Abstract
Natural killer (NK) cells are part of the innate immune system and are an alluring option for immunotherapy due to their ability to kill infected cells or cancer cells without prior sensitization. Throughout the past 20 years, different groups have been able to reproduce NK cell development in vitro, and NK cell ontogeny studies have provided the basis for the establishment of protocols to produce NK cells in vitro for immunotherapy. Here, we briefly discuss NK cell development and NK cell immunotherapy approaches. We review the factors needed for NK cell differentiation in vitro, which stem cell sources have been used, published protocols, challenges and future directions for Good Manufacturing Practice protocols.
Keywords: cord blood, natural killer cells, differentiation
Generalities
Natural killer (NK) cells were first discovered in mice more than 30 years ago by Kiessling1,2 and Herberman3,4 due to their ability to lyse target cells without prior sensitization, and the fact that NK cells were not restricted by the target's expression of major histocompatibility complex (MHC).1,5 Human NK cells are characterized by their surface expression of CD56 and CD16, and absence of CD3.6,7 Two distinct subsets of human NK cells have been identified according to the cell surface density of CD56 expression.8 The majority (90%) of human peripheral blood (PB) NK cells is CD56dim and expresses high levels of CD16, a minority (10%) being CD56bright and CD16dim/neg. These NK cell subsets are functionally distinct. CD56bright cells are cytokine producers, whereas CD56dim cells are cytolytic and function as efficient effectors of natural and antibody-dependent target cell lysis.9
NK cells play a key role in the elimination of compromised host cells, such as tumor or virus-infected cells. The recognition of such cells by NK cells occurs through a process described as the ‘missing self hypothesis', coined by Karre10,11 three decades ago. NK cell-mediated killing involves exocytosis of cytoplasmic granules containing perforin and granzyme through a metabolically active process. They also have the ability to kill target cells via death receptors such as TRAIL and FasL. An equally important function of NK cells is their capacity to produce cytokines, especially interferon (IFN)-γ, tumor-necrosis factor and in some cases, granulocyte macrophage colony-stimulating factor (GM-CSF) or interleukin (IL)-10. These functional properties equip NK cells with the tools to actively eliminate susceptible targets through multiple and non-redundant mechanisms.12
The mechanisms used by NK cells to recognize and lyse non-self cells have been studied for several decades. Early in the 1990s, Colonna et al.13 uncovered the alloreactivity potential of NK cells. Using different human leucocyte antigen (HLA)-C haplotype donors and cell lines, they observed that NK cells could expand in mismatched patients. Subsequently, several works using NOD-SCID mice proved that human alloreactive NK cells could eradicate previously transplanted human acute myeloid leukemia (AML) cells,14,15 and that KIR (killer cell immunoglobulin-like receptor) mismatches correlated with the ability of donor NK cells to kill cryopreserved leukemic cells from the recipient.16
The ability of NK cells to kill without prior sensitization has made them attractive for immunotherapy. Notably, the use of NK cells for immunotherapy relies on the availability of a great number of NK cells with optimal cytotoxic activity. Obtaining a large number of NK cells is an important, although difficult task that underlies the most significant challenge to the development of successful NK cell adoptive transfer protocols. NK cells can be isolated from PB or umbilical cord blood (UCB). These cells can be used directly for immunotherapy or after a short- or long-term expansion in vitro with interleukins such as IL-2. Another approach is the generation of NK cells from stem cells, from either mobilized PB stem cells17,18 or UCB stem cells.19
Here, we review the current applications of NK cell immunotherapy, the existing in vitro NK cell production protocols, as well as the future directions and challenges awaiting NK cell adoptive therapy. To note, protocols used to expand NK cells in vitro are not considered in this review, these have been previously described elsewhere.20
Adoptive immunotherapy
Cancer is a major cause of death around the world: accounting for 7.6 million deaths in 2008. The highest mortality rates are seen in Southern Africa, and the more developed regions of the world, such as the United Kingdom and the European Union.21 Thus, an enormous amount of resources have been designated to the study of cancer ontogenesis. Our better understanding of the molecular and biological basis of cancer has provided the opportunity to discover new tools to fight this terrible condition. The emerging knowledge in the field of immunology has enabled us to gain deeper insights into how immunological systems work.
The development of different therapeutic approaches based on components of the immune system has been widely explored. T cells have been shown to be tumor antigen-specific, and can mediate tumor regression.22,23,24 However, T cell-based therapies have demonstrated some limitations. There are numerous mechanisms that have been identified allowing a tumor to escape T cell-mediated immune recognition. These mechanisms were reviewed by Khong et al.,25 and include: loss of antigen expression by the transformed cells, loss of MHC class I,26 the local presence of immunosuppressive factors and/or immunosuppressive cells. Different from T cells, NK cells belong to the innate immune system and do not require prestimulation to exert their effector functions.27 In allogenic hematopoietic stem cell (HSC) transplantation settings, T cells have been shown to provide graft versus host disease (GVHD), a complication in which transplanted cells recognize the recipient's tissues as ‘foreign' and mount an immunological attack,28 whereas NK cells do not. As non-transformed tissues generally do not overexpress ligands for activating NK cell receptors, alloreactive NK cells do not cause GVHD.
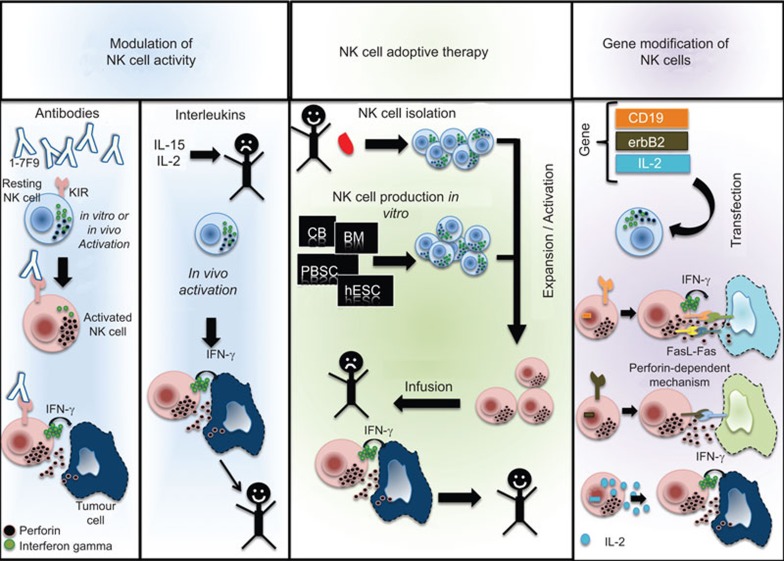
Currently, there are different approaches towards T cell-adoptive therapy; nevertheless, NK cell immunotherapy is rapidly progressing, exploring therapies as simple as manipulation of NK cell activity using antibodies or interleukins, or as complex as engineering gene-modified NK cells (Figure 1).
Figure 1.
NK cell immunotherapy. NK cells can be manipulated in order to augment their number and killing capacity. On the left-hand panel, NK cells can be modulated via antibodies or interleukin administration. NK cells are then activated and eliminate infected or cancer cells. The middle panel depicts NK cell adoptive therapy. NK cells can be isolated from healthy donors and undergo expansion or activation in vitro and be subsequently infused into the recipient. Similarly, NK cells can be produced from stem cells in vitro. The options to produce NK cells in vitro include cord blood, bone marrow, mobilized peripheral blood and embryonic stem cells. The right-hand panel shows the gene modification of NK cells. Target genes can be transfected into NK cells in order to re-direct NK cell specificity. Likewise, genes that boost NK cell cytotoxicity can also be transfected. NK, natural killer.
Modulation of NK cell activity
Use of antibodies.
In order to augment NK cell killing, the use of antibodies to block inhibitory receptors can mimic the missing self-environment. Recently, the development of human monoclonal antibodies that prevent signaling via KIR2DL1, KIR2DL2 and KIR2DL3 was developed. The monoclonal antibody 1-7F9 cross-reacts with KIR2DL1, KIR2DL2 and KIR2DL3 to increase NK cell-mediated lysis of tumors expressing HLA-C.29,30
IL-2 alone or in combination with other factors.
IL-2 affects many cell types of the immune system, including cytotoxic T cells, helper T cells, regulatory T cells, B cells and NK cells. IL-2 activation of NK cells can result in cytotoxic activity against targets that were previously NK cell-resistant.31 Observations of the interaction of autologous and allogenic NK cells with fresh tumor cells have also proved that IL-2 activation in vitro enhances the killing potential of NK cells.32 Throughout the years, several groups have assessed the effects of IL-2 administered intravenously, in order to treat solid tumors and hematological malignancies. Overall, reports clearly demonstrate that although promising outcomes have been observed, a low dose of IL-2 is not an optimal strategy for most indications. In most cases, a specific expansion of the CD56bright NK cell subset was observed.33 Other groups tried higher doses of IL-2, observing low remission when treating metastatic renal cell carcinoma.34 Additionally, a minor subset of treated patients showed remarkable toxicity, predominantly related to vascular leak syndrome,35 and adverse effects on the heart.36 The availability of new recombinant cytokines may offer new possibilities to overcome these obstacles. IL-2 can be used in combination with other factors: GM-CSF, IFN-α, IL-12 and IL-15. The overall experience of using IL-2 treatment with secondary factors seems to be promising. Future clinical trials may provide a solid basis for the use of IL-2 combined with other factors for the stimulation of endogenous NK cells against tumors.
