Abstract
In glomerulonephritis, the migration of inflammatory cells into the glomerulus is an important step in disease initiation and progression. The viral receptor Toll-like receptor 3 (TLR3) is known to play a role in virus-associated glomerulonephritis. Based on this knowledge, this study aimed to define the effects of the TLR3 ligand polyriboinosinic:polyribocytidylic acid (poly(I:C)) on the expression of adhesion molecules and macrophage colony-stimulating factor (M-CSF) on resident glomerular cells. Experiments in MCs demonstrated that the activation of viral receptors by poly(I:C) leads to a time- and dose-dependent induction of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1) and M-CSF at both the mRNA and protein levels; these results were confirmed by incubating MCs with HCV RNA. As shown in knockdown experiments, this effect is specifically mediated by TLR3. The prestimulation of MCs with proinflammatory cytokines increases the effects of poly(I:C), except for its induction of VCAM-1. Tumor-necrosis factor (TNF)-α, likewise, induces ICAM-1, VCAM-1 and M-CSF, and amplifies the mesangial response to poly(I:C). These results were confirmed by incubating MCs with HCV RNA. We thus provide evidence that human MCs represent a potential target of the leukocytes and monocytes that infiltrate the glomerulus in viral disease-associated GN, highlighting the possibility that MCs may act as resident antigen-presenting cells.
Keywords: adhesion molecules, glomerulonephritis, hepatitis C, Toll-like receptor 3
Introduction
In glomerulonephritis (GN), the infiltration of inflammatory cells is a critical event in disease initiation and progression. The type of infiltrating cell varies depending on the specific disease entity, but it is widely agreed that prolonged exposure to the cytokines, growth factors and matrix that infiltrating cells produce has deleterious effects. The expression of adhesion molecules on endothelial cells is a prerequisite for directing cell influx into the glomerulum, but their expression on other resident glomerular cells is less well characterized. However, once the vascular barrier is overcome, it is thought that mesangial cells (MCs), known for their ability to behave like inflammatory cells, could also be targeted by circulating leukocytes or monocytes. Moreover, MCs are known to express major histocompatibility complex (MHC) class II molecules,1, 2 which would allow them to function as antigen-presenting cells, using intercellular adhesion molecule 1 (ICAM-1) as a costimulatory molecule. This may be potentially relevant in glomerular diseases caused by pathogens that are able to elicit an immune response. In the case of hepatitis C virus (HCV)-associated GN, immune complexes containing viral RNA have been detected locally,3, 4, 5 supporting the hypothesis that a so-called ‘planted antigen' triggers glomerular disease. Both ICAM-1 and vascular cell adhesion molecule 1 (VCAM-1) have been found to be significantly increased in patients with severe cryoglobulinemic vasculitis associated with hepatitis C,6 and the cryoprecipitates are able to induce endothelial ICAM-1 and VCAM-1 expression in vitro.7
Based on these data, we intended to test whether the activation of the innate immune system viral receptors expressed on MCs leads to the amplification of antiviral responses and contributes to the establishment of glomerulopathy by regulating adhesion molecules or facilitating the intrarenal recruitment and activation of monocytes via macrophage colony-stimulating factor (M-CSF). Because the impact of mesangially expressed Toll-like receptor 3 (TLR3) on hepatitis C-associated GN had been demonstrated previously,8, 9, 10 in vitro assays were performed using established TLR3 ligands, namely, the synthetic viral dsDNA analogue polyriboinosinic:polyribocytidylic acid (poly(I:C))11 and HCV RNA isolated from patient sera.
Materials and methods
Cell culture of human MCs
Immortalized human MCs were grown as described previously and incubated with culture medium alone or medium containing the indicated test substances.12 The cytokine concentrations used were 25 ng/ml for tumor necrosis factor-α (TNF)-α, 10 ng/ml for IL-1β and 20 ng/ml for interferon (IFN)-γ. MCs were stimulated as indicated. For cytokine prestimulation experiments, MCs were incubated in culture medium with or without a mixture of TNF-α, IL-1β and IFN-γ for 12 h, washed with phosphate-buffered saline, incubated in culture medium for 6 h and washed again with phosphate-buffered saline. Subsequently, the MCs were incubated in culture medium with or without poly(I:C) (10 μg/ml) as indicated. For poly(I:C) prestimulation experiments, MCs were incubated in culture medium with or without poly(I:C) RNA (10 μg/ml) for 24 h, washed with phosphate-buffered saline, and subsequently incubated in culture medium with or without TNF-α for the indicated time intervals. For each stimulation experiment, controls were performed in parallel using culture medium alone. For mRNA level analyses, the extraction of total RNA was performed using an RNeasy Mini Kit (Qiagen, Hilden, Germany) with additional DNase digestion.
Quantitative reverse transcriptase-polymerase chain reaction (real-time PCR) analysis
Real-time PCR analysis was performed as described.8 A total of 2 μg isolated total RNA was subjected to random primed reverse transcription using MMLV-RT (Superscript; Life Technologies, Karlsruhe, Germany). In parallel, 2 μg aliquots were processed without reverse transcription to control for contaminating genomic DNA. Real-time PCR was performed on a TaqMan ABI 7700 sequence detection system (PE Applied Biosystems, Weiterstadt, Germany). rRNA was used as a reference gene. All water controls were negative for both the targets and rRNA. The following GenBank sequences were used for the design of the predeveloped Taq Man assay reagents or primers and probes, purchased from Applied Biosystems: NM_000201 (human ICAM-1), NM_080682, NM_001078.2, M30257.1, M603335.1 (human VCAM-1), NM_172210.2, NM_172211.2, NM_172212.2, NM_000757.4 (human M-CSF) and M33197 (human ).
Fluorescence-activated cell sorting (FACS) analysis
FACS analysis was performed as described.8 ICAM-1 and VCAM-1 were measured on the surfaces of cells fixed with 4% formaldehyde and stained with anti-ICAM-1-FITC or anti-VCAM-1-FITC or the corresponding FITC-labeled negative control. For the measurement of intracellular M-CSF expression, cells were permeabilized using 0.1% Triton for 2 min on ice and fixed with 4% formaldehyde prior to staining with anti-M-CSF-PE or the corresponding PE-labeled negative control. Anti-ICAM-1, anti-VCAM-1, anti-M-CSF and their respective negative controls were obtained from Biozol, Eching, Germany; Southern Biotech, Birmingham, AL, USA; R&D Systems, Minneapolis, MN, USA, respectively. A FACSCanto II flow cytometer (Becton Dickinson, San Jose, CA, USA) was used to measure the proportion of cells expressing each protein. The data were analyzed using FACSDiva software (Becton Dickinson).
