Abstract
Leptin is one of the most important hormones secreted by adipocytes, with a variety of physiological roles related to the control of metabolism and energy homeostasis. Since its discovery in 1994, leptin has attracted increasing interest in the scientific community for its pleiotropic actions. One of these functions is the relationship between nutritional status and immune competence. It structurally resembles proinflammatory cytokines, such as IL-6 and IL-12. The cytokine-like structural characteristic of leptin is implicative of its function in regulating immune responses. The role of leptin in regulating immune responses has been assessed in vitro as well as in clinical studies. It has been shown that disease conditions of reduced leptin production are associated with increased infection susceptibility. Conversely, immune-mediated disorders, such as autoimmune diseases, are associated with the increased secretion of leptin and the production of proinflammatory pathogenic cytokines. In this paper, we review the most recent advances of the role of leptin in immune-rheumatological diseases, and we discuss whether strategies aimed at modifying leptin levels could represent innovative and therapeutic tools for autoimmune disorders.
Keywords: adipokines, autoimmune diseases, leptin, rheumatic diseases
Introduction
The existence of a factor secreted by white adipose tissue (WAT), which acts to control feeding, weight and WAT mass, was first proposed by Kennedy1 and is supported by monogenic mutations resulting in obesity.2, 3, 4 WAT is composed of adipocytes filled mainly with triacylglycerol and embedded in loose connective tissue containing adipocyte precursors, fibroblasts, immune and other cells. Obesity, the condition that originally motivated the research on WAT, is characterized by low-grade systemic inflammation. It is thought that excess WAT can contribute to the maintenance of obesity through inflammation-inducing lipotoxicity by secreting factors that stimulate the synthesis of inflammatory agents in other organs and by secreting inflammatory agents itself.5 The current view of WAT states that it is an active contributor to body homeostasis by sending out and responding to signals that modulate appetite, energy expenditure, insulin sensitivity, the endocrine and reproductive systems, bone metabolism, inflammation and immunity.6 Several findings have converged to indicate that adipocytes share certain properties with immune cells, such as complement activation7 and proinflammatory cytokine production.8 Fat cell precursors also share features with macrophages. Numerous genes that code for transcription factors, cytokines, inflammatory signaling molecules, and fatty acid transporters are essential for adipocyte biology and are also expressed and functional in macrophages.9 Adipose tissue secrets a variety of factors: the term ‘adipokine' is generally given to any protein that can be synthesized and secreted by adipocytes. Among these factors, only leptin and adiponectin (and possibly resistin, adipsin and visfatin) are primarily produced by adipocytes, and they can therefore be properly classified as adipokines.10 In particular, leptin is considered the prototypical adipokine.
This review includes a critical discussion of what is known and what we hope will soon be known regarding the role of leptin in autoimmune diseases. In this regard, we will first discuss the leptin characteristic in general and its role in regulating the neuroendocrine function in particular. We will then review what is known about the effects of leptin on inflammation. Third, we will illustrate the interaction between leptin and innate and adaptive immunity. The emerging evidence on leptin in immune-rheumatological diseases will represent the focus of the present review. Finally, we will discuss the controversial issues of the development of leptin receptor mutants with antagonistic properties or various antibodies against leptin as therapeutic options for autoimmune disorders or other diseases.
Leptin
Leptin, from the Greek root leptos, meaning ‘thin', is a 16-kD, non-glycosylated peptide hormone encoded by the gene obese (ob), the murine homolog of the human gene LEP.11 The ob gene was cloned in 1994, and leptin was identified in 1995 as the product of the ob gene and a hormonal signal that regulates energy balance.12, 13, 14 The ob gene, located within the 7q31.3 locus, was identified by positional cloning as a 4.5-kb RNA that was expressed in adipose tissue. This RNA encoded a predicted 167-amino acid polypeptide with a signal sequence.11 Mice and humans homozygous for a leptin gene mutation (an extremely rare genetic disorder) develop increased appetite, obesity, insulin resistance, hypothalamic hypogonadism, a deficit of the thyroid and growth hormone axes, and immunosuppression.11, 15, 16 Leptin's three-dimensional structure, consisting of four interconnected antiparallel α-helices, is highly similar to members of the long-chain helical cytokines, such as interleukin-6 (IL-6), IL-11, IL-12, and granulocyte colony-stimulating factor.17, 18
As an endocrine hormone, leptin is synthesized mainly by adipose tissue and, more particularly, by differentiated mature adipocytes. Circulating levels and adipose tissue mRNA expression of leptin are proportionate to the body mass index (BMI) and the body fat mass.9, 19 Under certain circumstances, leptin is produced at low levels by tissues, such as the intestine, placenta, mammary and gastric fundic epithelium, skeletal muscle and brain.18, 20
Like the majority of neurohormones, leptin levels exhibit important circadian rhythms, peaking at night in humans, and its pulsatility characteristics are similar in lean and obese subjects with the only exception being pulse amplitude, which is higher in obese subjects.21, 22
Leptin synthesis is mainly regulated by food intake and different hormones, but it also depends on energy status; crucial factors in regulating serum leptin concentrations seem to be short-term caloric intake and the amount of energy stored in adipocytes.5 There is also a strong relationship between leptin patterns and meal timing. A shift in meal timing led to a shift in the plasma leptin peak in both humans and rodents.23, 24 Moreover, leptin levels are reduced during starvation and malnutrition.25 Leptin expression in adipocytes is induced by insulin, melanyl-CoA, adenosine triphosphate, glucosamine and short-chain fatty acids, but it is inhibited by cyclic adenosine 5'-monophosphate and long-chain fatty acid.18, 26
Furthermore, the expression of leptin can be directly upregulated by sex hormones and is inhibited by testosterone and increased by ovarian sex steroids.5 As a result of the effect of sex hormones, leptin levels are higher in women than in men, even when adjusted for BMI, which may be relevant to the influence of sex on the development or frequency of certain diseases.5, 27
Leptin expression is also regulated by a wide range of inflammation mediators (34). Through the mediation of these agents, leptin is increased by acute infection and sepsis, consistent with the findings that leptin mRNA expression is stimulated by lipopolysaccharide and cytokines, such as tumor necrosis factor (TNF)-α, IL-6 and IL-1β, during acute inflammatory reactions.18, 28 However, some studies have not found increased levels of leptin in acute inflammatory conditions, such as HIV infection in humans (Table 1).29
Table 1. Crucial factors in regulating serum leptin concentrations.
| Increased leptin production | Decreased leptin production |
|---|---|
| Obesity | Prolonged weight loss |
| Hyper-alimentation | Acute fasting |
| Insulin resistance | Catecholamines (β3 receptors) |
| Glucocorticoids | Androgens |
| Estrogens | Free fatty acids |
| Cytokines (TNF-α, IL-6 and IL-1) | Growth hormone |
| Infections | PPAR-γ antagonists (thiazoledinediones) |
| Chronic inflammation |
Abbreviations: IL, interleukin; PPAR, peroxisome proliferator-activated receptor; TNF, tumor necrosis factor.