IL-15.
IL-15 is a promising candidate for immunotherapy, as it activates not only NK cells, but also CD8+ T cells and NK T cells. In preclinical studies, IL-15 has demonstrated potentiated antitumor effects when administrated in combination with chemotherapy, adoptive therapy or monoclonal antibodies.37 A recent study after UCB transplantation demonstrated that IL-15 administration rapidly increased IFN-γ production, highlighting its therapeutic potential, as NK cell function booster to protect against infection and relapse after allogeneic HSC transplantation.38 Furthermore, there is currently a phase 1 study to treat refractory metastatic malignant melanoma and metastatic renal cell cancer using recombinant IL-15 (clinical trial NCT01021059).
Adoptive transfer of NK cells
NK cells can be isolated, expanded or produced in vitro to be used either in autologous or an allogeneic setting. In an autologous setting, NK cells have to undergo either a short- or long-term in vitro expansion. Based on clinical data, it is clear that short-term activation is not sufficient for NK cell augmentation of phenotypic and functional characteristics, it seems that infusion of long-term ex vivo activated NK cells may present a clinical benefit. On the other hand, given the current comprehension of NK cell regulation, the use of allogeneic NK cells is tentatively alluring. KIR ligand mismatch is a prerequisite for NK cell alloreactivity, and the recipient must lack one or more KIR ligands present in the donor so that a clinical response can be observed. Pioneered work by Ruggeri et al.14 revealed delayed relapse, better engraftment and protection from GVHD in leukemic patients, other reports include similar results39,40,41 treating different malignancies. Nonetheless, this approach relies on the availability of a great number of NK cells with optimal cytotoxic activity. Table 1 shows some malignancies currently treated with NK cells.
Table 1. Autologous/allogenic NK cell therapy.
| Cancer | NK cell therapy | Reference |
|---|---|---|
| MRCC | Autologous | Escudier et al.121 |
| Malignant glioma | Ishikawa et al.122 | |
| Metastatic breast cancer | deMagalhaes-Silverman et al.123 | |
| Lymphoma and breast cancer | Lister et al.124 | |
| AML | Allergenic | Miller et al.39 |
| ALL or AML | Koehl et al.125 | |
| AML and CML | Passweg et al.72 | |
| Refractory renal cell carcinoma and melanoma | Arai et al.45 |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; MRCC, metastatic renal cell carcinoma; NK, natural killer.
Gene modification of NK cells for cancer immunotherapy
Genetically modified NK cells hold great potential for cancer immunotherapy. Certain tumors, such as carcinomas or melanoma lacking MHC class I, are resistant to NK cell killing. Hence, different methodologies have been developed to modify genetically NK cells and overpass this hurdle. These modifications include different approaches, including induction of proliferation, survival through cytokine gene therapy and targeting NK cells to specific antigens or malignant cells.
Studies using isolated NK cells demonstrate that adoptive transfer of chimeric antigen-specific bearing NK cells in mice (chimeric receptor against ErbB2 for breast cancer42) or human (chimeric receptors directed against CD19, a molecule widely express in malignant B cells43) might be an efficient approach for cancer immunotherapy. For example, transfection of IL-2 in the IL-2-dependent NK cell line NK-92 increased cytotoxic activity against tumor cell lines in vitro. IL-2-transduced cells showed greater in vivo anti-tumor activity in mice,44 and currently clinical trials have shown promising results.45 Similarly, the hydrodynamic method of injection of naked DNA into mice's tail vein, to transfer an IL-15 gene plasmid, has shown to increase the number and functions of NK cells.46
NK cell production in vitro
NK cell adoptive transfer for the treatment of some malignancies, like AML, has successfully been performed in the past.14 The current obstacle in clinical studies with adoptive transfer of NK cells originates from the fact that they are present in low numbers in PB mononuclear cells. Obtaining a large number of NK cells is a difficult task, and represents the main drawback to the development of successful NK cell adoptive transfer protocols (reviewed by Suck et al.20). Current protocols include the generation of NK cells from HSCs derived either from bone marrow (BM) or UCB.47,48 The cultures use a combination of cytokines and stromal cell lines as ‘feeder layer' in order to commit stem cells to the NK cell lineage. In the next section, we will briefly review NK cell development, the current protocols of NK cell production, their challenges and future directions.
NK cell development
One unique feature of NK cells is that their development does not require any events associated with antigen receptor gene rearrangement as for T and B cells. NK cell development has been the subject of many studies for many years, and major contributions have been made during the last decade. NK cells are derived from CD34+ hematopoietic progenitor cells (HPCs);48 nevertheless, the site(s) of maturation and its details are just beginning to emerge. Some studies suggest that lymph nodes and secondary lymphoid tissues contain HPCs, and these compartments could be sites of NK cell development.49 Furthermore, Vacca et al.50 found that HPCs present in maternal decidua (the membrane lining the uterus during pregnancy) could undergo in vitro differentiation into functional NK cells. Additionally, Vosshenrich et al.51 described how NK cell development could occur in the thymus resulting in a specific NK cell subset (CD127+ NK cells). Nonetheless, NK cell development primarily occurs within the BM microenvironment.52 Neither the thymus53 nor the spleen54 seem to be vital for the generation of NK cells. Selective BM ablation studies in mice provided the first evidence in support of an important role of the BM for supporting NK cell maturation in vivo.55
The first step in NK cell development occurs when HSCs give rise to different hematopoietic lineages. One of these precursors, the common lymphoid progenitor (CLP), is known as the earliest lymphoid progenitor that will later differentiate into B and NK/T cell-restricted precursors. NK/T precursors will afterwards give rise to NK precursors (NKPs), and lastly immature NK (iNK) and mature NK cells. Nevertheless, there is currently a debate regarding other NK cell precursors; a recent study by Grzywacz et al.56 provided new insights into this subject; using in vitro cultures with cytokines and feeder layer combinations, this group demonstrated that NK cells could also be derived from myeloid precursors (common myeloid progenitors, granulocytic–monocytic precursors, as well as CD33+CD13+ and macrophage colony stimulating factor receptor progeny). Other reports suggest that the model of gradual restriction in the differentiation potential of hematopoietic cells does not always follow the pathway from pluripotent (HSCs) to oligopotent and to monopotent progenitors.57 The following description of NK cell development is based on the assumption that NK cells derives from CLPs.
CLPs give rise to NK, T and B cells but not to myeloid cell lineages. CLPs have to undergo differentiation into a bipotent NK/T progenitor. NK/T progenitors can develop exclusively into T and/or NK cells. The transition from NK/T to NKP is marked by the acquisition of the IL-2/15Rβ subunit (CD122 receptor).58 Expression of CD122 turns NKPs into IL-2/IL-15 responsive cells that are committed to the NK cell lineage. IL-15 has been described as a critical cytokine for NK cell development.59 Transcription factors control the developmental processes. ID2 and ID3 expression markedly favors the development of NK cells. In addition, E4BP4 (also called NFIL3) has been described in mice as an essential transcription factor for the generation of NK cells,60,61 and recent studies in humans confirm the exclusive expression of this factor by NK cells.50 E4BP4 allows the progression of NKPs to both immature and mature NK cells.
The next step of NK cell development is characterized by the differentiation of NKPs into iNK cells. The expression of some growth factor receptors, FMS-like tyrosine kinase 3 (FLT3) and IL-7Rα, decreases as cells proceed from NKP to iNK cells, whereas the expression of IL-2Rβ, CD2 and 2B4 (CD244) increases.62,63,64 iNK cells are characterized by CD161 expression. A population of CD3−CD56−CD161+ cells has previously been described in UCB; these cells can be generated in vitro from CD34+Lin− UCB cells, suggesting that the initial expression of CD161 identifies iNK cells.65
Maturation of iNK cells involves the acquisition of activation and inhibitory markers. The appearance of NK cell activating receptors occurs before the expression of HLA class I-specific inhibitory receptors; therefore, a mechanism needs to keep immature NK cells in control to avoid inopportune killing of normal autologous cells. Recent findings suggest that 2B4 expression occurs early in NK cell development working as an inhibitory receptor, preventing killing of normal autologous cells.63 NK cells go through a process in which acquisition of receptors, and control of cytokine production is still not well understood. An in vitro model of NK cell development using UCB stem cells showed that the acquisition of cytotoxic properties correlated with the expression of self-MHC-specific inhibitory receptors (CD94/NKG2A) in differentiating NK cells.47
NK cells undergo a last maturation step through the recognition of self-molecules. A prototypical example of this process is the MHC class I-induced NK cell ‘education' or ‘licensing'.66,67 Ever since the original observation of the ability of NK cells to detect ‘missing self' using their inhibitory receptors for self-MHC class I, it has been suggested that NK cells follow an education process to become both competent to recognize ‘missing self' and tolerant to self. This education process requires the recognition of self-MHC class I molecules by cognate inhibitory receptors such as KIRs. A recent report studying NK cell education after allogeneic transplantation has shown that there may be other receptors than KIRs involved in educating NK cells.38
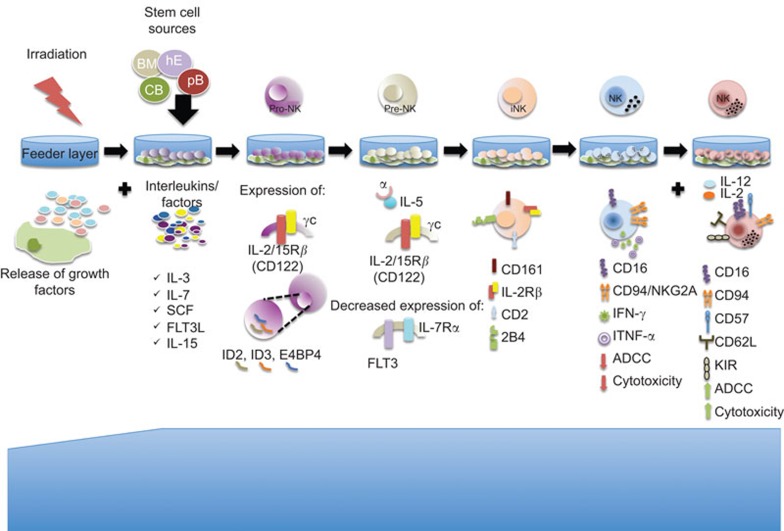
In summary, the current knowledge on NK cell development represents a guide to follow when producing NK cells in vitro (Figure 2). Deliberate usage of this knowledge should allow us to effectively produce NK cells in vitro with the appropriate activation state and respective repertoire.
