Abstract
Mannan-binding lectin (MBL) plays a key role in the lectin pathway of complement activation and can influence cytokine expression. Toll-like receptor 4 (TLR4) is expressed extensively and has been demonstrated to be involved in lipopolysaccharide (LPS)-induced signaling. We first sought to determine whether MBL exposure could modulate LPS-induced inflammatory cytokine secretion and nuclear factor-κB (NF-κB) activity by using the monocytoid cell line THP-1. We then investigated the possible mechanisms underlying any observed regulatory effect. Using ELISA and reverse transcriptase polymerase chain reaction (RT-PCR) analysis, we found that at both the protein and mRNA levels, treatment with MBL suppresses LPS-induced tumor-necrosis factor (TNF)-α and IL-12 production in THP-1 cells. An electrophoretic mobility shift assay and western blot analysis revealed that MBL treatment can inhibit LPS-induced NF-κB DNA binding and translocation in THP-1 cells. While the binding of MBL to THP-1 cells was evident at physiological calcium concentrations, this binding occurred optimally in response to supraphysiological calcium concentrations. This binding can be partly inhibited by treatment with either a soluble form of recombinant TLR4 extracellular domain or anti-TLR4 monoclonal antibody (HTA125). Activation of THP-1 cells by LPS treatment resulted in increased MBL binding. We also observed that MBL could directly bind to the extracellular domain of TLR4 in a dose-dependent manner, and this interaction could attenuate the binding of LPS to cell surfaces. Taken together, these data suggest that MBL may affect cytokine expression through modulation of LPS-/TLR-signaling pathways. These findings suggest that MBL may play an important role in both immune regulation and the signaling pathways involved in cytokine networks.
Keywords: cytokines, mannan-binding lectin, nuclear factor-κB, THP-1 cells, Toll-like receptor 4
Introduction
Mannan-binding lectin (MBL), a Ca2+-dependent (C-type) lectin, is an important serum component associated with innate immunity.1, 2 It has the overall ‘bundle-of-tulips' structure that was first described for C1q.3 The MBL polypeptide is composed of four domains, a cysteine-rich N-terminal domain, a collagen-like region containing Gly-X-Y repeats (where X is any amino acid and Y is often hydroxyproline or hydroxylysine), a neck region and a C-terminal carbohydrate recognition domain (CRD).4, 5, 6, 7, 8, 9 Human MBL is a homooligomer of ∼31 kDa peptides, where three peptides interact to form a subunit, and two to six subunits interact to form a variety of MBL molecules that are held together through disulfide linkages located at the amino termini of the peptides. Molecular mass can range from ∼200 to 600 kDa,10, 11 but only the more highly polymeric forms demonstrate biological activity and can function to fix complements.
MBL can recognize a diverse range of infectious agents, including bacteria, yeast, parasites and viruses through the action of its CRDs. The carbohydrate specificity of MBL is rather broad, including 𝒹-mannose, N-acetylglucosamine and ℓ-fucose. This carbohydrate is specificity thought to occur in accordance with the need for MBL to recognize a variety of pathogens. Upon binding to pathogens, MBL may activate the complement cascade through the function of the lectin pathway, after which microbes are killed by cellular lysis and indirect opsonization. When binding to the collectin receptor of effector cells, MBL can mediate direct opsonization and MBL-dependent, cell-mediated cytotoxicity.12 MBL also plays an important role in defense in surface mucous membranes.13 As such, MBL has been considered to be a key molecule in the regulation of innate immunity.14
In the past decade, basic scientific understanding of how the immune system functions has undergone a substantial paradigm shift. In addition to playing a role in first-line host defense, innate immunity also functions in the initiation of adaptive immune responses and in controlling the types of these responses through the process of pattern recognition.15 In the innate immune system, both soluble and membrane-bound pattern recognition molecules function to assess the level of danger caused by a particular intrusion, and they then initiate a host protection program. Monocytes, macrophages and dendritic cells (DCs), when activated by pathogens, often initiate the synthesis of proinflammatory cytokines.16 The cytokine environment then subsequently influences adaptive immune responses. Then, both soluble and cellular components of the innate system can remove degenerated or dead cells or tissue debris and then subsequently participate in mechanisms regulating tissue repair. Thus, in the absence of perceived danger, the innate system seems to be able to remove dying cells while avoiding the induction of an adaptive immune response, which in turn could lead to autoimmunity or further tissue damage.17
Pattern recognition receptors involved in the innate immune system, such as Toll-like receptors (TLRs), distinguish infectious nonself from noninfectious self receptors and control the initiation of adaptive responses.18 In this context, TLRs activation results in the recruitment of MyD88, a protein that interacts with IL-1 receptor-associated kinase. This interaction then leads to initiation of a signal transduction cascade that culminates in nuclear translocation of nuclear factor (NF)-κB family members resulting in altered expression of genes such as IL-6, tumor-necrosis factor (TNF)-α, IL-12, IL-1 and other important cytokines that function in inflammation and immune defense. The secreted inflammatory cytokines then stimulate macrophages and natural killer cells that can directly kill pathogens or control the initiation of adaptive responses.19 Lipopolysaccharide (LPS), the major constituent of the outer membrane of Gram-negative bacteria and a strong activator of the inflammatory response and immune regulation, can activate human monocytes and induce them to secrete TNF-α and IL-12.20 Among the TLR family, TLR4 is expressed very extensively (e.g., monocytes, macrophages, immature DCs, T cells and B cells) and plays a critical role in recognition and signaling of bacterial LPS.21
It was reported that MBL influences the cytokine network as the result of stimulation by various microorganisms.22, 23, 24, 25, 26 Recently, researchers have speculated that novel MBL functions may exist. MBL was shown to directly bind to apoptotic cell surfaces and apoptotic cell blebs through the action of its CRDs.27, 28 MBL was able to bind to healthy autologous cells in a specific and sugar-sensitive manner.29, 30 It has also been demonstrated that cytokine expression can be altered by interactions of these cells with MBL.31, 32 MBL and lung collectins could interact with both TLR4 and MD-2 through distinct mechanisms.33 MBL interacted directly with deglycosylated CD14;34 however, the underlying mechanism regulating the binding of MBL to various cell types and the mechanism by which MBL affects cytokine expression remain unknown. Recently, it has been reported that the C-type lectin DC-SIGN is able to modulate TLR signaling at the level of the transcription factor NF-κB to affect anti-inflammatory cytokine responses.35 As a key soluble pattern recognition molecule in the innate immune system, it is likely that MBL can interact with TLRs to affect their function. Thus, the investigation into how this member of the collectin family regulates LPS/TLR-mediated biological functions is of great importance.