Knockdown of gene expression with short interfering RNA (siRNA)
Predesigned siRNAs specific for TLR3 and RIG-I were purchased from Ambion (Tokyo, Japan). The siRNA was transfected into the cells using the siPORT-NeoFX transfection agent (Ambion), as described previously.13 Scrambled siRNA was used as the nonspecific negative control siRNA (Ambion). Cells were treated with siRNA for 24 h and washed once with cell culture medium to remove any remaining transfection agent.
Preparation of HCV RNA
Exosomes containing viral RNA from the sera of human patients with high viral loads were isolated by centrifugation, as described previously.14 Samples (20–50 ml) of clotted human whole blood were centrifuged at 1500g for 10 min. The supernatant was collected and centrifuged again at 10 000g for 30 min to remove solid remnants, and the resulting supernatant was centrifuged at 70 000g for 60 min in an SW28 rotor (Beckman Instruments Inc.). The pellet was dissolved in 1 ml cell culture medium without serum or antibiotics, and Lipofectamine was added. The concentration of HCV used for stimulation was 100×106 geq/ml, confirmed by RT-PCR. For HCV stimulation, confluent human MCs in 6-well plates were used; once the virus was added, the plates were centrifuged at 1000g for 45 min to allow efficient viral infection. Subsequent stimulation was performed as indicated.
Statistical analysis
All values are provided as the mean±SEM. Statistical analysis was performed using the unpaired t-test or ANOVA, as applicable. Significant differences in expression levels are indicated for P values <0.05 (*) or <0.01 (**), respectively.
Results
The effect of poly(I:C) on the expression of ICAM-1, VCAM-1 and M-CSF in human MCs
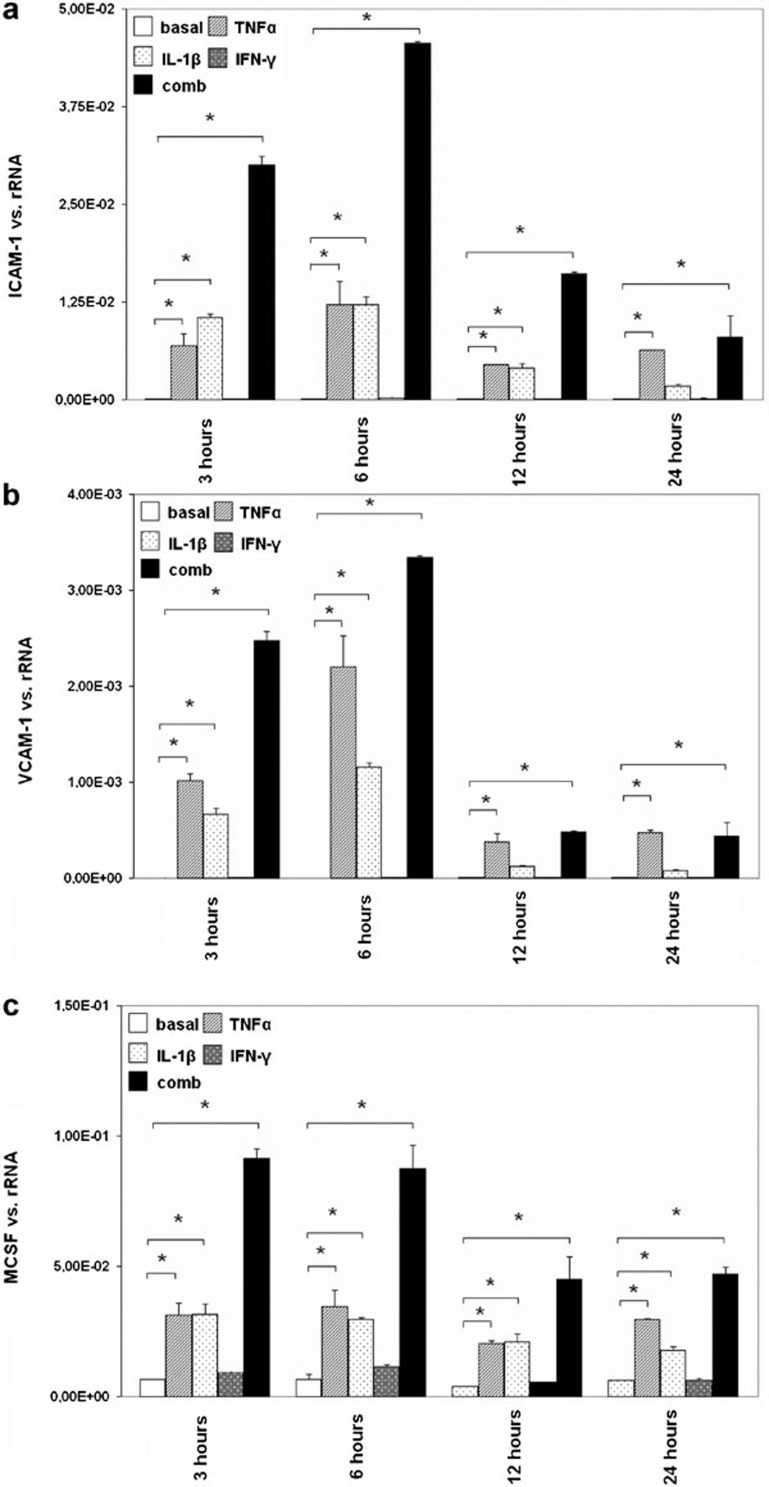
Because MCs play an important role in immune-mediated glomerular disease, and adhesion molecules and M-CSF are key mediators of leukocyte attraction to the site of inflammation, we examined the expression of ICAM-1, VCAM-1 and M-CSF in cultured human MCs upon exposure to the cytokines TNF-α, IL-1β and IFN-γ. These cytokines were chosen to simulate an inflammatory milieu similar to what would occur during virus-associated GN. MCs were stimulated with the cytokines TNF-α, IL-1β and IFN-γ alone or in combination for different lengths of time, and the expression of ICAM-1, VCAM-1 and M-CSF was subsequently analyzed by real-time PCR. The expression of these genes was significantly upregulated after 3, 6, 12 and 24 h of TNF-α treatment. IL-1β treatment increased the expression of ICAM-1 and VCAM-1 significantly after 3, 6 and 12 h and increased the expression of M-CSF after 3, 6, 12 and 24 h. IFN-γ had no effect on the basal expression of ICAM-1, VCAM-1 or M-CSF. The highest ICAM-1 expression was observed when MCs were stimulated with the combination of the cytokines for 3, 6 or 12 h; after 24 h, ICAM-1 expression levels were comparable to those induced by TNF-α. The maximal increases in both VCAM-1 and M-CSF expression were induced by the combination of cytokines after 3 and 6 h (Figure 1).
