. The differentiation of HSCs into immature human T and B cells and poor interactions between these cells were suggested based on cell phenotype analysis and the rare production of antigen-specific immunoglobulin G (IgG) class antibodies.25 These results suggest that current immunodeficient mice may be insufficient, and human factors that support the differentiation and maturation of cells and mediate cell-to-cell interactions must be introduced. To this end, a new series of immunodeficient mice has been generated by introducing human cytokine genes into NOD/SCID/γcnull, Rag2nullγcnull mice.
In this review, we describe the current knowledge of human hematopoietic cells developed in NOD/SCID/γcnull and Rag1/2nullγcnull mice and the strains derived from them. In addition, the humanized mouse models used in studies of various human diseases will be summarized.
Immunodeficient mice
Since the discovery of nude and SCID mice,5, 6 humanized mice have been generated by using various immunodeficient mice. SCID mice that received human T and B cells by transplantation of fetal liver and thymus, which were termed SCID-hu mice by McCune et al.,26, 27 provided an attractive humanized model for research in various fields; however, engraftment rates were not high. In 1998, Goldman et al.13 reported the enhanced engraftment of human cells in Rag2null mice possessing the IL-2Rγnull gene. In the 2000s, a series of immunodeficient mice was developed by combining the IL-2Rγnull gene with conventional SCID and Rag1/2null mice. These strains showed extremely high engraftment rates and differentiation of human cells, resulting in remarkable advances in the development of human disease models. These stains include the NOG (NOD/Shi-Prkdcscid Il2rgtm1Sug/Jic) mice reported in 2002;11 RG (BALB/cA-Rag2nullIl2rγnull (BRG) and C57BL/6-Rag2nullIl2rγnull (B6RG)) mice reported in 1997, 1998 and 2004;13, 14, 28 and NSG (NOD/LtSz-Prkdcscid Il2rgtm1Wjl/J) mice reported in 2005.12 Recently, immunodeficient BALB/c-Rag1nullIl2rgnull,16 NOD-Rag1nullIl2rgnull,15 and NOD/SCID-JaK3null mice have been established as alternatives to NOG/NSG mice. Data accumulated to date suggest that NOG/NSG mice are the best recipients for humanized tissue and human cell engraftment occurs in the following order: NSG=NOG>NRG>BRG>NOD/SCID>B6RG.15, 16 The disadvantages of SCID and NOD/SCID mice include the frequent occurrence of thymic lymphoma and the leakiness, in which T and B cells develop in aged mice.29, 30 However, NOG mice show no leakiness or spontaneous thymic lymphoma.31, 32 This may be attributed to inactivation of IL-2Rγ, which is shared by important cytokines, such as IL-2, IL-4, IL-7, IL-15 and IL-21, each of which is important for T- and B-cell growth.33 These results indicate that NOG/NSG mice are better recipients of human cells and tissues.
The most attractive feature of these humanized mice is the development of multilineage hematopoietic cells by transplantation of human HSCs. In particular, T-cell subpopulations, including CD4 and CD8 single positive cells, which could not be differentiated in NOD/SCID mice, successfully developed in these mice.14, 18, 21 These results suggest the usefulness of humanized mice for investigating human immune responses. Indeed, the utility of these humanized mice is well accepted by researchers worldwide. However, these humanized mice are insufficient, since human cells are not fully functional. For example, no or rare antigen-specific IgG production occurs after multiple injections of antigens.25
To overcome this issue, various improved strains based on these immunodeficient mice have been developed or are being developed by several groups, including our group.34, 35 In Figure 1, the history of the development of immunodeficient humanized mice and their respective improvements are summarized. These mice have been established in NOG/NSG or BRG backgrounds. Background strain selection is crucial for the improvement of immunodeficient mice. In 1996, we reported human granulocyte–macrophage colony-stimulating factor (hGM-CSF) and interleukin-3 (IL-3) cosecreting transgenic (Tg) B6-SCID mice.36 These strains were able to maintain human tumor cell lines possessing hGM-CSF and human IL-3 (hIL-3) receptors on the surface;37 human cells, however, could not engraft in these mice. We recently established hGM-CSF/IL-3 Tg NOG mice by the conversion of strain B6 to NOG by backcross mating. High human cell engraftment rates were observed in hGM-CSF/IL-3 Tg NOG mice. We also compared engraftment of HSCs in NOG, BRG and B6RG mice. Higher engraftment rates were obtained in NOG and BRG, but not B6RG mice. In particular, we were surprised to detect only a few human cells in B6RG mice (unpubl. data). Similar results were reported by Traggiai et al.,14 indicating that immunodeficient mice in a B6 background are not sufficient for generating humanized mice. Recent reports on signal regulatory protein (Sirpα) suggested that NOD mice are superior to other strains, because Sirpα in NOD strains is more similar to that in humans, compared with Sirpα in other mice strains.38, 39 Therefore, BRG transgenic mice with human Sirpα have been established to enhance human cell engraftment efficacy.20
Figure 1.
The history of the development on immunodeficient mice for humanized mice model. In retrospect, nude mice or SCID mice were the first immunodeficient strains. Subsequently, their congenic strains were generated to improve engraftment capacities. NOD/SCID mice established in 1995 have been a milestone in this field, because of the severer phenotype than nude and SCID mice. In early 2000s, NOG, NSG and BRG mice were established by introducing the IL-2Rγnull allele into NOD/SCID or BALB/cA RAG2null mice. Due to the complete loss of murine immune systems, human hematopoiesis has been enormously enhanced in these mice. Currently, these strains were further improved by introducing human genes for various cytokines or HLA class I and II, so as to recapitulate a human bona fide hematopoiesis and immune system. The superscripts represent the respective references. BRG, BALB/cA-Rag2nullIl2rγnull; HLA, histocompatibility leukocyte antigen; NOD, nonobese diabetic; NSG, NOD/LtSz-Prkdcscid Il2rgtm1Wjl/J; SCID, severe combined immunodeficiency.