Materials and methods
Preparation of MBL
MBL was isolated from human plasma according to Tan et al.,36 with modifications as described.37 Briefly, a pool of freshly frozen human plasma (2.5 liters, provided by Guangzhou General Hospital of Guangzhou Military Area Command of Chinese PLA, China) was thawed from −80 °C. After extraction and elimination of the majority of the unrelated proteins, the residual fraction was solubilized, and MBL was purified from the extract by a process involving three chromatographic steps. The first step, affinity chromatography on a mannan-agarose (Sigma, Poole, UK) column, was used to select for functionally active, carbohydrate-binding MBL with approximately 2000-fold purification. The subsequent steps were anion-exchange chromatography and gel filtration using a Mono-Q HR 5/5 column (Pharmacia Biotech Europe, Orsay, France) or a Superose 6 HR 10/30 column (Pharmacia Biotech Europe), respectively. The purified MBL was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and subsequent western blot (WB) methods.
The MBL preparations were determined to be free of endotoxin contamination through the use of a Limulus amebocyte lysate assay. Biotinylated MBL was prepared by coupling of N-hydroxysuccinimidobiotin to the purified MBL as described.38
Cell culture
THP-1 cells (a gift from Dr JH Han, Scripps, La Jolla, CA, USA) were maintained in endotoxin-free RPMI-1640 (Gibco BRL, Gaithersburg, MD, USA) with 10% (v/v) heat-inactivated fetal calf serum (Gibco BRL, Grand Island, CA, USA), penicillin (100 U/ml) and streptomycin (100 µg/ml) at 37 °C in a 5% (v/v) CO2 environment. Actively growing cells were harvested from the flasks (Nunc, Roskilde, Denmark) by centrifugation at 450 g for 5 min and then washed with RPMI-1640 prior to the experiments.
Cytokine measurements by ELISA
To study the effect of MBL on cytokine secretion, THP-1 cells (1×106/ml) were seeded in 12-well tissue culture plates (Corning-Costar, Cambridge, MA, USA) containing RPMI-1640 complete medium and maintained at 37 °C in a 5% (v/v) CO2 environment for 2 h after MBL was added at concentrations ranging from 0 to 20 µg/ml. Smooth LPS (100 ng/ml; from Escherichia coli O111:B4; List Biological, Campbell, CA, USA) was added to the complete medium and incubated overnight at 37 °C in a 5% (v/v) CO2 environment. The supernatants from the wells were harvested after centrifuging the cells at 450 g for 5 min, and they were stored at −80 °C pending analysis. Negative control cells were cultured in complete medium only. Levels of IL-12 p40+p70 and TNF-α were determined using ELISA Kits (Bender MedSystems, CA, USA), following the manufacturer protocol. To demonstrate the specificity of the MBL response, anti-MBL polyclonal antibody (pAb; R&D systems, MN, USA) was used.
Cytokine gene expression analysis by reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was isolated from 1×106 THP-1 cells for use in the above experiments by the TRIzol reagent method (Gibco BRL, Rockville, MD, USA). cDNA was synthesized with the Expand Reverse Transcriptase Kit (Roche Diagnostics, Lewes, UK). For PCR, Taq Supreme polymerase and buffers from Helena Biosciences (Sunderland, UK) were used. Reactions were performed with an initial denaturation of 2 min at 94 °C followed by cycles of denaturation for 30 s at 94 °C, subsequent annealing for 30 s and extension for 30 s at 68 °C. Primers and programs were performed as described.39, 40, 41, 42 Using the IL-12p40 sense primer, 5′-GGA CCA GAG CAG TGA GGT CTT-3′, and antisense primer, 5′-CTC CTT GTT GTC CCC TCT GA-3′, a product of 373 bp was amplified where primer annealing occurred at 52 °C for 35 cycles. For IL-12p35, using primers 5′-CTC CTC CTT GTG GCT ACC CT-3′ and 5′-CTG GAA TTT AGG CAA CTC TCAT-3′, a 281-bp product was amplified with annealing occurring at 55 °C for 33 cycles. Using TNF-α primers 5′-AAG CCT GTA GCC CAT GTT GT-3′ and 5′-CAG ATA GAT GGG CTC ATA CC-3′, a 330-bp product occurred when annealing was performed at 54 °C for 29 cycles. Finally, the β-actin primers 5′-CCA GAG CAA GAG AGG CAT CC-3′ and 5′-GTG GTG GTG AAG CTG TAG CC-3′ generated a 435-bp product when annealing conditions were performed at 56 °C for 35 cycles. PCR products were identified by 1% agarose gel electrophoresis, and the gray values of the DNA fragments were measured using the Gel Image Analysis System (UVP Inc., Upland, CA, USA). β-actin served as an internal control.
Analysis of NF-κB by electrophoretic mobility shift assay (EMSA) and WB analysis
THP-1 cells (5×105 cells/sample) were stimulated with LPS (100 ng/ml) in the presence of 15 µg/ml of either human serum albumin (HSA), MBL or MBL, along with exposure to anti-MBL pAb for 1 h. This was followed by cell harvesting for nuclear extraction. THP-1 cell nuclear extracts were prepared using the NucBuster Protein Extraction Kit (Novagen, Madison, WI, USA). Oligonucleotide probes were radiolabeled with [γ-32P]ATP using T4 Polynucleotide Kinase (Takara, Tokyo, Japan). For the binding reaction, 5 µg of nuclear extracts were incubated in 30 µl of total reaction buffer containing 10 mM Hepes (pH 7.9), 12.5% glycerol, 70 mM NaCl, 1 mM DTT, 1 mM EDTA and 2 µg poly (dI-dC). The 32P-labeled oligonucleotide was added to the reaction mixture and incubated for 20 min at room temperature. Samples were electrophoresed on 6% acrylamide gels (made using 50 mM Tris buffer containing 380 mM glycine and 2 mM EDTA). Then, autoradiography was performed.