Figure 1.
The effect of TNF-α, IL-1β and IFN-γ on the expression of ICAM-1, VCAM-1 and M-CSF in human MCs. MCs were grown under standard culture conditions (basal) or incubated with TNF-α, IL-1β and IFN-γ alone or in combination (comb) for different intervals (3, 6, 12 or 24 h), and the expression of ICAM-1 (a), VCAM-1 (b) and M-CSF (c) was analyzed by real-time PCR. The results are given as the means±SEM of two experiments conducted in parallel for each condition. rRNA served as the reference gene. Comparable results were obtained in two independent series of experiments. ICAM-1, intercellular adhesion molecule 1; IFN, interferon; MC, mesangial cell; M-CSF, macrophage colony-stimulating factor; real-time PCR, quantitative reverse transcriptase-polymerase chain reaction; TNF-α, tumor necrosis factor-α VCAM-1, vascular cell adhesion molecule 1.
The effect of poly(I:C) on the expression of ICAM-1, VCAM-1 and M-CSF in human MCs
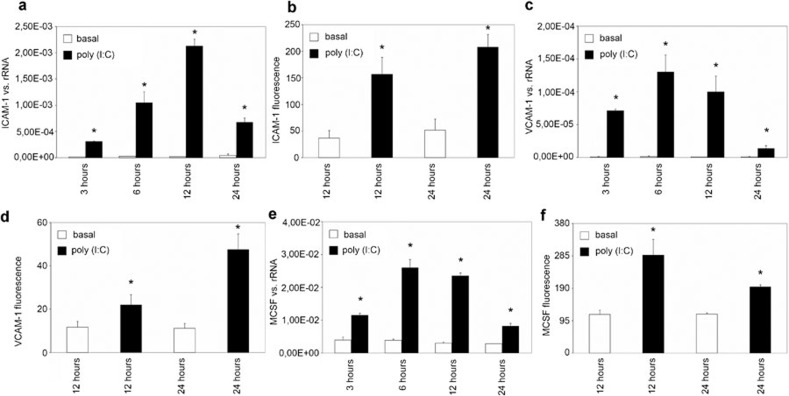
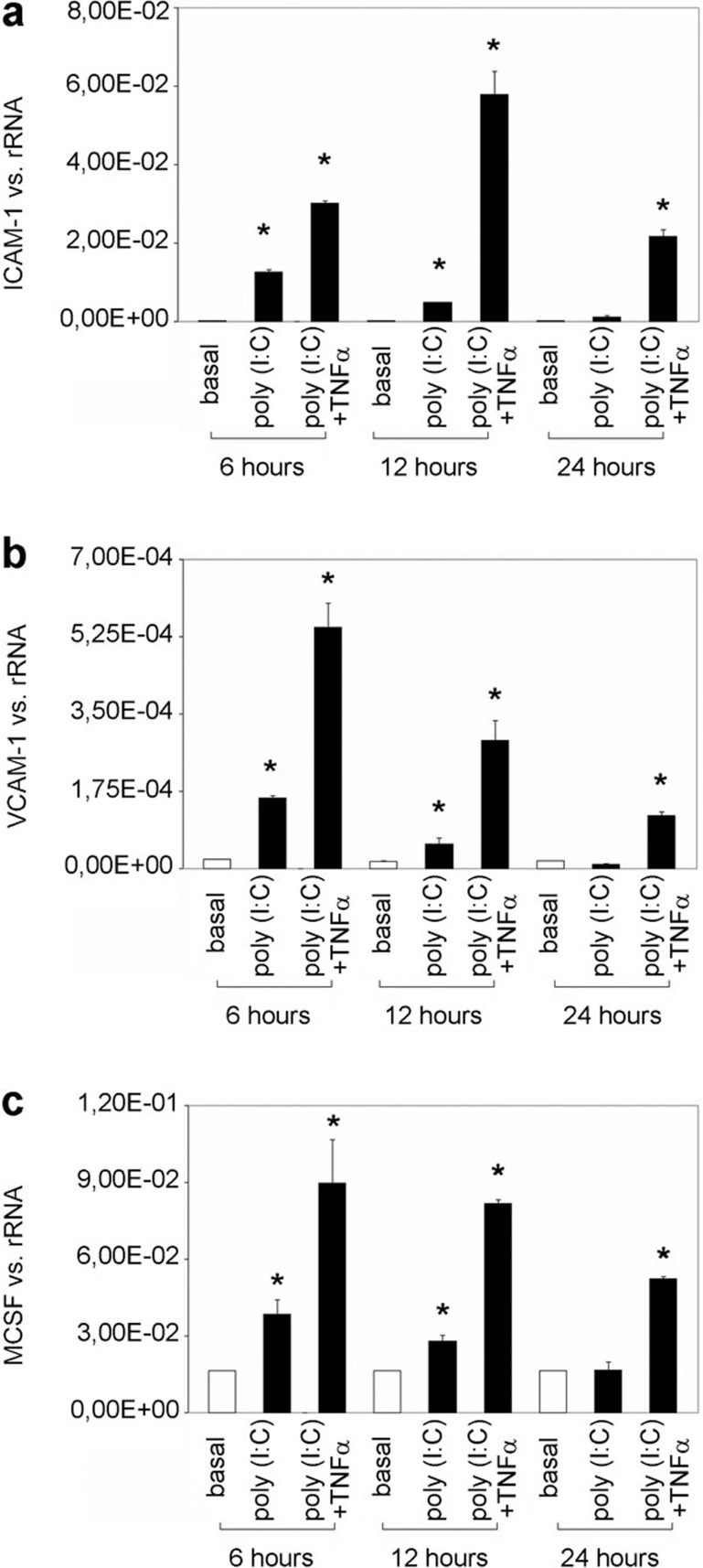
During viral infection, viral RNA is thought to reach the mesangium and therefore potentially activate receptors for dsRNA. Here, MCs were stimulated with poly(I:C) (10 μg/ml), mimicking viral RNA for different time intervals (3, 6, 12 or 24 h), and the expression of ICAM-1, VCAM-1 and M-CSF was analyzed by real-time PCR. Stimulation with poly(I:C) resulted in an increase in ICAM-1 expression after 3, 6, 12 and 24 h, with a maximal increase at 12 h (Figure 2a). VCAM-1 (Figure 2c) and M-CSF (Figure 2e) expression was also significantly induced at all stimulation time points, with peak expression at 6 h.