Improved strains were generated by injecting human DNA into pronuclear stage embryos, injecting genetically modified embryonic stem cells into blastocysts (knock-in (KI)), or introducing human genes by backcross mating with established transgenic mice. Specifically, human cell differentiation from HSCs in improved mice produced using the KI strategy has been analyzed by Flavell's group at the Yale University.20 They reported elevated HSC and myeloid cell numbers in thrombopoietin (TPO) KI mice and increased alveolar Mφ in hGM-CSF/IL-3 KI mice.40, 41 We also developed transgenic NOG mice that secreted human IL-2 or IL-4 by injecting DNA into NOG embryos. The suppression of graft-versus-host disease (GVHD) and dominant conversion to Th2 cells in hIL-4 Tα NOG mice and remarkable differentiation of human NK cells in hIL-2 Tα NOG mice, respectively, were observed (unpubl. data). Shultz's group generated human leukocyte antigen (HLA) class I transgenic NSG mice by backcross mating the respective transgenic mice into NSG mice. The successful generation of antigen-specific cytotoxic T cells42 was observed. Antigen-specific IgG was produced in HLA class II transgenic NSG mice.43
Real humanized mice are expected to be generated in the near future by improving immunodeficient mice.
Hematolymphoid humanized mice
The reconstitution of the human hematopoietic system is one of the most advanced areas in humanized mouse research. The use of NOG/NSG or BRG mice has greatly improved human hematopoiesis, as shown by the development of multiple human cell lineages, including B and T lymphocytes, NK cells, myeloid DC, plasmacytoid DC, Mφ and erythroblasts. Here, we discuss the current status and perspectives in this field.
Lymphoid cells
Two major subsets of lymphoid cells, i.e., B and T cells, were developed in NOG mice by simply transferring human HSCs after irradiation.
B cells.
Human B cells are detected in the PB 1 month after HSC transplantation and gradually increase in number over the following 3 months. In the BM, B-cell differentiation in humanized mice seems to consistently resemble that in humans, since several distinct precursor populations exist:25 CD19−CD38+CD10+CD34+ early-B cells, CD19+IgM−CD20−CD34+ pro-B cells, CD19+IgM−CD20−CD34− pre-B cells and CD19+IgM+IgD− immature B cells. The pro-B and pre-B populations were also characterized by the expression of intracellular VpreB and Cμ chains. In the spleen, one of the remarkable B-cell phenotypes in humanized mice is high CD5 expression, which is markedly different from genuine human B cells.23 The significance of this upregulated CD5 expression remains controversial, i.e., whether these are human B-1 cells or transitional 1 B cells.23, 25
Immunization of humanized mice with various exogenous substances induces antigen-specific IgM responses, suggesting that the B-cell repertoire is diverse and can cover a myriad of antigens.21, 23 Since antigen-specific IgG responses in conventional humanized mice are very weak,14, 21, 23 it has been speculated that these B cells have some intrinsic defects in the class switch machineries. Several in vitro experiments, however, demonstrated that these B cells do produce IgG in response to stimulation through their antigen receptors and CD40 in the presence of IL-21.25 In addition, recent reports have shown that new mouse strains that express HLA-DR mounted an antigen-specific IgG response upon immunization.43 Collectively, B cells in humanized mice maintain the ability to mediate humoral immune reactions.
T cells.
Human T cells can develop in humanized NOG/NSG or BRG mice in the thymus and accumulate in the spleen. This is one of the most important points of distinction from NOD/SCID mice, which only partially support the differentiation of human T cells. Typically, 3–5 months is necessary for T cells to colonize the spleen.44 In the thymus, human thymocytes show typical surface phenotypes,44 i.e., CD4−CD8−, CD4+CD8+, CD4−CD8+ and CD4+CD8− stages, suggesting that they follow the normal differentiation pathway. The thymus is indispensable for T-cell development, and selection is largely mediated by mouse major histocompatibility complex (MHC) molecules, given that Foxn1-deficient NOG mice (NOG nu/nu) cannot support T-cell development, and CD4+ and CD8+ T cells do not fully develop in NOG I-Aβnull or NOG β2mnull backgrounds,25 respectively.
Although development seems to be relatively normal, the functionality of T cells in humanized mice remains controversial. For example, in studies using Epstein–Barr virus (EBV), humanized mice showed antiviral T-cell responses in which interferon-γ producing CD8+ T cells were differentiated and protected mice from lymphoma development,14, 45 thus supporting the normal functions of human CD8+ T cells. However, in vitro experiments have suggested that human T cells have a limited ability to respond to antigenic stimulation.25 Although the mechanisms underlying abnormal T-cell functions remain unclear, mismatches between mouse MHC and the HLA of donor human cells may be involved. Indeed, HLA-A- or HLA-DR-expressing NSG mice showed normal cytotoxic reactions (cytotoxic T-lymphocyte responses) to viral infection or IgG responses against exogenous antigens, respectively.43, 46, 47
NK cells.
Human NK cells also develop from the early stage (4 weeks) of HSC transfer. However, the number of NK cells is not very high (a low percentage of human CD45+ cells), indicating the necessity for growth and differentiation factors in immunodeficient mice.48 Huntington et al.49 reported that the hIL-15/hIL-15Rα complex induces extensive proliferation and differentiation of CD16+KIR+ NK cells. Chen et al.50 also reported that administering IL-15 and the Flt-3/Flk-2 ligand by plasmid DNA injection into HSC-transferred mice leads to an increased number of NK cells. In IL-2 Tg NOG mice (Katano et al., manuscr. in prep.), human NK cells predominantly develop prior to B and T cells after HSC transfer, and consist of the largest population in human CD45+ cells. However, their function has not been well characterized.