Figure 2.
NK cell production in vitro. NK cells can be generated from stem cells. Isolated stem cells are plated over an irradiated feeder layer that creates the microenvironment for NK cell development. The addition of different interleukins (like IL-7, IL-15, SCF, FLT3L among others) drives the differentiation towards the NK cell lineage. Cells undergo different phases; the first stage (pro-NK) is characterized by the expression of important transcription factors like ID2, ID3 an E4BP4. In the second stage, SCF and FLT3L promote the expression of the IL-2/15β receptor, which increases the responsiveness to IL-15. The decreased expression of IL-7Rα and FLT3L are characteristic of the pre-NK stage. Afterwards, the expression of CD161, CD2 and 2B4 reflects the iNK stage. The coordinated acquisition of different activating and inhibitory markers is followed by the increased cytotoxicity capacity that can be enhanced by the addition of IL-12 or IL-2. FLT3L, FLT3 ligand; iNK, immature natural killer; NK, natural killer; SCF, stem cell factor.
Source of stem cells for NK cell production
NK cell immunotherapy has been developing rapidly during the past decade. The production of NK cells includes several approaches. NK cells can be directly isolated from PB or UCB; donor cells are expanded for short or long period of time in vitro, and subsequently infused to treat cancer or hematological diseases.68,69,70,71 The main advantage of obtaining NK cells from these sources is the immediate availability of cells even after long-term expansion in vitro, compared to de novo production of NK cells. However, the disadvantage of this technique relies in the fact that prolonged exposure of NK cells to cytokines in vitro induces cell exhaustion. Therefore, cells are ineffective at killing after infusion in the recipient, and die within a few days. High doses of NK cells are required in immunotherapy. It has been reported that 1×107–2×107 cells/kg is a safe dose, and further clinical studies on higher doses are currently under study.72 This would mean that donors would be required to donate multiple times. Furthermore, freezing NK cells is not an option as NK cell-killing capacity is reduced after freezing/thawing.73,74 Therefore, fresh samples should always be preferred for NK cell therapy.
To overcome these obstacles, the production of NK cells from CD34+ stem cells has become an alluring option. Stem cells can be isolated and frozen for further use. Due to their potential capacity of expansion, and low numbers of initial cells needed to produce NK cells, these cells could represent an option that provides NK cells for multiple infusions from the same donor. Production of NK cells in vitro from stem cells takes 3–5 weeks. BM stem cells or mobilized PB stem cells are promising sources of CD34+ cells. Studies show the successful generation of NK cells with mature properties,48,75 and similar results have been shown using human embryonic stem cells (hESCs).76 The disadvantage of obtaining HSC from these sources is that it is considered to be an invasive procedure and time consuming (finding correct HLA match/mismatch) compared to the off the shelf availability of UCB and decreased HLA restriction of this source. UCB stem cells, likewise, mobilized PB stem cells and BM stem cells, can be stored and used at different time points for NK cell generation. All stem cell sources have their advantages and disadvantages. Their use will depend mostly on the infrastructure and availability of resources in the local clinical setting.
hESCs.
Although controversial, hESCs have been used to generate NK cells. In order to avoid the periodic destruction of 5–7 days embryos, researchers have established cell lines from human blastocysts. Thomson et al.77 established the first hESC cell line in 1989. Since then, hESCs have offered a reliable cell source for regenerative medicine, and hundreds of cell lines have been produced (reviewed by Allegrucci78). Woll et al.79 worked with H9 hESC cell line in order to produce NK cells. They showed that produced NK cells expressed maturation markers including KIRs, natural cytotoxic receptors and CD16. In addition, these cells could lyse malignant cells by both direct cell-mediated cytotoxicity and antibody-dependent cellular cytotoxicity. Furthermore, in a following work, using an in vivo mouse model of human leukemia, they tested the anti-tumor activity of the generated NK cells in vivo.76 Interestingly, they also tested NK cells generated from UCB, finding a striking difference in the development status of the cells. According to the expression of certain markers, like perforin, granzyme B, KIRs and CD117, they found that hESC-derived NK cells were more mature and had better cytotoxic capacity. Nonetheless, derived NK cells from hESC cell lines therapy is limited by their potential to induce an allogeneic immune response, which could be solved by the development of hESCs banks to cover the genetic diversity of the population, as suggested by the authors.
Mobilized PB and BM stem cells.
In the 1950s, researchers discovered that BM contained two stem cell populations: HSCs and BM stromal cells. Different from the embryonic stem cells, these two populations are already programmed to differentiate into specific cell types. As NK cells differentiate from BM stem cells, certainly BM represents an alternative source of stem cells. The main disadvantages of working with BM stem cells are that there are limited in number, hard to maintain long term in culture, and most importantly, the harvesting procedure is difficult and painful. BM stem cells have been previously used to study NK cell ontogeny. Using irradiated BM mono nuclear cells or long-term BM cultures as feeder layers; sorted CD34+ populations were cultured, and studied for NK cell differentiation. The results suggest that intimate contact with stromal cells is needed for the most primitive progenitors to differentiate into NK cells, but is no longer required after the first step when commitment happens.48 Similarly, Shibuya et al.80 aimed to determine minimum lymphokine requirements for CD34+ HPC differentiation. They cultured BM stem cells for 28 days using IL-2 and stem cell factor (SCF, kit ligand or steel factor), CD34+CD33+ cells differentiated into CD16− NK cells. Although the group tried to demonstrate that stromal cells are not needed for NK cell generation, the percentages of NK cells achieved were very low, suggesting a critical role of stromal cells in NK cell differentiation in vitro.
Stem cells can be mobilized using granulocyte colony-stimulating factor for easier harvesting.81 Giuliani et al.82 used PB stem cells to study the importance of membrane-bound IL-15 in the commitment towards NK cell differentiation. Surprisingly, they found that membrane-bound IL-15 promotes the generation of a new subset of NK cells, NK-ireg. This subset expresses HLA-G, and secretes IL-10 and IL-21. Its generation is strictly dependent on reciprocal trans-presentation of bound IL-15 exclusively expressed by the PB hematopoietic progenitors.
Table 2 summarizes the current protocols of NK cell production from BM/PB stem cells. NK cell production has not been the main goal of these studies; perhaps the disadvantages of these sources of stem cells have prevented the development of protocols for NK cell immunotherapy.
Table 2. NK cells derived from UCB stem cells.
| Source | Feeder layer | Fold expansion or cell number | Phenotype | Functionality | Purity (end of culture) | Reference |
|---|---|---|---|---|---|---|
| CD133+ | None | 16×106 cells | CD56+ cells: no perforin, low expression of FasL | IFN-γ production (2.77 ng/ml) after IL-12/18 stimulation Cr51 assay: 27% killing of K562 at 5:1 E/T ratio | 54% | Kao et al.91 |
| CD34+ | EL08-1D2 and AFT024 | 123 852 cells | Not described | Not described | Not described | McCullar et al.88 |
| CD34+ | MS-5 stromal cells | 112- to 130-fold | Not described | Not described | 95% | Haddad et al.127 |
| CD34+ | EL08-1D2 | 2852-fold | CD56+CD117low: high expression of NKp44, CD161 NKp30, medium expression of NKG2A, NKG2D and CD94, low expression of CD16 and KIR | Cr51 assay: 48% killing of K562 at 5:1 E/T ratio | 99% | Grzywacs et al.47 |
| CD34+ | None | 910×106 cells | High expression of CD161, NKG2D, NKp46 and perforin, medium expression of CD16 and CD94, low expression of KIR | Cr51 assay: 78% killing of K562 at 5:1 E/T ratio | Not described | Perez et al.127 |
| CD34+ | Human BM stromal cells | Twofold | Medium expression of CD16 | Cr51 assay: 15% killing of K562 at 5:1 E/T ratio | 25.80% | Frias et al.93 |
| CD34+ | MS-5 stromal cells | 7- to 27-fold | High/medium expression of CD2, CD7 and CD8, low expression of CD16 | Not described | 72%±7% | Kobari et al.128 |
| CD34+ | None | Using IL-15+IL-21400-fold | UCB CD34+ derived: KIR−CD161−CD244+; UCB CD34−lin− derived: KIR−CD244+CD161+ | CD34−lin− derived NK cells: CD107a assay K562: 59.5% ratio 10:1 (IL-12+IL-15 culture) | 92% | Bonanno et al.92 |
Abbreviations: E/T, effector/target; IFN, interferon; NK, natural killer; UCB, umbilical cord blood.