Figure 2.
The effect of poly(I:C) on the synthesis of ICAM-1, VCAM-1 and M-CSF in human MCs. MCs were grown under standard culture conditions (basal) or incubated with poly(I:C) (10 μg/ml) for different intervals (3, 6, 12 or 24 h), and the synthesis of ICAM-1 (a, b), VCAM-1 (c, d) and M-CSF (e, f) was analyzed by real-time PCR (a, c, e) and FACS (b, d, f), as described in the section on ‘Materials and methods'. The results are given as the means±SEM of two experiments conducted in parallel for each condition. rRNA served as the reference gene. Comparable results were obtained in two independent series of experiments. ICAM-1, intercellular adhesion molecule 1; MC, mesangial cell; M-CSF, macrophage colony-stimulating factor; poly(I:C), polyriboinosinic:polyribocytidylic acid; real-time PCR, quantitative reverse transcriptase-polymerase chain reaction; VCAM-1, vascular cell adhesion molecule 1.
FACS analysis was performed to prove that the demonstrated effects of poly(I:C) were relevant at the protein level. Stimulation with poly(I:C) (10 μg/ml) for 12 and 24 h significantly increased the synthesis of ICAM-1 (Figure 2b), VCAM-1 (Figure 2d) and M-CSF (Figure 2f) at the protein level.
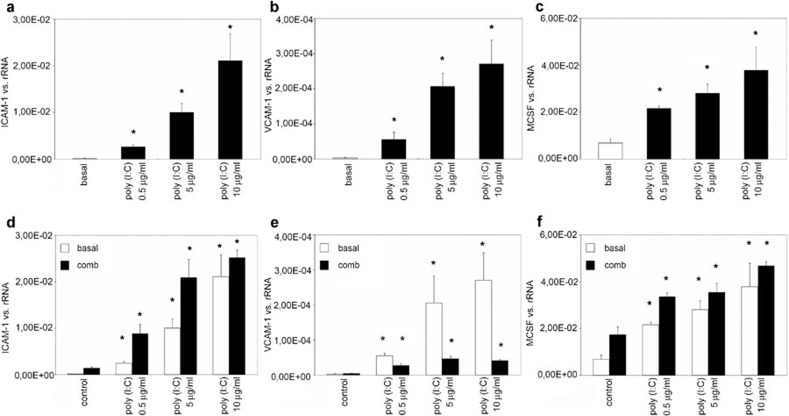
MCs were then incubated with culture medium alone (basal) or culture medium containing poly(I:C) at different concentrations (0.5, 5 or 10 μg/ml) for 24 h. The exposure of MCs to different concentrations of poly(I:C) increased the levels of ICAM-1 (Figure 3a), VCAM-1 (Figure 3b) and M-CSF (Figure 3c) mRNAs in a dose-dependent manner. Because an induction of the viral receptors TLR3 and RIG-I was previously observed after treatment with a combination of the cytokines TNF-α, IL-1β and IFN-γ,4, 6 MCs were pre-incubated with a combination of these cytokines and subsequently stimulated with different concentrations of poly(I:C) (0.5, 5 or 10 μg/ml) for 24 h. The concentration-dependent poly(I:C)-induced increase in ICAM-1 (Figure 3d) and M-CSF (Figure 3f) expression was further increased in cells pretreated with the cytokine combination. By contrast, cytokine pretreatment reduced the poly(I:C)-induced increase in VCAM-1 expression.
Figure 3.
The effect of poly(I:C) and cytokine treatment on mesangial ICAM-1, VCAM-1 and M-CSF expression. MCs were grown under standard culture conditions (basal) or incubated with different concentrations of poly(I:C) (0.5, 5 or 10 μg/ml) for 24 h, and the expression of ICAM-1 (a), VCAM-1 (b) and M-CSF (c) was analyzed by real-time PCR. MCs were then pretreated with a combination of cytokines (comb) and subsequently stimulated with different concentrations of poly(I:C) (0.5, 5 or 10 μg/ml) for 24 h, and the expression of ICAM-1 (d), VCAM-1 (e) and M-CSF (f) was analyzed. The results are given as the means±SEM of two experiments conducted in parallel for each condition. rRNA served as the reference gene. Comparable results were obtained in two independent series of experiments. ICAM-1, intercellular adhesion molecule 1; MC, mesangial cell; M-CSF, macrophage colony-stimulating factor; poly(I:C), polyriboinosinic:polyribocytidylic acid; real-time PCR, quantitative reverse transcriptase-polymerase chain reaction; VCAM-1, vascular cell adhesion molecule 1.
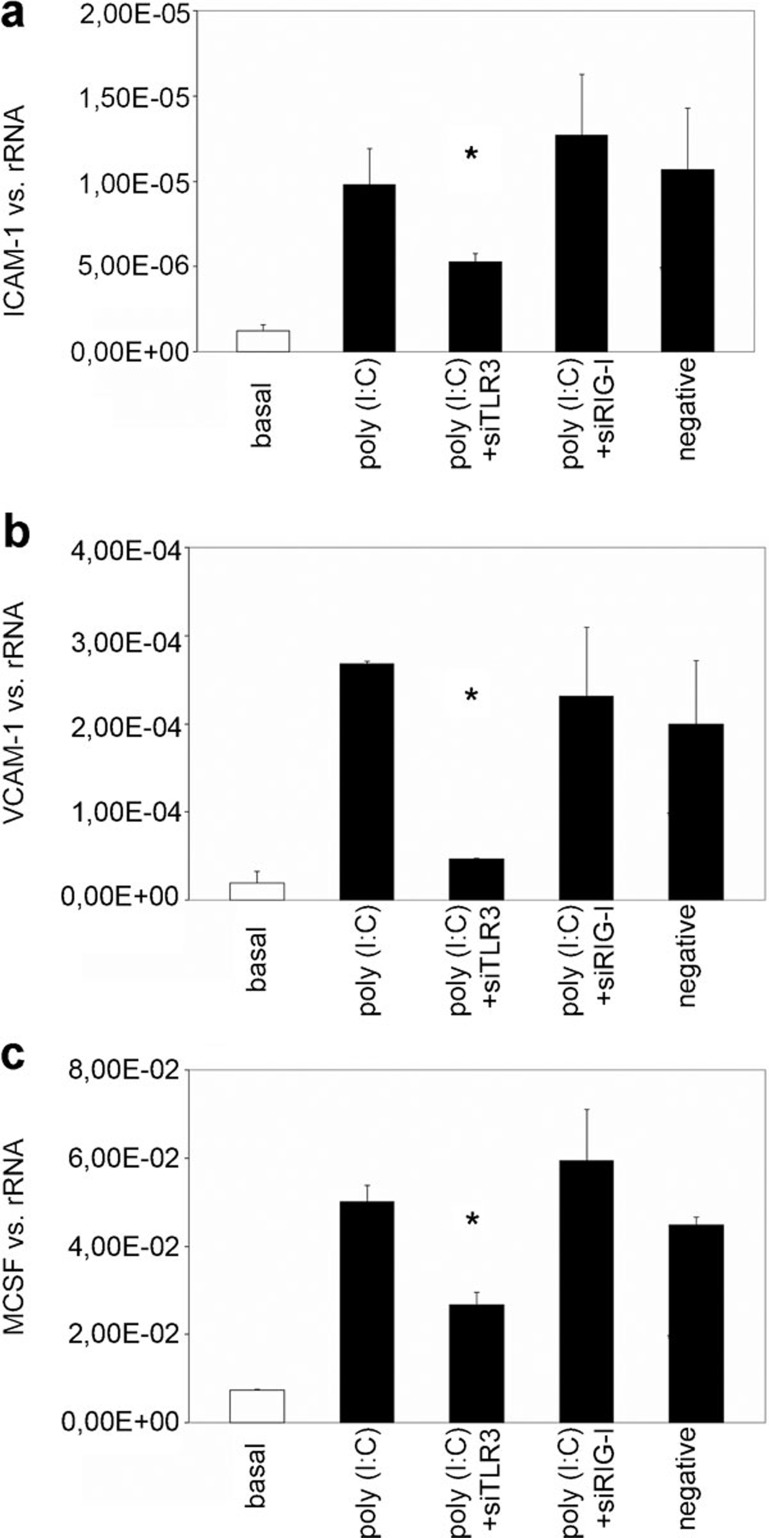
The effect of transfection with siRNA specific to TLR3 and RIG-I on the poly(I:C)-induced expression of ICAM-1, VCAM-1 and M-CSF
To test which viral receptors mediate the poly(I:C)-induced expression of adhesion molecules and M-CSF, MCs were transfected with siRNA targeting TLR3 or RIG-I and stimulated with poly(I:C) (10 μg/ml) for 12 h. The poly(I:C)-induced increases in ICAM-1 (Figure 4a), VCAM-1 (Figure 4b) and M-CSF (Figure 4c) expression were blocked by treatment with siRNA specific for TLR3. Neither siRNA targeting RIG-I nor the unspecific RNA used as a negative control had any effect.