As an alternative method for studying human NK cell functions, ex vivo isolated human NK cells from peripheral blood mononuclear cells (PBMCs) have been transplanted into NOG mice. Although the inoculated NK cells were not maintained in these mice for a long time, the cells exerted effective antibody-dependent cellular cytotoxicity and suppressed the growth of a Burkitt's lymphoma cell line (Daudi), following concomitant administration of an anti-CD20 antibody (rituximab).51
Myeloid cells
In conventional humanized mice, although human myeloid cells were shown to be differentiated, the efficiency was poor. Recently, however, the development of various lineages of myeloid cells has been improved by introducing human cytokines.
Monocytes.
Human monocytes/Mφ can be detected in the blood, lymphoid organs (spleen and BM) and some tissue organs (lung and liver) in conventional humanized mice. The frequency of human CD14+ cells among the total human CD45+ cell population is usually not more than 1%–2% in the spleen, while the frequency can reach 8% or 5% in the lungs or liver, respectively. The delivery of plasmid DNA encoding several human cytokine genes,50 i.e., IL-15 with Flt-3 ligand (Flt3L) or macrophage CSF, by hydrodynamic injection robustly induces the development of human monocytes/Mφ in various organs. In addition, in a novel mouse strain in which the TPO gene was replaced with the human homolog, the development of total human myeloid lineage cells was significantly improved.40 Accordingly, the frequency of monocytes was increased in the blood, but not in the BM. In our studies, the transgenic expression of hIL-3 and GM-CSF genes in NOG (hGM-CSF/IL-3 Tα NOG) mice also improved the development of whole human myeloid cells, including CD14+ monocytes (Ito et al., manuscr. submitt.).
DCs.
In conventional humanized mice, several reports have demonstrated the presence of both myeloid DCs (CD11c+HLA-DR+CD40+CD86+) and plasmacytoid DCs (CD123+HLA-DR+BDCA2+) in the BM, spleen and liver.14, 50 These CD11c+ or CD123+ DCs were functional, as demonstrated by their ability to induce activation of allogeneic human T cells or to produce interferon-α after stimulation.14 The frequency of these cells, however, is generally low in the spleen (typically less than 1% in our studies). Chen et al.50 demonstrated that the administration of plasmid DNA encoding human IL-4/GM-CSF/Flt3L or IL-5/Flt3L markedly increased the DC yield.50 Enhancements in DC development were also observed in our hGM-CSF/IL-3 Tα NOG mice, in terms of both number and frequency, in various lymphoid organs (Ito et al., manuscr. submitt.).
Granulocytes.
Although granulocytes comprise a large fraction of human leukocytes, their frequency in humanized mice is very low (less than 2%–3% of human leukocytes in PB and BM in our studies). To improve differentiation of this population, several groups have attempted to produce novel humanized mouse strains by providing human cytokines. Billerbeck et al.52 created a transgenic NSG strain that expressed the human stem cell factor, GM-CSF and IL-3 genes, and demonstrated a slight increased development of human CD15+ granulocytes in the BM. In TPO KI mice, as mentioned above, a large number of human CD66b+ granulocytes were produced in the BM.40 Moreover, this strain enabled the development of mature human neutrophils with lobulated nuclei, which has not been achieved before. Additionally, in our hGM-CSF/IL-3 Tα NOG mice, we confirmed significant increases in CD66b+ granulocyte numbers in the BM and PB. Furthermore, the presence of human basophils and eosinophils in the PB was detected by May–Giemsa staining (Ito et al., manuscr. submitt.). This is the first report to demonstrate the development of these cell populations in humanized mice. Collectively, the development of human granulocytes in humanized mice has been greatly improved by the addition of human cytokines.
Mast cells.
Mast cells play an important role in allergic responses by releasing intracellular granules containing histamine or various leukotrienes. Crosslinking of their surface Fc-epsilon receptor (FcεR) by IgE triggers a series of reactions.53, 54 Although there is little evidence suggesting the development of human mast cells, Kambe et al.55 demonstrated the presence of human mast cells in the skin, spleen and BM of humanized NOG mice. These cells were positive for c-kit and CD203c, but expression of FcεR was not determined. Recently, we detected FcεR positive mast cells in the BM, spleen and several non-lymphoid tissues of hGM-CSF/IL-3 Tα NOG mice (Ito et al., manuscr. submitt.). These data suggest that IL-3 and/or GM-CSF are important for inducing the differentiation of human mast cells.
Humanized models
Cancer
Due to their supply by the Central Institute for Experimental Animals, NOG mice have been predominantly used in this field. The characteristics of NOG mice include rapid growth of tumors and well-maintained characteristics after multiple passages. In a study by Machida et al.,56 100 HeLa S3 cells could be successfully engrafted in NOG mice; in contrast, 105 and 106 cells were required for engraftment in NOD/SCID and C.B-17-SCID mice, respectively. However, primary human tumors do not always engraft in NOG mice, even though these animals show higher engraftment than conventional immunodeficient mice. Some tumors, such as prostate carcinoma, which are difficult to engraft in SCID and nude mice, are also difficult to engraft in NOG mice. The growth of some tumor cell lines appears to be less than in conventional immunodeficient mice. The reason for this is unclear, but it may be explained by the differential adaptation of cell lines to conventional immunodeficient mice. IL-2Rγ gene inactivation may influence the growth of some tumors in NOG mice. Another characteristic of NOG mice is a high occurrence of metastasis. Genes responsible for metastasis have been investigated through the use of this characteristic.57 The high homing capacity of human cells also appears to be maintained in NOG mice. When U266 myeloma cells were intravenously injected into NOG mice, they grew only in the BM, resulting in paralysis.58
Various cancer models have been established using these advantages.51, 59 On the other hand, a model that can be used to investigate immune responses to tumors has only recently been developed. Mismatching of HLA between tumor cells and hematopoietic cells from HSCs of different donors may cause severe GVHD or a lack of response. To induce an effective immune response against tumors in mice, HLA matching is required. Recently, Shultz et al.42 reported that antigen-specific cytotoxic T lymphocytes were successfully induced in a newly established HLA class I (A-2) transgenic NSG mouse model by transfer of HLA-matched HSC. The development of these mice may lead to new immunotherapy models for cancer. The injection of human PBMCs (hPBMCs) into immunodeficient mice is known to cause severe GVHD; this provides a good model of GVHD.60 We recently found that NOG-I-Aβnullβ2mnull mice showed mild GVHD, although high engraftment rates were observed as compared with non-transgenic NOG mice after transfer of hPBMCs (unpubl. data). Cotransplantation of a patient's tumor and hPBMCs into such immunodeficient mice may facilitate analysis of the immunological responses to the tumor.