UCB stem cells.
Controversy still remains regarding the use of UCB stem cells with a variety of results showing very low NK cell yields to others demonstrating successful production of functional NK cells. A study using NOD/SCID mice receiving grafts of human hematopoietic cells revealed that NK cell production from UCB CD34+ cells was slower and less efficient compared to progenitors from BM and spleen cells (68%–98% vs. 17%).83 In addition, in an attempt to study NK cell development after transplantation, UCB CD34+ cells administered in mice differentiated into NK cells lacking the expression of maturation markers like CD158a (KIR2DL1), CD158b (KIR2DL2/DL3) and NKB1.83
Using UCB CD34+ cells and the feeder layer EL08.1D2, Grzywacz et al.47 described the coordinated acquisition of NK cell receptors that mark the transition from non-cytotoxic to cytotoxic CD56+ cells. The outcome in this study supports the notion of using feeder layer to complete NK cell maturation. In 2011, using UCB CD34+ cells and the feeder layer EL08.1D2, Grzywacz et al.56 investigated NK cell differentiation from myeloid precursors giving new insights into NK cell ontogeny. Even though the primary goal of their study was not the generation of NK cells for immunotherapy, the purity and yield reported, up to 94%, is optimal for this purpose.
Haddad et al.84 used MS5 stromal cells, a murine BM cell line established in 1989, to study NK cell ontology. Using UCB CD34+ cells, they characterized two early lymphoid progenitors, CD34+CD45RAhiCD7+ and CD34+CD45RAhiLin−CD10+, which correspond to T/NK or B lineage, respectively. Table 3 summarizes the current protocols of NK cell production from UCB stem cells.
Table 3. NK cell-derived from BM or PB stem cells.
| Source | Feeder layer | Fold expansion/number of cells | Phenotype | Functionality | Purity (end of culture) | Reference |
|---|---|---|---|---|---|---|
| H9 hESC line then isolation of CD34+ cells, also use of UCB as a comparison | For hESC line: M210-B4; for CD34+: AFT024 | 2 log expansion | UCB: high expression of CD16, NKp46, CD94 and granzyme, medium expression of Perforin, low or no expression of NKG2D, CD158a and D158b | Cr51 assay: UCB, 45% killing of K562 at 5:1 E/T ratio; hESC, 70% killing of K562 at 5:1 E/T ratio | Not reported | Woll et al.79 |
| hESC: high expression Cof CD16, NKp46, CD94, NKG2D, perforin and Cgranzyme, low expression Cof CD158a and CD158b | ||||||
| BM CD34+ | Stromal cells from irradiated BMMNC | 690-fold | CD56bright | Cr51 assay: 80% killing of K562 at 6.6:1 E/T ratio | 75% | Miller et al.48 |
| BM CD34+ | No feeder layer | 6.5-fold | CD56bright | Cr51 assay at 1:4 E/T ratio: IL-15 only. 45% killing of K562; IL-15+KL, 30% killing of K562 | 40%–85% | Mrozek et al.75 |
Abbreviations: BMMNC, irradiated bone marrow mononuclear cell; BM, bone marrow; E/T, effector/target; hESC, human embryonic stem cell; NK, natural killer; PB, peripheral blood; UCB, umbilical cord blood.
Feeder layer and cytokines used in NK cell cultures
Feeder layers.
Mouse embryonic fibroblasts are often used as ‘feeder layer' in human stem cell research. Oostendorp et al.85 undertook the task of uncovering, comparing and characterizing the hematopoietic microenvironment provided by midgestation mouse embryos. This group isolated stromal cell lines from embryonic liver and gastrointestinal region. The characteristics provided by these feeder layers are related to the developmental stage from which they are derived. The first hematopoiesis starts at day 10.5 after gestation in the aorta-gonads-mesonephros region. Subsequently, embryonic liver is colonized with hematopoietic cells, and the liver becomes the major hematopoietic organ during embryogenesis.
The cell line AFT024 was first described by Moore et al.,86 immortalized with temperature sensitive SV-40 T antigen and derived from murine fetal liver stromal cells. Subsequently, Miller et al.87 used AFT024 cell line to study NK cell ontogeny. In 2001, their work suggested that NK cell differentiation and receptor acquisition was contact dependent with the feeder layer AFT024 and IL-15. In this work, CD34+lin−CD38− single-cell progeny was analyzed to determine whether receptor acquisition was determined at a stem cell level. Their results show that NK cell receptor fate is acquired after NK cell commitment and does not require stromal presentation of MHC-1 by the stromal cells.87 Later in 2008, McCullar et al.88 investigated AFT024 and EL08-1D2 potential to generate NK cells, and found that EL08-1D2 is significantly better at recapitulating NK cell development. EL08-1D2 was cloned from embryonic liver at day 11, and was proven in this study to support the generation of NK cells from human hematopoietic precursors, and used also by other groups.56
M2-10B4 cell line is a clone derived from murine BM stromal cells. M2-10B4 has been used earlier for NK cell expansion.89 In addition, Pierson et al.90 tested different mouse cell line's (M2-10B4, NRK-49F and NIH-3T3) capacity and irradiated PB mononuclear cells to determine the factors controlling NK cell expansion. Remarkably, their findings suggest the need of stromal cell microenvironment, and the importance of direct contact with the feeder layer.
To summarize, the majority of published data indicates the need of a microenvironment produced by stromal cells. This microenvironment provides the necessary factors for the maturation of NK cells, and adequate acquisition of KIR receptors.
Serum-free culture.
Fetal bovine serum is usually used in hESC cultures due to its high content of grow factors. However, fetal bovine serum is a complex mixture and its contents vary from batch to batch, which constitutes a hurdle for Good Manufacturing Practice (GMP) protocols. In addition, it represents a major cost of cell culture. Some researchers have reduced their serum usage, by adding lipids, insulin, trace metals and other ingredients. Kao et al.91 isolated and expanded UCB CD133+ cells for 7 days following isolation of CD34+ cells in a serum-free media. Using an NK cell-inducing cytokine cocktail, they compared the ability of NK cell generation from expanded and freshly isolated CD34+ cells. The majority of generated NK cells were CD56bright, showing a significantly higher capacity of IFN-γ secretion and cytotoxicity than freshly isolated CD34+ cells.
Using serum-free, feeder cell-free culture system, Bonanno et al.92 showed the successful production of NK cells from UCB CD34−lin− cells and tested the impact of IL-21 on NK cell phenotype and functions. Their results showed high levels of NKG2D, IFN-γ, GM-CSF and tumor-necrosis factor-α expression, as well as resistance to IL-12 maturation. Along these lines, Frias et al.93 used a mesenchymal stem cell-MSC-based serum-free culture media to expand UCB HSCs. After expansion, they isolated CD34−CD7+ cells and cultured them in media supplemented with NK cell-inducing cytokine cocktail. After 2 weeks, NK cells were generated and displayed cytotoxic activity along with CD16 expression. In summary, serum-free systems are feasible and generate NK cells with cytotoxic potential that could be used for immunotherapy.
Cytokines.
Throughout the years, the cytokines playing a key role during NK cell production in vitro have been discovered. The combination and concentration of these cytokines has an impact on NK cell production. We briefly describe their properties in the next section.
More than 10 years ago, Williams et al.94 suggested that NK cell differentiation can be dissected in distinct phases. In the first phase, early acting cytokines, including IL-7, SCF and FLT3 ligand (FLT3L) could act upon HSCs to commit them to the NK lineage along with the induction of the β-subunit of the IL-15 receptor (IL-2Rβ) expression. Successively, the triggering of this receptor complex (IL-2Rβ/γc) with IL-15 acts to further expand, and differentiate these cells into mature NK cells.95 Table 4 shows the use of these cytokines in different NK cell differentiation protocols.