Figure 4.
The effect of transfection with siRNA targeting TLR3 or RIG-I on the poly(I:C)-induced expression of ICAM-1, VCAM-1 and M-CSF. MCs were transfected with siRNAs targeting TLR3 or RIG-I or with nonspecific RNA as a negative control and stimulated with poly(I:C) (10 μg/ml) for 12 h. The expression of ICAM-1, VCAM-1 and M-CSF was analyzed by real-time PCR. The results are given as the means±SEM of two experiments conducted in parallel for each condition. rRNA served as the reference gene. Comparable results were obtained in two independent series of experiments. ICAM-1, intercellular adhesion molecule 1; MC, mesangial cell; M-CSF, macrophage colony-stimulating factor; poly(I:C), polyriboinosinic:polyribocytidylic acid; real-time PCR, quantitative reverse transcriptase-polymerase chain reaction; siRNA, short interfering RNA; TLR3, Toll-like receptor 3; VCAM-1, vascular cell adhesion molecule 1.
The effect of TNF-α on the expression of ICAM-1, VCAM-1 and M-CSF in cultured human MCs
TNF-α is known to play an immunoregulatory role in inflammatory glomerular processes. To investigate this role, MCs were stimulated with TNF-α for different intervals (3, 6, 9, 12 or 24 h), and the expression of ICAM-1, VCAM-1 and M-CSF was analyzed by real-time PCR. TNF-α treatment led to a significant increase in ICAM-1 (Figure 4a), VCAM-1 (Figure 4b) and M-CSF (Figure 4c) expression at all time points, with an early maximum at 3 h for M-CSF and at 6 h for ICAM-1 and VCAM-1.
The poly(I:C)-mediated induction of ICAM-1, VCAM-1 and M-CSF is enhanced by TNF-α
We have previously shown that poly(I:C) increases the expression of the mesangial TNF-α receptor 2.14 In the present manuscript, we demonstrate that TNF-α induces the expression of the adhesion molecules ICAM-1, VCAM-1 and M-CSF (Figure 1). To test for an enhancement of the poly(I:C)-mediated induction of ICAM-1, VCAM-1 and M-CSF by TNF-α, MCs were incubated with poly(I:C) (10 μg/ml) for 24 h and then stimulated with TNF-α for different intervals (6, 12 and 24 h). The increase in the expression of ICAM-1, VCAM-1 and M-CSF stimulated by poly(I:C) persisted until 12 h of incubation with culture medium alone (poly(I:C)); after 24 h, the expression of the target genes was comparable to that in the control. Subsequent TNF-α stimulation significantly enhanced the poly(I:C)-induced expression of ICAM-1 (Figure 5a), VCAM-1 (Figure 5b) and M-CSF (Figure 5c), with maximum ICAM-1 expression at 12 h of TNF-α stimulation and maximum VCAM-1 and M-CSF expression at 6 h of TNF-α stimulation.
Figure 5.
The poly(I:C)-mediated induction of ICAM-1, VCAM-1 and M-CSF is amplified by TNF-α. MCs were incubated with poly(I:C) for 24 h and then grown in culture medium alone (poly(I:C)) or stimulated with TNF-α (poly(I:C)+TNF-α) for different intervals (6, 12 or 24 h). The expression of ICAM-1 (a), VCAM-1 (b) and M-CSF (c) was analyzed by real-time PCR. The results are given as the means±SEM of two experiments conducted in parallel for each condition. rRNA served as the reference gene. Comparable results were obtained in two independent series of experiments. ICAM-1, intercellular adhesion molecule 1; MC, mesangial cell; M-CSF, macrophage colony-stimulating factor; poly(I:C), polyriboinosinic:polyribocytidylic acid; real-time PCR, quantitative reverse transcriptase-polymerase chain reaction; TNF-α, tumor necrosis factor-α VCAM-1, vascular cell adhesion molecule 1.