Infectious diseases
Human lymphocytes, including T and B cells, predominantly develop in humanized mice transferred with HSCs. Therefore, appropriate models are provided for viruses that specifically infect lymphocytes and express their pathology, such as HIV-1, HTLV-1 and EBV. HIV-1 infection models have been widely used for the analysis of disease mechanisms and the development of anti-HIV-1 drugs,61 as HIV-1 infects human T cells in SCID-hu mice.26, 62, 63 This research is further accelerated through the use of HSC-transplanted immunodeficient mice, in which multilineage hematopoietic cells can be differentiated.64, 65, 66, 67 In this field, a unique model for HIV-168, 69, 70 reported by Garcia's group at the University of North Carolina and termed bone marrow–liver–thymus (BLT) mice, has attracted attention. As the name suggests, this model is generated by transplantation of fetal bone marrow, liver and thymus into a subcutaneous region of the kidney. The most attractive feature of BLT mice is reconstitution of human mucosal immunity; this has not yet been obtained in human immune system mice transferred with HSCs. The human mucosal lymphoid apparatus, including Peyer's patches and gut-associated lymphoid tissue, has been successfully reconstituted in BLT mice, resulting in the development of mucosal immunity. They reported that the IL-2Rγ gene was indispensable for development of the mucosal lymphoid system, indicating that mucosal immunity cannot develop in NOG/NSG and BRG mice that contain a mutant Il2rg gene (reported at the Third International Workshop of Humanized Mice (IWHM 2011) held in Pittsburgh in October 2011).
This mouse appears to provide a better HIV-1 model as compared with conventional humanized mice transferred with HSCs. However, this model cannot be investigated from the aspect of humoral immunity involving B cells, and cannot be used in some countries such as Japan because of ethical issues. Additional genetic modifications of current immunodeficient mice may be necessary to overcome this disadvantage.
EBV usually presents in healthy subjects as a latent infection; however, it expresses a variety of pathological features in the healthy, termed EBV-associated infectious mononucleosis, hemophagocytic lymphohistiocytosis, lymphoproliferative disease, Burkitt's lymphoma and Hodgkin's disease in those immunosuppressed, due to HIV-1 infection or BM transplantation.71 Since the report of EBV-associated lymphoproliferative disease by Traggiai et al.14 using humanized BRG mice, various humanized mouse models of these clinical pathologies have been reported.72, 73, 74
Humanized models of tuberculosis, salmonellosis, yellow fever and Dengue fever have been investigated.75, 76, 77
Animal models appropriate for developing a malaria vaccine are eagerly desired, as malaria is one of the most common infectious diseases worldwide.78 An interesting human malaria model uses immunodeficient mice with transplanted human liver. By injecting human hepatocytes into liver-damaged immunodeficient mice,79, 80, 81, 82 human hepatocytes replace the mouse hepatocytes. In these hu-liver mice, intrahepatic multiplication of Plasmodium falciparum has been observed.83 However, human erythrocytes from human blood must be successively injected into the mice intraperitoneally, because human erythrocytes cannot develop from HSCs.84 To establish the complete malaria life cycle in mouse models, it is necessary to develop mice in which human erythrocytes persist and flow in mouse peripheral blood. Hu-liver mice provide a good infection model for viruses specific to hepatocytes, including hepatitis C and B viruses.85, 86, 87
These models provide invaluable tools for analyzing the mechanisms of human infection and for developing chemotherapeutic agents such as antibodies.
GVHD
GVHD is a severe complication with a high mortality rate that often develops in patients who receive allogeneic BM transplantation for the treatment of acute/chronic leukemia, aplastic anemia or congenital immunodeficiency. Approximately 20 years ago, Mosier et al.63 first demonstrated that the induction of xenogenic GVHD was possible in immunodeficient mice (C.B-17-SCID) by transplanting hPBMCs. In this model, the transplanted human T cells may be activated and attack the recipient mouse tissue, thus resulting in the development of allogeneic GVHD-like symptoms.
Although C.B-17-SCID or NOD/SCID mice have been useful in GVHD research, there are several problems. For example, human cell engraftment is relatively low, due to the mouse endogenous innate immune system. It also requires sublethal dose total body irradiation, which results in large variances in disease onset. Furthermore, a relatively large number of hPBMCs have to be administered intraperitoneally, but not intravenously, to induce the disease.88 This does not reflect BM transplantation, where cells are infused intravenously. van Rijn et al.89 used H-2d-RAG2null IL2rγnull mice in which xeno-GVHD was induced by intravenous injection of hPBMCs; however, this model still depends on the infusion of large numbers of hPBMCs (3×107 cells/head) and total body irradiation. Our xeno-GVHD NOG mouse model has shown significant improvements over other models, such as the rapid onset of disease and uniform death of recipients. In addition, a smaller number of donor cells (2.5×106) is sufficient with intravenous injection, and total body irradiation is not always necessary.60 These results were confirmed by other studies using NSG mice.90
Collectively, NOG or NSG mice are the most suitable platforms for basic and preclinical GVHD research at this time.