For WB analysis, the extracts were separated by 10% SDS–PAGE and then transferred to a nitrocellulose membrane (BioRad, CA, USA). After blocking with 5% non-fat milk protein in triethanolamine-buffered saline (pH 7.5), the membrane was incubated with NF-κB-specific mouse antihuman monoclonal antibody (mAb) p65 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing, horseradish peroxidase (HRP)-conjugated secondary antibody was added to the membrane along with triethanolamine-buffered saline containing 0.1% Tween 20. Actin was used as an internal control. For visualization of the protein bands, an enhanced chemiluminescence detection system (Amersham Biosciences, Uppsala, Sweden) was applied.
To exclude the stimulatory signaling of MBL through binding to TLR4, an experiment using cells cultured in the presence MBL alone was performed as the negative control.
Analysis of the effect of MBL on the binding of LPS to THP-1 cells using flow cytometry (FCM)
THP-1 cells were collected and pre-incubated at 1×106 cells/tube in the absence or presence of 15 µg/ml of MBL at 37 °C for 30 min. The cells were then further incubated in a concentration of 100 ng/ml Alexa488-labeled smooth LPS (E. coli O111:B4; Molecular Probes, Eugene, OR, USA) at 4 °C for 30 min. After washing, the cells were analyzed by FCM using FACSCalibur (Becton Dickinson, Mountain View, CA, USA). To demonstrate the specificity of the MBL response, anti-MBL pAb was added to MBL preparations for a period of 10 min prior to cellular incubation.
Analysis of MBL binding to THP-1 cells by FCM
Washed THP-1 cells (2×105) were resuspended in Tris-buffered saline, pH 7.4, containing 5 mM CaCl2 and 1% bovine serum albumin (buffer A). Three types of Tris-buffered saline with differing concentrations of calcium ion were used for binding assays. Alternatively, as the Ca2+-free control, 5 mM EDTA was substituted for CaCl2. To identify if a possible MBL binding site (ligand) for MBL was expressed on the cell membrane, anti-TLR4 mAb HTA125 (30 µg/ml; eBioscience, San Diego, CA, USA) was employed as an inhibitor and was pre-incubated with the cells for 10 min. Mouse isotype IgG (Sigma, Madrid, Spain) was used as a control. Each cell suspension (0.2 ml) was incubated for 30 min on ice in the presence of biotinylated MBL (10 µg/ml). The cells were then further incubated for 30 min on ice in the presence of ExtrAvidin-FITC (Sigma) at a final dilution of 1∶100. After washing, the cells were analyzed by FCM.
A soluble form of recombinant TLR4 extracellular domain (sTLR4) protein, consisting of the putative extracellular domain (Met1-Lys631) and a 6 His tag at the C-terminal end, was prepared in our lab as previously described.43 sTLR4 protein (30 µg/ml), a molecule that may also function as an inhibitor of MBL, was pre-incubated with biotinylated MBL (10 µg/ml) at 37 °C for 30 min. HSA was used as a negative control. The mixture was then incubated with the cells in the previously described buffer A for 30 min on ice, after which biotinylated MBL was added. The cells were incubated further for 30 min on ice with ExtrAvidin-FITC. After washing, the cells were analyzed by FCM.
In certain experiments, cells were stimulated for 2 h using smooth LPS (100 ng/ml) dissolved in RPMI-1640 complete medium supplemented with a physiological calcium concentration (1.3 mM, supplemented with Ca(NO3)2). After washing, the binding of MBL to cells was analyzed by FCM.
Analysis of TLR4 expression in LPS-stimulated THP-1 cells by RT-PCR and northern blot analysis
For RT-PCR, total RNA was isolated from untreated THP-1 cells or LPS-stimulated THP-1 cells using a Qiagen Kit (Valencia, CA, USA) following the manufacturer's instructions. The isolated RNA was then treated with RNase-free DNase I. For the RT reaction, the MMLV preamplification system (Life Technologies, Carlsbad, CA, USA) was applied. PCR amplification was performed with Taq Gold polymerase (Perkin Elmer, Foster City, CA, USA) for 32 cycles at 95 °C for 45 s, then 54 °C for 45 s, followed by a temperature of 72 °C for 1 min. The primers used for RT-PCR were 5′-TGG ATA CGT TTC CTT ATA AG-3′ and 5′-GAA ATG GAG GCA CCC CTT C-3′. Glyceraldehyde-3-phosphate dehydrogenase primers were obtained from Clontech (Palo Alto, CA, USA).
For northern blot analysis, total RNA was analyzed by electrophoresis through a 1% agarose/formaldehyde gel, followed by northern blot transfer to Gene Screen Plus membranes (NEN Life Science Products, Boston, MA, USA). The plasmids containing human TLR4 or actin cDNAs were labeled with [α-32P]dCTP (3000 Ci/mmol; Amersham Pharmacia Biotech, Buckinghamshire, UK). Membranes were pretreated and hybridized in 50% formamide (Merck, Rahway, NJ, USA) with 10% dextran sulfate (Sigma, St. Louis, MO, USA) and washed twice with 2× SSC (1× SSC: 0.15 M NaCl, 0.015 M sodium citrate) and 1% SDS at 60 °C for 30 min. Then, the membranes were washed twice with 0.1× SSC at room temperature for 30 min. Membranes were exposed for 4–48 h at −80 °C under intensifying screens.
Analyses of binding of MBL to sTLR4
For deglycosylation, sTLR4 (4 µg) was incubated with 2 unit of N-glycosidase F (Roche Diagnostics, Mannheim, Germany) at 37 °C for 2 h in 10 mM Tris buffer (pH 7.4) containing 10 mM EDTA, 2% (v/v) β-mercaptoethanol, 0.1% (w/v) SDS and 1% (v/v) Nonidet P-40. The removal of oligosaccharides was confirmed using a Carbohydrate Detection Kit (G. P. Sensor, J-Oil Mills, Yokohama, Japan) according to the manufacturer's instructions.
