Abstract
Salmeterol is a long-acting β2-agonist that activates adenylate cyclase, causing long-lasting bronchodilation and has been used for many years to control asthma. However, little information is available about the immunoregulatory effects of salmeterol. We found that salmeterol decreases the production of pro-inflammatory cytokines in a model of allergen-challenged mice that expressed tumor-necrosis factor-alpha, interleukin-1 and interleukin-6. Dendritic cells (DCs) are antigen-presenting cells and act as sentinels in the airway. We found that salmeterol (10−5 mol/l) reduced the inflammation caused by lipopolysaccharide (0.1 µg/ml) in activated murine bone marrow-derived DCs. Moreover, western blots demonstrated that this protective effect was mediated partially by inhibiting signaling through the nuclear factor-kappa B (NF-κB), mitogen-activated protein kinase (MAPK) pathways and dramatically decreased levels of p-ERK. We suggest that salmeterol regulates the inflammation of allergen-induced asthma by modulating DCs. In conclusion, we provide evidence that DCs are the target immune cells responsible for the action of salmeterol against asthma.
Keywords: asthma, dendritic cells, pro-inflammatory cytokines, salmeterol
Introduction
Asthma, a response of the respiratory mucosal immune system, is a chronic airway inflammatory disease characterized by airway hyper-responsiveness (AHR) to a variety of specific and nonspecific stimuli and reversible airflow obstruction due to pulmonary eosinophilia, mucus hypersecretion and increased serum immunoglobulin E levels.1 Because of its potent bronchodilating effects, the long-acting β2-adrenoreceptor (β2-AR) agonist salmeterol is traditionally prescribed in addition to therapies involving inhaled corticosteroids for treatment of moderate to severe asthma.2 Salmeterol is highly lipophilic and diffuses through the lipid bilayer in muscle cell membranes to reach the β2-ARs, explaining its slow onset and long duration of action.3 β2-ARs are widely distributed not only in the smooth muscle tissue in the airway but also on some immunological cell membranes,4 implicating β2-ARs in the underlying immunological processes.
Dendritic cells (DCs) are antigen-presenting cells that serve as sentinels in the airway and induce primary immune responses.1, 5 DCs develop from bone marrow precursors and reside as immature DCs throughout the airway. When aeroallergens invade or exposure to inflammatory cytokines occurs, immature DCs undergo differentiation and maturation. Upon maturation, DCs on mucosal surfaces migrate into mediastinal lymph nodes6, 7 where they stimulate naive T cells and initiate adaptive immune responses. During this interaction with naive T cells, DCs not only report their earlier microenvironment but also alter the polarization of T helper (Th) 1 or Th2 cells.8 In asthma, DCs are critical for the development of the Th2 immune response.9 DCs also secrete high levels of pro-inflammatory cytokines when activated by the binding of the Toll-like receptor (TLR) 4 ligand. It has been demonstrated that pro-inflammatory cytokines in asthmatic subjects are higher than in normal subjects and play a key role in the development of asthma.10, 11
DCs form an interface between the innate and adaptive immune systems and orchestrate both primary and secondary immune responses. Despite the key role that DCs play in the development of asthma, few researchers have investigated the contribution of DCs to the action of anti-asthmatic agents in airway inflammation. We suggest that DCs may be the targets of anti-asthmatic drugs because DCs express β2-ARs, histamine receptors and cysteinyl leukotriene receptors.12, 13
The purpose of this study was to examine murine bone marrow-derived dendritic cells (BMDCs) cultured in the presence of salmeterol and lipopolysaccharide (LPS) to assess the effects of this β2-agonist on the function of DCs in vitro and in vivo.
Materials and methods
Mice and reagents
Female C57BL/6 mice, 6–8 weeks of age, were purchased from Joint Ventures Sipper BK Experimental Animal (Shanghai, China) and kept in a specific pathogen-free environment (Hangzhou, China). The animal protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Zhejiang University, and the animals were cared for in accordance with guidelines from Zhejiang University and the National Institutes of Health. LPS derived from Escherichia coli 0111:B4 and ovalbumin (OVA) were obtained from Sigma Chemical Co. (St Louis, MO, USA). Recombinant murine granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-4 were purchased from PeproTech (Rocky Hill, NJ, USA). Antibodies specific for total and phosphorylated forms of ERK1/2 (Thr202/Tyr204), JNK1/2 (Thr183/Tyr185) and p38 (Thr180/Tyr182) were purchased from Cell Signaling Technology (Boston, MA, USA). The phospho-IκBα and IκB kinase complex (IKKα/β) and P65 were from purchased Thermo (Rockford, IL, USA). The antibody used to detect β-actin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). FITC-conjugated anti-mouse CD86, PE-conjugated anti-mouse CD11c, CD80, CD40, MHC-II and the enzyme-linked immunosorbent assay (ELISA) kits for murine TNF-α, IL-1 and IL-6 were purchased from eBioscience (San Diego, CA, USA). Salmeterol was purchased from West Woods Business Park (Elisville, MO, USA), dissolved in 100% DMSO (Sigma Chemical Co., St Louis, MO, USA) and diluted with RPMI 1640 medium to the desired concentration with a final DMSO concentration of 0.1% for the in vitro studies. DMSO was added to untreated cultures at 0.1% (v/v) as a solvent control.
Generation of DCs
Murine BMDCs were prepared as previously described, with minor modifications.14 Briefly, bone marrow mononuclear cells were prepared from mouse (6–8 weeks old) tibia and femur suspensions by depletion of red cells and were cultured at a density of 2×106 cells/ml in six-well plates in complete RPMI 1640 medium supplemented with 10 ng/ml rmGM-CSF and 1 ng/ml IL-4. Non-adherent cells were gently washed out after 48 h of culture; the remaining loosely adherent clusters were cultured for an additional 48 h and harvested for treatment with salmeterol and LPS. To analyze the effects of salmeterol on DC development, salmeterol (10−8–10−4 mol/l) was added on day 6 with or without LPS (0.1 µg/ml).