Humanized liver models
Humanized liver models, in which the mouse liver is replaced with a human liver, are useful for evaluating drug metabolism in the human liver, as there are numerous differences in liver enzymes between humans and mice. In the first human liver model developed by Mercer et al.,81 SCID/bg mice carrying a urokinase-type plasminogen activator transgene (Alb-uPA) entered a profound hypofibrinogenemic state, which caused hepatocyte death. They transplanted human hepatocytes into the inferior splenic pole and demonstrated that human hepatocytes could be engrafted over 50% in the liver of these mice. To improve xenoengraftment of human hepatocytes, NOG-uPA82 and FRG (fumarylacetoacetate hydrolasenull/RAG2null/IL-2Rγnull)79 mice, in which liver damage is induced by adenovirus-mediated uPA expression, were developed and showed markedly high rates of replacement by human hepatocytes (over 80%). Nevertheless, several problems limit their utility, such as poor breeding efficiency in the mouse colony, development of renal disease, and a very narrow time window for transplantation. Recently, Hasegawa et al.80 established a novel NOG substrain that expresses the herpes simplex virus type 1 thymidine kinase (TK) transgene under the control of a mouse albumin promoter. Administration of ganciclovir, which is non-toxic to human and mouse tissues, ablated TK-expressing liver parenchymal cells. Herpes simplex virus type 1 TK NOG mice allowed high engraftment of human hepatocytes (over 80%) and did not develop systemic morbidity (liver disease, renal disease and bleeding diathesis) as seen in other uPA-dependent models. Stable, long-term humanization of TK NOG mice will facilitate studies of drug metabolism, toxicology and the virology of hepatitis viruses.
Future perspectives
Over the last 10 years, remarkable progress has been achieved in humanized mouse models using NOD/SCID/γcnull, Rag1/2nullγcnull mice, especially for hematology and immunology. Various humanized mouse models have been established that enable direct research of human diseases, which was previously impossible in immunocompetent animals. These models will also contribute to the analysis of mechanisms underlying human immune disorders and the development of vaccines against infectious diseases through the use of humanized mice that contain a wide variety of functional human hematopoietic cells.
However, several issues remain to be overcome, such as the rare differentiation of certain cell lineages from HSCs, immature differentiation and insufficient intercellular relationships. To overcome these problems, the inclusion of other immunodeficient mouse genes or human genes responsible for cell differentiation and interaction has been investigated. These attempts may result in more appropriate immunodeficient humanized mice.
Recently, progress in the field of regenerative medicine has drawn our attention, following the establishment of human embryonic stem and inducible pluripotent stem cells. In the future, artificial human organs or HSCs developed from embryonic stem or inducible pluripotent stem cells may be available. Although these techniques have not yet impacted the field of humanized mice, new models will likely result from transplantation of artificial human organs and HSCs.
Acknowledgments
This research was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), by a grant (22A-9) from the National Center for Child Health and Development, and by a grant from Research on Emerging and Re-emerging Infectious Diseases from Ministry of Health, Labour and Welfare, Japan
References
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- Ito M, Kobayashi K, Nakahata T. NOD/Shi-scid IL2rγnull (NOG) mice more appropriate for humanized mouse models. Curr Top Microbiol Immunol. 2008;324:53–76. doi: 10.1007/978-3-540-75647-7_3. [DOI] [PubMed] [Google Scholar]
- Legrand N, Weijer K, Spits H. Experimental models to study development and function of the human immune system in vivo. . J Immunol. 2006;176:2053–2058. doi: 10.4049/jimmunol.176.4.2053. [DOI] [PubMed] [Google Scholar]
- Zhang B, Duan Z, Zhao Y. Mouse models with human immunity and their application in biomedical research. J Cell Mol Med. 2009;13:1043–1058. doi: 10.1111/j.1582-4934.2008.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issacson J, Cattanach B. Report. Mouse News Lett. 1962;27:31. [Google Scholar]
- Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- Koyanagi Y, Tanaka Y, Tanaka R, Misawa N, Kawano Y, Tanaka T, et al. High levels of viremia in hu-PBL-NOD-scid mice with HIV-1 infection Leukemia11997Suppl 3109–112. [PubMed]
- Christianson SW, Greiner DL, Hesselton RA, Leif JH, Wagar EJ, Schweitzer IB, et al. Enhanced human CD4+ T cell engraftment in β2-microglobulin-deficient NOD-scid mice. J Immunol. 1997;158:3578–3586. [PubMed] [Google Scholar]
- Shultz LD, Banuelos S, Lyons B, Samuels R, Burzenski L, Gott B, et al. NOD/LtSz-Rag1nullPfpnull mice: a new model system with increased levels of human peripheral leukocyte and hematopoietic stem-cell engraftment. Transplantation. 2003;76:1036–1042. doi: 10.1097/01.TP.0000083041.44829.2C. [DOI] [PubMed] [Google Scholar]
- Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, et al. NOD/SCID/γcnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2Rγnull mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- Goldman JP, Blundell MP, Lopes L, Kinnon C, Di Santo JP, Thrasher AJ. Enhanced human cell engraftment in mice deficient in RAG2 and the common cytokine receptor gamma chain. Br J Haematol. 1998;103:335–342. doi: 10.1046/j.1365-2141.1998.00980.x. [DOI] [PubMed] [Google Scholar]
- Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- Pearson T, Shultz LD, Miller D, King M, Laning J, Fodor W, et al. Non-obese diabetic-recombination activating gene-1 (NOD-Rag1null) interleukin (IL)-2 receptor common gamma chain (IL2rγnull) null mice: a radioresistant model for human lymphohaematopoietic engraftment. Clin Exp Immunol. 2008;154:270–284. doi: 10.1111/j.1365-2249.2008.03753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J, et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rγnull mutation. Clin Immunol. 2010;135:84–98. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata T, Ando K, Nakamura Y, Ueyama Y, Shimamura K, Tamaoki N, et al. Functional human T lymphocyte development from cord blood CD34+ cells in nonobese diabetic/Shi-scid, IL-2 receptor γnull mice. J Immunol. 2002;169:204–209. doi: 10.4049/jimmunol.169.1.204. [DOI] [PubMed] [Google Scholar]
- Hiramatsu H, Nishikomori R, Heike T, Ito M, Kobayashi K, Katamura K, et al. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/γcnull mice model. Blood. 2003;102:873–880. doi: 10.1182/blood-2002-09-2755. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Shimazu H, Shultz LD, Fukata M, Nakamura R, Lyons B, et al. Purified human hematopoietic stem cells contribute to the generation of cardiomyocytes through cell fusion. FASEB J. 2006;20:950–952. doi: 10.1096/fj.05-4863fje. [DOI] [PubMed] [Google Scholar]
- Willinger T, Rongvaux A, Strowig T, Manz MG, Flavell RA. Improving human hemato-lymphoid-system mice by cytokine knock-in gene replacement. Trends Immunol. 2011;32:321–327. doi: 10.1016/j.it.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chainnull mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepus CM, Gibson TF, Gerber SA, Kawikova I, Szczepanik M, Hossain J, et al. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/γc−/−, Balb/c-Rag1−/−γc−/−, and C.B-17-scid/bg immunodeficient mice. Hum Immunol. 2009;70:790–802. doi: 10.1016/j.humimm.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura T, Kametani Y, Ando K, Hirano Y, Katano I, Ito R, et al. Functional CD5+ B cells develop predominantly in the spleen of NOD/SCID/γcnull (NOG) mice transplanted either with human umbilical cord blood, bone marrow, or mobilized peripheral blood CD34+ cells. Exp Hematol. 2003;31:789–797. doi: 10.1016/s0301-472x(03)00193-0. [DOI] [PubMed] [Google Scholar]
- Hayakawa J, Hsieh MM, Uchida N, Phang O, Tisdale JF. Busulfan produces efficient human cell engraftment in NOD/LtSz-Scid IL2Rγnull mice. Stem Cells. 2009;27:175–182. doi: 10.1634/stemcells.2008-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Takahashi T, Okajima A, Shiokawa M, Ishii N, Katano I, et al. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/γcnull (NOG) mice (hu-HSC NOG mice) Int Immunol. 2009;21:843–858. doi: 10.1093/intimm/dxp050. [DOI] [PubMed] [Google Scholar]
- McCune J, Kaneshima H, Krowka J, Namikawa R, Outzen H, Peault B, et al. The SCID-hu mouse: a small animal model for HIV infection and pathogenesis. Annu Rev Immunol. 1991;9:399–429. doi: 10.1146/annurev.iy.09.040191.002151. [DOI] [PubMed] [Google Scholar]
- McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- Kirberg J, Berns A, von Boehmer H. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex-encoded molecules. J Exp Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer RP, Bosma GC, Bosma MJ. Severe combined immunodeficiency (SCID) in the mouse. Pathology, reconstitution, neoplasms. Am J Pathol. 1985;120:464–477. [PMC free article] [PubMed] [Google Scholar]
- Bosma MJ. B and T cell leakiness in the scid mouse mutant. Immunodefic Rev. 1992;3:261–276. [PubMed] [Google Scholar]
- Kato C, Fujii E, Chen YJ, Endaya BB, Matsubara K, Suzuki M, et al. Spontaneous thymic lymphomas in the non-obese diabetic/Shi-scid, IL-2Rγnull mouse. Lab Anim. 2009;43:402–404. doi: 10.1258/la.2009.009012. [DOI] [PubMed] [Google Scholar]
- Katano I, Ito R, Eto T, Aiso S, Ito M. Immunodeficient NOD-scid IL-2Rγnull mice do not display T and B cell leakiness. Exp Anim. 2011;60:181–186. doi: 10.1538/expanim.60.181. [DOI] [PubMed] [Google Scholar]
- Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, et al. The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- Okada S, Harada H, Ito T, Saito T, Suzu S. Early development of human hematopoietic and acquired immune systems in new born NOD/Scid/Jak3null mice intrahepatic engrafted with cord blood-derived CD34+ cells. Int J Hematol. 2008;88:476–482. doi: 10.1007/s12185-008-0215-z. [DOI] [PubMed] [Google Scholar]
- Sato Y, Takata H, Kobayashi N, Nagata S, Nakagata N, Ueno T, et al. Failure of effector function of human CD8+ T cells in NOD/SCID/JAK3/ immunodeficient mice transplanted with human CD34+ hematopoietic stem cells. PLoS One. 2010;5:e13109. doi: 10.1371/journal.pone.0013109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa Y, Fukuchi Y, Ito M, Kobayashi K, Kuramochi T, Ikeda Y, et al. Establishment of human granulocyte–macrophage colony stimulating factor produing transgenic SCID mice. Br J Haematol. 1996;95:437–442. doi: 10.1046/j.1365-2141.1996.8012423.x. [DOI] [PubMed] [Google Scholar]
- Fukuchi Y, Miyakawa Y, Kobayashi K, Kuramochi T, Shimamura K, Tamaoki N, et al. Cytokine dependent growth of human TF-1 leukemic cell line in human GM-CSF and IL-3 producing transgenic SCID mice. Leuk Res. 1998;22:837–843. doi: 10.1016/s0145-2126(98)00084-8. [DOI] [PubMed] [Google Scholar]
- Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- Takizawa H, Manz MG. Macrophage tolerance: CD47-SIRP-α-mediated signals matter. Nat Immunol. 2007;8:1287–1289. doi: 10.1038/ni1207-1287. [DOI] [PubMed] [Google Scholar]