Leptin exerts its biological actions by binding to its receptors. The leptin receptor (OB-R), a product of the diabetes (db) gene, belongs to the class I cytokine receptor superfamily, which includes receptors for IL-6, granulocyte colony-stimulating factor and gp130. It was identified biochemically and was subsequently shown to be broadly expressed.30, 31
Six alternatively spliced isoforms of OB-R were identified, which contain identical extracellular binding domains but differ by the length of their cytoplasmic domains: a long isoform (OB-Rb), four short isoforms (OB-Ra, OB-Rc, OB-Rd and OB-Rf), and a soluble isoform (OB-Re).24 OB-Rb is the only receptor isoform that expresses all the protein motifs required for cytokine receptor signaling. More important, although the other receptor isoforms were expressed broadly, OB-Rb is highly enriched in the hypothalamus in nuclei that modify body weight when altered.31 It is now known that the leptin receptor also signals at other central nervous system sites outside the hypothalamus, and its expression at these sites contributes to the leptin's effects.31
In addition to regulation of energy balance, leptin is a crucial factor for normal development of the reproductive system. Congenital leptin deficiency has been associated with hypogonadotropic hypogonadism and infertility, which was corrected by exogenous leptin administration. However, these effects of leptin depend upon functional hypothalamic neurons of the arcuate nucleus.24, 32
Different splice variants of the leptin receptors may differ in relation to signaling pathways and sites of expression, as with OB-Rb.33
Such ubiquitous expression of the leptin receptors in humans and widespread binding of leptin in various organs indicates its role in a constellation of vital processes, including growth, metabolic control, insulin sensitivity regulation and reproduction. These aspects of leptin actions have been briefly illustrated in Figure 1 and have been extensively reviewed elsewhere.9, 4, 33, 34
Figure 1.
Leptin functions in humans. Leptin, mainly secreted by white adipose tissue, travels through the blood, crossing the hematoencephalic barrier. Leptin binds the OB-Rb isoform on neurons in the hypothalamus, activating CART interneurons and inhibiting NPY- and AgRP-interneurons to regulate appetite and energy expenditure. Furthermore, the adipokine modulates sympathetic tone and activity of the hypothalamus–hypophisis–thyroid axis and hypothalamus–hypophisis–adrenal gland axis. Leptin action on the cardiovascular system is complex. Leptin promotes endothelial dysfunction (by immune cell recruitment and interference with endothelium-mediated vasodilatation), plaque formation (by oxidative stress promotion and foam cell formation), plaque destabilization and consequent thrombosis (metalloproteases production and abnormal vascular repair). Leptin exerts a pivotal role in modulating energy expenditure. The adipokine acts on smooth muscles, pancreas and liver by increasing glucose and free fatty acid uptake and promoting fatty acid β-oxidation. In hepatocytes, leptin inhibits lipogenesis and modulates gluconeogenesis. Thereby, leptin is involved in the control of the insulin-resistance/insulin-sensitivity imbalance. Leptin regulation of food intake also exerts its actions on the gastrointestinal tract. AgRP, agouti-related protein; CART, cocaine- and amphetamine-regulated transcript peptide; NPY, neuropeptide-Y.
Although it is highly expressed in the brain, the functional long isoform of the leptin receptor OB-Rb is expressed in both human and murine hematopoietic stem cells and in human B-cell progenitors.18, 35 The capacity of bone marrow stromal cells to express leptin provides strong evidence for leptin in development of hematopoietic stem cells. Modulation of the immune system by leptin is exerted at the development, proliferation, antiapoptotic, maturation and activation levels.24, 36 In fact, leptin receptors can also be found in different tissues and immune cell types, including various subpopulations of T and B lymphocytes, dendritic cells, monocytes, neutrophils, macrophages and natural killer (NK) cells.18, 37, 38, 39, 40, 41 The cytokine-like structural characteristic of leptin is implicative of its function in regulating immune responses. This concept is discussed in detail in the present review.
Leptin, inflammation and immune response
Leptin's role in regulating immunity has been fueled by different observations. The primary amino acid sequence of leptin indicated that it could belong to the long-chain helical cytokine family,36, 42 with members such as IL-2 and IL-12. The cytokine-like structural characteristic of leptin is implicative of its function in regulating immune response. In fact, a main aspect of the effects of leptin on the immune system is its action as a proinflammatory cytokine. The full length functional leptin receptor isoform OB-Rb shows sequence homology to members of class I cytokine receptor (gp130) superfamily,30, 36 which includes the receptor for IL-6, leucocyte inhibitory factor and granulocyte colony-stimulating factor. This receptor lacks intrinsic tyrosine kinase activity, is involved in several downstream signal transduction pathways, and has been identified in immune cells of both animals and humans.43, 44 OB-Rb has been shown to have the signaling capabilities of IL-6-type cytokine receptors, activating janus kinases and signal transducers and activators of transcription (STAT), phosphatidylinositol 3-kinase and the mitogen-activated protein kinase (MAPK) signaling pathways.36, 41, 45 Like other cytokine receptor family members, OB-Rb is internalized upon ligand binding via clathrin-coated vesicles into endosomes.46 The extended intracellular domain in the distal part of OB-Rb is required for the induction of STAT signaling.47 Leptin has been shown to activate various isoforms of STATs, including STAT1, STAT3, STAT5 and STAT6, in a variety of cell types.18, 48 Among the various STAT proteins activated by OB-Rb, STAT3 has been shown to mediate the leptin signal in activated macrophages and in promoting the survival and activation of lymphocytes and peripheral blood mononuclear cells (PBMCs).18, 45, 49, 50 In NK cells, leptin is involved in all processes of cell development, differentiation, proliferation, activation and cytotoxicity, and this effect is mediated by STAT3 activation.51 Based on the complex activation network of the STAT proteins, further analyses of their regulation by leptin signaling in immune cells will merit a better understanding of leptin-mediated immune modulation.18 The MAPK, the insulin receptor substrate 1 and the phosphatidylinositol 3-kinase pathways are also important to mediate leptin's action on immune T cells. In neutrophils, leptin activates chemotaxis via the p38 MAPK pathway.52 Moreover, in PBMCs, the MAPK pathway seems to mediate antiapoptotic effects.53, 54 The phosphatidylinositol 3-kinase pathway is the upstream regulator for a number of effectors, including the anti-apoptotic transcription factor NF-κB. NF-κB plays a key role in mediating different signaling systems to regulate the immune response. Leptin activates NF-κB in dendritic cells, whereas a leptin-signaling deficiency leads to enhanced levels of the inhibitor protein of NF-κB (Figure 2).37
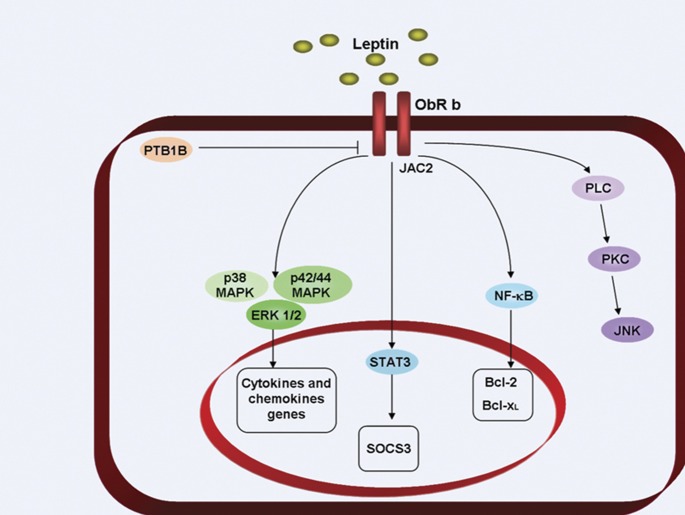
Figure 2.