Table 4. Cytokines used in NK cell differentiation in vitro.
| Media for | Feeder layer/media | SCF (ng/ml) | IL-2 (ng/ml) | IL-3 (ng/ml) | IL-7 (ng/ml) | IL-15 (ng/ml) | IL-21 (ng/ml) | Flt-3L (ng/ml) | GM-CSF (ng/ml) | Others (ng/ml) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD34 expansion | IMDM | 15 | — | 4.1 | — | — | — | 6.7 | 1.3 | TPO: 8.5 , IL-6: 0.8 | Kao et al.91 |
| NK cell differentiation | IMDM | 50 | 12.5 | — | 20 | 40 | — | 50 | — | — | |
| NK cell differentiation | AFT024/ EL08-1D2 | 20 | — | 5 | 20 | 10 | 10 ng/ml | 10 | — | — | McCullar et al.88 |
| NK cell differentiation | MS-5/ RPMI 1640 | 50 | 100 IU/ml | — | 20 | 20 | — | 50 | — | — | Haddad et al.126 |
| NK cell differentiation | EL08-1D2/Ham F12+DMEM 1:2 | 20 | — | 5 | 20 | 10 | — | 10 | — | — | Grzywacs et al.47 |
| NK cell differentiation | MyeloCult TM H5100 | — | — | — | — | 20 | 30 | 25 | — | HC: 10–6M | Perez et al.127 |
| NK cell differentiation | Stro-1+ cells/HAM F12 | 10 | 1000 IU/ml | — | 10 | 10 | — | — | — | — | Frias et al.93 |
| NK cell differentiation | MS-5/IMDM | 50 | 5 | 10 | 20 | 10 | — | 50 | — | MGDF: 50 | Kobari et al.128 |
| NK cell differentiation | OP9/RPMI | — | — | — | 10 | 10 | — | 10 | — | — | Beck et al.129 |
| NK cell differentiation | MyeloCultTM H5100 | 20 | — | — | — | 50 | 50 | 20 | — | HC: 10–6M | Bonanno et al.92 |
| hESCs | M210-B4/RPMI 1640 | — | — | — | — | — | — | — | — | — | Woll et al.79 |
| NK cell differentiation | AFT024/DMEM:Ham F12 | 20 mg/l | — | 5 | 20 mg/l | 10 | — | 10 | — | — | |
| NK cell differentiation | dSC/RPMI-1640 | 20 | — | — | 20 | 20 | 20 | 20 | 20 | — | Vacca et al.130 |
| NK cell differentiation | BMMNC/ LTBMC/RPMI | — | 10 or 1000 IU/ml | — | — | — | — | — | — | HC: 10–6M | Miller et al.48 |
| NK cell differentiation | RPMI-1640 | 100 | 10 U | — | — | 100 | — | — | — | Mrozek et al.75 |
Abbreviations: AFT024, murine fetal liver stromal cells; BMMNC, irradiated bone marrow mononuclear cells; EL08-1D2, embryonic liver murine stromal cells; dSC, decidual stromal cells; DMEM/Ham F12 , Dulbecco-modified Eagle medium (high glucose without sodium pyrouvate)/Nutrient Mixture F-12 Ham; FLT-3L, FLT-3 ligand; GM-CSF, granulocyte macrophage colony-stimulating factor; HC, hydrocortisone; hESCs, human embryonic stem cells; IL-3, 5 ng/ml was added once at culture initiation; LTBMC, long-term bone marrow cultures; MGDF, megakaryocyte growth and development factor; MS-5, murine bone marrow stromal cells; myelocult H5100, MyeloCult H5100 is optimized for the initiation and maintenance of myeloid long-term cultures of human hematopoietic cells and stromal cell feeder layers; M210-B4, murine bone marrow stromal cell; NK, natural killer OP9, murine bone marrow stromal cells; SCF, stem cell factor, C-kit ligand; Stro+1, human marrow-derived STRO-1+ cells.
SCF is the ligand for c-kit receptor.96 Both SCF and FLT3L are expressed by HSCs and by subsets of lineage committed cells including NK cells.97 It has been demonstrated that neither FLT3L nor SCF by itself can induce NK cell differentiation from CD34+ cells, instead these two factors are able to stimulate the proliferation of NK cell intermediates CD122+ that are responsive to IL-15.62 A study from Colucci et al.98 in mice showed that SCF interactions in vivo are not only required for the commitment of HSC to the NK cell lineage, but also provide the necessary signalling for the generation of normal numbers of fully mature NK cells.
IL-7.
IL-7 is essential for the homeostasis of the immune system. It regulates NK cell as well as T- and B-lymphoid cell development.99 Stromal and BM cells are major producers of IL-7. Lymphoid progenitor cell differentiation, proliferation and survival of HSCs are supported by IL-7. In addition, although it is clear that NK cell differentiation occurs mainly in the BM, there are reports suggesting that early NK cell precursors can migrate to the thymus,100 secondary lymphoid organs,101 and mucosae tissues like the gut102 where they can differentiate into mature NK cells. Even though mature NK cells in the blood do not express IL-7Rα, there is evidence that murine thymic NK cells do express this receptor, and that IL-7 is needed for their homeostasis. This specific subset exhibits low cytotoxic activity but high cytokine secretion.51 Similarly, another NK cell subset, termed ‘NK-22', localized in human tonsils and mucosae gut was recently described.102 These cells are able to secrete IL-22 in response to IL-23. IL-7 is able to support NK-22 survival, and, in combination with IL-1β, mediates NK-22 proliferation.
IL-15.
IL-15 produced in the BM, thymus and secondary lymphoid organs is a crucial element to drive NK cell development and survival.103 IL-15 and its specific receptor IL-15Rα are essential for NK cell generation and maintenance, as it mediates NK cell development from committed NK cell precursors, promotes the differentiation of immature NK cells, and supports the survival of mature NK cells in peripheral lymphoid organs.33,59,75 Moreover, IL-15 can facilitate the conversion of poor cytolytic NK cells into highly cytolytic NK cells.104 In vivo, IL-15 can be either secreted or trans-presented to NK cells by different accessory cells.105 Studies in mice showed that levels of trans-presentation of IL-15 vary throughout maturation.106
Overall, NK cell differentiation is likely to be even more complex in vivo. There is an ongoing effort to characterize the growth factors and their receptors on hematopoietic cells and their progeny as this knowledge will give additional insights into this amazing biological process.
Challenges
Definition of activated NK cells
Another important factor to consider is the phenotype of the produced cells. It is clear that not every NK cell production protocol will generate NK cells with similar phenotype and function. Hence, the characterization, not only of the phenotype, but also of the functionality of these cells, is of great importance. Such differences in NK cell characteristics resulting from production protocols will have a significant impact on the clinical application and efficiency.
We still do not fully understand what are the requirements to generate functional maturation of NK cells. There is an intrinsic ability of NK cells to respond to stimuli. Observations in mice by Yokoyama et al.67 gave rise to the licensing hypothesis. Following the observations that NK cells from mice lacking MHC class I respond poorly, the licensing theory has become an interesting subject explored by other researchers. Given different terms, licensing or education,66 it has become obvious that this complex system of KIRs plays a key role in recognizing neighboring cells expressing MHC class I antigens, determining NK cell effector functions.
In addition, the NK cell subset dichotomy into CD56bright and CD56dim is slowly evolving. The finding of a new intermediate between CD56bright and CD56dim cells was described in the past,107 and recently, the characterization of this subset has unraveled its phenotypic and functional features.108 Likewise, Bjorkstrom et al. recently proposed that CD56dim cells continue to differentiate. During this process, NK cells lose the expression of NKG2A and later acquire KIRs and CD57 along with a change in their homing molecules expression pattern.109
Does NK cell number matter?
Today, cell purity is highly important as protocols define that samples should be at least 90% so that the clinical effect can be attributed to the product in question.110 T cells represent the major concern, as it is well known that they play a critical role in the pathophysiology of GVHD.28 Nonetheless, recent work has shown the need of T cells in NK cell education after allergenic UCB transplantation,38 albeit more clinical data may be needed to confirm this finding. NK cell yield is very important as well, since the dose-dependent effect of NK cells used in immunotherapy has been previously reported.111
Potential risks of NK cell immunotherapy
Although we have seen that NK cell immunotherapy holds promise in the clinics, we are aware that every single therapy entails certain risks. The main concerns regarding NK cell immunotherapy are the detrimental side effects. As mentioned before, purity of the final products plays a major role; the existence of T cells will most likely provide a GVHD effect, though one report suggests that this may not be the case.112 Another important factor to consider is the phenotype of the generated NK cells; producing cells with an activating phenotype and expressing few inhibitory receptors could increase the risk of NK cell autoreactivity, where the lack of inhibitory signals in the generated NK cells or host's cells can cause severe tissue destruction (as exemplified by transported associated protein deficiency in patients).113 Furthermore, NK cells are known to play an important role in autoimmune diseases, due to their interaction with dendritic cells and macrophages; NK cells are able to participate in the induction/maintenance of cell tissue injuries.114 Current research groups are conducting clinical trials to address these potential risks. There are several phase I clinical trials using either KIR–HLA mismatch NK cells115 or expanded allogenic NK cells.116 These trials have shown so far that NK cell immunotherapy is safe, where no side effects were observed after the infusion of high or low doses of NK cells.
GMP procedures
Before the ‘bench to the bedside' translation, each of the protocols described have to comply with the good manufacturing practices. Ex vivo expansion of NK cells, as previously mentioned, is one source of NK cells for immunotherapy. Progress has been made in this area: using CliniMACS-purified NK cells, Siegler et al.117 was able to expand single KIR-positive alloreactive NK cells for AML patients. In addition, Sutlu et al. have designed an automated reactor able to expand NK cells from PB mononuclear cells and demonstrated that large amounts of high reactive NK cells can be produced and used for immunotherapy.68 Furthermore, the expansion of UCB NK cells using tacrolimus and a low molecular weight heparin-media has been developed by Tanka et al.118 The results showed high NK cell fold expansion and high level of cytotoxicity activity against leukemic cell line K562 and patient's primary AML and chronic myeloid leukemia cells. On the other hand, Spanholtz et al.119,120 have recently published a high log-scale expansion of NK cells from UCB CD34+ cells using clinical grade protocols. This work shows the feasibility of using fresh and even frozen UCB stem cells to produce NK cells able to lyse melanoma cell lines, and mediate cytolysis of primary leukemia cells. To our knowledge there are not many published protocols using stem cells complying with GMP procedures to date. This is a big challenge as the use of clinical grade reagents is limited. The development of these products will speed up the chances of developing clinical grade protocols able to produce NK cells for immunotherapy.