Apoptosis assay
After treating with salmeterol (10−8–10−4 mol/l) for 24 h, DCs were labeled with annexin V and propidium iodide (PI) purchased from BD PharMingen (San Diego, CA, USA), following the manufacturer's instructions. Apoptotic cells (annexin V- and PI-positive) were detected using flow cytometry.
Measurement of cytokines
DCs were cultured at a concentration of 5×105 cells/ml in 24-well microplates (Costar) and were activated with LPS (0.1 µg/ml) in the presence or absence of salmeterol in complete RPMI 1640 medium supplemented with 10 ng/ml rmGM-CSF and 1 ng/ml IL-4. Concentrations of tumor necrosis factor-alpha (TNF-α), IL-1β and IL-6 in cell culture supernatants or in bronchoalveolar lavage fluid (BALF) were measured using murine cytokine-specific Quantikine ELISA kits (eBioscience).
Real-time RT-PCR
The effects of salmeterol on the kinetics of mRNA expression of TNF-α, IL-1β and IL-6 in DCs activated by LPS were investigated. Total RNA was extracted from DCs with TRIzol reagent, and then, following the instructions provided by the manufacturer, cDNAs were synthesized using a Revertaid First-Strand cDNA Synthesis Kit (Toyobo, Northpoint, UK). PCR amplification reactions were conducted using a SYBR Green Supermix (TaKaRa; Dalian, China) in a 20 µl reaction containing 200 nM primers and 5 ng cDNA. Thermal cycling was initiated with a 2-min denaturation at 95 °C, followed by 40 cycles at 95 °C for 34 s, 60 °C for 15 s and 72 °C for 30 s. All measurements were performed in duplicate. The level of inflammatory cytokine mRNA expression was assessed relative to that of the β-actin housekeeping gene. The following primer pairs (forward and reverse, respectively) were used: 5′-CTGGGACAGTGACCTGGACT-3′ and 5′-GCACCTCAGGGAAGAGTCTG-3′ for TNF-α 5′-AGTTGCCTTCTTGGGACT GA-3′ and 5′-TCCACGATTTCCCAGAGAAC-3′ for IL-6; and 5′-GCCCATCCTCTGTGACTCAT-3′ and 5′-AGGCCACAGGTATTTTGTCG-3′ for IL-1β.
FACS analysis
DCs were harvested and washed twice with phosphate-buffered saline (PBS) containing 0.1% sodium azide and 2% heat-inactivated fetal calf serum (wash buffer). Cells were then incubated with Fc receptor-blocking antibodies (PharMingen) for 5 min. Fluorescence antibodies were then added at a concentration of one mg per 1×106 cells per 100 µl, and cells were incubated at 4 °C for 30 min. The cells were washed twice with ice-cold PBS and then analyzed by flow cytometry using a FACSCalibur flow cytometer and Cell Quest software (Becton Dickinson, Mountain View, CA, USA).
Western blotting
For analysis of mitogen-activated protein kinase (MAPK) and signaling pathways, phospho-antibodies against ERK1/2, JNK/SAPK and p38 were used to detect expression levels of these molecules in whole-cell lysates using western blot. To examine the effects of salmeterol on nuclear factor-kappa B (NF-κB) nuclear translocation, cell lysates were analyzed using Western blotting to detect phospho-IκBα, IKKα/β and P65. Cell extracts were subjected to SDS-PAGE, transferred onto nitrocellulose membranes and were blotted as described previously.15
Protocol for the model of allergen-challenged mice
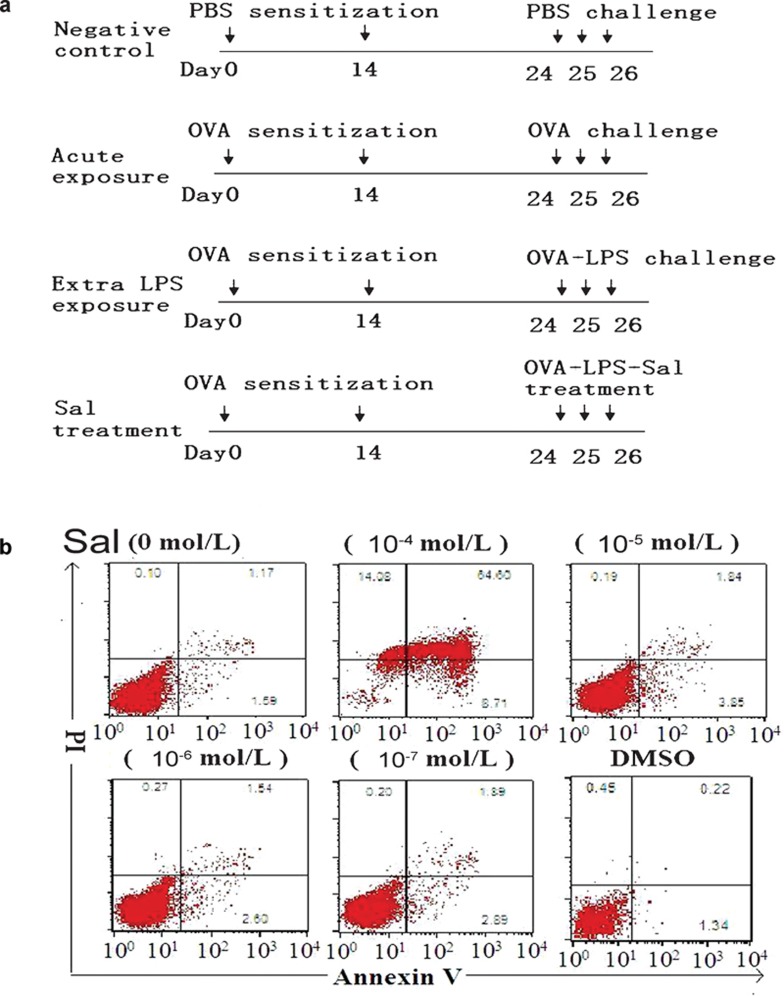
The protocols and schedules used for OVA, LPS or PBS administration to mice are summarized in Figure 1a. All mice were sensitized on days 0 and 14 by intraperitoneal injection of either PBS or 0.08 mg OVA and 0.1 ml aluminum hydroxide (alum) in 0.1 ml of PBS (pH 7.4). After sensitization, animals were exposed to aerosolized PBS-only (negative control), 1% OVS/PBS (acute exposure), 1% OVA/0.01% LPS/PBS (extra LPS exposure) or 1% OVA/0.01% LPS/salmeterol/PBS (sal treatment) for 40 min, once per day for 3 consecutive days (days 24–26). On day 27, the mice were killed and lungs were divided into two groups for analysis: the left lung lobes were lavaged three times with 1 ml of PBS with 1% fetal calf serum and 5 U/ml heparin, and the right halves were fixed by 4% paraformaldehyde for histological analysis.