Leptin signal transduction. The long receptor isoform of leptin is connected to the JAK-STAT intracellular signaling system. As a consequence of letpin binding to the leptin receptor, the following steps occur: (i) JAK2 is activated with a consequent autophosphorylation and phosphorylation of the tyrosine residues on the intracellular domains of the receptor. STAT1, STAT2 and STAT5 bind tyrosine residues. STAT3 proteins form dimers and translocate to the nucleus and modulate c-fos, c-jun, erg1, SOCS3 and AP1 gene expression; (ii) Src homology domains of receptor (SHP2) activates MAPK pathways, including p38, p42/44 and ERK1/2. These pathways control cytokine and chemokine genes; and (iii) leptin activates PLC, leading to PKC activation, followed by JNK stimulation. → refers to activation; ⊣ refers to inhibition. Bcl, B-cell lymphoma; ERK, extracellular signal-regulated kinase; JAK, c-Jun N-terminal kinase-associated kinase; JNK, c-Jun N-terminal kinases; MAPK, mitogen-activated protein kinase; NF-kB, nuclear factor-kappa B; ObR, Ob receptor; PKC, protein kinase C; PLC, phospholipase C; SOCS3, suppressor of cytokine signaling-3; STAT, signal transducer and activator of transcription.
The above-described role of leptin in the function of the immune system could be relevant in both cellular and humoral immunity.
The main effects of leptin in innate immunity involve the activation of proliferation and the phagocytosis of monocytes/macrophages, the chemotaxis of neutrophils, the release of oxygen radicals by these cells, and the activation of NK cells.25 In macrophages, leptin also upregulates the secretion of proinflammatory cytokines, such as TNF-α, IL-6 and IL-12.
The effect of leptin on adaptive immunity is also well studied. Leptin markedly stimulates the proliferation of naive T cells and the secretion of IL-2 by these cells. Studies in humans have further delineated the role of leptin in the activation of lymphocytes. Leptin alone is unable to induce the proliferation and activation of mature human peripheral blood lymphocytes unless it is co-administered with other nonspecific immunostimulants, in which case leptin results in the induction of early (CD69) and late activation markers (CD25 and CD71) in both CD4 and CD8 lymphocytes.16, 44, 55
Mice with a mutation in the leptin receptor (db/db mice) gene have a marked reduction in the size and cellularity of the thymus and exhibit defective T cell-mediated immunity. In experimental animals, inflammatory stimuli acutely induces leptin mRNA and increases serum leptin levels. In this setting, leptin deficiency is associated with reduced inflammation in animal models of autoimmune diseases but also with increased susceptibility to bacterial and virus infections.56
Similarly, humans with a congenital leptin deficiency have a much higher incidence of infection-related death during childhood. Leptin deficiency is also associated with increased susceptibility to the toxicity of proinflammatory stimuli, such as endotoxin and TNF-α.6 Furthermore, starvation and malnutrition, two conditions characterized by low leptin levels, are also associated with alterations in immune responses and thymic atrophy, which can be reversed by leptin administration.57 Similarly, low circulating CD4+ cells, impaired T-cell proliferation and impaired release of T-cell cytokines exhibited by patients with congenital leptin deficiency are ameliorated by the administration of recombinant human leptin.5
Further effects of leptin in immunity involves the suppression of CD4+CD25+ regulatory T-cell (Treg) proliferation.5, 58 It has been recently shown that freshly isolated Tregs produce leptin. Moreover, the leptin receptor is highly expressed on the cell surface of Tregs. In vitro neutralization with a leptin monoclonal antibody during stimulation with anti-CD3 and anti-CD28 antibodies resulted in Treg proliferation, which was IL-2 dependent.36, 58 These findings indicate that leptin signaling is clearly involved in maintaining the anergic state of human Tregs. In agreement with this, both leptin-deficient (ob/ob mice) and leptin receptor-deficient mice (db/db mice) were associated with a marked increase in the number of Tregs (Figure 3).53, 59
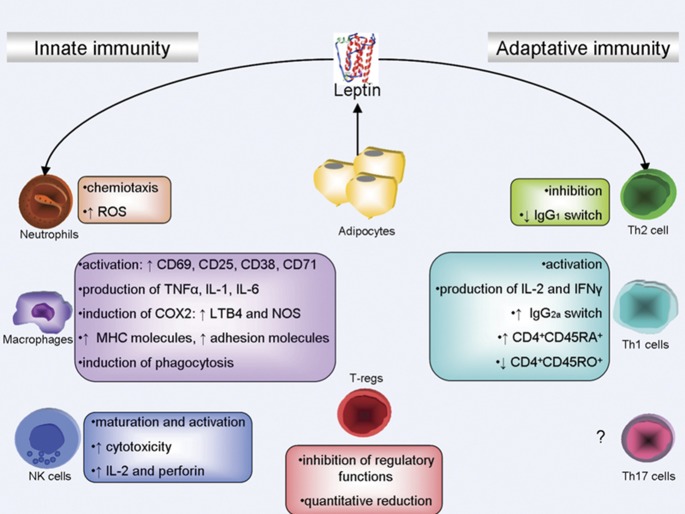
Figure 3.
Leptin and the immune system. Leptin affects both innate and adaptive immunity. Moreover, leptin promotes immune cell development through sequential steps of myelopoiesis and lymphopoiesis. In innate immunity, leptin modulates the activity and function of neutrophils by increasing chemotaxis and the secretion of oxygen radicals. Leptin promotes monocyte/macrophage activation by inducing CD69, CD25, CD38, CD71 and adhesion molecules expression. Moreover, leptin induces the production of chemokines and proinflammatory cytokines, such as TNF-α, IL-1 and IL-6. Macrophages stimulated by leptin release NO and LTB4. The adipokine promotes the maturation, activation and cytotoxic capacity of NK cells. In adaptative immunity, leptin affects the generation, maturation and survival of thymic T cells by reducing their rate of apoptosis. In particular, leptin promotes the lymphocyte phenotype switch to CD4+CD45RA+. Moreover, leptin induces IL-2 and INF-γ production. Recent data suggest the involvement of leptin in the quantitative and qualitative inhibition of naturally occurring T regulatory cells. → refers to activation; ⊣ refers to inhibition. CD, cluster of differentiation; IFN, interferon; Ig, immunoglobulins; IL, interleukin; LT, leukotriene; NK, natural killer; NOS, nitric oxide synthase; ROS, reactive oxygen species; Th, T helper; TNF, tumor necrosis factor.
Although leptin is well known for its regulatory effects on immune cells, its expression and release is reciprocally under the control of different inflammatory stimuli. It has been shown that acute inflammation and proinflammatory cytokines, such as TNF-α, IL-1, IL-6 and leucocyte inhibitory factor, positively regulate leptin expression in adipose tissue and circulating leptin levels,24, 60 whereas long-term exposure to IL-1 or TNF-α negatively regulates leptin levels.24, 61
Leptin in immuno-rheumatological diseases
Autoimmunity results from a failure or breakdown of the mechanisms responsible for maintaining self-tolerance in B cells, T cells, or both cell types. Self-tolerance may be induced in generative lymphoid organs as a consequence of immature self-reactive lymphocytes recognizing self-antigens, called central tolerance, or in peripheral sites as a result of mature self-reactive lymphocytes encountering self-antigens during particular conditions, called peripheral tolerance.62
As reported previously, leptin plays an important role in the control of immune balance. This role is apparent in the autoimmunity-resistance phenotypes of mice deficient in either the leptin gene (ob/ob) or the leptin receptor (db/db).