Future directions and conclusions
Up to now, several clinical strategies exploiting NK cell alloreactivity to treat cancer have been developed. NK cell expansion in vitro from PB or UCB cells has been carried out. However, NK cell expansion in vivo is transient, and NK cell populations may be heterogeneous due different factors.
Our understanding of NK cell effector functions and responses continues to flourish, along with the opportunities of designing better therapeutic tools using strategies that target these mechanisms. Even thought the majority of the published protocols do not aim to produce NK cells for immunotherapy, they have greatly contributed to the establishment of the few already existing. We still have a long journey, with obstacles that are not impossible to override. Research groups from the clinics, pharmaceutical companies and academic institutions with basic research are now more than ever collaborating in order to facilitate the incorporation of novel technology that will finally bring help fighting this disease.
Acknowledgments
We would like to thank Dr Sergio Querol and Professor Salim Khakoo for their precious advices.
References
- Kiessling R, Klein E, Pross H, Wigzell H. ‘Natural' killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5:117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Kiessling R, Klein E, Wigzell H. ‘Natural' killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16:216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975;16:230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- Lanier LL, Phillips JH, Hackett J, Jr, Tutt M, Kumar V. Natural killer cells: definition of a cell type rather than a function. J Immunol. 1986;137:2735–2739. [PubMed] [Google Scholar]
- Lanier LL, Testi R, Bindl J, Phillips JH. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989;169:2233–2238. doi: 10.1084/jem.169.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz J, Schmidt RE, Michon J, Hercend T, Schlossman SF. Characterization of functional surface structures on human natural killer cells. Adv Immunol. 1988;42:181–211. doi: 10.1016/s0065-2776(08)60845-7. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Karre K. NK cells, MHC class I molecules and the missing self. Scand J Immunol. 2002;55:221–228. doi: 10.1046/j.1365-3083.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol. 2006;24:257–286. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- Colonna M, Brooks EG, Falco M, Ferrara GB, Strominger JL. Generation of allospecific natural killer cells by stimulation across a polymorphism of HLA-C. Science. 1993;260:1121–1124. doi: 10.1126/science.8493555. [DOI] [PubMed] [Google Scholar]
- Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–339. [PubMed] [Google Scholar]
- Takenaka K, Mizuno SI, Harada M, Nagafuji K, Miyamoto T, Iwasaki H, et al. Generation of human natural killer cells from peripheral blood CD34+ cells mobilized by granulocyte colony-stimulating factor. Br J Haematol. 1996;92:788–794. doi: 10.1046/j.1365-2141.1996.408950.x. [DOI] [PubMed] [Google Scholar]
- Sconocchia G, Provenzano M, Rezvani K, Li J, Melenhorst J, Hensel N, et al. CD34+ cells cultured in stem cell factor and interleukin-2 generate CD56+ cells with antiproliferative effects on tumor cell lines. J Transl Med. 2005;3:15. doi: 10.1186/1479-5876-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao IT, Yao CL, Kong ZL, Wu ML, Chuang TL, Hwang SM. Generation of natural killer cells from serum-free, expanded human umbilical cord blood CD34+ cells. Stem Cells Dev. 2007;16:1043–1051. doi: 10.1089/scd.2007.0033. [DOI] [PubMed] [Google Scholar]
- Suck G, Koh MB. Emerging natural killer cell immunotherapies: large-scale ex vivo production of highly potent anticancer effectors. Hematol Oncol Stem Cell Ther. 2010;3:135–142. doi: 10.1016/s1658-3876(10)50024-4. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong HT, Restifo NP. Natural selection of tumor variants in the generation of ‘tumor escape' phenotypes. Nature immunology. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido F, Ruiz-Cabello F, Cabrera T, Perez-Villar JJ, Lopez-Botet M, Duggan-Keen M, et al. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today. 1997;18:89–95. doi: 10.1016/s0167-5699(96)10075-x. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- Coghill JM, Sarantopoulos S, Moran TP, Murphy WJ, Blazar BR, Serody JS. Effector CD4+ T cells, the cytokines they generate, and GVHD: something old and something new. Blood. 2011;117:3268–3276. doi: 10.1182/blood-2010-12-290403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagne F, Andre P, Spee P, Zahn S, Anfossi N, Gauthier L, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–2677. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DM, Jr, Bakan CE, Zhang S, Collins SM, Liang J, Srivastava S, et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood. 2011;118:6387–6391. doi: 10.1182/blood-2011-06-360255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BW, Morstyn G. Natural killer (NK)-resistant human lung cancer cells are lysed by recombinant interleukin-2-activated NK cells. Cell Immunol. 1987;106:215–222. doi: 10.1016/0008-8749(87)90165-1. [DOI] [PubMed] [Google Scholar]
- Torelli GF, Guarini A, Palmieri G, Breccia M, Vitale A, Santoni A, et al. Expansion of cytotoxic effectors with lytic activity against autologous blasts from acute myeloid leukaemia patients in complete haematological remission. Br J Haematol. 2002;116:299–307. [PubMed] [Google Scholar]
- Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. 2005;86:209–239. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- Cao S, Wang YL, Ren XB, Yu JP, Ren BZ, Zhang XW, et al. Efficacy of large doses of IL-2-activated human leukocyte antigen haploidentical peripheral blood stem cells on refractory metastatic renal cell carcinoma. Cancer Biother Radiopharm. 2011;26:503–510. doi: 10.1089/cbr.2011.0982. [DOI] [PubMed] [Google Scholar]
- Rosenstein M, Ettinghausen SE, Rosenberg SA. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986;137:1735–1742. [PubMed] [Google Scholar]
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- Jakobisiak M, Golab J, Lasek W. Interleukin 15 as a promising candidate for tumor immunotherapy. Cytokine Growth Factor Rev. 2011;22:99–108. doi: 10.1016/j.cytogfr.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, et al. NK-cell education after allogeneic transplantation: dissociation between recovery of cytokine producing and cytotoxic functions. Blood. 2011;118:2784–2792. doi: 10.1182/blood-2011-04-347070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- Ren XB, Yu JP, Cao S, Ren BZ, Li H, Liu H, et al. Antitumor effect of large doses IL-2-activated HLA haploidentical peripheral blood stem cells on refractory metastatic solid tumor treatment. Cancer Biother Radiopharm. 2007;22:223–234. doi: 10.1089/cbr.2007.334. [DOI] [PubMed] [Google Scholar]
- Koehl U, Sorensen J, Esser R, Zimmermann S, Gruttner HP, Tonn T, et al. IL-2 activated NK cell immunotherapy of three children after haploidentical stem cell transplantation. Blood Cells Mol Dis. 2004;33:261–266. doi: 10.1016/j.bcmd.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Pegram HJ, Jackson JT, Smyth MJ, Kershaw MH, Darcy PK. Adoptive transfer of gene-modified primary NK cells can specifically inhibit tumor progression in vivo. . J Immunol. 2008;181:3449–3455. doi: 10.4049/jimmunol.181.5.3449. [DOI] [PubMed] [Google Scholar]
- Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima S, Mailliard R, Kashii Y, Reichert TE, Herberman RB, Robbins P, et al. Stable transduction of the interleukin-2 gene into human natural killer cell lines and their phenotypic and functional characterization in vitro and in vivo. . Blood. 1998;91:3850–3861. [PubMed] [Google Scholar]
- Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625–632. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- Arina A, Murillo O, Dubrot J, Azpilikueta A, Gabari I, Perez-Gracia JL, et al. Interleukin-15 liver gene transfer increases the number and function of IKDCs and NK cells. Gene Ther. 2008;15:473–483. doi: 10.1038/gt.2008.4. [DOI] [PubMed] [Google Scholar]
- Grzywacz B, Kataria N, Sikora M, Oostendorp RA, Dzierzak EA, Blazar BR, et al. Coordinated acquisition of inhibitory and activating receptors and functional properties by developing human natural killer cells. Blood. 2006;108:3824–3833. doi: 10.1182/blood-2006-04-020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Alley KA, McGlave P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma-based long-term culture system: identification of a CD34+7+ NK progenitor. Blood. 1994;83:2594–2601. [PubMed] [Google Scholar]
- Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev. 2006;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- Vacca P, Vitale C, Montaldo E, Conte R, Cantoni C, Fulcheri E, et al. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc Natl Acad Sci USA. 108:2402–2407. doi: 10.1073/pnas.1016257108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Richard-Le Goff O, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer. Nat Rev Immunol. 2003;3:413–425. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- Ramos SB, Garcia AB, Viana SR, Voltarelli JC, Falcao RP. Phenotypic and functional evaluation of natural killer cells in thymectomized children. Clin Immunol Immunopathol. 1996;81:277–281. doi: 10.1006/clin.1996.0189. [DOI] [PubMed] [Google Scholar]
- Passlick B, Izbicki JR, Waydhas C, Nast-Kolb D, Schweiberer L, Ziegler-Heitbrock HW. Posttraumatic splenectomy does not influence human peripheral blood mononuclear cell subsets. J Clin Lab Immunol. 1991;34:157–161. [PubMed] [Google Scholar]
- Haller O, Wigzell H. Suppression of natural killer cell activity with radioactive strontium: effector cells are marrow dependent. J Immunol. 1977;118:1503–1506. [PubMed] [Google Scholar]
- Grzywacz B, Kataria N, Blazar BR, Miller JS, Verneris MR. Natural killer cell differentiation by myeloid progenitors. Blood. 2011;117:3548–3558. doi: 10.1182/blood-2010-04-281394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T. Differentiation plasticity of hematopoietic cells. Blood. 2002;99:3089–3101. doi: 10.1182/blood.v99.9.3089. [DOI] [PubMed] [Google Scholar]
- Ikawa T, Kawamoto H, Fujimoto S, Katsura Y. Commitment of common T/natural killer (NK) progenitors to unipotent T and NK progenitors in the murine fetal thymus revealed by a single progenitor assay. J Exp Med. 1999;190:1617–1626. doi: 10.1084/jem.190.11.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. . J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamizono S, Duncan GS, Seidel MG, Morimoto A, Hamada K, Grosveld G, et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. . J Exp Med. 2009;206:2977–2986. doi: 10.1084/jem.20092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoyne DM, Long E, Veiga-Fernandes H, de Boer J, Williams O, Seddon B, et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- Yu H, Fehniger TA, Fuchshuber P, Thiel KS, Vivier E, Carson WE, et al. Flt3 ligand promotes the generation of a distinct CD34+ human natural killer cell progenitor that responds to interleukin-15. Blood. 1998;92:3647–3657. [PubMed] [Google Scholar]
- Sivori S, Falco M, Marcenaro E, Parolini S, Biassoni R, Bottino C, et al. Early expression of triggering receptors and regulatory role of 2B4 in human natural killer cell precursors undergoing in vitro differentiation. Proc Natl Acad Sci USA. 2002;99:4526–4531. doi: 10.1073/pnas.072065999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IM, Zatsepina O, Zamai L, Azzoni L, Mikheeva T, Perussia B. Definition of a natural killer NKR-P1A+/CD56−/CD16− functionally immature human NK cell subset that differentiates in vitro in the presence of interleukin 12. J Exp Med. 1996;184:1845–1856. doi: 10.1084/jem.184.5.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LL, Chang C, Phillips JH. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J Immunol. 1994;153:2417–2428. [PubMed] [Google Scholar]
- Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- Sutlu T, Stellan B, Gilljam M, Quezada HC, Nahi H, Gahrton G, et al. Clinical-grade, large-scale, feeder-free expansion of highly active human natural killer cells for adoptive immunotherapy using an automated bioreactor. Cytotherapy. 2010;12:1044–1055. doi: 10.3109/14653249.2010.504770. [DOI] [PubMed] [Google Scholar]
- Alici E, Sutlu T, Bjorkstrand B, Gilljam M, Stellan B, Nahi H, et al. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood. 2008;111:3155–3162. doi: 10.1182/blood-2007-09-110312. [DOI] [PubMed] [Google Scholar]
- Doskali M, Tanaka Y, Ohira M, Ishiyama K, Tashiro H, Chayama K, et al. Possibility of adoptive immunotherapy with peripheral blood-derived CD3CD56+ and CD3+CD56+ cells for inducing antihepatocellular carcinoma and antihepatitis C virus activity. J Immunother. 2011;34:129–138. doi: 10.1097/CJI.0b013e3182048c4e. [DOI] [PubMed] [Google Scholar]
- Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passweg JR, Tichelli A, Meyer-Monard S, Heim D, Stern M, Kuhne T, et al. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18:1835–1838. doi: 10.1038/sj.leu.2403524. [DOI] [PubMed] [Google Scholar]
- Voshol H, Dullens HF, Den Otter W, Vliegenthart JF. Human natural killer cells: a convenient purification procedure and the influence of cryopreservation on cytotoxic activity. J Immunol Methods. 1993;165:21–30. doi: 10.1016/0022-1759(93)90102-d. [DOI] [PubMed] [Google Scholar]
- Fujiwara S, Akiyama M, Yamakido M, Seyama T, Kobuke K, Hakoda M, et al. Cryopreservation of human lymphocytes for assessment of lymphocyte subsets and natural killer cytotoxicity. J Immunol Methods. 1986;90:265–273. doi: 10.1016/0022-1759(86)90084-0. [DOI] [PubMed] [Google Scholar]
- Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–2640. [PubMed] [Google Scholar]
- Woll PS, Grzywacz B, Tian X, Marcus RK, Knorr DA, Verneris MR, et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113:6094–6101. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Allegrucci C, Young LE. Differences between human embryonic stem cell lines. Hum Reprod Update. 2007;13:103–120. doi: 10.1093/humupd/dml041. [DOI] [PubMed] [Google Scholar]
- Woll PS, Martin CH, Miller JS, Kaufman DS. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J Immunol. 2005;175:5095–5103. doi: 10.4049/jimmunol.175.8.5095. [DOI] [PubMed] [Google Scholar]
- Shibuya A, Nagayoshi K, Nakamura K, Nakauchi H. Lymphokine requirement for the generation of natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1995;85:3538–3546. [PubMed] [Google Scholar]
- Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nature Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- Giuliani M, Giron-Michel J, Negrini S, Vacca P, Durali D, Caignard A, et al. Generation of a novel regulatory NK cell subset from peripheral blood CD34+ progenitors promoted by membrane-bound IL-15. PLoS One. 2008;3:e2241. doi: 10.1371/journal.pone.0002241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalberer CP, Siegler U, Wodnar-Filipowicz A. Human NK cell development in NOD/SCID mice receiving grafts of cord blood CD34+ cells. Blood. 2003;102:127–135. doi: 10.1182/blood-2002-07-2024. [DOI] [PubMed] [Google Scholar]
- Itoh K, Tezuka H, Sakoda H, Konno M, Nagata K, Uchiyama T, et al. Reproducible establishment of hemopoietic supportive stromal cell lines from murine bone marrow. Exp Hematol. 1989;17:145–153. [PubMed] [Google Scholar]
- Oostendorp RA, Harvey KN, Kusadasi N, de Bruijn MF, Saris C, Ploemacher RE, et al. Stromal cell lines from mouse aorta-gonads-mesonephros subregions are potent supporters of hematopoietic stem cell activity. Blood. 2002;99:1183–1189. doi: 10.1182/blood.v99.4.1183. [DOI] [PubMed] [Google Scholar]
- Moore KA, Ema H, Lemischka IR. In vitro maintenance of highly purified, transplantable hematopoietic stem cells. Blood. 1997;89:4337–4347. [PubMed] [Google Scholar]
- Miller JS, McCullar V. Human natural killer cells with polyclonal lectin and immunoglobulinlike receptors develop from single hematopoietic stem cells with preferential expression of NKG2A and KIR2DL2/L3/S2. Blood. 2001;98:705–713. doi: 10.1182/blood.v98.3.705. [DOI] [PubMed] [Google Scholar]
- McCullar V, Oostendorp R, Panoskaltsis-Mortari A, Yun G, Lutz CT, Wagner JE, et al. Mouse fetal and embryonic liver cells differentiate human umbilical cord blood progenitors into CD56-negative natural killer cell precursors in the absence of interleukin-15. Exp Hematol. 2008;36:598–608. doi: 10.1016/j.exphem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson BA, McGlave PB, Hu WS, Miller JS. Natural killer cell proliferation is dependent on human serum and markedly increased utilizing an enriched supplemented basal medium. J Hematother. 1995;4:149–158. doi: 10.1089/scd.1.1995.4.149. [DOI] [PubMed] [Google Scholar]
- Pierson BA, Gupta K, Hu WS, Miller JS. Human natural killer cell expansion is regulated by thrombospondin-mediated activation of transforming growth factor-beta 1 and independent accessory cell-derived contact and soluble factors. Blood. 1996;87:180–189. [PubMed] [Google Scholar]
- Kao IT, Yao CL, Kong ZL, Wu ML, Chuang TL, Hwang SM. Generation of natural killer cells from serum-free, expanded human umbilical cord blood CD34+ cells. Stem Cells Dev. 2007;16:1043–1051. doi: 10.1089/scd.2007.0033. [DOI] [PubMed] [Google Scholar]
