Abstract
Objective:
Arthritis and valvular carditis co-exist in several human rheumatic diseases, including systemic lupus erythematosus, rheumatic fever, and rheumatoid arthritis. K/BxN T-cell receptor transgenic mice develop spontaneous, autoantibody-associated arthritis and valvular carditis. The common Fc receptor gamma signaling chain (FcRγ) is required for carditis in K/BxN mice. FcRγ pairs with numerous receptors in a variety of cells. Here we sought to identify the FcRγ-associated receptors and FcγR-expressing cells that mediate valvular carditis in this model.
Methods:
We bred K/BxN mice lacking the genes encoding the activating Fc gamma receptors (FcγRI, III, and IV) and assessed for valvular carditis. We similarly assessed complement component C3-deficient K/BxN mice. Immunohistochemistry, bone marrow transplantation, and macrophage depletion were used to define the key FcRγ-expressing cell type.
Results:
Genetic deficiency of only one of the activating FcγRs did not prevent carditis, whereas deficiency of all three activating FcγRs did. Further analysis demonstrated that FcγRIII and FcγRIV were the key drivers of valve inflammation; FcγRI was dispensable. C3 was not required. FcRγ expression by radioresistant cells was critical for valvular carditis to develop, and further analysis pointed to macrophages as the key candidate FcγR-expressing effectors of carditis.
Conclusion:
FcγRIII and FcγRIV acted redundantly to promote valvular carditis in the K/BxN mouse model of systemic autoantibody-associated arthritis. Macrophage depletion reduced the severity of valve inflammation. These findings suggest that pathogenic autoantibodies engage FcγRs on macrophages to drive valvular carditis and provide new insight into the pathogenesis of cardiovascular inflammation in the setting of autoantibody-associated chronic inflammatory diseases.
Several systemic autoimmune diseases characterized by autoantibody production affect both the synovial joints and the heart, including systemic lupus erythematosus (SLE), anti-phospholipid syndrome, acute rheumatic fever, and rheumatoid arthritis (RA) (1-3). Most notable is the increased cardiovascular morbidity and mortality among patients with SLE and RA due to atherosclerotic coronary artery disease. This increased risk is not fully explained by traditional risk factors, strongly suggesting that the chronic inflammatory diseases themselves contribute to poor cardiovascular outcomes (4-6). Cardiac manifestations of these diseases also include valvular carditis (2). The immune mechanisms by which these diseases provoke inflammation of the joints and also the cardiovascular system remain poorly understood.
T cell receptor (TCR) transgenic K/BxN mice develop spontaneous, fully penetrant, autoantibody-associated arthritis and valvular carditis (7, 8). The valve inflammation in these mice shares several pathologic features with the valvular carditis found in rheumatic conditions. Specifically, it affects left-sided valves, is characterized by immunoglobulin G (IgG) and complement C3 binding to the valves and a cellular infiltrate comprising predominantly T cells and mononuclear myeloid cells (2, 7, 9, 10). Neutrophils are not found in the inflamed valve in these mice (7). This model system is therefore well-suited to address how systemic autoimmune inflammatory diseases drive cardiac pathology.
Autoimmunity in K/BxN mice is initiated by a breach of immunological self-tolerance when T lymphocytes bearing the transgene-encoded “KRN” TCR recognize peptides derived from the ubiquitously expressed antigen glucose-6-phosphate isomerase (GPI) presented by the major histocompatibility complex (MHC) class II molecule I-Ag7 (8, 11). This ultimately leads to the sustained production of anti-GPI IgG autoantibodies. Transfer of anti-GPI autoantibodies causes arthritis in recipient mice (12). Interruption of any of the events leading to the production of autoantibodies abrogates the development of both arthritis and valvular carditis (7). However, the downstream immune effector mechanisms responsible for arthritis and carditis in this model differ. Specifically, arthritis requires complement component C5 but not the common Fc receptor gamma signaling chain (FcRγ). Conversely, valvular carditis requires FcRγ but not C5 (7).
FcRγ is an immunoreceptor tyrosine-based activation motif (ITAM)-containing signaling molecule that pairs with activating Fc gamma receptors (FcγRs) and several other types of receptors; it is required for cell surface expression of the receptors and for signal transduction (13-15). FcγRs bind the Fc portion of IgG (13, 15). There are two general categories of FcγRs: activating and inhibitory. In mice, the activating FcγRs are FcγRI, III and IV. These receptors have unique IgG-binding alpha chains, but share the common gamma signaling chain, FcRγ. The inhibitory receptor, FcγRII, does not associate with FcRγ. The activating FcγRs have distinct cellular expression patterns, predominantly on myeloid cells. They bind the different IgG subtypes with varying affinities: FcγRI and IV predominantly bind IgG2a/c and IgG2b, whereas FcγRIII binds IgG1 more strongly than it binds IgG2a/c and IgG2b (13, 15). The knowledge that the common gamma signaling chain FcRγ is required for valvular carditis in K/BxN mice led to two hypotheses: 1) that one or more of the activating Fc gamma receptors is required or 2) that a different FcRγ-associated receptor is at work. Here we used a genetic approach to dissect these possibilities and also explored what FcRγ-expressing cells are the key drivers of valvular carditis in K/BxN mice.
Materials and Methods
Mice
KRN TCR transgenic mice on the C57BL/6 (B6) background and B6 mice congenic for H-2g7 (B6.g7) were gifts from Diane Mathis and Christophe Benoist (Harvard Medical School, Boston, MA, USA and the Institut de Génétique et de Biologie Moléculaire et Cellulaire, Strasbourg, France) (7, 8). C5-deficient B6 mice congenic for the non-obese diabetic (NOD)-derived Hc allele (encoding non-functional C5) were also a gift from Drs. Mathis and Benoist (7, 16). C3-deficient mice on the B6 background were a gift from Michael Carroll (Harvard Medical School, Boston, MA, USA) (17). FcRγ (Fcer1g)-deficient mice on the B6 background were purchased from Taconic (18). Mice with the targeted deletion of Fcgr1 have been previously described (19). Mice with the targeted deletion of Fcgr4 were a gift from Jeffrey Ravetch (The Rockefeller University, New York, NY, USA) and have been previously described (19, 20). Generation and characterization of the FcγR II/III/IV and FcγRI/II/III/IV deficient mice will be described elsewhere (J. Sjef Verbeek, Ph.D., unpublished data, 2013). Notably, Fcgr2, Fcgr3, and Fcgr4 are in close proximity on mouse chromosome 1, and this entire region was targeted; Fcgr1 is on mouse chromosome 3. FcγRIII (Fcgr3)-deficient mice on the B6 background (Fcgr3tm1Sjv, stock no. 003171), B6 mice congenic for CD90.1 (B6.PL-Thy1a/Cy3, stock no. 000406) and Rag1-deficient B6 mice (Rag1tm1Mom, stock no. 002216) were purchased from the Jackson Laboratory (21, 22).