The ligand blot analysis was performed using a previously described method.44 In brief, glycosylated and deglycosylated sTLR4 proteins (2 µg) were electrophoresed on a 13% polyacrylamide gel in the presence of SDS under denaturing conditions and then transferred onto polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). The membrane was incubated with 5 mM Tris buffer (pH 7.4) containing 0.15 M NaCl, 5 mM CaCl2 and 5% (w/v) bovine serum albumin (buffer B) to block nonspecific binding. The membrane was then incubated with MBL or HSA (5 µg/ml) in buffer B for 3 h at room temperature. The membrane was washed and incubated with anti-MBL mAb HYB 131–11 (1∶2000; Abcam, Cambridge, UK) for 90 min, followed by incubation with HRP-labeled goat antimouse IgG (1∶ 1500; Sigma) for 60 min. The binding of human MBL to sTLR4 was finally visualized by chemiluminescence (Super Signal; Pierce, Rockford, IL, USA).
The binding of MBL to sTLR4 was also examined using microtiter wells. sTLR4 or HSA (10 µg/ml, 50 µl/well) was coated onto microtiter wells, which were then incubated with 10 mM Hepes buffer (pH 7.4) containing 0.15 M NaCl, 5 mM CaCl2 and 5 mg/ml bovine serum albumin (buffer C) to block nonspecific binding. The indicated concentrations of MBL in buffer C were added and incubated at 37 °C for 5 h. In some experiments, 5 mM EDTA was substituted in place of the CaCl2 in buffer C. After washing, the wells were incubated with anti-MBL mAb HYB 131-11 (1∶5000). Binding was detected with HRP-labeled goat antimouse IgG (1∶1500), and the absorbance at 450 nm was measured.
Statistical analysis
The mean and SD were calculated using Excel software (Microsoft). ANOVA was used for statistical analysis, and P<0.05 was considered to represent a statistically significant difference between two sample means.
Results
Purification of MBL
MBL was purified from pooled human plasma samples that contained high protein concentrations (approximately 2500 µg MBL/L on average). SDS–PAGE followed by WB indicated that this highly purified MBL was a functional multimer composed of 32-kDa peptide chains (Figure 1). It was highly bioactive, as demonstrated by a ligand-binding assay and yeast coagulation (data not shown).
Figure 1.
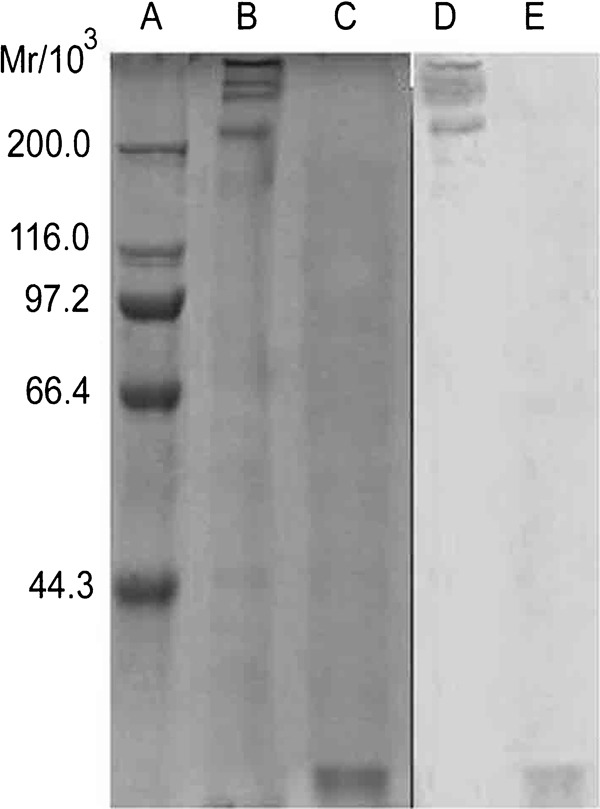
Analysis of purified MBL protein by SDS–PAGE and WB. (a) Protein marker; (b) purified MBL under non-reducing conditions; (c) purified MBL under reducing conditions; (d) WB analysis of purified MBL under non-reducing conditions; (e) WB analysis of purified MBL under reducing conditions. MBL, mannan-binding lectin; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; WB, western blot.
Suppression of LPS-induced TNF-α levels and IL-12 p40+p70 levels by MBL
THP-1 cells stimulated with LPS reached a maximal response in the secretion of proinflammatory cytokines such as IL-12 and TNF-α (data not shown). Cytokines produced by monocytes are known to play important roles in immune response regulation. To investigate whether MBL affects cytokines produced by LPS-stimulated THP-1 cells, THP-1 cells were treated with LPS (100 ng/ml) in the presence of increasing concentrations of MBL (0, 1, 10 and 20 µg/ml). Following 24-h incubation, the supernatants were analyzed by ELISA. As shown in Figure 2, TNF-α and IL-12 p40+p70 induction after LPS treatment was profoundly inhibited by exposure to high concentrations of MBL (10–20 µg/ml), as compared with THP-1 cells that were not treated with MBL (P<0.05). Additionally, HSA (20 µg/ml) or low-concentration MBL treatment (1 µg/ml) resulted in no significant effects. Addition of anti-MBL pAb, while cells were pre-incubated with MBL, restored the secretion of TNF-α and IL-12 p40+p70, indicating that MBL specifically inhibits the interaction between LPS and THP-1 cells.
Figure 2.