In human autoimmune settings, the role of leptin seems to be more complicated. However, numerous relevant aspects of the biological activity of leptin in the immune system have been recently revealed by different authors, providing new insights about the role of leptin in autoimmunity (Table 2).
Table 2. Autoimmune diseases and leptin.
| Systemic lupuserythematosus | Leptin | Garcia-Gonzales A. Rheumatolm Int (2002) | Plasma |
| Sada KE. J Rheum (2006) | Plasma | ||
| Adiponectin | Sada KE. J Rheum (2006) | Plasma | |
| Rovin BH. Kidney Int (2005) | Urine | ||
| Rheumatoid arthritis | Leptin | Toussirout E. Rheumatology (2005) | Plasma |
| Otero M. Ann Rheum Dis (2006) | Plasma | ||
| Bokarewa M et al. Ann Rheum Dis (2003) | Synovium | ||
| Shafller. JAMA (2003) | Synovium | ||
| Adiponectin | Otero M. Ann Rheum Dis (2006) | Plasma | |
| Shafller et al. JAMA (2003) | Synovium | ||
| Senolt L et al. Cytokine (2006) | Synovium | ||
| Visfatin | Otero M. Ann Rheum Dis (2006) | Plasma | |
| Resistin | Senolt L et al. Ann Rheum Dis (2006) | Synovium | |
| Multiple sclerosis | Leptin | Matarrese G. PLOS (2006) | Plasma+CSF |
| IBD | Leptin | Tuzun A. J Gastroenterol Hepatol (2004) | Plasma |
| Barbier M. Gastroenterol Clin Biol (2003) | Omentum | ||
| Asthma | Leptin | Shore SA. J Allergy Clin Immunol (2005) | Plasma |
| Gurkan F. Ann Allergy Athsma Immunol (2005) | Plasma |
Leptin and systemic lupus erythematosus (SLE)
SLE is a prototype of autoimmune diseases in humans, characterized by a multisystem disorder, widespread inflammation and immune complex deposition in key target organs.
Systemic inflammation has been shown to modulate adipocyte metabolism and, consequently, leptin levels. On the other hand, there is a strong relationship between leptin and the immune system. Thus, some authors studied leptin levels in patients affected by SLE. Harle et al. showed that leptin might provide an important link between chronic inflammation and the hypoandrogenic state in SLE patients.63
Higher leptin concentrations in SLE patients, with respect to controls, have been reported by different authors,64, 65, 66 suggesting a relationship between leptin and lupus disease-correlated factors. The same data have been recently reported in Korean SLE patients. Moreover, it has been reported that although leptin was strongly associated with obesity in SLE, the difference in leptin concentrations between patients and controls was independent of sex and BMI, suggesting that additional factors drive leptin production in lupus.65
In a recent study, our group reported data from 50 SLE patients that were stratified according to fertile or menopausal status. Our results showed a hyperexpression of leptin notably among fertile SLE patients. In SLE, leptin correlated with metabolic syndrome and different cardiovascular risk factors. Moreover, we found a positive correlation between leptin levels and SLE-specific disease activity indexes.64
Some authors did not confirm higher levels of leptin in SLE patients.67, 68 The differences between the reported data may be due to small patient groups, different disease severity and steroids and immunosuppressive treatments. Further studies on homogeneous and numerous groups of patients are required to clarify the role of leptin in SLE immune imbalance.
Leptin, rheumatoid arthritis (RA) and different models of arthritis
Experimental antigen-induced arthritis is a model of immune-mediated joint inflammation induced by the administration of methylated bovine serum albumin into the knees of immunized mice. Antigen-induced arthritis is less severe in leptin deficient ob/ob mice than in wild-type mice.69 The milder form of antigen-induced arthritis seen in ob/ob and db/db mice, as compared with their controls, was accompanied by decreased synovial levels of IL-1 and TNF-α and a reduced proliferative response to antigen by lymph node cells in vitro.57
RA is a chronic systemic inflammatory disease of the synovial tissue. Increased serum levels of several inflammatory cytokines have been found to correlate with disease activity and progression.
Significant evidence demonstrate that circulating leptin levels are high in patients with RA.70, 71 In these subjects, it has been reported that acute fasting induces an improvement of different clinical and biological measures of disease activity associated with a fall in circulating leptin, which is related to CD4 lymphocyte hyporeactivity and increased IL-4 secretion.72 However, the same investigators showed that after a 7-day ketogenic diet in patients with RA, there were no significant changes in any clinical or biological measurements of disease activity, despite a significant increase in serum leptin concentrations.57, 72
Serum levels of leptin have generally been reported to be higher in patients with RA than in control subjects.70, 71, 73 Bokarewa et al. also observed that circulating plasma concentrations of leptin were significantly higher than matched synovial fluid sample leptin levels. Local consumption of leptin in the joint cavity was associated with nonerosive joint disease, suggesting that leptin has a protective role against the destructive course of RA.71
However, the information regarding the relationship between leptin, inflammation and disease activity is conflicting,74 and little is known about their roles in radiographic joint damage.
In one study,75 serum leptin concentrations were higher in patients with erosive RA. Another study has shown that concentrations of leptin in synovial fluid were lower than those found in the plasma of patients with nonerosive RA, but not in those with erosive disease. This was thought to indicate that the consumption of leptin in the joints was associated with protection against erosions, and therefore that leptin protected against bone erosion.71 Recently, leptin concentrations were described to be higher in patients with RA, and the association between increased concentrations of leptin and RA was not affected by a statistical adjustment for BMI. The authors found that higher serum leptin concentrations were associated with reduced joint damage, and this relationship was attenuated by an adjustment for BMI but was enhanced by an adjustment for inflammation.73 This finding suggests that the beneficial effects of leptin and BMI on radiographic damage may be related.
Leptin is a relevant regulator of bone remodeling, a homeostatic function involved in maintaining bone mass consistency. Indeed, after binding to its receptors or hyphothalamic neurons, leptin regulates osteoblasts, bone formation and osteoclast differentiation.76
Nitric oxide has well-documented proinflammatory effects on joint cartilage, triggering the loss of the chondrocyte phenotype, chondrocyte apoptosis and the activation of metalloproteinases. In cultured chondrocytes, nitric oxide synthase is activated by leptin plus interferon (INF)-γ and by the combination of leptin plus IL-1 (Figure 4).5
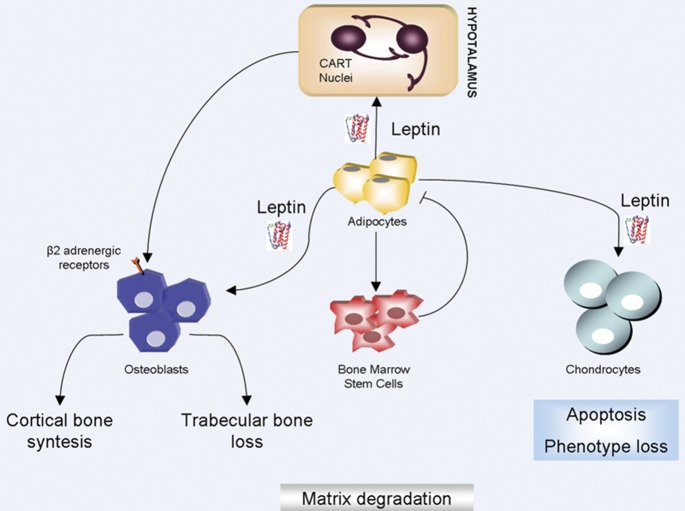
Figure 4.