Mice with the described targeted alleles of the genes encoding the various FcγRs and complement components were interbred with KRN mice and B6.g7 congenic mice to generate mice described in the study. For ease of nomenclature, we refer to KRN+ H-2b/g7 mice as ‘K/BxN’ as we have done previously (7). Genotype was determined by PCR for all mice and confirmed by flow cytometry for the FcγR-deficient mice. Mice were bred in our specific-pathogen-free colonies and maintained under Institutional Animal Care and Use Committee-approved protocols at the University of Minnesota (protocols 0909A72086 and 1207A17481).
Antibodies
Antibodies used for flow cytometry included anti-CD3 (145-2C11) and anti-CD4 (RM4-5) from Biolegend (San Diego, CA, USA); anti-CD4 (RM4-5), anti-CD90.1 (HIS51), anti-CD44 (IM7), anti-IFNγ (XMG1.2), anti-IL17 (eBioTc11-18H10.1), anti-CD62L (MEL-14), anti-CD16/32 (clone 93), anti-Gr-1 (RB6-8C5), anti-F4/80 (BM8), and anti-B220 (RA3-6B2) from eBioscience (San Diego, CA, USA); anti-Vβ6 (RR4-7), anti-CD11b (M1/70) and anti-CD64 (X54-5/7.1) from BD Biosciences Pharmingen (San Jose, CA, USA); and anti-Armenian hamster IgG from Jackson ImmunoResearch Laboratories (West Grove, PA). The anti-FcγRIV specific antibody (9E9) was generously provided by Jeffrey Ravetch and has been previously described (23).
Additional antibodies used for immunohistochemistry included anti-CD64 (N-19) from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-CD16/32 (clone 93) and biotinylated anti-F4/80 (BM8) from eBioscience; anti-CD11c (N418) and anti-CD31 (clone 390) from Biolegend; and anti-Langerin (929F3.098) from Imgenex (San Diego, CA, USA). Biotinylated-SP-conjugated antibodies recognizing IgG1, IgG2b, and IgG2c were from Jackson ImmunoResearch Laboratories. Secondary antibodies included bovine anti-goat-DyLight 594 and strepavidin-AF488 from Jackson ImmunoLaboratories; goat anti-rabbit-DyLight 594 (Poly4054) and goat anti-Armenian hamster-DyLight 594 (Poly4055) from Biolegend; and strepavidin-DyLight 550 from Thermo Scientific (Rockford, IL, USA).
Assessment of arthritis, anti-GPI titers, and histology
Arthritis was assessed as previously described (24, 25). Serum anti-GPI total IgG and IgG subtype (IgG1, IgG2b, IgG2c and IgG3) titers were measured as described (25). Except where indicated, 8-10 week old mice were used for analysis of cardiac valve histopathology.
Immunohistochemistry and toluidine blue staining
Frozen sections were first blocked for Fcγ receptors and with avidin/biotin (Invitrogen Corporation, Carlsbad, CA, USA), when necessary. For the detection of the Fcγ receptors, sections were incubated with the unconjugated primary antibody recognizing the Fcγ receptor of interest (anti-CD64, anti-CD16/32 or the FcγRIV specific antibody) or appropriate isotype controls followed by detection with fluorescent-conjugated secondary antibodies. For the detection of CD11c, CD31 and langerin, frozen sections were stained with AF488-conjugated antibodies described above or appropriate isotype controls. For the detection of F4/80, frozen sections were stained with biotinylated anti-F4/80 followed by fluorescent strepavidin. Nuclei were counterstained with DAPI. Immunohistochemistry for IgG1, IgG2b and IgG2c was performed using biotinylated primary antibodies from Jackson Immunolaboratories or the appropriate isotype controls. The primary antibodies were detected by application of ImmPACT DAB peroxidase substrate with the ABC peroxidase kit (Vector Laboratories, Burlingame, CA, USA). Mast cells were detected in frozen sections by staining with toluidine blue per the Toluidine Blue Staining Protocol from IHC World, LLC (Woodstock, MD, USA). Slides were viewed on an Olympus BX51 microscope equipped with a digital camera and DP-BSW software (Olympus).
Flow cytometry
Intracellular cytokine staining for IL-17 was performed according to the instructions of the manufacturer (eBioscience). Flow cytometry was performed using a FACSCalibur and an LSRII (BD Biosciences, San Jose, CA, USA), and cells were analyzed using FlowJo version 8.8.7 software (Tree Star, Inc., Ashland, OR, USA).
Bone marrow transplantation
Nine- to 15-week-old Rag1-deficient recipient mice were irradiated with 300 Rad. Four hours following irradiation, 3 × 106 donor bone marrow cells were injected intravenously. Hearts were harvested 9 weeks following bone marrow transplantation.
Adoptive transfer
After labeling with carboxyfluorescein succinimidyl ester (CFSE), 5x106 donor cells were injected intravenously into H-2g7-expressing recipient mice. Lymphocytes were harvested from the recipient mouse 40 hours later and analyzed by flow cytometry.
Macrophage depletion
Three-week-old K/BxN mice were treated with clodronate liposomes or control PBS liposomes 100μL/10 g body weight injected intraperitoneally weekly from weeks 3-7 (Dr. Nico Van Rooijen, ClodronateLiposomes.com, The Netherlands). Mice were assessed for arthritis weekly. Hearts were harvested at 8 weeks and assessed for carditis. The presence of splenic macrophages was analyzed by flow cytometry.
Serum-transferred arthritis
Pooled serum (150 μL/dose) from K/BxN mice was injected intraperitoneally into recipient mice on days 0 and 2. The mice were monitored for the development of arthritis for 10 days.