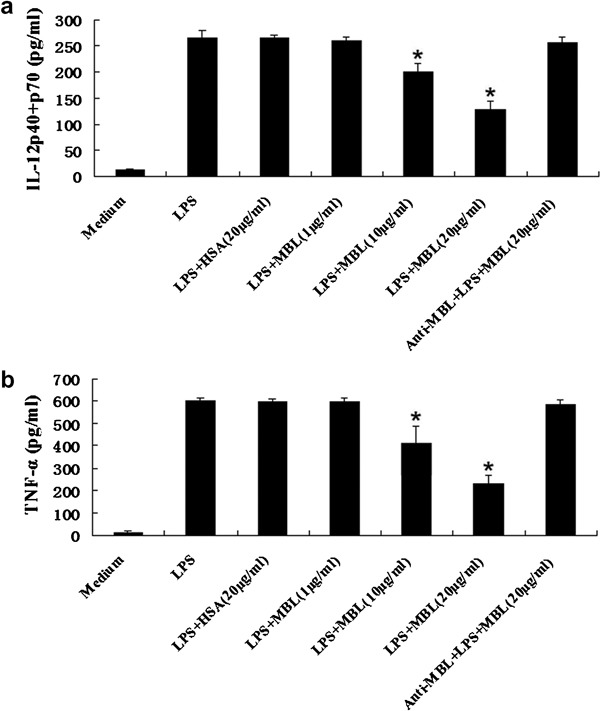
Inhibition of LPS-induced cytokine production from THP-1 cells by MBL. THP-1 cells were stimulated with LPS (100 ng/ml) in the presence of the indicated concentrations of HSA, anti-MBL pAb and MBL, or MBL alone for 24 h. Supernatants were harvested and subjected to ELISA for IL-12 p40+p70 (a) and TNF-α (b). *P<0.05 as compared to the LPS-stimulated group. Similar results were observed in three independent experiments. HSA, human serum albumin; LPS, lipopolysaccharide; MBL, mannan-binding lectin; pAb, polyclonal antibody; TNF, tumor-necrosis factor.
Suppression of mRNA levels of TNF-α and IL-12 p40+p70 by MBL
To determine whether MBL affects the expression of TNF-α and IL-12 at the mRNA level, TNF-α, IL-12p35 and IL-12p40 mRNA levels were examined by RT-PCR, and a representative experiment was analyzed by Gel Imaging System scanning. As shown in Figure 3, LPS treatment strongly induced expression of all three genes; however, their levels were significantly downregulated in the presence of a high concentration of MBL (20 µg/ml), as compared with the corresponding groups that were not treated with MBL. Additionally, HSA (20 µg/ml) treatment did not inhibit induction of these genes. Addition of anti-MBL pAb, while these cells were pre-incubated with MBL, restored the gene levels of TNF-α, IL-12p35 and IL-12p40, suggesting a specific inhibitory effect caused by MBL. Together, these data suggest that MBL treatment at high concentrations can inhibit the LPS-induced expression of TNF-α and IL-12 mRNA.
Figure 3.
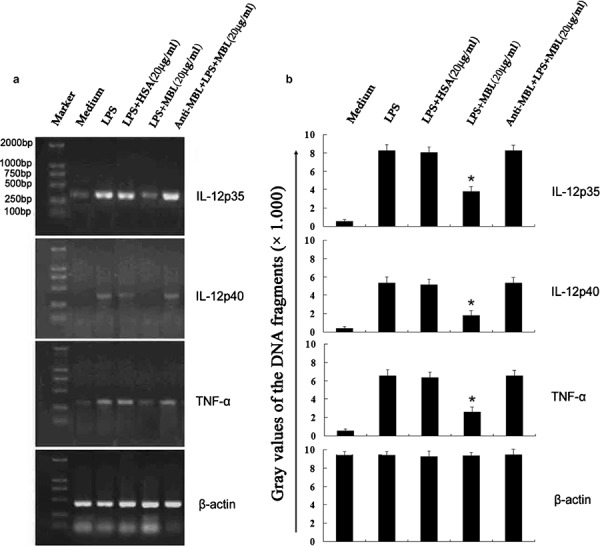
Inhibition of LPS-induced TNF-α and IL-12 mRNA expression in THP-1 cells by MBL. THP-1 cells were stimulated with LPS (100 ng/ml) in the presence of the indicated concentrations of HSA, anti-MBL pAb and MBL, or MBL alone for 24 h. Samples were taken for RNA extraction from various groups, and the amplification and electrophoresis of TNF-α, IL-12p35 and IL-12p40 genes was carried out simultaneously. (a) Phosphorimage from an individual experiment representing three independent experiments. (b) Gray values of the DNA fragments. *P<0.05 as compared to the LPS-stimulated group. Similar results were observed in three independent experiments. β-actin was used as an internal control. HSA, human serum albumin; LPS, lipopolysaccharide; MBL, mannan-binding lectin; pAb, polyclonal antibody; TNF, tumor-necrosis factor.
Inhibition of NF-κB DNA binding and its translocation in THP-1 cells by MBL
We next chose to investigate whether MBL treatment affects LPS-induced signaling through interactions with surface molecules on THP-1 cells. We determined the effects of MBL treatment on LPS-induced NF-κB activation in THP-1 cells using both EMSA and WB analysis. For EMSA, nuclear extracts from LPS-stimulated THP-1 cells were incubated with p40 NF-κB probe. Our results indicate that NF-κB binding was enhanced within 1 h following LPS stimulation, and that MBL (15 µg/ml) treatment could reduce this DNA-binding activity. DNA binding was restored by treatment with anti-MBL pAb (Figure 4b). The binding of this complex was determined to be specific, and it could be competitively reduced by treatment with a 100-fold excess of unlabeled consensus NF-κB oligonucleotide (Figure 4a). WB analysis of corresponding nuclear fractions using anti-p65 mAb confirmed that in THP-1 cells, LPS treatment can significantly increase NF-κB translocation from the cytoplasm to the nucleus. This effect was strongly inhibited by treatment with MBL (15 µg/ml) (Figure 4c). Addition of anti-MBL pAb restored NF-κB translocation from the cytoplasm to the nucleus (Figure 4c), further demonstrating the specific inhibitory effect of MBL on the interaction between smooth LPS and THP-1 cells.
Figure 4.