Figure 1.
Experimental design and salmeterol concentration. (a) Experimental protocol. Mice were sensitized with PBS or OVA plus alum on days 0 and 14 and then were challenged with an aerosolized form of different drugs for 3 consecutive days. The extra-LPS exposure group of mice was exposed to 1% OVA and 0.01% LPS aerosol for 3 days. The salmeterol treatment group of mice was exposed to 1% OVA, 0.01% LPS and 10−5 mol/l salmeterol aerosol for 3 days. (b) The highest concentration of salmeterol increases apoptosis in DCs. On day 6, DCs cultured in GM-CSF and IL-4 were treated with different concentrations of salmeterol for 24 h. DCs treated with salmeterol were then harvested and labeled with annexin V/PI. The numbers indicate percentages of PI- or annexin V-positive cells. DC, dendritic cell; GM-CSF, granulocyte/macrophage colony-stimulating factor; LPS, lipopolysaccharide; OVA, ovalbumin; PBS, phosphate-buffered saline; PI, propidium iodide.
Analysis of BALF
Cell counts were determined on BALF smear slides stained with Wight and Giemsa (Beyotime, Nantong, China). Numbers of eosinophils were calculated as the percentage of eosinophils multiplied by the total number of cells in the BALF samples. The collected BALF was then centrifuged at 800g, and the supernatant was collected for analysis of cytokine levels.16
Lung histology
Paraffin-embedded sections (4 µm) were stained with hematoxylin and eosin to evaluate lung infiltration, as described previously.17 A semiquantitative scoring system was used to grade the size of lung infiltrates, whereby +5 signifies a large (>3 cells–deep) widespread infiltrate around the majority of vessels and bronchioles, and +1 signifies a small number of inflammatory foci. Goblet cells were counted on periodic acid-Schiff-stained lung sections using an arbitrary scoring system. In brief, periodic acid-Schiff-stained goblet cells in airway epithelium were measured in a double-blinded manner using a numerical scoring system (0, <5% 1, 5–25% 2, 25–50% 3, 50–75% and 4, >75% goblet cells). The sum of the airway scores from each lung was divided by the number of airways examined (20–30 per mouse) and expressed as a mucus score in arbitrary units.18
Airway hyper-responsivity in response to methacholine (MeCh)
AHR was assessed by whole-body plethysmography (Buxco Electronics, Troy, NY, USA), inducing airflow obstruction with a MeCh aerosol.19, 20 Each group of mice was exposed for 3 min to aerosolized saline followed by exposure to increasing concentrations of aerosolized MeCh (0, 6, 12.5, 25, 50 and 100 mg/ml) dissolved in isotonic saline. Following each nebulization, the enhanced pause (Penh) was recorded for 3 min. The Penh values measured during each 3 min sequence were averaged and expressed for each dose of MeCh. Penh data were plotted as the change from baseline per dose.
Statistical analysis
All experiments were independently performed three times in triplicate. Results are given as the mean±s.d. Results were compared using the Student's t-test and ANOVA. Values of P<0.05 were considered to be statistically significant.
Results
Salmeterol induces apoptosis of DCs
As an inhalant, the clinical concentration of salmeterol in the plasma is very low.21 To exclude the possibility that the impairment of DC function caused by salmeterol was due to a reduction of DCs via apoptosis, we first detected the apoptotic sensitivity of BMDCs to salmeterol. On day 6 of DC culture, salmeterol was added at different concentrations (10−7–10−4 mol/l) and the cultures were incubated for 24 h. We found that the highest concentration of salmeterol (10−4 mol/l) caused apoptosis of DCs, as shown by annexin V and PI labeling (Figure 1b). This observation demonstrated that the highest concentration of salmeterol that should be used in BMDCs is 10−5 mol/l, as salmeterol could not affect the differentiation and maturation of DCs at this concentration (data not shown).