Statistical analysis
A repeated-measures analysis of variance (ANOVA) was used to compare arthritis severity scoring. Post-hoc Tukey’s multiple comparison test was used when more than two groups were compared. Mitral valve thickness data were compared using a two-tailed Mann-Whitney U test. All other statistical differences between the mean values for groups were calculated using unpaired Student’s two-tailed t test. P values <0.05 were considered significant.
Results
Antibody production, T cell activation and antigen presentation occur normally in FcRγ-deficient K/BxN mice
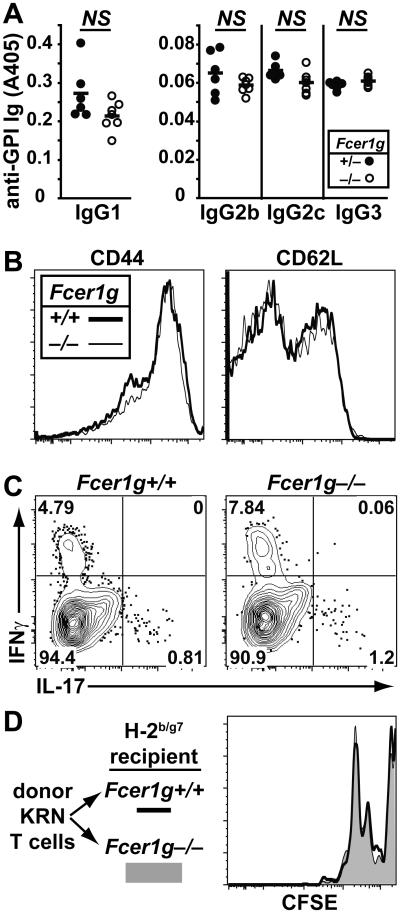
We have previously shown that K/BxN mice lacking FcRγ (Fcer1g) are protected from valvular carditis despite normal production of anti-GPI IgG antibodies (7). We further found no difference in the production of the anti-GPI IgG subtypes IgG1, IgG2b, IgG2c and IgG3 titers in FcRγ-deficient K/BxN mice compared with wild type K/BxN mice (Figure 1A). Because CD4+ T cells are key contributors to the valve pathology in this model (26), we asked whether these cells were activated normally in FcRγ-deficient K/BxN mice. Indeed, we found no difference in T cell expression of CD44 and CD62L or in intracellular cytokine production (IFNγ and IL-17) (Figure 1B and 1C). KRN T cells transferred into FcRγ-deficient or FcRγ-sufficient B6.g7 mice proliferated equivalently (Figure 1D). We therefore concluded that the protection from valvular carditis afforded by FcRγ deficiency in K/BxN mice is not due to impaired T or B cell activation or autoantibody synthesis, but rather to later effector events.
Figure 1. Normal adaptive immunity in FcRγ-deficient K/BxN mice.
(A) The titers of anti-GPI IgG1, IgG2b, IgG2c and IgG3 antibodies in serum from FcRγ-sufficient (n = 6) and FcRγ-deficient (n = 7) K/BxN mice are shown. P values calculated using a Student’s t-test were not significantly different (NS). (B) Representative flow cytometric assessment of CD44 and CD62L expression on CD4+Vβ6+ splenocytes and lymph node cells from FcRγ-sufficient and FcRγ-deficient K/BxN mice. Data are representative of 1 experiment, n = 3 FcRγ−/− K/BxN mice and n= 2 K/BxN control mice. Vβ6 is the KRN transgene-encoded TCRβ-chain. Activated T cells are CD44high and CD62Llow. (C) Representative flow cytometric assessment of the percentage of CD4+Vβ6+ splenocytes expressing intracellular IFNγ and IL-17 from the indicated mice. Data are representative of 2 experiments with a total of 5 FcRγ-deficient K/BxN mice and 3 K/BxN control mice. (D) Congenically-marked CFSE-labeled lymphocytes from KRN mice were transferred into FcRγ-sufficient or –deficient recipient mice expressing H-2g7. Cells are gated on CD3+CD4+90.1+Vβ6+ cells. Data represent two experiments with 3 FcRγ-deficient recipient mice and 2 FcRγ-sufficient control mice.
Complement is not required for the development of valvular carditis
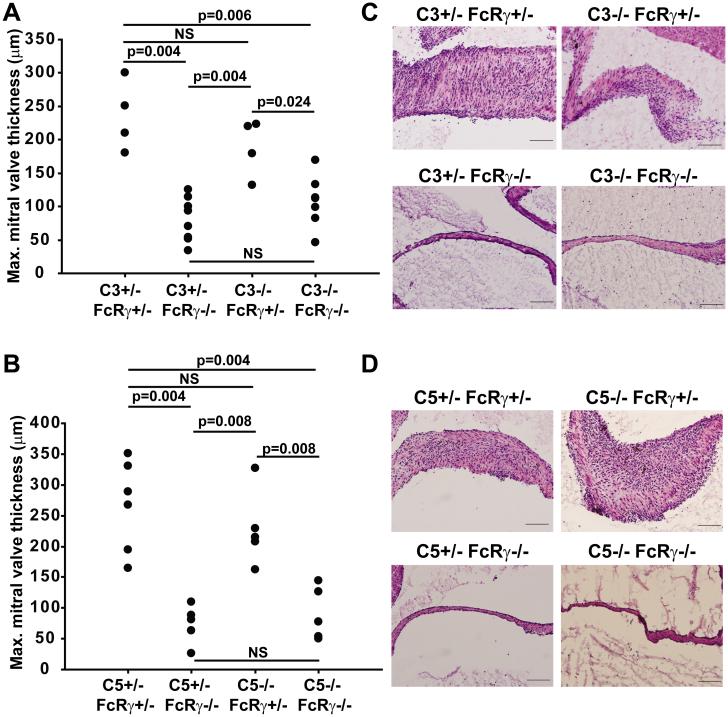
Because autoantibodies appear critical for the effector phase of valvular carditis, we investigated whether antibody-mediated activation of the complement system was pathogenically important. Previously we have shown that complement component C5 is not required for valvular carditis in this model (7). It remained possible that the upstream complement component C3 could be at work, for instance by binding to receptors for C3 split products, particularly since C3 is bound to the inflamed valves (7). We therefore bred K/BxN mice containing null alleles of the genes encoding FcRγ and/or complement components C3 or C5. We found that C3, like C5, was not necessary for the development of valvular carditis (Figure 2). These findings confirm that the autoantibody-associated valve inflammation in this model depends on FcRγ and not the complement system.
