Natural killer (NK) cells, like T and B cells, are an important subset of immune cells, and contribute to innate immunity based on quick and direct cytotoxicty to tumors, viral infected cells and stressed cells without pre-stimulation.1, 2 Great advances have been made in dividing immune cells into distinct developmental and functional subgroups by transcription factors and typical surface markers during the last 20 years, but the developmental pathway of NK cells is still not as clear as that of T or B cells.3, 4, 5
In a recent issue of Immunity, Gordon et al. defined transcription factors T-bet and Eomes as two sequential, genetically separable checkpoints of NK cell maturation.6 T-bet was essential for developmental stability of immature NK cells, while Eomes was required for NK cell maturation. Thus single deficiency of T-bet or Eomes leads to loss of non-overlapping NK cell subsets, respectively, and NK cells were absolutely deficient in T-bet and Eomes double knockout mice though NK precursor (NKP) seems to be unaffected. The research unveiled distinctive roles of T-bet and Eomes in NK cell development, but also raised some questions for further discussion.
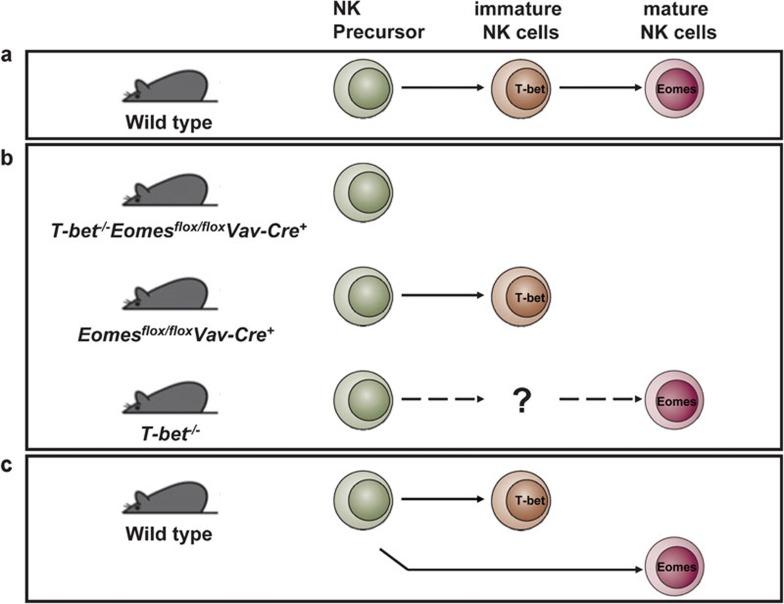
If both Eomes and T-bet control NK cell maturation, how do the two factors cooperate? Though this paper did not present exact expressions of the two factors on NK cells at different developmental stages, results of temporal deletion studies may give some clues. T-bet should be continuously expressed on immature and mature NK cells, while Eomes is expressed strictly on mature NK cells. As mentioned above, T-bet stabilizes immature NK cell, whereas Eomes promotes NK cell maturation. A reasonable hypothesis is that T-bet may partially inhibit Eomes induction and NK cell maturation is accompanied by downregulation of T-bet and upregulation of Eomes. But the fact is that mature NK cells kept on expressing a high level of T-bet. There seems to be some upstream factors that intrinsically trigger NK cell maturation by inducing Eomes expression. The signal provided by Eomes maybe stronger than that of T-bet; thus, NK cells become mature. Hence, T-bet seems dispensable in mature NK cells. This assumption is strongly supported by T-bet-deficient mice, which still have nearly half NK cells compared to normal mice. But NK cells in Eomes−/− mice can stably exists, and double deficiency of T-bet and Eomes leads NK cells to lose the NK antigens such as NK1.1 and NKp46 (Figure 1), suggesting that T-bet is not only a molecule to maintain immature NK cell phenotypes, but also critical for differentiation from NKP to immature NK cells. Then it seems that unintelligible mature NK cells exist and even increase, while immature NK cells are absent in T-bet-deficient mice. Further study is required to determine whether there are parts of DX5+ mature NK cells which generate directly from NKPs and are also independent on DX5− NK cells.
Figure 1.
Working models for the developmental pathways of natural killer (NK) cells. Two hypotheses on NK cell development are proposed. (a) ‘Single line' model, also a conventional model, proposes that immature NK cells derive from NK precursor (NKP) and subsequently differentiate into mature NK cells during NK cell development in wild-type mice. (b) However, ‘single line' model can not explain compositions of NK cells in T-bet or Eomes single deficient mice and double knockout mice. (c) ‘Parallel lines' model proposes that differentiation pathways for two subsets of NK cells diverge after NKP stage.