Salmeterol attenuates the inflammation of asthma induced by OVA and LPS
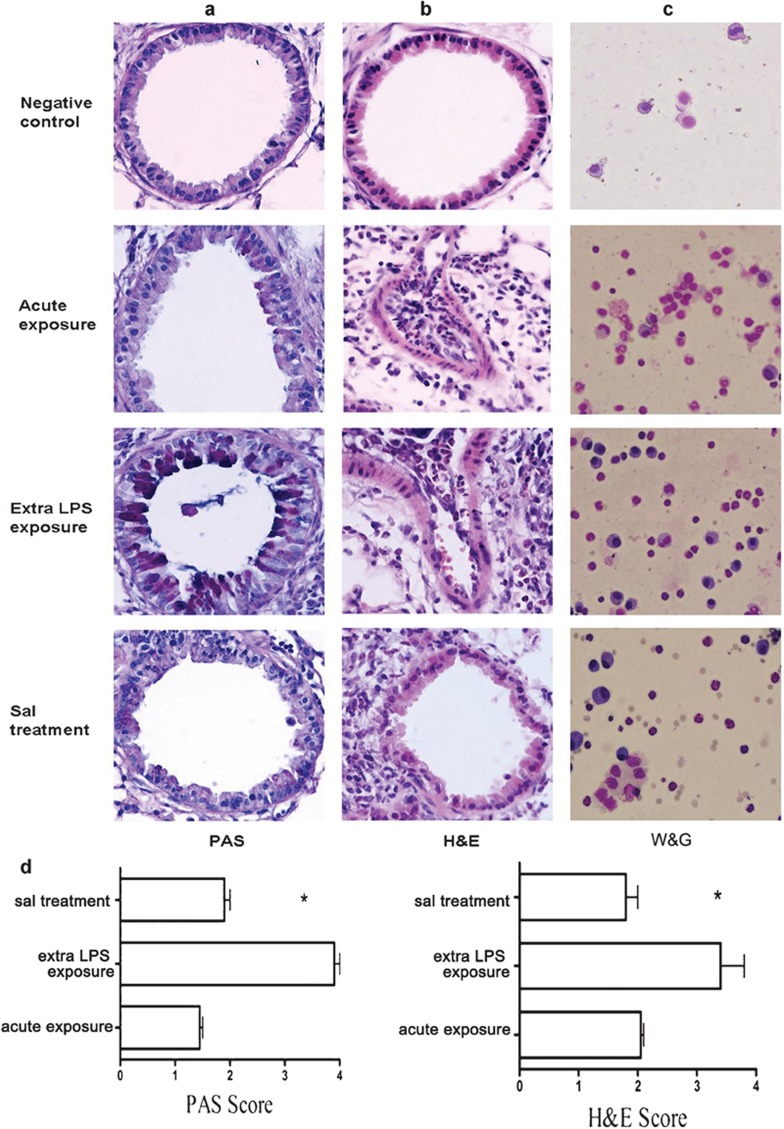
Salmeterol stimulates the β2-receptors located in the smooth muscle tissue in the airways, resulting in smooth muscle relaxation.22 Salmeterol is used in combination with inhaled corticosteroids for long-term prevention and control of symptoms in moderate or severe, persistent asthma.23 We investigated whether salmeterol could regulate the inflammation of asthma. Microscopically, the lung tissue of allergen-challenged mice showed peribronchial cell recruitment, together with hyperplasia of bronchial smooth muscle. Figure 2 shows the representative lung pathology of four groups of allergen-challenged mice. In all groups, the extra-LPS-exposed mice developed the most severe allergic airway inflammation, characterized by goblet cell hyperplasia (Figure 2a), peribronchovascular eosinophilic infiltration (Figure 2b) and a large number of eosinophils in the BALF (Figure 2c). Salmeterol reduced peribronchial eosinophilic infiltration and mucus hypersecretion and hyperplasia of goblet cells compared with the extra-LPS exposure group (Figure 2d). These results demonstrated the remedial effect of salmeterol on asthma.
Figure 2.
Salmeterol attenuates the inflammation of asthma induced by OVA and LPS. Lung sections of OVA-sensitized C57BL/6 mice killed 1 day after the third challenge with PBS, OVA, OVA with LPS or OVA with LPS plus salmeterol are shown. The formalin-fixed tissue sections were stained with PAS reagent to visualize mucus (a) and with H&E to visualize cell recruitment (b). In c, the BALF cells were stained with W&G. Original magnification, ×400. In d, *P<0.05; extra-LPS exposure group vs. salmeterol treatment group; data are representative of three independent experiments (n=10). BALF, bronchoalveolar lavage fluid; H&E, hematoxylin and eosin; LPS, lipopolysaccharide; OVA, ovalbumin; PAS, periodic acid-Schiff; W&G, Wight and Giemsa.
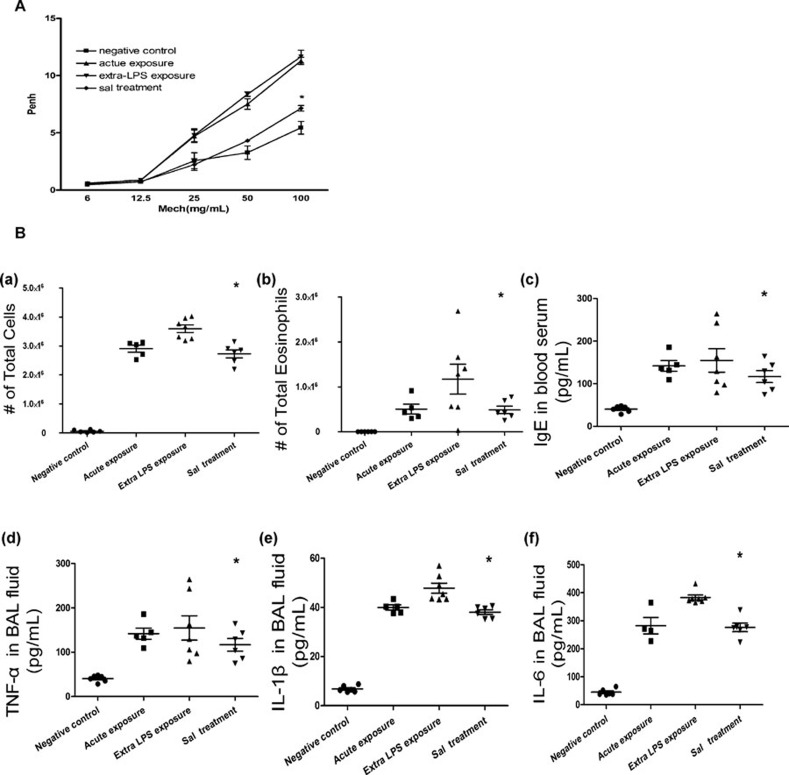
Salmeterol decreased OVA/LPSinduced AHR in response to MeCh
As a long-acting β2-AR agonist, salmeterol has been used for bronchodilation for many years in clinical settings. As shown in Figure 3A, salmeterol decreased the allergic mice after challenge with OVA/LPS. Whole-body plethysmography was carried out to examine the AHR in response to MeCh. In comparison with the control group of mice, the groups treated with OVA and extra-LPS developed a greater Penh in response to MeCh at the doses of 25 and 50 mg/ml. Treating the OVA/LPS groups with salmeterol resulted in a significant decrease in the enhanced AHR in allergic mice in a dose-dependent manner. As it is already known that salmeterol decreases the AHR via bronchodilation,24 the data on the group treated with OVA/salmeterol are not shown.