Figure 2. Complement components C3 and C5 are not necessary for valvular carditis.
The maximum mitral valve thickness in 8-week old K/BxN mice of the indicated genotypes for the genes encoding (A) C3 and FcRγ and (B) C5 and FcRγ is plotted. Each point represents one mouse. P-values were calculated using the Mann-Whitney U test. (C, D) Representative photomicrographs of mitral valves from K/BxN mice of the indicated genotypes stained with hematoxylin and eosin. (Objective 20x; Scale bars are 100 μm).
Presence of IgG and FcγR expressing cells in the inflamed K/BxN mitral valve
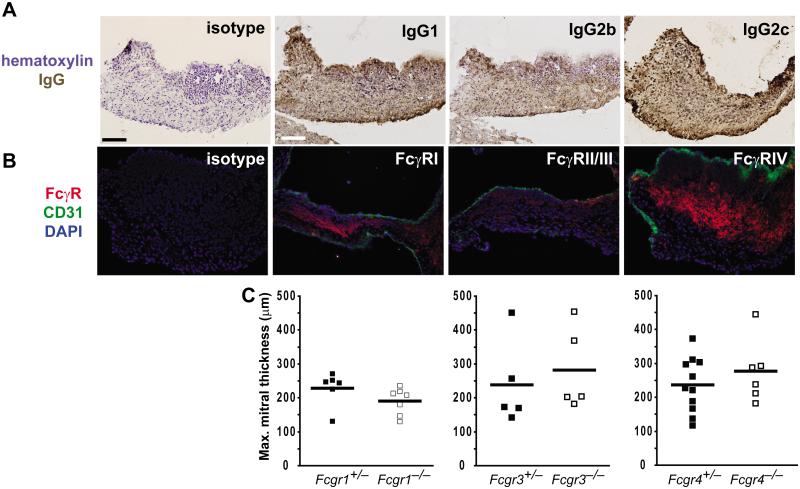
Given the requirement for FcRγ in the development of valvular carditis, we probed the inflamed mitral valve for the presence of the individual activating FcγRs and for the various IgG subtypes using immunohistochemistry. Although IgG1 is the predominant autoantibody subtype produced in K/BxN mice, we found that IgG1, 2b and 2c were all bound to the inflamed mitral valve (Figure 3A) (8). We also detected expression of each of the activating FcγRs in the inflamed cardiac valve (Figure 3B). These findings made it possible that any or all of the activating FcγRs could be the key driver(s) of valvular carditis in K/BxN mice.
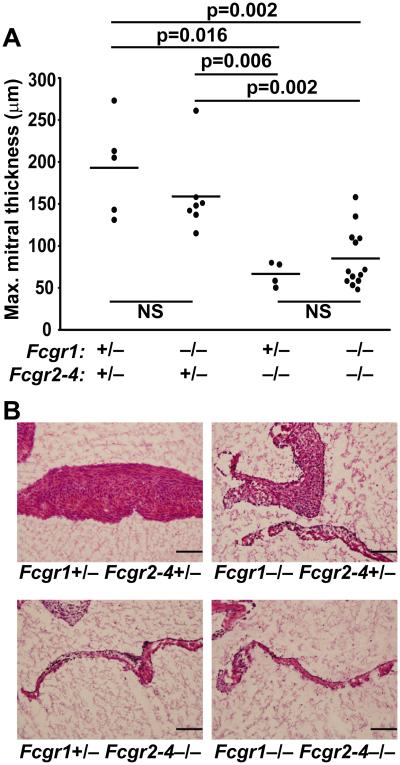
Figure 3. All activating FcγRs are expressed in inflamed valves, but deletion of single activating FcγRs does not prevent valvular carditis.
Sections through the inflamed mitral valve of an arthritic K/BxN mice were probed via immunohistochemistry for the indicated antigens. (A) Brown staining indicates the presence of the indicated IgG subtype. Sections were counterstained with hematoxylin (blue). (B) The indicated FcγRs are shown in red and the endothelial marker CD31 in green. Nuclei were counterstained with DAPI (blue). No staining was observed with isotype control antibodies (top panels). (Objective 20x; Scale bars are 100 μm). (C) K/BxN mice expressing null alleles of the genes encoding FcγRI (Fcgr1), FcγRIII (Fcgr3), or FcγRIV (Fcgr4) as indicated were bred and assessed for valvular carditis. The development of valvular carditis was not affected by the deficiency of any one activating FcγR as measured by maximal mitral valve thickness. Each point represents one mouse and bars represent means; in each case, p-values were not significant (Mann-Whitney U test).
K/BxN mice lacking single activating FcγRs are not protected from valvular carditis
We therefore asked if the absence of any one of the activating FcγRs protected against valve inflammation. To answer this question, we bred null alleles of the genes encoding the alpha chains of each of the activating Fcγ receptors (Fcgr1−/−, Fcgr3−/−, and Fcgr4−/−) into the K/BxN system and assessed for the development of valvular carditis. We found that K/BxN mice lacking expression of only one of the activating FcγRs developed valvular carditis equivalent in severity to controls (Figure 3C). These mice also developed arthritis comparable in severity to controls (data not shown), as expected since genetic absence of the common signaling chain FcRγ did not reduce the severity of spontaneous arthritis in K/BxN transgenic mice (7).