- Rongvaux A, Willinger T, Takizawa H, Rathinam C, Auerbach W, Murphy AJ, et al. Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. . Proc Natl Acad Sci USA. 2011;108:2378–2383. doi: 10.1073/pnas.1019524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willinger T, Rongvaux A, Takizawa H, Yancopoulos GD, Valenzuela DM, Murphy AJ, et al. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proc Natl Acad Sci USA. 2011;108:2390–2395. doi: 10.1073/pnas.1019682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M, et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2rγnull humanized mice. Proc Natl Acad Sci USA. 2010;107:13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner R, Chaudhari SN, Rosenberger J, Surls J, Richie TL, Brumeanu TD, et al. Expression of HLA class II molecules in humanized NOD.Rag1KO.IL2RgcKO mice is critical for development and function of human T and B cells. PLoS One. 2011;6:e19826. doi: 10.1371/journal.pone.0019826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu H, Nishikomori R, Heike T, Ito M, Kobayashi K, Katamura K, et al. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/γcnull mice model. Blood. 2003;102:873–880. doi: 10.1182/blood-2002-09-2755. [DOI] [PubMed] [Google Scholar]
- Yajima M, Imadome K, Nakagawa A, Watanabe S, Terashima K, Nakamura H, et al. T cell-mediated control of Epstein–Barr virus infection in humanized mice. J Infect Dis. 2009;200:1611–1615. doi: 10.1086/644644. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M, et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2rγnull humanized mice. Proc Natl Acad Sci USA. 2010;107:13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Gurer C, Ploss A, Liu YF, Arrey F, Sashihara J, et al. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J Exp Med. 2009;206:1423–1434. doi: 10.1084/jem.20081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanai H, Wilcox MA, Mathis D, Benoist C.A defective Il15 allele underlies the deficiency in natural killer cell activity in nonobese diabetic mice Proc Natl Acad Sci USA01;1079305–9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. . J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Khoury M, Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc Natl Acad Sci USA. 2009;106:21783–21788. doi: 10.1073/pnas.0912274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiokawa M, Takahashi T, Murakami A, Kita S, Ito M, Sugamura K, et al. In vivo assay of human NK-dependent ADCC using NOD/SCID/γcnull (NOG) mice. Biochem Biophys Res Commun. 2010;399:733–737. doi: 10.1016/j.bbrc.2010.07.145. [DOI] [PubMed] [Google Scholar]
- Billerbeck E, Barry WT, Mu K, Dorner M, Rice CM, Ploss A. Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocytenmacrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rγnull humanized mice. Blood. 2011;117:3076–3086. doi: 10.1182/blood-2010-08-301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol. 2000;105:847–859. doi: 10.1067/mai.2000.106485. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Wershil BK. The two faces of the mast cell. Nature. 1996;381:21–22. doi: 10.1038/381021a0. [DOI] [PubMed] [Google Scholar]
- Kambe N, Hiramatsu H, Shimonaka M, Fujino H, Nishikomori R, Heike T, et al. Development of both human connective tissue-type and mucosal-type mast cells in mice from hematopoietic stem cells with identical distribution pattern to human body. Blood. 2004;103:860–867. doi: 10.1182/blood-2003-04-1160. [DOI] [PubMed] [Google Scholar]
- Machida K, Suemizu H, Kawai K, Ishikawa T, Sawada R, Ohnishi Y, et al. Higher susceptibility of NOG mice to xenotransplanted tumors. J Toxicol Sci. 2009;34:123–127. doi: 10.2131/jts.34.123. [DOI] [PubMed] [Google Scholar]
- Suemizu H, Monnai M, Ohnishi Y, Ito M, Tamaoki N, Nakamura M. Identification of a key molecular regulator of liver metastasis in human pancreatic carcinoma using a novel quantitative model of metastasis in NOD/SCID/γcnull (NOG) mice. Int J Oncol. 2007;31:741–751. [PubMed] [Google Scholar]
- Miyakawa Y, Ohnishi Y, Tomisawa M, Monnai M, Kohmura K, Ueyama Y, et al. Establishment of a new model of human multiple myeloma using NOD/SCID/γcnull (NOG) mice. Biochem Biophys Res Commun. 2004;313:258–262. doi: 10.1016/j.bbrc.2003.11.120. [DOI] [PubMed] [Google Scholar]
- Ninomiya M, Kiyoi H, Ito M, Hirose Y, Naoe T. Retinoic acid syndrome in NOD/scid mice induced by injecting an acute promyelocytic leukemia cell line. Leukemia. 2004;18:442–448. doi: 10.1038/sj.leu.2403284. [DOI] [PubMed] [Google Scholar]
- Ito R, Katano I, Kawai K, Hirata H, Ogura T, Kamisako T, et al. Highly sensitive model for xenogenic GVHD using severe immunodeficient NOG mice. Transplantation. 2009;87:1654–1658. doi: 10.1097/TP.0b013e3181a5cb07. [DOI] [PubMed] [Google Scholar]
- Zhang L, Meissner E, Chen J, Su L. Current humanized mouse models for studying human immunology and HIV-1 immuno-pathogenesis. Sci China Life Sci. 2010;53:195–203. doi: 10.1007/s11427-010-0059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namikawa R, Kaneshima H, Lieberman M, Weissman IL, McCune JM. Infection of the SCID-hu mouse by HIV-1. Science. 1988;242:1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y, Tanaka Y, Ito M, Yamamoto N. Humanized mice for human retrovirus infection. Curr Top Microbiol Immunol. 2008;324:133–148. doi: 10.1007/978-3-540-75647-7_9. [DOI] [PubMed] [Google Scholar]
- Sato K, Nie C, Misawa N, Tanaka Y, Ito M, Koyanagi Y.Dynamics of memory and naive CD8+ T lymphocytes in humanized NOD/SCID/IL-2Rγnull mice infected with CCR5-tropic HIV-1 Vaccine82010Suppl 2B32–37. [DOI] [PubMed]
- Watanabe S, Ohta S, Yajima M, Terashima K, Ito M, Mugishima H, et al. Humanized NOD/SCID/IL2Rγnull mice transplanted with hematopoietic stem cells under nonmyeloablative conditions show prolonged life spans and allow detailed analysis of human immunodeficiency virus type 1 pathogenesis. J Virol. 2007;81:13259–13264. doi: 10.1128/JVI.01353-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Terashima K, Ohta S, Horibata S, Yajima M, Shiozawa Y, et al. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rγnull mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood. 2007;109:212–218. doi: 10.1182/blood-2006-04-017681. [DOI] [PubMed] [Google Scholar]