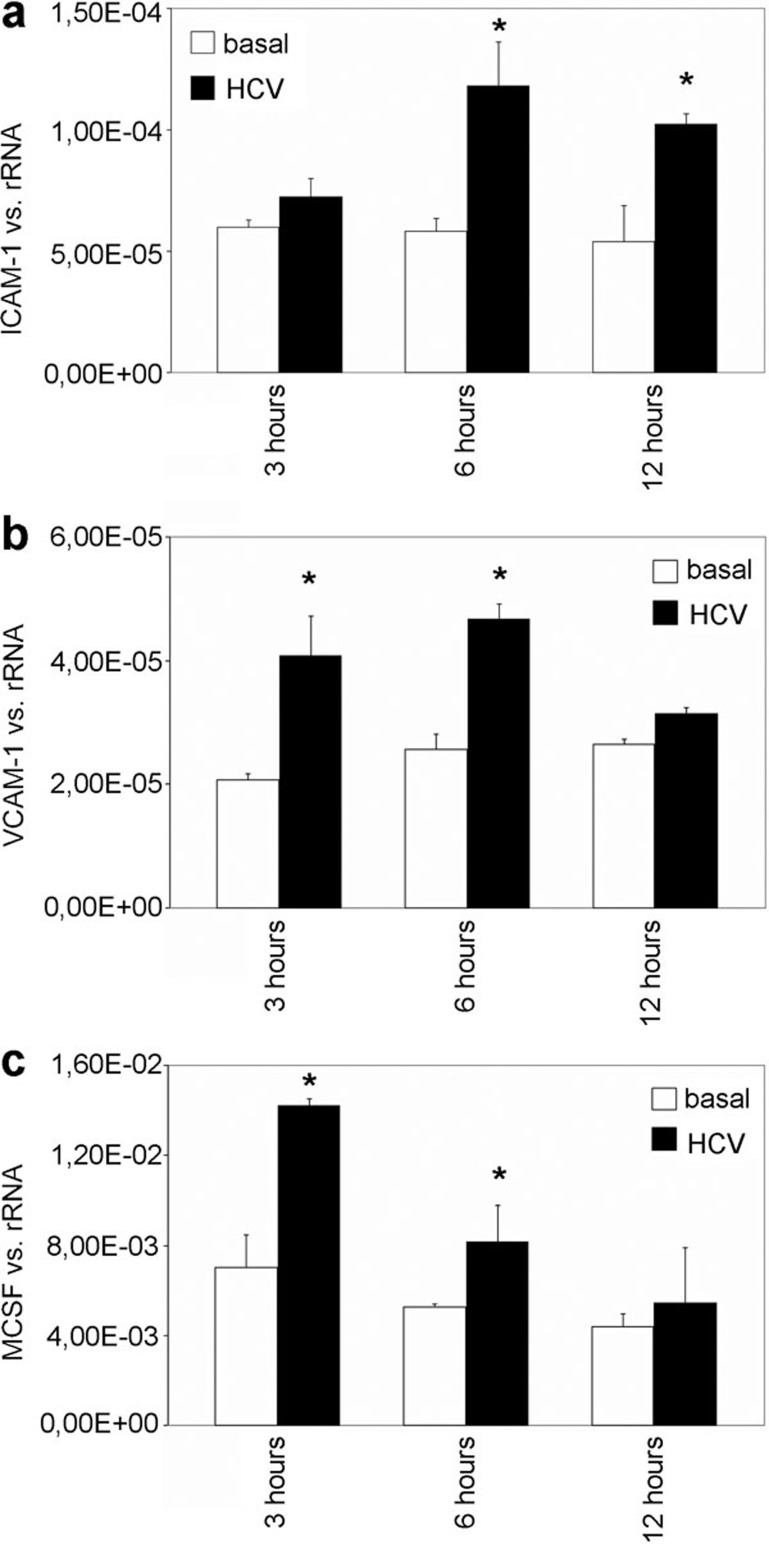
The effect of HCV RNA on the expression of ICAM-1, VCAM-1 and M-CSF in cultured human MCs
To confirm the relevance of the experimental results obtained upon the stimulation of human MCs with poly(I:C) in HCV infection, MCs were transfected with lipofectamine and stimulated with HCV RNA isolated as described for different intervals (3, 6 and 12 h). The expression of ICAM-1, VCAM-1 and M-CSF was analyzed. An increase in ICAM-1 expression was observed after 6 and 12 h of HCV RNA stimulation (Figure 6a). Upregulation of VCAM-1 and M-CSF expression by HCV RNA was significant compared with that in controls after 3 and 6 h (Figure 6b and c).
Figure 6.
The effect of HCV RNA on the mesangial expression of ICAM-1, VCAM-1 and M-CSF. MCs were transfected with lipofectamine and stimulated with HCV RNA for different intervals (3, 6 or 12 h). The expression of ICAM-1 (a), VCAM-1 (b) and –M-CSF (c) was analyzed by real-time PCR. The results are given as the means±SEM of two experiments conducted in parallel for each condition. rRNA served as the reference gene. Comparable results were obtained in two independent series of experiments. HCV, hepatitis C virus; ICAM-1, intercellular adhesion molecule 1; MC, mesangial cell; M-CSF, macrophage colony-stimulating factor; real-time PCR, quantitative reverse transcriptase-polymerase chain reaction; VCAM-1, vascular cell adhesion molecule 1.
Discussion
The infiltration of inflammatory cells into the glomerulum is a feature shared by the majority of glomerulopathies and has been suggested to play a substantial part in the initiation and progression of local immune and inflammatory responses. Both ICAM-1 and VCAM-1 are known to be of pivotal importance in the transendothelial migration of leukocytes by mediating the firm adhesion of leukocytes to endothelium,15 while ICAM-1 has the principal role during the transmigration stage.16 M-CSF facilitates monocyte survival, monocyte-to-macrophage conversion and macrophage proliferation.17
As we have shown previously, the activation of the viral receptor TLR3 expressed on human MCs amplifies antiviral and inflammatory responses by inducing the expression of cytokines and chemokines involved in hepatitis C-associated GN, which is characterized by the deposition of immune complexes containing viral RNA.8, 9, 10 With the intent to define further immune regulatory functions of MCs, we analyzed the mesangial expression of adhesion molecules and M-CSF in response to viral receptor activation. These experiments provide evidence of a time- and dose-dependent induction of ICAM-1, VCAM-1 and M-CSF in human MCs by poly(I:C), a synthetic analogue of viral dsRNA, on both the mRNA and protein levels of these molecules; this effect is mediated specifically by the viral receptor TLR3. These experimental findings were confirmed by the incubation of MCs with HCV RNA, thereby underscoring the potential clinical impact of the results presented. Correspondingly, in IgA nephropathy, a glomerulopathy that is likewise induced and aggravated by viral infections, increased ICAM-1 expression was found in the mesangium in patients with advanced disease,18, 19 and enhanced glomerular and interstitial granulocyte macrophage colony-stimulating factor expression correlated with the progression of tissue injury.20 Moreover, our in vitro results match earlier clinical observations implicating ICAM-1 and VCAM-1 in active inflammation in immune complex and crescentic GN.21, 22 Of particular interest, we found that the prestimulation of cells with cytokines to simulate an inflammatory milieu enhanced the poly(I:C)-dependent induction of ICAM-1 and M-CSF but significantly suppressed VCAM-1. Again, our experimental results are consistent with the finding of differential expression of ICAM-1 and VCAM-1 in cellular versus fibrous crescents and in subtypes of ANCA-associated vasculitis.23 We infer that mesangially expressed VCAM-1 is relevant for the limitation of inflammatory reactions.