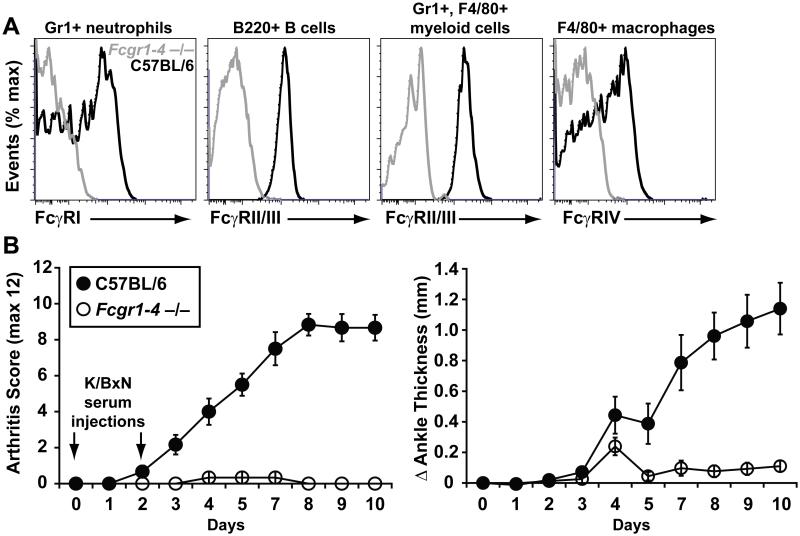
Because no single activating FcγR was solely responsible for the development of valvular carditis, we considered the possibilities that more than one activating FcγR was contributing (i.e., redundancy) or that an alternative FcRγ-associated receptor, not in the FcγR family, was responsible. To discriminate between these hypotheses, we took advantage of recently-developed mice lacking all of the FcγR alpha chain genes (Fcgr1, 2, 3 and 4) (Figure 4A). Although the common signaling chain FcRγ is not required for spontaneous arthritis in K/BxN transgenic mice (7), it is absolutely required for arthritis induced by passive transfer of serum from K/BxN mice into naïve recipients (27, 28). Similarly, the new mice lacking all of the FcγR alpha chain genes were completely protected from serum-transferred arthritis (Figure 4B), confirming the expected functional defect that the absence of activating FcγRs engenders in the serum transfer model.
Figure 4. Characterization of FcgR1-4-deficient mice.
(A) Flow cytometric assessment of FcγR expression on the indicated cell types in C57BL/6 background mice lacking all of the FcγRs (Fcgr1-4−/−, gray histograms) and C57BL/6 control mice (black histograms). (B) Pooled serum from arthritic K/BxN mice (150 μL/dose on days 0 and 2) was injected intraperitoneally into 6-week-old C57BL/6 background Fcgr1-4-deficient recipient mice and controls (n = 6 for both groups). The mice were monitored for the development of arthritis for 10 days and assessed by arthritis severity score (left) and change in ankle thickness (right). Data are mean ± SEM.
FcγRIII and FcγRIV are key mediators of valvular carditis
We bred K/BxN mice lacking FcγRI, FcγRII, FcγRIII and FcγRIV and found that they were protected from valvular carditis (Figure 5). Furthermore K/BxN mice lacking only FcγRs II/III/IV were also protected, whereas FcγRI deficiency had no effect (Figure 5). Arthritis severity scores were not different between these various groups with one exception: the Fcgr1 +/−, Fcgr2-4 −/− group had slightly lower scores than the Fcgr1 +/−, Fcgr2-4 +/− group (p = 0.044, data not shown). K/BxN mice deficient for Fcgr2-4 also had slightly less total IgG anti-GPI IgG than those sufficient for Fcgr2-4 (data not shown), a somewhat unexpected finding since absence of the inhibitory receptor FcγRII often, but not always, leads to increased autoantibody titers (29, 30). These decreases in arthritis severity and antibody titers were slight compared with the dramatic protection from valvular carditis. In sum, these findings demonstrate that the FcRγ-associated activating receptors FcγRIII and FcγRIV are the key drivers of cardiac valve inflammation in this model — there is no need to invoke roles for other FcRγ-associated receptors.
Figure 5. K/BxN mice lacking FcγRIII and FcγRIV are protected from valvular carditis.
8-week old K/BxN mice expressing null alleles of FcγRI (Fcgr1) and/or FcγRII, III and IV (Fcgr2-4) in heterozygosity or homozygosity were evaluated for valvular carditis. (A) The maximum mitral valve thickness is plotted for K/BxN mice of the indicated genotypes. Each point represents one mouse, bars represent means. P-values were calculated using the Mann-Whitney U test. (B) Representative photomicrographs of mitral valves from the same mice stained with hematoxylin and eosin. (Objective 20x; Scale bars are 100 μm).
FcRγ expression on radio-resistant cells promotes valvular carditis
To explore what FcRγ expressing cells are required for the development of valvular carditis, we performed a reciprocal bone marrow transplantation experiment. We transplanted bone marrow from FcRγ-sufficient or –deficient K/BxN mice into sublethally-irradiated FcRγ-sufficient or –deficient Rag1-deficient hosts. We found that recipient mice lacking FcRγ developed less severe valvular carditis than controls, whereas the FcRγ status of the donor did not influence carditis severity (Figure 6A). Therefore, FcRγ chain expression by radio-resistant host cells, rather than by radiosensitive, bone marrow-derived cells, contributes to the development of valvular carditis in K/BxN mice. Candidate cells included mast cells, Langerhans cells, dendritic cells (DCs), macrophages, and endothelial cells based on the described radio-resistance of subpopulations of these cell types (31-36).
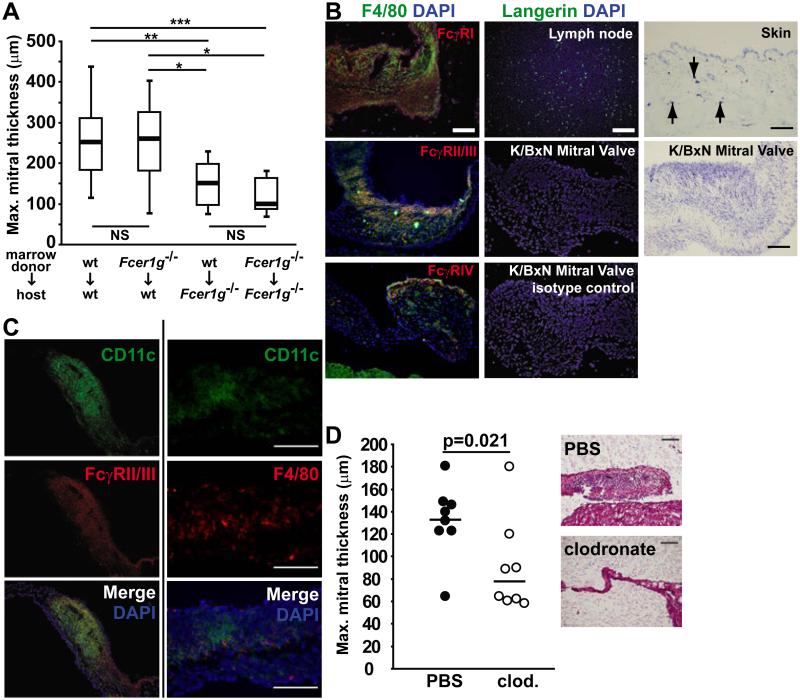
Figure 6. Macrophages are the critical FcRγ-expressing cell type mediating valvular carditis.