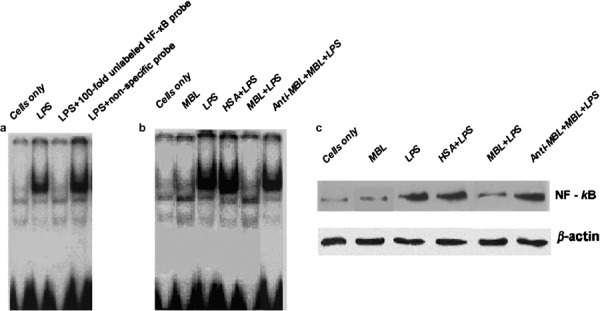
Suppression of LPS-stimulated NF-κB activity by MBL. (a) Specific and non-specific competitive assays of NF-κB. THP-1 cells were stimulated with LPS for 1 h, followed by cell harvesting for nuclear extraction. The nuclear extracts were mixed with a radiolabeled NF-κB oligonucleotide probe and 100-fold molar excess of unlabeled consensus NF-κB oligonucleotide and then analyzed by EMSA. A non-specific probe for NF-κB was used as the control. (b) LPS-induced NF-κB DNA-binding activity is inhibited by MBL. THP-1 cells were stimulated with LPS in the presence of 15 µg/ml HSA, anti-MBL pAb and MBL, or MBL for 1 h, followed by cell harvesting to prepare nuclear extracts. The nuclear extracts were mixed with radiolabeled NF-κB oligonucleotide probe and analyzed with EMSA. (c) MBL inhibits NF-κB translocation in THP-1 cells. THP-1 cells were stimulated with LPS in the presence of 15 µg/ml HSA, anti-MBL pAb and MBL, or MBL alone for 1 h, then the cells were harvested to prepare nuclear extracts. The proteins in the nuclei-free supernatants were separated by 10% SDS–PAGE, followed by transfer to a nitrocellulose membrane. After blocking, the membrane was incubated with NF-κB-specific mouse antihuman mAb p65, followed by incubation with HRP-conjugated secondary antibody. ECL was applied for visualization of the protein bands. β-actin was used as an internal control. ECL, enhanced chemiluminescence; EMSA, electrophoretic mobility shift assay; HRP, horseradish peroxidase; HSA, human serum albumin; LPS, lipopolysaccharide; mAb, monoclonal antibody; MBL, mannan-binding lectin; NF, nuclear factor; pAb, polyclonal antibody; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Inhibition of binding of LPS to THP-1 cells by MBL
To investigate possible mechanisms underlying the regulatory effect of MBL on cytokine secretion, we examined whether MBL could alter the binding of LPS to THP-1 cells. When cells were incubated with labeled LPS at 4 °C, significant LPS binding at the cell surface was observed (Figure 5a, dotted line). Pre-incubation with MBL (10 µg/ml) significantly attenuated this cell-surface binding (Figure 5a, black solid line). Addition of anti-MBL pAb during pre-incubation of cells with MBL restored the binding of LPS to the cell surface (Figure 5b), again demonstrating that the inhibitory effect of MBL is specific.
Figure 5.
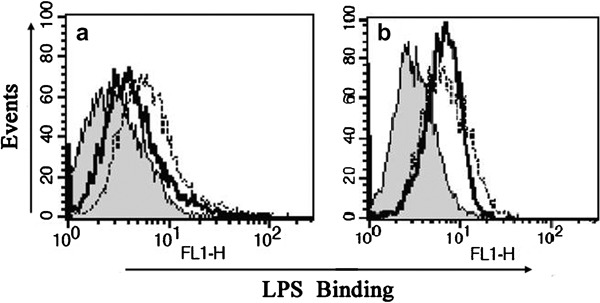
Suppression of the binding of smooth LPS to THP-1 cells by MBL. THP-1 cells were pre-incubated with (black solid line) or without (dotted line) MBL for 30 min (a) or with anti-MBL pAb and MBL (black solid line) for 30 min (b) and then further incubated at 4 °C for 30 min with Alexa488-labeled smooth LPS (E. coli O111:B4). The binding of LPS on the cell surface was determined by FCM. The histograms shown are representatives of three experiments. Shaded curves represent the negative control without labeled LPS. FCM, flow cytometry; LPS, lipopolysaccharide; MBL, mannan-binding lectin; pAb, polyclonal antibody.
Binding of MBL to THP-1 cells occurs in a Ca2+-dependent manner
To study the possible mechanism underlying the effect of MBL treatment on the interaction between LPS and THP-1 cells, cellular MBL-binding experiments were performed. When THP-1 cells were incubated with biotinylated MBL in the presence of physiological calcium concentrations, MBL was able to definitively bind to cells, as demonstrated by FCM (Figure 6a). At higher Ca2+ concentrations, this binding was markedly increased (Figure 6b, c), whereas binding was markedly reduced in the absence of Ca2+ (Figure 6d). These results indicate that MBL interacts with THP-1 cells in a Ca2+-dependent manner.
Figure 6.
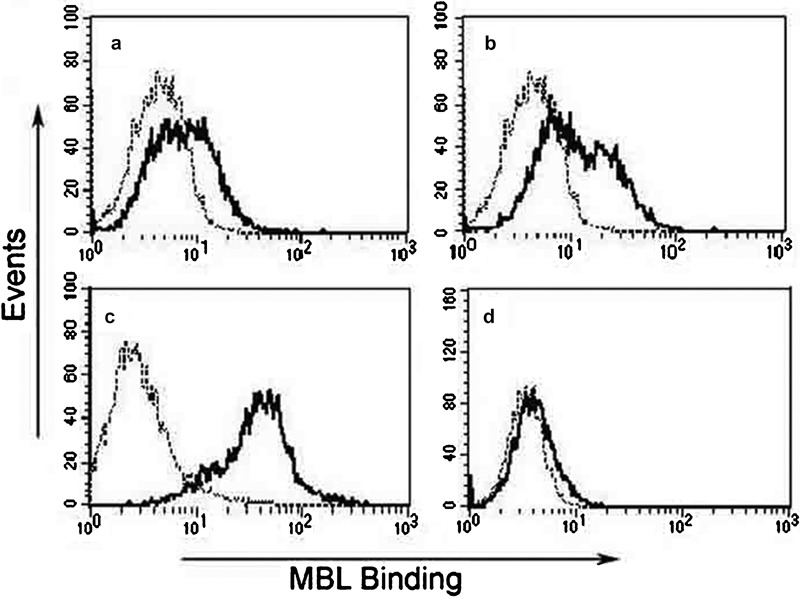
Ca2+-dependent binding of MBL with THP-1 cells. (a) Tris-buffered saline with 1.3 mM CaCl2; (b) Tris-buffered saline with 5 mM CaCl2; (c) Tris-buffered saline with 10 mM CaCl2; (d) Tris-buffered saline with 5 mM EDTA. Each solution of THP-1 cells was incubated with biotinylated MBL for 10 min before the addition of ExtrAvidin-FITC. After incubation for 30 min at 37 °C, binding of MBL to cells was analyzed by FCM, as shown in the representative histograms (a–d) (black lines, biotinylated MBL binding; dotted lines, negative cell-only controls). These data are representative of four independent experiments. FCM, flow cytometry; MBL, mannan-binding lectin.