- Bonanno G, Mariotti A, Procoli A, Corallo M, Scambia G, Pierelli L, et al. Interleukin-21 induces the differentiation of human umbilical cord blood CD34−lineage− cells into pseudomature lytic NK cells. BMC Immunol. 2009;10:46. doi: 10.1186/1471-2172-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias AM, Porada CD, Crapnell KB, Cabral JM, Zanjani ED, Almeida-Porada G. Generation of functional natural killer and dendritic cells in a human stromal-based serum-free culture system designed for cord blood expansion. Exp Hematol. 2008;36:61–68. doi: 10.1016/j.exphem.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NS, Klem J, Puzanov IJ, Sivakumar PV, Schatzle JD, Bennett M, et al. Natural killer cell differentiation: insights from knockout and transgenic mouse models and in vitro systems. Immunol Rev. 1998;165:47–61. doi: 10.1111/j.1600-065x.1998.tb01229.x. [DOI] [PubMed] [Google Scholar]
- Williams NS, Moore TA, Schatzle JD, Puzanov IJ, Sivakumar PV, Zlotnik A, et al. Generation of lytic natural killer 1.1+, Ly−49− cells from multipotential murine bone marrow progenitors in a stroma-free culture: definition of cytokine requirements and developmental intermediates. J Exp Med. 1997;186:1609–1614. doi: 10.1084/jem.186.9.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DE, Eisenman J, Baird A, Rauch C, van Ness K, March CJ, et al. Identification of a ligand for the c-kit proto-oncogene. Cell. 1990;63:167–174. doi: 10.1016/0092-8674(90)90297-r. [DOI] [PubMed] [Google Scholar]
- Matos ME, Schnier GS, Beecher MS, Ashman LK, William DE, Caligiuri MA. Expression of a functional c-kit receptor on a subset of natural killer cells. J Exper Med. 1993;178:1079–1084. doi: 10.1084/jem.178.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci F, Di Santo JP. The receptor tyrosine kinase c-kit provides a critical signal for survival, expansion, and maturation of mouse natural killer cells. Blood. 2000;95:984–991. [PubMed] [Google Scholar]
- Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T−B+NK+ severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- Mingari MC, Vitale C, Cantoni C, Bellomo R, Ponte M, Schiavetti F, et al. Interleukin-15-induced maturation of human natural killer cells from early thymic precursors: selective expression of CD94/NKG2-A as the only HLA class I-specific inhibitory receptor. Eur J Immunol. 1997;27:1374–1380. doi: 10.1002/eji.1830270612. [DOI] [PubMed] [Google Scholar]
- Freud AG, Becknell B, Roychowdhury S, Mao HC, Ferketich AK, Nuovo GJ, et al. A human CD34+ subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- Gamero AM, Ussery D, Reintgen DS, Puleo CA, Djeu JY. Interleukin 15 induction of lymphokine-activated killer cell function against autologous tumor cells in melanoma patient lymphocytes by a CD18-dependent, perforin-related mechanism. Cancer Res. 1995;55:4988–4994. [PubMed] [Google Scholar]
- Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205:1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GA, Liou YH, Wang SW, Ko KL, Jiang ST, Liao NS. Different NK cell developmental events require different levels of IL-15 trans-presentation. J Immunol. 2011;187:1212–1221. doi: 10.4049/jimmunol.1100331. [DOI] [PubMed] [Google Scholar]
- Dulphy N, Haas P, Busson M, Belhadj S, Peffault de Latour R, Robin M, et al. An unusual CD56brightCD16low NK cell subset dominates the early posttransplant period following HLA-matched hematopoietic stem cell transplantation. J Immunol. 2008;181:2227–2237. doi: 10.4049/jimmunol.181.3.2227. [DOI] [PubMed] [Google Scholar]
- Beziat V, Duffy D, Quoc SN, Le Garff-Tavernier M, Decocq J, Combadiere B, et al. CD56brightCD16+ NK cells: a functional intermediate stage of NK cell differentiation. J Immunol. 2011;186:6753–6761. doi: 10.4049/jimmunol.1100330. [DOI] [PubMed] [Google Scholar]
- Bjorkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 116:3853–3864. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- Berg M, Childs R. Ex-vivo expansion of NK cells: what is the priority—high yield or high purity. Cytotherapy. 2010;12:969–970. doi: 10.3109/14653249.2010.536216. [DOI] [PubMed] [Google Scholar]
- Alici E, Konstantinidis KV, Sutlu T, Aints A, Gahrton G, Ljunggren HG, et al. Anti-myeloma activity of endogenous and adoptively transferred activated natural killer cells in experimental multiple myeloma model. Exp Hematol. 2007;35:1839–1846. doi: 10.1016/j.exphem.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Sutlu T, Alici E. Ex vivo expansion of natural killer cells: a question of function. Cytotherapy. 2011;13:767–768. doi: 10.3109/14653249.2011.563295. [DOI] [PubMed] [Google Scholar]
- Moins-Teisserenc HT, Gadola SD, Cella M, Dunbar PR, Exley A, Blake N, et al. Association of a syndrome resembling Wegener's granulomatosis with low surface expression of HLA class-I molecules. Lancet. 1999;354:1598–1603. doi: 10.1016/s0140-6736(99)04206-3. [DOI] [PubMed] [Google Scholar]
- Schleinitz N, Vely F, Harle JR, Vivier E. Natural killer cells in human autoimmune diseases. Immunology. 2010;131:451–458. doi: 10.1111/j.1365-2567.2010.03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkholt L, Alici E, Conrad R, Sutlu T, Gilljam M, Stellan B, et al. Safety analysis of ex vivo-expanded NK and NK-like T cells administered to cancer patients: a phase I clinical study. Immunotherapy. 2009;1:753–764. doi: 10.2217/imt.09.47. [DOI] [PubMed] [Google Scholar]
- Siegler U, Meyer-Monard S, Jorger S, Stern M, Tichelli A, Gratwohl A, et al. Good manufacturing practice-compliant cell sorting and large-scale expansion of single KIR-positive alloreactive human natural killer cells for multiple infusions to leukemia patients. Cytotherapy. 2010;12:750–763. doi: 10.3109/14653241003786155. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Sugita J, Shiratori S, Shigematu A, Asanuma S, Fujimoto K, et al. Expansion of NK cells from cord blood with antileukemic activity using GMP-compliant substances without feeder cells. Leukemia; in press. [DOI] [PubMed]
- Spanholtz J, Tordoir M, Eissens D, Preijers F, van der Meer A, Joosten I, et al. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. PLoS One. 2010;5:e9221. doi: 10.1371/journal.pone.0009221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanholtz J, Preijers F, Tordoir M, Trilsbeek C, Paardekooper J, de Witte T, et al. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS One. 2011;6:e20740. doi: 10.1371/journal.pone.0020740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B, Farace F, Angevin E, Charpentier F, Nitenberg G, Triebel F, et al. Immunotherapy with interleukin-2 (IL2) and lymphokine-activated natural killer cells: improvement of clinical responses in metastatic renal cell carcinoma patients previously treated with IL2. Eur J Cancer. 1994;30A:1078–1083. doi: 10.1016/0959-8049(94)90460-x. [DOI] [PubMed] [Google Scholar]
- Ishikawa E, Tsuboi K, Saijo K, Harada H, Takano S, Nose T, et al. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004;24:1861–1871. [PubMed] [Google Scholar]
- deMagalhaes-Silverman M, Donnenberg A, Lembersky B, Elder E, Lister J, Rybka W, et al. Posttransplant adoptive immunotherapy with activated natural killer cells in patients with metastatic breast cancer. J Immunother. 2000;23:154–160. doi: 10.1097/00002371-200001000-00018. [DOI] [PubMed] [Google Scholar]
- Lister J, Rybka WB, Donnenberg AD, deMagalhaes-Silverman M, Pincus SM, Bloom EJ, et al. Autologous peripheral blood stem cell transplantation and adoptive immunotherapy with activated natural killer cells in the immediate posttransplant period. Clin Cancer Res. 1995;1:607–614. [PubMed] [Google Scholar]
- Koehl U, Sorensen J, Esser R, Zimmermann S, Gruttner HP, Tonn T, et al. IL-2 activated NK cell immunotherapy of three children after haploidentical stem cell transplantation. Blood Cells Molec Dis. 2004;33:261–266. doi: 10.1016/j.bcmd.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Haddad R, Guardiola P, Izac B, Thibault C, Radich J, Delezoide AL, et al. Molecular characterization of early human T/NK and B-lymphoid progenitor cells in umbilical cord blood. Blood. 2004;104:3918–3926. doi: 10.1182/blood-2004-05-1845. [DOI] [PubMed] [Google Scholar]
- Perez SA, Mahaira LG, Sotiropoulou PA, Gritzapis AD, Iliopoulou EG, Niarchos DK, et al. Effect of IL-21 on NK cells derived from different umbilical cord blood populations. Int Immunol. 2006;18:49–58. doi: 10.1093/intimm/dxh348. [DOI] [PubMed] [Google Scholar]
- Kobari L, Pflumio F, Giarratana M, Li X, Titeux M, Izac B, et al. In vitro and in vivo evidence for the long-term multilineage (myeloid, B, NK, and T) reconstitution capacity of ex vivo expanded human CD34+ cord blood cells. Exp Hematol. 2000;28:1470–1480. doi: 10.1016/s0301-472x(00)00557-9. [DOI] [PubMed] [Google Scholar]
- Beck RC, Padival M, Yeh D, Ralston J, Cooke KR, Lowe JB. The Notch ligands Jagged2, Delta1, and Delta4 induce differentiation and expansion of functional human NK cells from CD34+ cord blood hematopoietic progenitor cells. Biol Blood Marrow Transplant. 2009;15:1026–1037. doi: 10.1016/j.bbmt.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca P, Vitale C, Montaldo E, Conte R, Cantoni C, Fulcheri E, et al. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc Natl Acad Sci US A. 2011;108:2402–2407. doi: 10.1073/pnas.1016257108. [DOI] [PMC free article] [PubMed] [Google Scholar]