Figure 3.
Salmeterol attenuates the AHR and inflammatory response in asthma (A) Salmeterol decreased OVA/LPSinduced AHR in response to methacholine. Airway reactivity in response to increasing doses of nebulized methacholine was assessed by whole-body plethysmography (n=10). The data are expressed as the mean±s.e.m. *P<0.05; extra-LPS exposure group vs. salmeterol treatment group. (B) Salmeterol regulates the inflammatory response in asthma. Asthma was induced as described in the section on ‘Materials and methods'. Total cells (a) and eosinophils (b) from BALF were enumerated to evaluate lung airspace inflammation. (c–f) The levels of IgE in blood serum and the levels of pro-inflammatory cytokines in BALF were measured by ELISA. Group comparisons were analyzed by one-way ANOVA with the Dunnett post hoc t-test. *P<0.05; data are representative of three independent experiments (n=5). AHR, airway hyper-responsiveness; BALF, bronchoalveolar lavage fluid; IgE, immunoglobulin E; LPS, lipopolysaccharide; OVA, ovalbumin; TNF, tumor-necrosis factor; Penh, enhanced pause.
Salmeterol regulates the response of inflammation in asthma
TLR4 signaling plays a key role during the inflammatory process in the lung,25 and LPS is a natural ligand of TLR4. We used an in vivo asthma model to determine whether salmeterol could influence the inflammation caused by TLR4 signaling. After aerosolizing different groups of mice, we determined the total inflammatory cell and eosinophil count in the BALF (Figure 3B(a) and (b)) and compared these results with those in the group challenged with OVA and LPS only. We found that the number of total inflammatory cells decreased after treatment with salmeterol. We also collected serum to measure the concentration of immunoglobulin E (Figure 3B(c)). Pro-inflammatory cytokines were measured in the group treated with salmeterol and then compared to the group challenged with OVA and LPS only (Figure 3B(d–f)). Levels of TNF-α, IL-1β and IL-6 were significantly lower in samples from salmeterol-treated mice, as compared to measurements from the extra-LPS exposure group, but these levels were not different from those in the acute exposure group. These results suggested that salmeterol contends with asthma via regulating the inflammation of the airway.
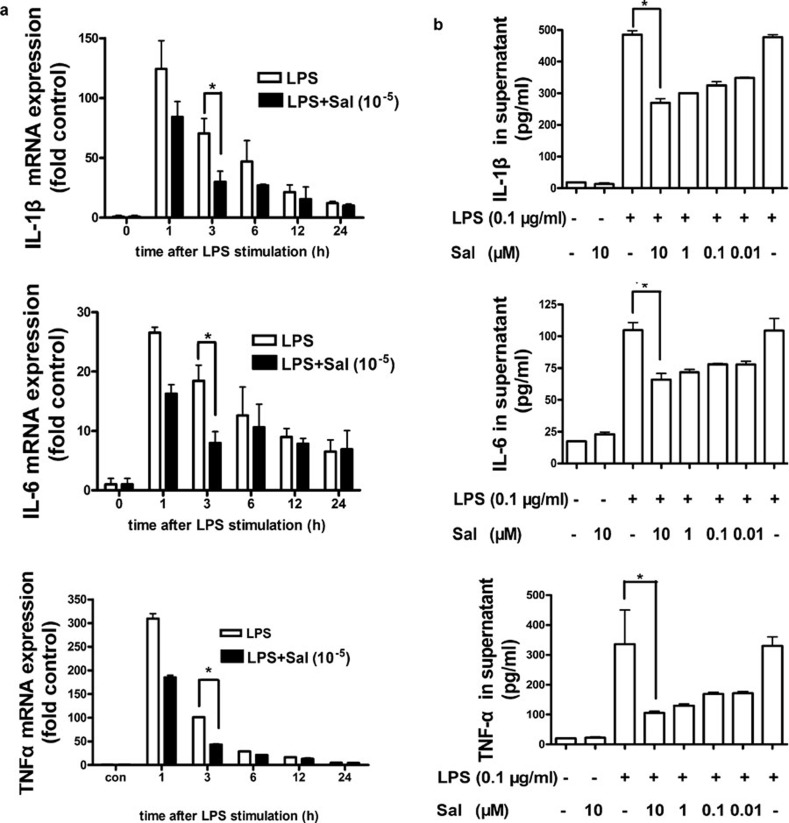
Salmeterol decreases mRNA and protein expression levels of pro-inflammatory cytokines in LPS-activated DCs
Because salmeterol can alleviate inflammation associated with asthma, we wanted to determine whether salmeterol can decrease the expression of pro-inflammatory cytokines in DCs. First, mRNA expression of pro-inflammatory cytokines from LPS-activated DCs after treatment with salmeterol (10−5 mol/l) was analyzed by real-time PCR. As shown in Figure 4a, the mRNA levels of TNF-α, IL-1β and IL-6 were significantly lower in treated DCs than in the control (P<0.05). We then detected the levels of protein expression of pro-inflammatory cytokines. Salmeterol treatment significantly inhibited production of pro-inflammatory cytokines, including TNF-α, IL-1β and IL-6 from LPS-stimulated DCs. As shown in Figure 4b, salmeterol decreased the expression of TNF-α, IL-1β and IL-6 in a concentration-dependent manner. After treatment for 9 h with a final salmeterol concentration of 10−5 mol/l, the levels of TNF-α, IL-1β and IL-6 were significantly lower in the supernatants of salmeterol-treated LPS-stimulated DCs than in LPS-stimulated DCs.
Figure 4.