Leptin and bone homeostasis. Leptin acts as a regulator of bone and cartilage homeostasis. Leptin acts on osteoblasts via two pathways: an indirect pathway and a direct pathway. The first pathway involves sympathetic nervous system activation. In the second pathway, leptin acts directly on osteoblasts. The combination of these actions promotes cortical bone synthesis and trabecular bone loss. Moreover, leptin induces apoptosis and phenotype loss of condrocytes. → refers to activation; ⊣ refers to inhibition. CART, cocaine- and amphetamine-regulated transcript peptide.
Bone is the target of many inflammatory rheumatic diseases, as in RA. The effect of inflammation on bone is associated with an increased risk of fractures and deformities, which may be the result of a decreased quality of bone.
Recently, preliminary data presented by our group showed a hyperexpression of leptin in patients with different rheumatic diseases, such as RA, psoriatic arthritis and ankylosing spondylitis (AS). We demonstrated that leptin levels were higher in all patients with respect to normal subjects. Moreover, we evaluated body mass density. Patients with rheumatic diseases presented a lower BMD at hip and spine lumbar levels. In RA and AS patients, a positive correlation between leptin values and hip T scores was shown. However, in patients with psoriatic arthritis, we observed a positive correlation between leptin values and lumbar spine T scores. In each studied disease, we observed a positive correlation among leptin values, inflammatory markers and disease activity indexes.77
To our knowledge, there is little data regarding the role of leptin in other human models of arthritis in the literature. In 2009, it was demonstrated that severely obese patients affected by psoriatic arthritis showed higher leptin serum levels.78 Different data have been recently published on AS. Park et al. demonstrated that leptin levels and IL-6 and TNF-α mRNA expression of PMBCs from patients with AS were significantly higher than in those from controls. Moreover, the authors showed that the stimulation of PBMCs by exogenous leptin from AS patients significantly increased the production of IL-6 and TNF-α in a dose-dependent fashion, and these increases were observed as compared with controls.79 On the contrary, others showed that AS patients had lower leptin levels compared with controls, even after an adjustment for fat mass.80 All reported findings question the suggested key role of leptin in inflammation and in regulating bone metabolism in different models of arthritis.
Leptin and multiple sclerosis (MS)
Mice in which experimental autoimmune encephalomyelitis (EAE) has been induced by inoculation of appropriate self-antigens constitute an animal model of human MS. Ob/ob mice do not develop EAE in response to EAE-inducing antigens, but this resistance is abolished by administration of leptin.5, 81 The onset of EAE in wild-type mice is preceded by an increase in circulating leptin and is delayed by acute starvation.5, 82 During the active phase of EAE, leptin is secreted by both macrophages and T cells that have infiltrated the central nervous system, and leptin secretion by activated T cells appears to constitute an autocrine loop, sustaining their proliferation.5, 44, 82
MS is a chronic, immune-mediated disorder of the central nervous system. The disease is characterized by autoreactive T cells that traffic to the brain and spinal cord and injure the myelin sheaths of the central nervous system, resulting in chronic or relapsing-remitting paralysis.55
In human MS, leptin levels are high in serum and cerebrospinal fluid of patients never treated for their disease (MS) and are positively correlated with the secretion of INF-γ and inversely correlated with Tregs.53, 55
Of note, the increase in leptin in the cerebrospinal fluid is higher than in the serum, suggesting the possible secondary in situ synthesis of leptin in the central nervous system in humans or an increase in transport across the blood–brain barrier following enhanced systemic production.55
Recent reports have shown an increased secretion of serum leptin before relapses in MS patients following treatment with IFN-β and a capacity of leptin to enhance the in vitro secretion of TNF-α, IL-6 and IL-10 from PBMCs in MS patients during the acute phase of the disease, but not in patients with stable disease.83, 84
In view of all these considerations, Matarese et al.55 suggested that leptin could be one of the many proinflammatory factors that act in concert to promote pathogenic T-cell responses targeting neuroantigens in MS.
Leptin, connective tissue diseases and organ-specific autoimmune diseases
Anecdotal data support a role for leptin in other connective tissue and immune-rheumatological diseases.
To our knowledge, no data have been reported on Sjogren's syndrome. In a preliminary study, our group demonstrated that 42 patients affected by Sjogren's syndrome display higher levels of leptin compared with control subjects. In these patients, leptin was related to metabolic syndrome factors and to a number of cardiovascular risk factors.85
In another study, serum leptin levels were determined in 31 women with systemic sclerosis. Patients were divided into premenopausal and postmenopausal subgroups. Decreased serum leptin levels were found in the patients with systemic sclerosis. The premenopausal patients and controls had higher serum leptin levels compared with those in the postmenopausal subgroups.86
There are two recent studies regarding the effects of glucocorticoids on leptin concentrations in patients with polymyalgia rheumatica. In untreated patients, leptin levels did not differ from the levels of the controls.87 However, treatment with steroids significantly increased leptin concentration.
Leptin secretion by T cells seems to have, at most, a marginal role in experimentally induced hepatitis, in which no differences have been found in the ability of T cells from ob/ob and wild-type mice to induce inflammation.6 Other authors observed protection of ob/ob mice from autoimmunity in experimentally induced hepatitis.88 The activation of T cells and macrophages is one of the initial events during viral or autoimmune hepatitis. Activated T cells are directly cytotoxic toward hepatocytes and release proinflammatory cytokines, which mediate hepatocyte damage. TNF-α is a crucial cytokine in the acute disease process, and the neutralization of this cytokine reduces liver damage. Siegmund et al.88 showed that ob/ob mice were protected from experimentally induced hepatitis. TNF-α and IFN-γ levels were not elevated in these mice following stimulation, suggesting that their resistance was associated with reduced levels of those proinflammatory cytokines and low percentages of intrahepatic NK cells.55, 88
In an animal model of intestinal autoimmune inflammation, it was demonstrated that T cells from leptin resistant db/db mice showed a reduced capacity to induce colitis upon passive transfer in T cell-deficient mice. A histological observation of the colon revealed marked inflammation in mice injected with wild-type cells, whereas no inflammation was observed in mice receiving db/db cells.44, 89
Serum leptin levels are not altered in human inflammatory bowel diseases, although they might increase during the acute stages of ulcerative colitis. Furthermore, leptin mRNA is upregulated in mesenteric adipose tissue of these patients.6
The role of leptin has also been investigated in spontaneous models of autoimmunity, such as nonobese diabetic (NOD) mice. The NOD mouse strains have increased basal serum leptin before disease onset.44 Leptin administration augments inflammatory infiltrate, increases INF-γ production by peripheral T cells, and speeds up the destruction of pancreatic β cells.90
A recent study has shown that a spontaneous mutation of the leptin receptor in type 1 diabetes-prone NOD mice suppresses type 1 diabetes development in the NOD mice by inhibiting activation of T cells, demonstrating the important role of leptin signaling in the disease pathogenesis.91
The influence of leptin deficiency has been examined in immune-mediated renal disease and accelerated nephrotoxic nephritis in the ob/ob mice, which were found to be strongly protected from the disease.92 In this immune complex-mediated inflammatory disease, the authors observed renal protection of ob/ob mice associated with reduced glomerular crescent formation, reduced macrophage infiltration and glomerular thrombosis. These protective effects were associated with concomitant defects of both adaptive and innate immune responses.55, 92
Future prospects
Exogenous leptin administration in children with a congenital leptin deficiency normalized the absolute numbers of Tregs, enhanced CD4+ counts, restored proliferative responses, and promoted the cytokine release profile from lymphocytes.15 Furthermore, it has been demonstrated that the exogenous administration of recombinant methionyl human leptin in subjects with acquired leptin deficiency improves their circulating cytokine levels.44
However, there is a huge amount of data on the promotion of inflammation by high circulating leptin levels. As previously described, the leptin receptor is highly expressed on the cell surface of Tregs. In vitro neutralization with leptin monoclonal antibody during stimulation with anti-CD3 and anti-CD28 antibodies, resulted in Treg proliferation, which was IL-2 dependent.