- Denton PW, Estes JD, Sun Z, Othieno FA, Wei BL, Wege AK, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008;5:e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen R, Wahl A, Denton PW, Garcia JV. Immune reconstitution of the female reproductive tract of humanized BLT mice and their susceptibility to human immunodeficiency virus infection. J Reprod Immunol. 2011;88:195–203. doi: 10.1016/j.jri.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary S, Archin N, Cheema M, Dahl N, Garcia JV, Margolis D. Latent HIV-1 infection of resting CD4+ T cells in the humanized Rag2−/−γc−/− mouse. J Virol. 2012;86:114–120. doi: 10.1128/JVI.05590-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson AB, Kieff E.Epstein–Barr virusIn: Knipe DM, Howley PM (eds.)Fields virology. Philadelphia; Lippincott Williams & Wilkins; 20112575–2628. [Google Scholar]
- Yajima M, Imadome K, Nakagawa A, Watanabe S, Terashima K, Nakamura H, et al. A new humanized mouse model of Epstein–Barr virus infection that reproduces persistent infection, lymphoproliferative disorder, and cell-mediated and humoral immune responses. J Infect Dis. 2008;198:673–682. doi: 10.1086/590502. [DOI] [PubMed] [Google Scholar]
- Sato K, Misawa N, Nie C, Satou Y, Iwakiri D, Matsuoka M, et al. A novel animal model of Epstein–Barr virus-associated hemophagocytic lymphohistiocytosis in humanized mice. Blood. 2011;117:5663–5673. doi: 10.1182/blood-2010-09-305979. [DOI] [PubMed] [Google Scholar]
- Imadome K, Yajima M, Arai A, Nakazawa A, Kawano F, Ichikawa S, et al. Novel mouse xenograft models reveal a critical role of CD4+ T cells in the proliferation of EBV-infected T and NK cells. PLoS Pathog. 2011;7:e1002326. doi: 10.1371/journal.ppat.1002326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Willinger T, Rongvaux A, Eynon EE, Stevens S, Manz MG, et al. A mouse model for the human pathogen Salmonella typhi. . Cell Host Microbe. 2010;8:369–376. doi: 10.1016/j.chom.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby SJ, Brehm MA, Greiner DL, Shultz LD, McClelland M, Smith KD, et al. Humanized nonobese diabetic-scid IL2rγnull mice are susceptible to lethal Salmonella typhi infection. Proc Natl Acad Sci USA. 2010;107:15589–15594. doi: 10.1073/pnas.1005566107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firoz Mian M, Pek EA, Chenoweth MJ, Ashkar AA. Humanized mice are susceptible to Salmonella typhi infection. Cell Mol Immunol. 2011;8:83–87. doi: 10.1038/cmi.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerwein RW, Roestenberg M, Moorthy VS. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol. 2011;11:57–64. doi: 10.1038/nri2902. [DOI] [PubMed] [Google Scholar]
- Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, et al. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat Biotechnol. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Kawai K, Mitsui T, Taniguchi K, Monnai M, Wakui M, et al. The reconstituted ‘humanized liver' in TK-NOG mice is mature and functional. Biochem Biophys Res Commun. 2011;405:405–410. doi: 10.1016/j.bbrc.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, et al. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- Suemizu H, Hasegawa M, Kawai K, Taniguchi K, Monnai M, Wakui M, et al. Establishment of a humanized model of liver using NOD/Shi-scid IL2Rgnull mice. Biochem Biophys Res Commun. 2008;377:248–252. doi: 10.1016/j.bbrc.2008.09.124. [DOI] [PubMed] [Google Scholar]
- Mikolajczak SA, Sacci JB, Jr, de la Vega P, Camargo N, VanBuskirk K, Krzych U, et al. Disruption of the Plasmodium falciparum liver-stage antigen-1 locus causes a differentiation defect in late liver-stage parasites. Cell Microbiol. 2011;13:1250–1260. doi: 10.1111/j.1462-5822.2011.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Diaz MB, Mulet T, Viera S, Gomez V, Garuti H, Ibanez J, et al. Improved murine model of malaria using Plasmodium falciparum competent strains and non-myelodepleted NOD-scid IL2Rγnull mice engrafted with human erythrocytes. Antimicrob Agents Chemother. 2009;53:4533–4536. doi: 10.1128/AAC.00519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissig KD, Wieland SF, Tran P, Isogawa M, Le TT, Chisari FV, et al. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J Clin Invest. 2010;120:924–930. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haridass D, Yuan Q, Becker PD, Cantz T, Iken M, Rothe M, et al. Repopulation efficiencies of adult hepatocytes, fetal liver progenitor cells, and embryonic stem cell-derived hepatic cells in albumin-promoter-enhancer urokinase-type plasminogen activator mice. Am J Pathol. 2009;175:1483–1492. doi: 10.2353/ajpath.2009.090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn ML, Bility MT, Zhang L, Kovalev GI, Buntzman A, Frelinger JA, et al. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology. 2011;140:1334–1344. doi: 10.1053/j.gastro.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino G, Anastasi J, Feng J, Mc Shan C, DeGroot L, Quintans J, et al. The fate of human peripheral blood lymphocytes after transplantation into SCID mice. Eur J Immunol. 1993;23:1023–1028. doi: 10.1002/eji.1830230506. [DOI] [PubMed] [Google Scholar]
- van Rijn RS, Simonetti ER, Hagenbeek A, Hogenes MC, de Weger RA, Canninga-van Dijk MR, et al. A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mononuclear cells in RAG2−/− γc−/− double-mutant mice. Blood. 2003;102:2522–2531. doi: 10.1182/blood-2002-10-3241. [DOI] [PubMed] [Google Scholar]
- King MA, Covassin L, Brehm MA, Racki W, Pearson T, Leif J, et al. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009;157:104–118. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]