Considering these facts, the author still preferably concluded that T-bet and Eomes are sequential checkpoints of NK cell maturation. Key evidence is provided by adoptive transfer assays demonstrating that purified Eomes− NK cells can differentiate into Eomes+ NK cells after adoptive transfer into Il2rg−/−Rag2−/− mice.6 However, the result of adoptive transfer is quite different when the recipients are sublethally-irradiated mice. After being transferred into irradiated mice, donor derived Eomes− NK cells failed to acquire Eomes and remained immature in the spleen, which is identical with result from our lab. A proper explanation is that cytokines redundancy in Il2rg−/−Rag2−/− mice would induce Eomes in donor cells and events occurring in irradiated recipients may be closer to the natural condition. Thus, adoptive transfer experiments did not provide sufficient evidence that T-bet and Eomes sequentially work on NK cell maturation.
Besides, we notice that hepatic Eomes−DX5−TRAIL+ NK cells were thought to be immature NK cells and the DX5+ NK cells that arose in recipient organs were considered the same as mature NK cells in periphery for adoptive transfer experiments. Although hepatic Eomes− NK cells show immature phenotypes,6 it is doubtful to equate them to immature NK cells. An adoptive transfer study performed in our lab showed that purified hepatic DX5− NK cells preferentially accumulate to liver, and donor cells are nearly undetectable in other organs which excludes the possibility that NK cells change their phenotypes in other organs. Numbers of donor derived cells varied significantly between liver and other organs. Together with the fact that the stable enrichment of DX5−TRAIL+ NK cells in the liver of normal adult mice,7, 8 ‘immature' is not a sufficient reason to explain NK cell unique tissue reconstruction. Therefore, hepatic DX5−TRAIL+ NK cells may be different from the immature intermediate. Mount of Eomes−DX5−TRAIL+ immature NK cells occurred in spleen and bone marrow (BM) of Eomesflox/floxVav-Cre+ mice, and the expressions of TRAIL, DX5 and several members of the ly49 family on the intermediate resembled those on hepatic Eomes−DX5−TRAIL+ NK cells. However, more precise comparison will be helpful to clarify the difference between the two subsets. Whether transfer of splenic DX5− NK cells into recipient mice will lead to a stable existence of Eomes−DX5−TRAIL+ NK cells in liver remains to be determined. To investigate the origin of hepatic Eomes−DX5−TRAIL+ NK cells, the author sorted BM NK cells with the phenotype of CD27loCD11blo and CD27hiCD11blo cells separately, and demonstrated that both of them can give rise to Eomes−DX5−TRAIL+ NK cells in the liver 1 week after transfer. However, we can also see that Eomes−DX5−TRAIL+ NK cells appeared in the spleen and BM of recipient mice 1 week after hepatic Eomes−DX5−TRAIL+ NK transfer, but the frequency of Eomes−DX5−TRAIL+ NK cells decreased 2 weeks after transfer. Hence, 1 week may be not long enough to define the ability of BM precursors to contribute to the existence of stable DX5−TRAIL+ NK cells in the liver.
Stability of Eomes− NK cells in the liver is probably due to the hepatic environment which appears non-permissive for Eomes induction.6 Given that about half of NK cells in the liver are still Eomes+, it seems that hepatic NK cells may consist of NK cells originally from the liver and conventional NK cells originally from BM. Chemokines and adhesion molecules expressed on hepatic NK cells may contribute to the diversity of liver NK cells.
References
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell. 2010;142:847–856. doi: 10.1016/j.cell.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslein DG, Lanier LL. Transcriptional control of natural killer cell development and function. Adv Immunol. 2011;109:45–85. doi: 10.1016/B978-0-12-387664-5.00002-9. [DOI] [PubMed] [Google Scholar]
- Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol. 2006;24:257–286. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8 T cells. Nat Rev. 2006;11:645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36:55–67. doi: 10.1016/j.immuni.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Cretney E, Hayakawa Y, Ota T, Akiba H, Ogasawara K, et al. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood. 2005;105:2082–2089. doi: 10.1182/blood-2004-08-3262. [DOI] [PubMed] [Google Scholar]
- Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, et al. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]