Salmeterol decreases the mRNA and protein levels of pro-inflammatory cytokines in LPS-activated DCs. (a) Effect of salmeterol on mRNA levels of pro-inflammatory cytokines in LPS-activated DCs. Murine BMDCs (day 6) were treated as indicated, and then they were harvested and the total RNA was extracted. Real-time PCR was performed to examine relative levels of TNF-α, IL-1β and IL-6 mRNA expression. DCs (day 6) were treated with salmeterol (10 µM) and LPS (0.1 µg/ml) for 3 h and then analyzed using real-time PCR. *P<0.05; extra-LPS exposure group vs. salmeterol treatment group; data are representative of three independent experiments. (b) Effect of salmeterol on secretion of pro-inflammatory cytokines in LPS-activated DCs. Day 6 DCs were treated as indicated and were then analyzed for cytokine production by ELISA. The LPS concentration in the samples was 0.1 µg/ml. Samples were incubated with different concentrations of salmeterol for 9 h. *P<0.05; LPS (0.1 µg/ml) vs. LPS/salmeterol (10 µM). Data are representative of three independent experiments. BMDC, bone marrow-derived dendritic cell; DC, dendritic cell; LPS, lipopolysaccharide; TNF, tumor-necrosis factor.
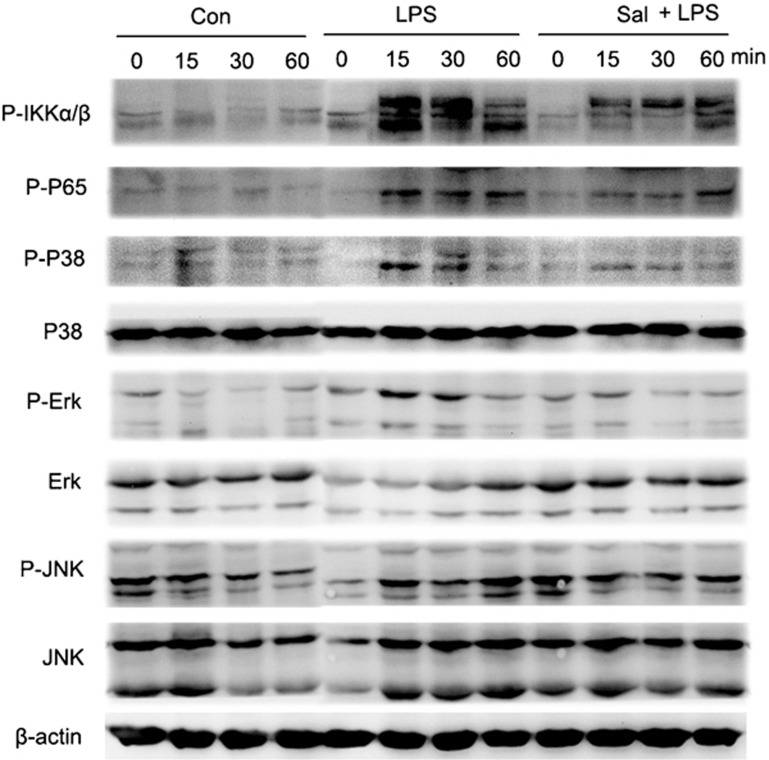
Salmeterol inhibits the activation of MAPK and NF-κB pathways induced by TLR4 signaling
LPS interacts with DCs and is recognized by TLR4.26, 27 MAPK signaling pathways play important roles during DC maturation, differentiation and cytokine secretion. We examined whether MAPK signaling pathways are involved in the salmeterol-mediated impairment of pro-inflammatory cytokine expression in DCs. Upon LPS stimulation of DCs, the ERK, p38 and JNK pathways were rapidly activated, whereas salmeterol-treated DCs showed decreased activation, as evidenced by alteration in the levels of phosphorylated ERK, p38 and JNK. Under normal conditions, most NF-κB subunits are sequestered in the cytoplasm by IκBα and IKKα/β. Following IκBα and IKKα/β phosphorylation, NF-κB subunits translocate into the nucleus where they regulate the expression of various genes. We found that phospho-IκBα and IKKα/β levels in total cell lysates of LPS-stimulated, salmeterol-treated DCs were considerably lower than those in control DCs (Figure 5). Phospho-p65 subunit levels showed corresponding changes. Surprisingly, LPS stimulation for 30 min significantly decreased the total levels of IκBα and IKKα/β. Thus, we observed the dynamics of IκBα and IKKα/β over a 2-h LPS stimulation period. We found that salmeterol could partially inhibit LPS-induced activation and degradation of IκBα and IKKα/β. The salmeterol-mediated alterations in the secretion of pro-inflammatory cytokines in mature DCs may be partially due to inhibition of the MAPK and NF-κB signaling pathways.
Figure 5.
Salmeterol inhibits MAPK and NF-κB activation. Day 6 DCs were treated with LPS (0.1 µg/ml) and salmeterol (10 µM) for varying amounts of time (0, 15, 30 and 60 min), and total cell lysates were prepared and analyzed for phospho-ERK (1/2) (p-ERK), phospho-JNK (p-JNK) and phospho-p38 (p-p38) expression. Cell lysates were prepared and blotted with the indicated anti-phospho antibodies. Total ERK, JNK and p38 were probed as quantitative controls. Salmeterol inhibits LPS-induced IKKα/β activation and NF-κB nuclear translocation. All proteins were extracted from cells, treated as above, and were western blotted for NF-κB p65. Phosphorylation levels of the proteins were quantified by measuring the band intensities. The results represent three independent experiments. DC, dendritic cell; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-kappa B.
Discussion
Asthma affects 300 million people worldwide28 and is increasing in prevalence, particularly in Western Europe and the United States. Asthma is characterized by chronic inflammation of the airway. Generally, the inflammation associated with asthma is directed by Th2 cytokines; these cytokines engage in positive feedback mechanisms to promote the production of more inflammatory mediators. DCs, the primary antigen-presenting cell, play a unique role in initiating and regulating primary immune responses and are pivotal to the Th1/Th2 balance.1 Salmeterol is known to be a long-acting β-agonist and is a powerful agent used to relieve the bronchoconstriction associated with asthma.29 However, the impact of salmeterol on DCs has not been well studied.