Together, these results suggest that leptin may be considered as a therapeutic target in some clinical situations, such as proinflammatory states or autoimmune diseases.
Moreover, recently reported clinical studies on autoimmune disease patients demonstrate that high serum leptin levels may serve as a diagnostic marker for clinical application.
Strong evidence suggests that high circulating leptin levels promote inflammation. Thus, it might be plausible to control bioavailable, circulating leptin by means of a soluble, high-affinity leptin-binding molecule, similar to the strategy used with soluble TNF-α receptors in the treatment of arthritis and inflammatory bowel diseases. In addition, another way to target leptin activity might consist of blocking the leptin receptor with humanized monoclonal antibodies or mutant leptin analogues that are able to bind to the receptor without activating it.76
In addition, recombinant methionyl human leptin replacement could prove to be a new and potentially useful therapy to be added to our therapeutic armamentarium for the treatment of disease states of absolute or relative leptin deficiency and to restore immune function.
Moreover, as suggested by others, it will be interesting to determine whether leptin might serve as a natural adjuvant in vaccinations. Used in a localized rather than systemic manner, leptin might simultaneously stimulate T helper 1 responses while down-modulating regulatory T cells. It might also be possible in the future to expand or contract antigen-specific subsets of regulatory T cells through the use of bivalent leptin receptor agonists or antagonists.93
Future studies are needed to identify the precise relationship among leptin, the metabolic state, and Tregs in the context of autoimmune disease susceptibility.
The results may open up new ways of thinking about leptin-targeting therapies for the treatment of inflammatory and autoimmune diseases.
Conclusions
Without doubt, leptin is an important modulator of T-cell function. Great progress has been achieved in understanding leptin's role in animal studies. Both in vitro and in vivo studies support the involvement of leptin in the pathophysiology of autoimmune diseases. Moreover, it is clear that leptin plays a pleiotropic role in the body, and it is evident that there is intricate leptin-mediated interplay among WAT, metabolic disorders and autoimmune inflammatory disorders. Nevertheless, more work is needed to fully elucidate the role of leptin in the immune system and to further delineate its position in the treatment of autoimmune diseases.
References
- Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci. 1953;140:578–596. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973;9:294–298. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Qi Y, Singhal NS, Jackson MB, Scherer PE. Brain adipocytokine action and metabolic regulation. Diabetes. 2006;55:145–154. doi: 10.2337/db06-s018. [DOI] [PubMed] [Google Scholar]
- Lago F, Dieguez C, Gomez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune response. Cytokine Growth Factor Rev. 2007;18:313–325. doi: 10.1016/j.cytogfr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Rosen BS, Cook KS, Yaglom J, Groves DL, Volanakis JE, Damm D, et al. Adipsin and complement factor D activity: an immune-related defect in obesity. Science. 1989;244:1483. doi: 10.1126/science.2734615. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- Fantuzzi G, Sennello JA, Batra A, Fedke I, Lehr AH, Zeitz M, et al. Defining the role of T cell derived leptin in the modulation of hepatic or intestinal inflammation in mice. Clin Exp Immunol. 2005;142:31–38. doi: 10.1111/j.1365-2249.2005.02898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Cheetham CH, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam QLK, Lu L. Role of Leptin in Immunity. Cell Mol Immunol. 2007;4:2–13. [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immune-reactine leptin concentrations in normal weight and obese humans. N Engl J Med. 1996;334:292. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- Chan JL, Matarese G, Shetty GK, Raciti P, Kelesidis I, Aufiero D, et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci USA. 2006;103:8481–8486. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluher S, Mantsoros CS. Leptin in humans: lessons from translational research. Am J Clin Nutr. 2009;89:991S–997S. doi: 10.3945/ajcn.2008.26788E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Jr, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1071–R1079. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- Stofkova A. Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. Endocrine Regul. 2009;43:157–168. [PubMed] [Google Scholar]
- La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- Lee KN, Jeong IC, Lee SJ, Oh SH, Cho MY. Regulation of leptin gene expression by insulin and growth hormone in mouse adipocytes. Exp Mol Med. 2001;33:234–239. doi: 10.1038/emm.2001.38. [DOI] [PubMed] [Google Scholar]
- Blum WF, Englaro P, Hanitsch S, Juul A, Hertel NT, Muller J, et al. Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. Clin Endocrinol Metab. 1997;82:2904–2910. doi: 10.1210/jcem.82.9.4251. [DOI] [PubMed] [Google Scholar]
- Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, 3rd, et al. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarasheski KE, Zachwieja JJ, Horgan MM, Powderly WG, Santiago JV, Landt M. Serum leptin concentrations in human immunodeficiency virus-infected men with low adiposity. Metabolism. 1997;46:303–305. doi: 10.1016/s0026-0495(97)90258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Friedman JM. Obesity: causes and control of excess body fat. Nature. 2009;459:340–342. doi: 10.1038/459340a. [DOI] [PubMed] [Google Scholar]
- Gamba M, Pralong FP. Control of GnRH neuronal activity by metabolic factors: the role of leptin and insulin. Mol Cell Endocrinol. 2006;133:254–255. doi: 10.1016/j.mce.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Mangalat D, Korbut R. Adipocytokines. A novel link between inflammation and vascular function. J Physiol Pharmacology. 2006;57:505–528. [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol. 1996;6:1170–1180. doi: 10.1016/s0960-9822(02)70684-2. [DOI] [PubMed] [Google Scholar]