Enhanced MBL binding to THP-1 cells after LPS stimulation
To determine whether the observed MBL binding is influenced by inflammatory stimuli, THP-1 cells were cultured for 2 h in the presence of LPS (100 ng/ml) in physiological calcium concentrations. Significant enhancement of the binding of MBL to THP-1 cells was observed after LPS stimulation (Figure 7b), as compared to that achieved without LPS stimulation (Figure 7a). These data demonstrate that LPS treatment causes increased MBL binding to THP-1 cells.
Figure 7.
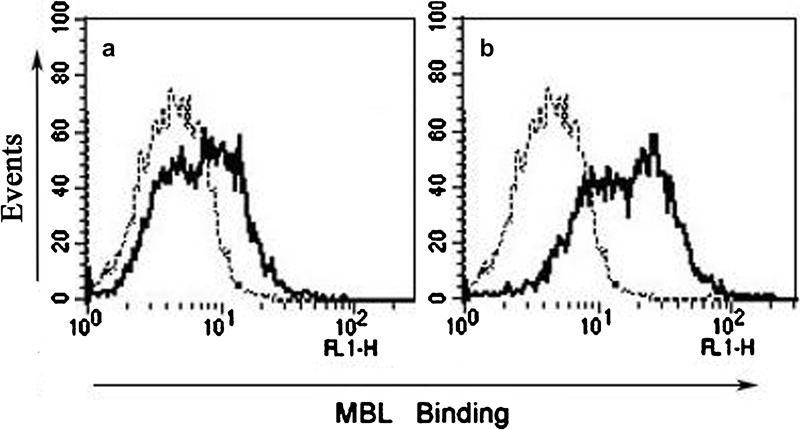
Enhanced MBL binding after LPS stimulation. THP-1 cells were stimulated for 2 h without (a) or with (b) LPS in complete 1640 medium before addition of biotinylated MBL under conditions of physiological calcium concentrations (1.3 mM). After washing, the cells (2×105) were resuspended. The cell suspension (0.2 ml) was first incubated with biotinylated MBL in Tris-buffered saline containing 1.3 mM CaCl2 for 30 min at room temperature. The cells were then incubated with ExtrAvidin-FITC for 30 min at room temperature. After washing, the cells were analyzed by FCM (black lines, biotinylated MBL binding; dotted lines, negative cell-only controls). These data are representative of four independent experiments. FCM, flow cytometry; LPS, lipopolysaccharide; MBL, mannan-binding lectin.
Enhanced TLR4 expression in LPS-stimulated THP-1 cells
TLR4 is extensively expressed and functions to mediate recognition of a wide variety of microbial products. To determine whether enhanced MBL binding is correlated with TLR4 expression in LPS-stimulated THP-1 cells, we treated THP-1 cells for 2 h with LPS and then examined rRNA expression using both RT-PCR and northern blot analysis. As shown in Figure 8, TLR4 mRNA was detected in THP-1 cells after 2 h; however, this expression was significantly enhanced after 2-h stimulation with LPS. These data suggest that LPS treatment results in increased TLR4 expression in THP-1 cells.
Figure 8.

Enhanced TLR4 expression in THP-1 cells after LPS stimulation. (a) Expression of TLR4 in THP-1 cells was analyzed using RT-PCR. Total RNA was isolated from THP-1 cells cultured in medium (with or without 100 ng/ml LPS) for 2 h using a Qiagen Kit. GAPDH was used as an internal control. (b) Northern blot analysis. Total RNA was prepared from THP-1 cells cultured in medium with or without 100 ng/ml LPS for 2 h. Blots were hybridized with probes specific for TLR4. β-actin was used as an internal control. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LPS, lipopolysaccharide; RT-PCR, reverse transcriptase polymerase chain reaction; TLR4, Toll-like receptor 4.
Inhibition of binding of MBL to THP-1 cells by anti-TLR4 mAb and sTLR4
Due to our observation that MBL could bind to THP-1 cells and attenuate the binding of LPS to the cells, we decided to investigate whether TLR4 on the surface of THP-1 cells is the binding site (ligand) for MBL. Our experimental results indicated that both anti-TLR4 mAb (HTA125) (Figure 9a) and sTLR4 protein (Figure 9b) were capable of independently inhibiting the cellular binding of MBL, indicating that MBL binds to TLR4 expressed on THP-1 cells.
Figure 9.