(A) Bone marrow was transplanted in the indicated donor:host combinations. Eight weeks later, the hearts were analyzed. Box plots depict the median (bold line), 1st and 3rd quartiles (box) and minimum and maximum values (whiskers) for each. Data comprise two experiments with 8-11 mice/group. P-values were calculated using the Mann-Whitney U test; * p<0.05, ** p = 0.01, *** p = 0.001. (B) Left: K/BxN mitral valves were probed for the indicated activating FcγRs (red) and the macrophage marker F4/80 (green). Nuclei were stained with DAPI (blue). Middle: Lymph node and K/BxN mitral valve sections were stained with anti-Langerin antibody (green). Nuclei were stained with DAPI (blue). Right: Toluidine blue-positive mast cells are detected in skin (positive control, arrows indicate mast cells). No mast cells were identified in the K/BxN mitral valve. (Objective 20x; scale bars = 100 μm). (C) Staining of inflamed K/BxN mitral valves for (left) CD11c plus anti-FcγRII/III and (right) CD11c plus F4/80 demonstrates co-localization in the bottom merged panels. (Objective 40x; Scale bar 100 microns). (D) Mitral valves of K/BxN mice treated with clodronate liposomes or control PBS liposomes were assessed at 8 weeks of age. Each point represents one mouse, bar indicates mean; p-value was calculated using the Mann-Whitney U test. Representative sections stained with H&E are shown to the right. (Objective 20x; Scale bars are 100 μm).
FcγR expressing cells in inflamed K/BxN mitral valves
We next explored which of the candidate cell types were present in the inflamed K/BxN mitral valve and co-localized with one or more of the activating FcγRs. We found that FcγRI (CD64), FcγRII/III (CD16/32) and FcγRIV all co-localized with F4/80, a marker for macrophages, on the inflamed mitral valve (Figure 6B). We could not detect Langerhans cells or mast cells in the inflamed mitral valves (Figure 6B). None of the FcγRs co-localized with CD31, an endothelial cell marker, as shown in Figure 3. CD11c, a marker of activated macrophages and dendritic cells, co-localized with FcγRII/III and also with F4/80 (Figure 6C), but not with FcγRI or IV (data not shown).These data indicated that the most likely FcγR-expressing cells mediating valvular carditis are radio-resistant macrophages and/or closely-related “macrophage-like” inflammatory DCs. Initial activation of KRN T cells depends on conventional DCs (cDCs) and other professional antigen presenting cells (12). Our finding that KRN T cells were activated normally in the absence of FcRγ (see Figure 1) therefore suggests that FcγR expression by cDCs is not required for KRN T cell activation, pointing to a key role for FcγR-expressing macrophages or similar cells in effecting carditis, as discussed below.
Clodronate liposome administration reduces valvular carditis severity
We used clodronate liposomes to deplete macrophages from K/BxN mice, starting at 3 weeks of age, and assessed the mice for the development of valvular carditis at 8 weeks of age. K/BxN mice treated with clodronate liposomes developed less severe valvular carditis compared with mice treated with control PBS liposomes (Figure 6D). We confirmed the efficiency of macrophage depletion by flow cytometric enumeration of CD11b+, F4/80+ splenic macrophages, which averaged 4.55 × 105 in the clodronate-treated mice and 2.75 × 106 in PBS treated mice (p < 0.0001). The clodronate liposome treatment had no effect on arthritis severity or anti-GPI antibody titers (data not shown). These data suggest that macrophages or similar clodronate-sensitive cells are important cellular effectors of valve inflammation. Collectively, our findings are consistent with a model in which pathogenic autoantibodies engage FcγRIII and FcγRIV on macrophages to drive valvular carditis in K/BxN mice.
Discussion
Studies of the pathogenesis of valvular carditis in rheumatic heart disease, anti-phospholipid antibody syndrome, and Libman-Sacks endocarditis in both humans and in rodent models have demonstrated the deposition of IgG and complement bound in the subendothelial connective tissue of the valve as well as the presence of CD4+ T cells and macrophages (2, 9, 10, 37, 38). The K/BxN mouse model of inflammatory arthritis and valvular carditis recapitulates these characteristics. We have demonstrated here the power of the K/BxN model to investigate the immunologic mechanisms mediating cardiovascular pathology in the setting of a systemic autoantibody-associated inflammatory disease. Despite the presence of complement C3 bound to the inflamed valves in K/BxN mice, we found that C3 was not necessary for valvular carditis to develop. Rather, our results suggest that pathogenic antibodies produced by the adaptive immune system engage activating FcγRs on innate immune effector cells to drive valvular carditis.
In this study, we found that no single activating FcγR (FcγRI, III or IV) was solely responsible for the development of valvular carditis in K/BxN mice. This is not surprising given the diversity of FcγR-expressing cells and the presence of multiple IgG subtypes in the inflamed mitral valves. Our findings are consistent with other examples of autoimmune pathology requiring the contribution of more than one activating FcγR. For example, in a passive mouse model of autoimmune hemolytic anemia induced by IgG2a and IbG2b subclasses of the anti-RBC antibody, FcγRI, III and IV all contributed to a severe anemia phenotype induced by IgG2a antibodies, while FcγRIII and IV but not FcγRI were required for both the mild and severe anemia phenotypes induced by IgG2b antibodies (39). Similarly, in a murine model of acute glomerular inflammation induced by switch variant monoclonal antibodies, FcγRIII and IV were required, whereas FcγRI was dispensable (40). Our results show that FcγRIII and IV drive valvular carditis in K/BxN mice, whereas FcγRI is not necessary. Because FcγRIV does not bind to IgG1 (23), these findings suggest that although IgG1 is the predominant IgG subtype produced in K/BxN mice, other IgG subtypes (specifically IgG2b and/or 2c) are also pathogenically important mediators of carditis.