- Fernandez-Riejos P, Najib S, Santos-Alvarez J, Martın-Romero C, Perez-Perez A, Gonzalez-Yanes C, et al. Role of leptin in the activation of immune cells. Mediators Inflamm. 2010;2010:568343. doi: 10.1155/2010/568343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam QL, Liu S, Cao X, Lu L. Involvement of leptin signaling in the survival and maturation of bone marrow-derived dendritic cells. Eur J Immunol. 2006;36:3118–3130. doi: 10.1002/eji.200636602. [DOI] [PubMed] [Google Scholar]
- Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–6828. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Sun R, You L, Gao C, Tian Z. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem Biophys Res Commun. 2003;300:247–252. doi: 10.1016/s0006-291x(02)02838-3. [DOI] [PubMed] [Google Scholar]
- Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176:7745–7752. doi: 10.4049/jimmunol.176.12.7745. [DOI] [PubMed] [Google Scholar]
- Sanchez-Margalet V, Martin-Romero C, Gonzalez-Yanes C, Goberna R, Rodriguez-Bano J, Muniain MA. Leptin receptor (Ob-R) expression is induced in peripheral blood mononuclear cells by in vitro activation and in vivo in HIV-infected patients. Clin Exp Immunol. 2002;129:119–124. doi: 10.1046/j.1365-2249.2002.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madej T, Boguski MS, Bryant SH. Threading analysis suggests that the obese gene product may be a helical cytokine. FEBS Letters. 1995;373:13–18. doi: 10.1016/0014-5793(95)00977-h. [DOI] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. 2005;173:3137–3142. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- Sanchez-Margalet V, Martin-Romero C. Human leptin signaling in human peripheral blood mononuclear cells: activation of the JAK-STAT pathway. Cell Immunol. 2001;211:30–36. doi: 10.1006/cimm.2001.1815. [DOI] [PubMed] [Google Scholar]
- Belouzard S, Rouille Y. Ubiquitylation of leptin receptor OB-Ra regulates its clathrin-mediated endocytosis. EMBO J. 2006;25:932–942. doi: 10.1038/sj.emboj.7600989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Yamashita T, Iida M, Kuwajima M, Shima K. A short form of leptin receptor performs signal transduction. Biochem Biophys Res Commun. 1997;231:26–29. doi: 10.1006/bbrc.1996.6030. [DOI] [PubMed] [Google Scholar]
- Hekerman P, Zeidler J, Bamberg-Lemper S, Knobelspies H, Lavens D, Tavernier J, et al. Pleiotropy of leptin receptor signalling is defined by distinct roles of the intracellular tyrosines. FEBS J. 2005;272:109–119. doi: 10.1111/j.1742-4658.2004.04391.x. [DOI] [PubMed] [Google Scholar]
- Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176:7745–7752. doi: 10.4049/jimmunol.176.12.7745. [DOI] [PubMed] [Google Scholar]
- Mansour E, Pereira FG, Araujo EP, Amaral ME, Morari J, Ferraroni NR, et al. Leptin inhibits apoptosis in thymus through a janus kinase-2-independent, insulin receptor substrate-1/phosphatidylinositol-3 kinase-dependent pathway. Endocrinology. 2006;147:5470–5479. doi: 10.1210/en.2006-0223. [DOI] [PubMed] [Google Scholar]
- Tian Z, Sun R, Wei H, Gao B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem Biophys Res Commun. 2002;298:297. doi: 10.1016/s0006-291x(02)02462-2. [DOI] [PubMed] [Google Scholar]
- Montecucco F, Bianchi G, Gnerre P, Bertolotto M, Dallegri F, Ottonello L. Induction of neutrophil chemotaxis by leptin: crucial role for p38 and Src kinases. Ann NY Acad Sci. 2006;1069:463–471. doi: 10.1196/annals.1351.045. [DOI] [PubMed] [Google Scholar]
- Matarese G, Carrieri PB, La Cava A, Perna F, Sanna V, de Rosa V, et al. Leptin increase in multiple sclerosis associates with reduced number of CD4(þ)CD25þ regulatory T cells. Proc Natl Acad Sci USA. 2005;102:5150–5155. doi: 10.1073/pnas.0408995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Margalet V, Martın-Romero C, Santos-Alvarez C, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clin Exp Immun. 2003;133:11–19. doi: 10.1046/j.1365-2249.2003.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese G, Leiter EH, La Cava A. Leptin in autoimmunity: many questions, some answers. Tissue Antigens. 2007;70:87–95. doi: 10.1111/j.1399-0039.2007.00886.x. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation and hematopoiesis. J Leukoc Biol. 2000;68:437–446. [PubMed] [Google Scholar]
- Palmer G, Gabay C. A role for leptin in rheumatic diseases. Ann Rheum Dis. 2003;62:913–915. doi: 10.1136/ard.62.10.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rosa V, Procaccini C, Calì G, Pirozzi G, Fontana S, Zappacosta S, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Taleb S, Herbin O, Ait-Oufella H, Verreth W, Gourdy P, Barateau V, et al. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2691–2698. doi: 10.1161/ATVBAHA.107.149567. [DOI] [PubMed] [Google Scholar]
- Gualillo O, Eiras S, Lago F, Dieguez C, Casanueva FF. Elevated serum leptin concentrations induced by experimental acute inflammation. Life Sci. 2000;67:2433–2441. doi: 10.1016/s0024-3205(00)00827-4. [DOI] [PubMed] [Google Scholar]
- Sato T, Laviano A, Meguid MM, Chen C, Rossi-Fanelli F, Hatakeyama K. Involvement of plasma leptin, insulin and free tryptophan in cytokine-induced anorexia. Clin Nutr. 2003;22:139–146. doi: 10.1054/clnu.2002.0609. [DOI] [PubMed] [Google Scholar]
- Firestein GS, Budd RC, Harris ED, McInnes IB, Ruddy S, Sergent JS.Kelley's textbook of rheumatology. 8th edn Philadelphia, PA; Saunders; 2009. [Google Scholar]
- Harle P, Pangratz G, Weidler C, Büttner R, Schölmerich J, Straub RH. Possible role of leptin in hypoandrogenicity in patients with SLE and RA. Ann Rheum Dis. 2004;63:809–816. doi: 10.1136/ard.2003.011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadacca M, Margiotta D, Rigon A, Cacciapaglia F, Coppolino G, Amoroso A, et al. Adipokines and systemic lupus erythematosus: relationship with metabolic syndrome and cardiovascular disease risk factors. J Rheumatol. 2009;36:295–297. doi: 10.3899/jrheum.080503. [DOI] [PubMed] [Google Scholar]
- Chung CP. Adipocytokines in SLE: relationship to inflammation, insulin resistance and coronary atherosclerosis. Lupus. 2009;18:799–806. doi: 10.1177/0961203309103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalez A, Gonzalez-Lopez L, Valera-Gonzalez IC, Cardona-Muñoz EG, Salazar-Paramo M, González-Ortiz M, et al. Serum leptin levels in women with systemic lupus erythematosus. Rheumatol Int. 2002;22:138–141. doi: 10.1007/s00296-002-0216-9. [DOI] [PubMed] [Google Scholar]
- Wislowska M, Rok M, Stepien K. Serum leptin in SLE. Rheumatol Int. 2008;28:467–473. doi: 10.1007/s00296-008-0526-7. [DOI] [PubMed] [Google Scholar]