Because TNF-α is a cytokine known to balance proinflammatory and immunosuppressive effects in inflammation and has been attributed an important role in renal injury in various disease entities, we next analyzed the effect of TNF-α treatment on the mesangial expression of adhesion molecules and M-CSF. Given that the activation of TLR3 induces mesangially expressed TNF-α and that MCs exhibit functional TNF-α receptors of both subtypes,14 its regulatory role in further inflammatory responses was predicted. Indeed, TNF-α upregulates mesangial ICAM-1, VCAM-1 and M-CSF expression, and is able to amplify their induction by TLR3, thereby modulating the antiviral response.
The results presented might well be relevant beyond viral disease-associated glomerulopathies. Indeed, not only is high glucose known to induce mesangial ICAM-1 expression24 and ICAM-1-deficient mice show less glomerular hypertrophy and mesangial matrix expression upon induction of diabetes,25 but certain ICAM-1 gene polymorphisms confer susceptibility to the development of type 1 diabetes and diabetic nephropathy.26 The increase in ICAM-1 induction promoted by viral RNA might thus explain the worse prognosis of diabetic nephropathy in patients with concomitant hepatitis C infection.27 Moreover, gene expression profiling of aging glomeruli demonstrates the activation of pathways that likely use NFκB as a transcriptional regulator;28 in that NFκB represents an integral component of the signaling pathway downstream of TLR3,29 viral infections could confer a risk for the development of glomerulosclerosis independent of the inflammatory tissue injury. Furthermore, the prognosis of any known glomerulopathy is made worse by hypertension, which might be partly explained by the amplification of shared pathways in pathogenesis, as demonstrated in hypertensive animal models that show an increased expression of adhesion molecules and upregulation of NF-κB,30 apart from cyclic stretch of MCs directly induce the expression of functional ICAM-1.31
In summary, we provide evidence that human MCs are a potential target of leukocytes and monocytes infiltrating the glomerulus in viral disease-associated GN. In this context, it is tempting to speculate that MCs may act as resident antigen presenting cells. Indeed, MCs are able to elicit an MHC class II-dependent proliferative lymphocyte response,32 and could conceivably present viral fragments or cell debris, making use of ICAM-1 as a costimulatory molecule and thereby potentiating immune processes, including the infiltration of inflammatory cells. Particularly in glomerulonephritides that involve locally deposited immune complexes with a corresponding activation of complement, this hypothesis is substantiated by the observation of a concomitant induction of MHC class II molecules, ICAM-1 and complement receptors in human MC by interferon gamma.33 This specific etiology needs further attention.
Acknowledgments
This work was supported by grants from the Deutsche Vereinigung zur Bekämpfung von Viruskrankheiten, the Else Kröner-Fresenius-Stiftung, the Wilhelm-Vaillant-Stiftung and the Fritz Bender Stiftung to MW.
References
- Chadban SJ, Tesch GH, Foti R, Lan HY, Atkins RC, Nikolic-Paterson DJ. Interleukin-10 differentially modulates MHC class II expression by mesangial cells and macrophages in vitro and in vivo. . Immunology. 1998;94:72–78. doi: 10.1046/j.1365-2567.1998.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeke HH, Resch K. The inflammatory function of renal glomerular mesangial cells and their interaction with the cellular immune system. Clin Investig. 1992;70:825–842. doi: 10.1007/BF00180754. [DOI] [PubMed] [Google Scholar]
- Stokes MB, Chawla H, Brody RI, Kumar A, Gertner R, Goldfarb DS, et al. Immune complex glomerulonephritis in patients coinfected with human immunodeficiency virus and hepatitis C virus. Am J Kidney Dis. 1997;29:514–525. doi: 10.1016/s0272-6386(97)90332-2. [DOI] [PubMed] [Google Scholar]
- Okada K, Takishita Y, Shimomura H, Tsuji T, Miyamura T, Kuhara Ts, et al. Detection of hepatitis C virus core protein in the glomeruli of patients with membranous glomerulonephritis. Clin Nephrol. 1996;45:71–76. [PubMed] [Google Scholar]
- Yamabe H, Inuma H, Osawa H, Kaizuka M, Tamura N, Tsunoda S, et al. Glomerular deposition of hepatitis C virus in membranoproliferative glomerulonephritis. Nephron. 1996;72:741. doi: 10.1159/000188987. [DOI] [PubMed] [Google Scholar]
- Lamprecht P, Moosig F, Gause A, Herlyn K, Csernok E, Hansen H, et al. Immunological and clinical follow up of hepatitis C virus associated cryoglobulinaemic vasculitis. Ann Rheum Dis. 2001;60:385–390. doi: 10.1136/ard.60.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplanski G, Maisonobe T, Marin V, Grès S, Robitail S, Farnarier C, et al. Vascular cell adhesion molecule-1 (VCAM-1) plays a central role in the pathogenesis of severe forms of vasculitis due to hepatitis C-associated mixed cryoglobulinemia. J Hepatol. 2005;42:334–340. doi: 10.1016/j.jhep.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Wörnle M, Schmid H, Banas B, Merkle M, Henger A, Roeder M, et al. Novel role of Toll-like receptor 3 in hepatitis C-associated glomerulonephritis. Am J Pathol. 2006;168:370–385. doi: 10.2353/ajpath.2006.050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wörnle M, Roeder M, Sauter M, Merkle M, Ribeiro A. Effect of dsRNA on mesangial cell synthesis of plasminogen activator inhibitor type 1 and tissue plasminogen activator. Nephron Exp Nephrol. 2009;113:57–65. doi: 10.1159/000228409. [DOI] [PubMed] [Google Scholar]