In this study, we found that salmeterol could not alter the phenotype of DCs. Salmeterol was also unable to affect the ability of DCs to stimulate the proliferation of allogeneic T lymphocytes or their ability to polarize T cells (data not shown). Using an asthma mouse model, we found that salmeterol not only exerts powerful therapeutic effects, but also is able to largely alleviate the symptoms of inflammation (Figures 2 and 3). We hypothesize that this phenomenon is related to the ability of salmeterol to change the pattern of pro-inflammatory cytokine secretion. Salmeterol greatly reduced the secretion of the pro-inflammatory cytokines TNF-α, IL-1β and IL-6 at both the mRNA and protein levels in DCs (Figure 4). The decreases in cytokine levels were dependent on the concentration of salmeterol used, providing strong evidence for the effect of salmeterol on the DC cytokine secretory profile. Recent studies have indicated that pro-inflammatory cytokines may be essential inflammatory mediators in the development of asthma.30, 31, 32 Pro-inflammatory cytokines are important for the development of airway inflammation by the innate immune system before activation of the adaptive immune system. They also serve as chemoattractants for neutrophils and eosinophils, increasing the AHR. Our results provide evidence that salmeterol inhibits the secretion of pro-inflammatory cytokines by DCs, potentially contributing to the total decrease of pro-inflammatory cytokines in asthma mouse models. Therefore, although salmeterol does not affect the ability of DCs to polarize T cells, the drug is still able to relieve asthma by altering the function of DCs.
DCs express both TLR433 and β2-AR,12 so the activation of β2-AR signaling may be involved in downregulating DC signaling through TLR4. It is well known that MAPK and NF-κB are the main pathways activated by TLR4 signaling.34, 35 In our study, we found that salmeterol reduced the levels of phosphorylated IκBα and nuclear NF-κB, proteins that are normally induced during DC maturation, suggesting that the ability of NF-κB to regulate gene expression may be inhibited (Figure 5). As expected, we also found that in salmeterol-treated DCs, the ERK, p38 and JNK pathways were rapidly activated at 15 min, whereas LPS-stimulated DCs treated with salmeterol showed dramatically decreased activation. β2-AR is a guanine nucleotide-binding protein-coupled receptor. The intracellular pathways activated by guanine nucleotide-binding protein-coupled receptors include the cyclic adenosine monophosphate/protein kinase A pathway, the MAPK/ERK1 pathway, the p43/p44MAPK pathway, the p38 MAPK pathway and the Ras pathway.36 Ras is a membrane-associated guanine nucleotide-binding protein that is normally activated in response to the binding of extracellular signals.37 We suggest that the phosphorylation of MAPK, which can be regulated by the Ras–Raf and Ras–PI3K signaling cascades, may be the node of those two signaling pathways, and this potential mechanism requires further study.
In summary, the data presented here provide initial evidence that salmeterol can suppress the inflammation associated with asthma, which may occur by action on the DCs. We also characterized some steps involved in its action mechanism, although additional studies are required to provide a more thorough understanding.
Acknowledgments
This work was supported by the Science and Technology Commission of Shanghai research project topics (2006073) and the National Key Basic Research Program of China (2007CB512403).
References
- Kuipers H, Lambrecht BN. The interplay of dendritic cells, Th2 cells and regulatory T cells in asthma. Curr Opin Immunol. 2004;16:702–708. doi: 10.1016/j.coi.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Nguyen LP, Lin R, Parra S, Omoluabi O, Hanania NA, Tuvim MJ, et al. Beta2-adrenoceptor signaling is required for the development of an asthma phenotype in a murine model. Proc Natl Acad Sci USA. 2009;106:2435–2440. doi: 10.1073/pnas.0810902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. Pharmacology of long-acting beta-agonists. Ann Allergy Asthma Immunol. 1995;75:177–179. [PubMed] [Google Scholar]
- Johnson M. Effects of beta2-agonists on resident and infiltrating inflammatory cells. J Allergy Clin Immunol. 2002;110 6 Suppl:S282–S290. doi: 10.1067/mai.2002.129430. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Lambrecht BN, Salomon B, Klatzmann D, Pauwels RA. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000;106:551–559. doi: 10.1172/JCI8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht BN, Pauwels RA, Fazekas de St Groth B. Induction of rapid T cell activation, division, and recirculation by intratracheal injection of dendritic cells in a TCR transgenic model. J Immunol. 2000;164:2937–2946. doi: 10.4049/jimmunol.164.6.2937. [DOI] [PubMed] [Google Scholar]
- Kuipers H, Heirman C, Hijdra D, Muskens F, Willart M, van Meirvenne S, et al. Dendritic cells retrovirally overexpressing IL-12 induce strong Th1 responses to inhaled antigen in the lung but fail to revert established Th2 sensitization. J Leukoc Biol. 2004;76:1028–1038. doi: 10.1189/jlb.0604325. [DOI] [PubMed] [Google Scholar]
- Schipf A, Heilmann A, Boue L, Mossmann H, Brocker T, Röcken M. Th2 cells shape the differentiation of developing T cell responses during interactions with dendritic cells in vivo. . Eur J Immunol. 2003;33:1697–1706. doi: 10.1002/eji.200323809. [DOI] [PubMed] [Google Scholar]
- Barkhordari E, Rezaei N, Ansaripour B, Larki P, Alighardashi M, Ahmadi-Ashtiani HR, et al. Proinflammatory cytokine gene polymorphisms in irritable bowel syndrome. J Clin Immunol. 2010;30:74–79. doi: 10.1007/s10875-009-9342-4. [DOI] [PubMed] [Google Scholar]
- Subratty AH, Hooloman NK. Role of circulating inflammatory cytokines in patients during an acute attack of bronchial asthma. Indian J Chest Dis Allied Sci. 1998;40:17–21. [PubMed] [Google Scholar]