- De Sanctis JB, Zabaleta M, Bianco NE, Garmendia J. Serum adipokine levels in patients with SLE. Autoimmunity. 2009;42:272–274. doi: 10.1080/08916930902828031. [DOI] [PubMed] [Google Scholar]
- Busso N, So A, Chobaz V, Morad C, Martinez-Soria E, Talabot D, et al. Leptin sygnaling deficiency impairs humoral and cellular responses and attenuates experimental arthritis. J Immunol. 2002;168:875–882. doi: 10.4049/jimmunol.168.2.875. [DOI] [PubMed] [Google Scholar]
- Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez Rejino JJ, et al. Changes in fat derives hormones plasma concentrations: adiponectin, leptin, resistin and visfatinin rheumatoid arthritis subjects. Ann Rheum Dis. 2006;65:1198–1201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokarewa M, Bokarew D, Hultgren O, Tarkowski A. leptin consumption in the inflamed joints of patients with RA. Ann Rheum Dis. 2003;62:952–956. doi: 10.1136/ard.62.10.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser DA, Thoen J, Reseland JE, Forre O. Decreased CD4 lymphocyte activation and increased Il-4 production in peripheral blood of RA patients after acute starvation. Clin Rheumatol. 1999;18:394–401. doi: 10.1007/s100670050125. [DOI] [PubMed] [Google Scholar]
- Young HR, Solus J, Sokka T, Oeser A, Chung CP, Gebretsadik A, et al. Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis. Arthritis Rheum. 2009;60:1906–1914. doi: 10.1002/art.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders HJ, Rihl M, Heufelder A, Loch O, Schattenkirchner M. Leptin serum levels are not correlated with disease activity in patients with rheumatoid arthritis. Metabolism. 1999;48:745–748. doi: 10.1016/s0026-0495(99)90174-9. [DOI] [PubMed] [Google Scholar]
- Targonska-Stepniak B, Majdan M, Dryglewska M. Leptin serum levels in rheumatoid arthritis patients: relation to disease duration and activity. Rheumatol Int. 2008;28:585–591. doi: 10.1007/s00296-007-0480-9. [DOI] [PubMed] [Google Scholar]
- Gomez R, Lago F, Gomez-Reino D, Dieguez C, Gualillo O. Adipokines in the skeleton: influence on cartilage function and joint degenerative diseases. J Mol Endocrinology. 2009;43:11–18. doi: 10.1677/JME-08-0131. [DOI] [PubMed] [Google Scholar]
- Vadacca M, Arcarese L, Taccone A, Rigon A, Buzzulini F, Fiori E, et al. Circulating leptin, bone metabolism and inflammation in rheumatic diseases. Ann Rheum Dis. 2010;69 Suppl3:521. [Google Scholar]
- Saeki H, Shibata S, Tada Y, Karakawa M, Minatani Y, Tamaki K. Psoriasis arthropathica associated with severe obesity showing high serum leptin level. J Dermatol. 2009;36:364–366. doi: 10.1111/j.1346-8138.2009.00656.x. [DOI] [PubMed] [Google Scholar]
- Park MC, Chung SJ, Park YB, Lee SK. Pro-inflammatory effect of leptin on peripheral blood mononuclear cells of patients with ankylosing spondylitis. Joint Bone Spine. 2009;76:170–175. doi: 10.1016/j.jbspin.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Toussirot E, Streit G, Nguyen NU, Dumoulin G, Le Huédé G, Saas P, et al. Adipose tissue, serum adipokines, and ghrelin in patients with ankylosing spondylitis. Metabolism. 2007;56:1383–1389. doi: 10.1016/j.metabol.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Matarese G, Di Giacomo A, Sanna V, Lord GM, Koward JK, Di Tuoro A, et al. Requirement for leptin in the induction and progression of acute encephalomyelitis. J Immunol. 2001;166:5909–5916. doi: 10.4049/jimmunol.166.10.5909. [DOI] [PubMed] [Google Scholar]
- Sanna V, Di Giacomo A, La Cava A, Lechler RI, Fontana S, Zappacosta S, et al. Leptin surge precede onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell response. J Clin Invest. 2003;111:241–250. doi: 10.1172/JCI16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese G, Procaccini C, de Rosa V. The intricate interface between immune and metabolic regulation: a role for leptin in the pathogenesis of multiple sclerosis. J Leuk Biol. 2008;84:893–899. doi: 10.1189/jlb.0108022. [DOI] [PubMed] [Google Scholar]
- Batocchi AP, Rotondi M, Caggiula M, Frisullo G, Odoardi F, Nociti V, et al. Leptin as a marker of multiple sclerosis activity in patients treated with interferon. J. Neuroimmunol. 2003;139:150–154. doi: 10.1016/s0165-5728(03)00154-1. [DOI] [PubMed] [Google Scholar]
- Vadacca M, Margiotta D, Buzzulini F, Sambataro G, Vernuccio A, Afeltra A. Leptin levels in patients with Sjogren's syndrome and systemic lupus erythematosus: relationship with metabolic syndrome and cardiovascular disease risk factors. Lubijana, Slovenia; Proceedings of the 7th International Congress on Autoimmunity. 2010 May;5–9 [Google Scholar]
- Kotulska A, Kucharz EJ, Brzezińska-Wcisło L, Wadas U. A decreased serum leptin level in patients with systemic sclerosis. Clin Rheumatol. 2001;20:300–302. doi: 10.1007/s100670170053. [DOI] [PubMed] [Google Scholar]
- Kreiner F, Galbo H. Insulin sensitivity and related cytokines, chemokines, and adipokines in polymyalgia rheumatica. Scand J Rheumatol. 2010;39:402–408. doi: 10.3109/03009741003631479. [DOI] [PubMed] [Google Scholar]
- Siegmund B, Lear-Kaul KC, Faggioni R, Fantuzzi G. Leptin deficiency, not obesity, protects mice from Con A-induced hepatitis. Eur J Immunol. 2002;32:552–560. doi: 10.1002/1521-4141(200202)32:2<552::AID-IMMU552>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Siegmund B, Sennello J, Jones-Carson J, Gamboni-Robertson F, Lehr HA, Batra A, et al. Leptin receptor expression on T lymphocytes modulates chronic intestinal inflammation in mice. Gut. 2004;53:965. doi: 10.1136/gut.2003.027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese G, Sanna V, Lecher RI, Sarvetinik N, Fontana S, Zappacosta S, et al. Leptin accelerates autoimmune diabetes in female NOD mice. Diabetes. 2002;51:1356–1361. doi: 10.2337/diabetes.51.5.1356. [DOI] [PubMed] [Google Scholar]
- Lee CH, Chen YG, Chen J, Reifsnyder PC, Serreze DV, Clare-Sakzker M, et al. Novel leptin receptor mutation in NOD/LtJ mice suppresses type 1 diabetes progression: II. Immunologic analysis. Diabetes. 2006;55:171–178. [PubMed] [Google Scholar]
- Tarzi RM, Cook HT, Jackson I, Pusey CD, Lord GM. Leptin-deficient mice are protected from accelerated nephrotoxic nephritis. Am J Pathol. 2004;164:385–390. doi: 10.1016/S0002-9440(10)63128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HA, Choi GS, Jeon JY, Yoon JM, Sung JM, Suh CH. Leptin and ghrelin in Korean systemic lupus erythematosus. Lupus. 2010;19:170–174. doi: 10.1177/0961203309350321. [DOI] [PubMed] [Google Scholar]