- Wörnle M, Roeder M, Sauter M, Ribeiro A. Role of matrix metalloproteinases in viral-associated glomerulonephritis. Nephrol Dial Transplant. 2009;24:1113–1121. doi: 10.1093/ndt/gfn627. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Banas B, Wörnle M, Berger T, Nelson PJ, Cohen CD, Kretzler M, et al. Roles of SLC/CCL21 and CCR7 in human kidney for mesangial proliferation, migration, apoptosis, and tissue homeostasis. J Immunol. 2002;168:4301–4307. doi: 10.4049/jimmunol.168.9.4301. [DOI] [PubMed] [Google Scholar]
- Matsukura S, Kokubu F, Kurokawa M, Kawaguchi M, Ieki K, Kuga H, et al. Role of RIG-I, MDA-5, and PKR on the expression of inflammatory chemokines induced by synthetic dsRNA in airway epithelial cells. Int Arch of Allergy Immunol. 2007;143 Suppl 1:80–83. doi: 10.1159/000101411. [DOI] [PubMed] [Google Scholar]
- Merkle M, Ribeiro A, Wörnle M. TLR3 dependent regulation of cytokines in human mesangial cells: a novel role for IP-10 and TNFa in hepatitis C associated glomerulonephritis. Am J Physiol Renal Physiol. 2011;301:57–69. doi: 10.1152/ajprenal.00083.2011. [DOI] [PubMed] [Google Scholar]
- Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009;105:223–230. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen ES. Endothelial signaling in paracellular and transcellular leukocyte transmigration. Front Biosci. 2009;14:2522–2545. doi: 10.2741/3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986;67:257–267. [PubMed] [Google Scholar]
- Nguyen TT, Shou I, Funabiki K, Shirato I, Kubota K, Tomino Y. Correlations among expression of glomerular intercellular adhesion molecule 1 (ICAM-1), levels of serum soluble ICAM-1, and renal histopathology in patients with IgA nephropathy. Am J Nephrol. 1999;19:495–499. doi: 10.1159/000013505. [DOI] [PubMed] [Google Scholar]
- Arrizabalaga P, Solé M, Abellana R, de las Cuevas X, Soler J, Pascual J, et al. Tubular and interstitial expression of ICAM-1 as a marker of renal injury in IgA nephropathy. Am J Nephrol. 2003;23:121–128. doi: 10.1159/000068920. [DOI] [PubMed] [Google Scholar]
- Ashizawa M, Miyazaki M. Detection of nuclear factor-kappaB in IgA nephropathy using Southwestern histochemistry. Am J Kidney Dis. 2003;42:76–86. doi: 10.1016/s0272-6386(03)00411-6. [DOI] [PubMed] [Google Scholar]
- Lefkowith JB. Leukocyte migration in immune complex glomerulonephritis: role of adhesion receptors. Kidney Int. 1997;51:1469–1475. doi: 10.1038/ki.1997.201. [DOI] [PubMed] [Google Scholar]
- Moon KC, Park SY, Kim HW, Hong HK, Lee HS. Expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in human crescentic glomerulonephritis. Histopathology. 2002;41:158–165. doi: 10.1046/j.1365-2559.2002.01446.x. [DOI] [PubMed] [Google Scholar]
- Arrizabalaga P, Solé M, Iglesias C, Escaramís G, Ascaso C. Renal expression of ICAM-1 and VCAM-1 in ANCA-associated glomerulonephritis—are there differences among serologic subgroups. Clin Nephrol. 2006;65:79–86. doi: 10.5414/cnp65079. [DOI] [PubMed] [Google Scholar]
- Park CW, Kim JH, Lee JH, Kim YS, Ahn HJ, Shin YS, et al. High glucose-induced intercellular adhesion molecule-1 (ICAM-1) expression through an osmotic effect in rat mesangial cells is PKC-NF-kappa B-dependent. Diabetologia. 2000;43:1544–1553. doi: 10.1007/s001250051567. [DOI] [PubMed] [Google Scholar]
- Okada S, Shikata K, Matsuda M, Ogawa D, Usui H, Kido Y, et al. Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes. 2003;52:2586–2593. doi: 10.2337/diabetes.52.10.2586. [DOI] [PubMed] [Google Scholar]
- Ma J, Möllsten A, Prázny M, Falhammar H, Brismar K, Dahlquist G, et al. 2006. Genetic influences of the intercellular adhesion molecule 1 (ICAM-1) gene polymorphisms in development of Type 1 diabetes and diabetic nephropathy. Diabet Med. 2005;23:1093–1099. doi: 10.1111/j.1464-5491.2006.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook ED, Penumalee S, Gavini B, Filippova K. Hepatitis C is a predictor of poorer renal survival in diabetic patients. Diabetes Care. 2005;28:2187–2191. doi: 10.2337/diacare.28.9.2187. [DOI] [PubMed] [Google Scholar]
- Wiggins JE, Patel SR, Shedden KA, Goyal M, Wharram BL, Martini S, et al. NFkappaB promotes inflammation, coagulation, and fibrosis in the aging glomerulus. J Am Soc Nephrol. 2010;21:587–597. doi: 10.1681/ASN.2009060663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Zamanian-Daryoush M, Nie H, Silva AM, Williams BR, Li X. Poly(I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NFkappa B and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. J Biol Chem. 2003;278:16713–16719. doi: 10.1074/jbc.M300562200. [DOI] [PubMed] [Google Scholar]
- Luft FC, Mervaala E, Müller DN, Gross V, Schmidt F, Park JK, et al. Hypertension-induced end-organ damage: A new transgenic approach to an old problem. Hypertension. 1999;33:212–218. doi: 10.1161/01.hyp.33.1.212. [DOI] [PubMed] [Google Scholar]
- Riser BL, Varani J, Cortes P, Yee J, Dame M, Sharba AK. Cyclic stretching of mesangial cells upregulates intercellular adhesion molecule-1 and leukocyte adherence: a possible new mechanism for glomerulosclerosis. Am J Pathol. 2001;158:11–17. doi: 10.1016/S0002-9440(10)63938-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeke HH, Emmendörffer A, Uciechowski P, von der Ohe J, Schwinzer B, Resch K. Activation of autoreactive T-lymphocytes by cultured syngeneic glomerular mesangial cells. Kidney Int. 1994;45:763–774. doi: 10.1038/ki.1994.101. [DOI] [PubMed] [Google Scholar]
- Uciechowski P, Schwarz M, Gessner JE, Schmidt RE, Resch K, Radeke HH. IFN-gamma induces the high-affinity Fc receptor I for IgG (CD64) on human glomerular mesangial cells. Eur J Immunol. 1998;28:2928–2935. doi: 10.1002/(SICI)1521-4141(199809)28:09<2928::AID-IMMU2928>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]