Our conclusion that macrophages are the critical FcγR-expressing cell type driving valvular carditis is based on our findings that a radioresistant FcγR-expressing cell type is at play, that clodronate liposomes deplete this key cell type, and that macrophage markers co-localize with the activating FcγRs in the inflamed mitral valves. Could dendritic cells also be involved? This question is complicated by the fact that the cell surface markers CD11b, F4/80, and CD11c can be expressed both by activated macrophages and by monocyte-derived DCs; our histologic studies cannot distinguish these cell types (see Figure 6) (7). Furthermore, clodronate liposomes can deplete some DC populations (41-43). We consider a role for cDCs unlikely, based on our finding that professional APC-mediated activation of KRN T cells occurred normally in the absence of FcRγ (see Figure 1). The absence of inflammatory cells in the native mitral valve strongly suggests that the CD11b+ F4/80+ CD11c+ cells present in the inflamed valve are recruited from the circulation. Circulating monocytes give rise not only to macrophages but also to cells termed “inflammatory DCs” or “TNF/iNOS-producing (TIP) DCs”, whose phenotypic and functional characteristics overlap considerably with those of activated macrophages (44), leading some investigators to argue that the distinction between these subsets is artificial (45). Our data support the conclusion that the key FcγR-expressing cell types driving valvular carditis in K/BxN mice are macrophages and/or phenotypically and functionally similar monocyte-derived DCs. We therefore postulate that autoantibodies engage FcγRIII and IV on these cells to drive valvular carditis in K/BxN mice. We are currently investigating how activating FcγR engagement influences macrophage function, with particular attention to defining which of their pro-inflammatory products are the critical mediators of carditis.
We have previously shown that CD4+ T cells are key effectors of valvular carditis in this model (26). Our new findings support a model in which CD4+ T cells and macrophages cooperate to provoke cardiac inflammation. Future investigations will focus on how these cells types are recruited into the valve tissue and on characterizing their molecular interactions.
Our findings provide important insight to guide the rational choice of therapeutic agents to treat cardiovascular inflammation in the setting of systemic autoantibody-associated diseases. Interventions that modulate Fc receptor function, such as intravenous immunoglobulin, may be attractive (46). Alternatively, the FcγR-expressing effector cells could be eliminated. Additionally, the key FcγRs themselves or their downstream signaling intracellular signaling molecules could be targeted. Because the activating FcγRs rely on the spleen tyrosine kinase (Syk) for signal transduction, Syk inhibitors such as fostamatinib (R788) or R406 might be good candidates to treat autoantibody-associated cardiovascular inflammation (47, 48). Future investigations of which pro-inflammatory pathways are activated by FcγR engagement on macrophages (or other cell types) to drive valvular carditis in this model are expected to point to additional therapeutic targets.
In summary, our findings delineate several new key points in the pathogenesis of autoimmune valvular carditis in K/BxN mice. FcRγ deficiency does not impact the T- and B-cell-dependent initiation phase in the development of valvular carditis, but rather the downstream effector phase. The activating receptors FcγRIII and FcγRIV drive valvular carditis, while FcγRI is dispensable. Moreover, macrophages contribute critically to the development of carditis in this model. Our findings provide new insight into the pathogenesis of cardiovascular inflammation in the setting of autoantibody-associated, chronic inflammatory diseases and offer new directions to pursue regarding basic pathogenic mechanisms and rational therapeutic approaches.
Acknowledgements
We thank Drs. Diane Mathis, Christophe Benoist and Michael Carroll for mice. We thank Jeffrey Ravetch for mice and the anti-FcγRIV specific antibody (9E9).
Funding sources
This work was supported by the Rheumatology Research Foundation, NIH grant R03AR057101, the Viking Children’s Fund, and the University of Minnesota Department of Pediatrics (to BAB). Dr. Binstadt was also supported by NIH grant K08AR054317.
Footnotes
Disclosures
None.
References
- 1.Asanuma Y, Oeser A, Shintani AK, Turner E, Olsen N, Fazio S, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–15. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 2.Blank M, Aron-Maor A, Shoenfeld Y. From rheumatic fever to Libman-Sacks endocarditis: is there any possible pathogenetic link? Lupus. 2005;14:697–701. doi: 10.1191/0961203305lu2203oa. [DOI] [PubMed] [Google Scholar]
- 3.Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52:3045–53. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 4.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–7. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 5.Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol. 2011;7:399–408. doi: 10.1038/nrrheum.2011.75. [DOI] [PubMed] [Google Scholar]
- 6.Gustafsson JT, Simard JF, Gunnarsson I, Elvin K, Lundberg IE, Hansson LO, et al. Risk factors for cardiovascular mortality in patients with systemic lupus erythematosus, a prospective cohort study. Arthritis Res Ther. 2012;14:R46. doi: 10.1186/ar3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binstadt BA, Hebert JL, Ortiz-Lopez A, Bronson R, Benoist C, Mathis D. The same systemic autoimmune disease provokes arthritis and endocarditis via distinct mechanisms. Proc Natl Acad Sci U S A. 2009;106:16758–63. doi: 10.1073/pnas.0909132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–22. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan MH, Bolande R, Rakita L, Blair J. Presence of bound immunoglobulins and complement in the myocardium in acute rheumatic fever. Association with cardiac failure. N Engl J Med. 1964;271:637–45. doi: 10.1056/NEJM196409242711301. [DOI] [PubMed] [Google Scholar]
- 10.Tenedios F, Erkan D, Lockshin MD. Cardiac involvement in the antiphospholipid syndrome. Lupus. 2005;14:691–6. doi: 10.1191/0961203305lu2202oa. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732–5. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 12.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–61. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 13.Boross P, Verbeek JS. The complex role of Fcgamma receptors in the pathology of arthritis. Springer Semin Immunopathol. 2006;28:339–50. doi: 10.1007/s00281-006-0049-9. [DOI] [PubMed] [Google Scholar]