- Seiffert K, Hosoi J, Torii H, Ozawa H, Ding W, Campton K, et al. Catecholamines inhibit the antigen-presenting capability of epidermal Langerhans cells. J Immunol. 2002;168:6128–6135. doi: 10.4049/jimmunol.168.12.6128. [DOI] [PubMed] [Google Scholar]
- Machida I, Matsuse Y, Kondo T, Kawano S, Saeki S, Tomari Y, et al. Cysteinyl leukotrienes regulate dendritic cell functions in a murine model of asthma. J Immunol. 2004;172:1833–1838. doi: 10.4049/jimmunol.172.3.1833. [DOI] [PubMed] [Google Scholar]
- Zhang M, Tang H, Guo Z, An H, Zhu X, Song W, et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- Wang D, Guo MX, Hu HM, Zhao ZZ, Qiu HL, Shao HJ, et al. Human T-cell leukemia virus type 1 oncoprotein tax represses ZNF268 expression through the cAMP-responsive element-binding protein/activating transcription factor pathway. J Biol Chem. 2008;283:16299–16308. doi: 10.1074/jbc.M706426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Lou J, Ouyang C, Chen W, Liu Y, Liu X, et al. Ras-related protein Rab10 facilitates TLR4 signaling by promoting replenishment of TLR4 onto the plasma membrane. Proc Natl Acad Sci USA. 2010;107:13806–13811. doi: 10.1073/pnas.1009428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthou G, Alissafi T, Semitekolou M, Simoes DC, Economidou E, Gaga M, et al. Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets. Nat Med. 2007;13:570–578. doi: 10.1038/nm1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semitekolou M, Alissafi T, Aggelakopoulou M, Kourepini E, Kariyawasam HH, Kay AB, et al. Activin-A that induces regulatory T cells that suppress T helper cell immune responses and protect from allergic airway disease. J Exp Med. 2009;206:1769–1785. doi: 10.1084/jem.20082603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JH, Irvin CG. Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol. 2003;94:1297–1306. doi: 10.1152/japplphysiol.00706.2002. [DOI] [PubMed] [Google Scholar]
- Shen HH, Ochkur SI, McGarry MP, Crosby JR, Hines EM, Borchers MT, et al. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol. 2003;170:3296–3305. doi: 10.4049/jimmunol.170.6.3296. [DOI] [PubMed] [Google Scholar]
- Capka V, Carter SJ. Minimizing matrix effects in the development of a method for the determination of salmeterol in human plasma by LC/MS/MS at low pg/mL concentration levels. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856:285–293. doi: 10.1016/j.jchromb.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Callaerts-Vegh Z, Evans KL, Dudekula N, Cuba D, Knoll BJ, Callaerts PF, et al. Effects of acute and chronic administration of beta-adrenoceptor ligands on airway function in a murine model of asthma. Proc Natl Acad Sci USA. 2004;101:4948–4953. doi: 10.1073/pnas.0400452101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates CJ, Lasserson TJ.Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events Cochrane Database Syst Rev 2010(1): CD007694. [DOI] [PMC free article] [PubMed]
- Storms W, Chervinsky P, Ghannam AF, Bird S, Hustad CM, Edelman JM, et al. A comparison of the effects of oral montelukast and inhaled salmeterol on response to rescue bronchodilation after challenge. Respir Med. 2004;98:1051–1062. doi: 10.1016/j.rmed.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch NA, Menges M, Rossner S, Schuler G. Culture of bone marrow cells in GM-CSF plus high doses of lipopolysaccharide generates exclusively immature dendritic cells which induce alloantigen-specific CD4 T cell anergy in vitro. . Eur J Immunol. 2000;30:1048–1052. doi: 10.1002/(SICI)1521-4141(200004)30:4<1048::AID-IMMU1048>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Saitoh S. Chaperones and transport proteins regulate TLR4 trafficking and activation. Immunobiology. 2009;214:594–600. doi: 10.1016/j.imbio.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Masoli M, Weatherall M, Ayling J, Williams M, Beasley R. The 24 h duration of bronchodilator action of the salmeterol/fluticasone combination inhaler. Respir Med. 2005;99:545–552. doi: 10.1016/j.rmed.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Machida I, Matsuse H, Kondo Y, Kawano T, Saeki S, Tomari T, et al. Effects of various anti-asthmatic agents on mite allergen-pulsed murine bone marrow-derived dendritic cells. Clin Exp Allergy. 2005;35:884–888. doi: 10.1111/j.1365-2222.2005.02262.x. [DOI] [PubMed] [Google Scholar]
- Berry M, Brightling C, Pavord I, Wardlaw A. TNF-alpha in asthma. Curr Opin Pharmacol. 2007;7:279–282. doi: 10.1016/j.coph.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Doganci A, Saver K, Karwot R, Finotto S. Pathological role of IL-6 in the experimental allergic bronchial asthma in mice. Clin Rev Allergy Immunol. 2005;28:257–270. doi: 10.1385/CRIAI:28:3:257. [DOI] [PubMed] [Google Scholar]
- Johnson VJ, Yucesoy B, Luster MI. Prevention of IL-1 signaling attenuates airway hyperresponsiveness and inflammation in a murine model of toluene diisocyanate-induced asthma. J Allergy Clin Immunol. 2005;116:851–858. doi: 10.1016/j.jaci.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Lakhani SA, Bogue CW. Toll-like receptor signaling in sepsis. Curr Opin Pediatr. 2003;15:278–282. doi: 10.1097/00008480-200306000-00009. [DOI] [PubMed] [Google Scholar]
- Zaru R, Ronkina N, Gaestel M, Arthur JS, Watts C. The MAPK-activated kinase Rsk controls an acute Toll-like receptor signaling response in dendritic cells and is activated through two distinct pathways. Nat Immunol. 2007;8:1227–1235. doi: 10.1038/ni1517. [DOI] [PubMed] [Google Scholar]
- Pomerantz JL, Baltimore D. Two pathways to NF-kappaB. Mol Cell. 2002;10:693–695. doi: 10.1016/s1097-2765(02)00697-4. [DOI] [PubMed] [Google Scholar]
- Fang Y, Lahiri J, Picard L. G protein-coupled receptor microarrays for drug discovery. Drug Discov Today. 2003;8:755–761. doi: 10.1016/s1359-6446(03)02779-x. [DOI] [PubMed] [Google Scholar]
- Kolch W. Ras/Raf signalling and emerging pharmacotherapeutic targets. Expert Opin Pharmacother. 2002;3:709–718. doi: 10.1517/14656566.3.6.709. [DOI] [PubMed] [Google Scholar]