- 14.Humphrey MB, Lanier LL, Nakamura MC. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol Rev. 2005;208:50–65. doi: 10.1111/j.0105-2896.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 15.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 16.Wetsel RA, Fleischer DT, Haviland DL. Deficiency of the murine fifth complement component (C5). A 2-base pair gene deletion in a 5'-exon. J Biol Chem. 1990;265:2435–40. [PubMed] [Google Scholar]
- 17.Wessels MR, Butko P, Ma M, Warren HB, Lage AL, Carroll MC. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci U S A. 1995;92:11490–4. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–29. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 19.Ioan-Facsinay A, de Kimpe SJ, Hellwig SM, van Lent PL, Hofhuis FM, van Ojik HH, et al. FcgammaRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity. 2002;16:391–402. doi: 10.1016/s1074-7613(02)00294-7. [DOI] [PubMed] [Google Scholar]
- 20.Nimmerjahn F, Lux A, Albert H, Woigk M, Lehmann C, Dudziak D, et al. FcgammaRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proc Natl Acad Sci U S A. 2010;107:19396–401. doi: 10.1073/pnas.1014515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity. 1996;5:181–8. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 22.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–77. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 23.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–92. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen LT, Jacobs J, Mathis D, Benoist C. Where FoxP3-dependent regulatory T cells impinge on the development of inflammatory arthritis. Arthritis Rheum. 2007;56:509–20. doi: 10.1002/art.22272. [DOI] [PubMed] [Google Scholar]
- 26.Haasken S, Auger JL, Binstadt BA. Absence of beta2 integrins impairs regulatory T cells and exacerbates CD4+ T cell-dependent autoimmune carditis. J Immunol. 2011;187:2702–10. doi: 10.4049/jimmunol.1000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corr M, Crain B. The role of FcgammaR signaling in the K/B x N serum transfer model of arthritis. J Immunol. 2002;169:6604–9. doi: 10.4049/jimmunol.169.11.6604. [DOI] [PubMed] [Google Scholar]
- 28.Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–68. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 29.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–9. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 30.Sharp PE, Martin-Ramirez J, Mangsbo SM, Boross P, Pusey CD, Touw IP, et al. FcgammaRIIb on myeloid cells and intrinsic renal cells rather than B cells protects from nephrotoxic nephritis. J Immunol. 2013;190:340–8. doi: 10.4049/jimmunol.1202250. [DOI] [PubMed] [Google Scholar]
- 31.Fukuzumi T, Waki N, Kanakura Y, Nagoshi J, Hirota S, Yoshikawa K, et al. Differences in irradiation susceptibility and turnover between mucosal and connective tissue-type mast cells of mice. Exp Hematol. 1990;18:843–7. [PubMed] [Google Scholar]
- 32.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, et al. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–8. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 33.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–41. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bogunovic M, Ginhoux F, Wagers A, Loubeau M, Isola LM, Lubrano L, et al. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J Exp Med. 2006;203:2627–38. doi: 10.1084/jem.20060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oghiso Y, Yamada Y. Heterogeneity of the radiosensitivity and origins of tissue macrophage colony-forming cells. J Radiat Res. 1992;33:334–41. doi: 10.1269/jrr.33.334. [DOI] [PubMed] [Google Scholar]
- 36.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts S, Kosanke S, Terrence Dunn S, Jankelow D, Duran CM, Cunningham MW. Pathogenic mechanisms in rheumatic carditis: focus on valvular endothelium. J Infect Dis. 2001;183:507–11. doi: 10.1086/318076. [DOI] [PubMed] [Google Scholar]
- 38.Guilherme L, Kalil J, Cunningham M. Molecular mimicry in the autoimmune pathogenesis of rheumatic heart disease. Autoimmunity. 2006;39:31–9. doi: 10.1080/08916930500484674. [DOI] [PubMed] [Google Scholar]
- 39.Baudino L, Nimmerjahn F, Azeredo da Silveira S, Martinez-Soria E, Saito T, Carroll M, et al. Differential contribution of three activating IgG Fc receptors (FcgammaRI, FcgammaRIII, and FcgammaRIV) to IgG2a- and IgG2b-induced autoimmune hemolytic anemia in mice. J Immunol. 2008;180:1948–53. doi: 10.4049/jimmunol.180.3.1948. [DOI] [PubMed] [Google Scholar]
- 40.Giorgini A, Brown HJ, Lock HR, Nimmerjahn F, Ravetch JV, Verbeek JS, et al. Fc gamma RIII and Fc gamma RIV are indispensable for acute glomerular inflammation induced by switch variant monoclonal antibodies. J Immunol. 2008;181:8745–52. doi: 10.4049/jimmunol.181.12.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atibalentja DF, Murphy KM, Unanue ER. Functional redundancy between thymic CD8alpha+ and Sirpalpha+ conventional dendritic cells in presentation of blood-derived lysozyme by MHC class II proteins. J Immunol. 2011;186:1421–31. doi: 10.4049/jimmunol.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weisser SB, van Rooijen N, Sly LM. Depletion and reconstitution of macrophages in mice. J Vis Exp. 2012:4105. doi: 10.3791/4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward NL, Loyd CM, Wolfram JA, Diaconu D, Michaels CM, McCormick TS. Depletion of antigen-presenting cells by clodronate liposomes reverses the psoriatic skin phenotype in KC-Tie2 mice. Br J Dermatol. 2011;164:750–8. doi: 10.1111/j.1365-2133.2010.10129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–74. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hume DA. Macrophages as APC and the dendritic cell myth. J Immunol. 2008;181:5829–35. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- 46.Durandy A, Kaveri SV, Kuijpers TW, Basta M, Miescher S, Ravetch JV, et al. Intravenous immunoglobulins--understanding properties and mechanisms. Clin Exp Immunol. 2009;158(Suppl 1):2–13. doi: 10.1111/j.1365-2249.2009.04022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braselmann S, Taylor V, Zhao H, Wang S, Sylvain C, Baluom M, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319:998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- 48.Bahjat FR, Pine PR, Reitsma A, Cassafer G, Baluom M, Grillo S, et al. An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression and prolongs survival in murine lupus. Arthritis Rheum. 2008;58:1433–44. doi: 10.1002/art.23428. [DOI] [PubMed] [Google Scholar]