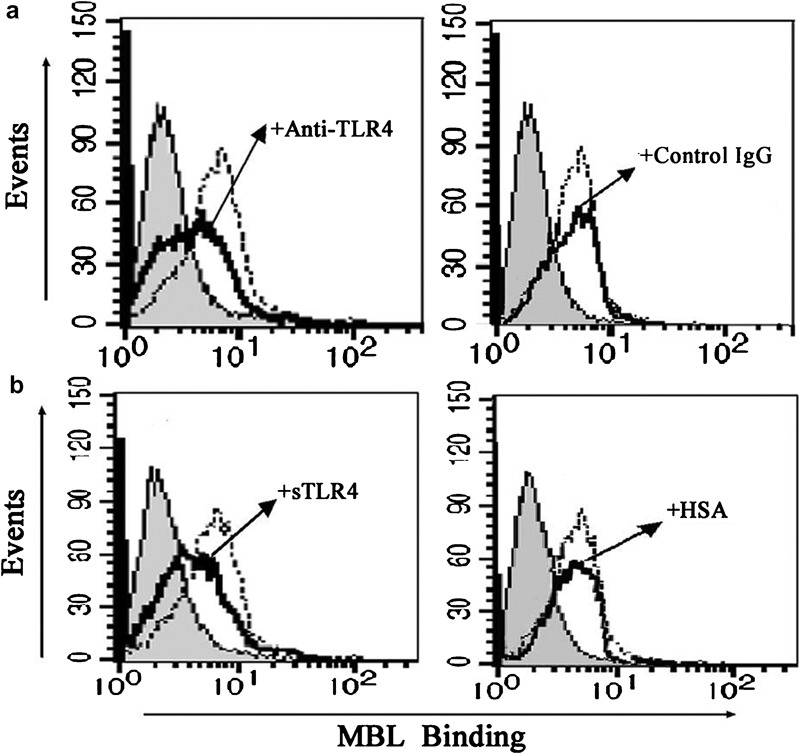
Attenuated MBL binding to THP-1 cells by anti-TLR4 mAb or sTLR4. (a) Anti-TLR4 mAb attenuates the binding of MBL to THP-1 cells. THP-1 cells were incubated with anti-TLR4 mAb or mouse isotype IgG (as a control) for 10 min before the addition of biotinylated MBL, and the binding of MBL was detected by ExtrAvidin-FITC (shaded curves, cells only; black solid line, anti-TLR4 mAb or mouse isotype IgG; dotted line, without anti-TLR4 mAb or mouse isotype control IgG). Data are representative of four independent experiments. (b) sTLR4 attenuates the binding of MBL to THP-1 cells. sTLR4 protein or HSA (used as a control) was pre-incubated with biotinylated MBL at 37 °C for 30 min, and the mixture was then incubated with the cells for 30 min on ice before the addition of ExtrAvidin-FITC. Binding of MBL was analyzed by FCM (shaded curves, the cells only; black solid line, sTLR4 or HSA; dotted line, without sTLR4 or HSA). Data shown are representative of four independent experiments. FCM, flow cytometry; HSA, human serum albumin; mAb, monoclonal antibody; MBL, mannan-binding lectin; sTLR4, soluble form of Toll-like receptor 4; TLR4, Toll-like receptor 4.
MBL binding to the extracellular domain of TLR4 occurs in a concentration- and Ca2+-dependent manner
We next investigated the direct interaction of MBL with purified sTLR4. Following gel electrophoresis, both glycosylated and deglycosylated sTLR4 proteins were visualized as a band at ∼75 kDa by Coomassie blue staining. For ligand blot analysis, polyvinylidene difluoride membrane was incubated with human MBL or HSA and then probed with anti-MBL mAb. Membrane-bound MBL was detected as a band corresponding to that of sTLR4, demonstrating that MBL binds to TLR4. Additionally, our results clearly demonstrate that MBL interacts with both glycosylated and deglycosylated forms of sTLR4 (Figure 10a). As such, we concluded that MBL directly interacts with the peptide portion of sTLR4. These results are consistent with our previous results indicating that carbohydrate binding specificity is not required for MBL to bind to sTLR4.
Figure 10.
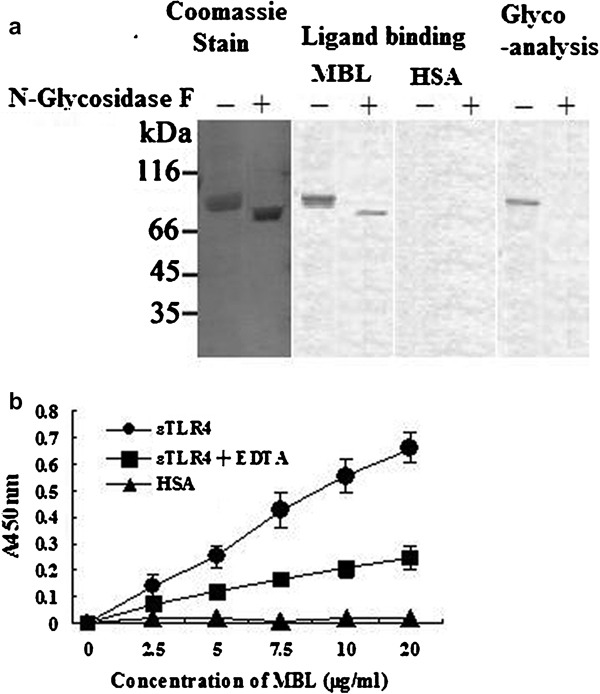
Binding of MBL to the extracellular domain of TLR4. (a) Ligand blot analysis. Glycosylated and deglycosylated sTLR4 proteins were electrophoresed and transferred onto a PVDF membrane. The proteins were visualized by Coomassie blue staining. The membrane was also incubated with human MBL or HSA, and membrane-bound MBL was detected by anti-MBL mAb, as described in the ‘Materials and methods' section. The oligosaccharide moieties of sTLR4 were visualized using a Carbohydrate Detection Kit (Glycoanalysis). (b) sTLR4 or HSA was coated onto microtiter wells and incubated with the indicated concentrations of MBL. The binding of MBL to sTLR4 was detected by anti-MBL mAb, as described in the ‘Materials and methods' section. The data shown are mean±SE of three experiments. HSA, human serum albumin; mAb, monoclonal antibody; MBL, mannan-binding lectin; PVDF, polyvinylidene difluoride; sTLR4, soluble form of Toll-like receptor 4; TLR4, Toll-like receptor 4.
To further examine the binding properties of MBL, we employed sTLR4-coated microtiter wells. As shown in Figure 10b, MBL bound to the solid phase sTLR4, but not HSA, in a concentration-dependent manner. Additionally, this observed binding appears to be Ca2+-dependent because substitution of EDTA for Ca2+ significantly inhibited this process. These results suggest that the lectin property of MBL plays an important role in regulating these interactions. Taken together, these results indicate that MBL binds not only to the peptide portion, but also the oligosaccharide moieties of sTLR4.
Discussion
As a major soluble pattern-recognition receptor in the innate immune system, MBL has long been known to recognize pathogens or autologous apoptotic cells via its CRD and interact with autologous normal cells via its collagen-like region. It has been presumed that MBL-CRD does not interact with autologous normal cells, but recently, Downing et al.29 reported that MBL could bind to autologous B lymphocytes, monocytes and immature DCs in a calcium-dependent manner through the function of its C-type lectin-binding sites , suggesting a new role for MBL in the immune system.
Our study showed that treatment with MBL at high concentrations (10–20 µg/ml), but not at a low concentration (1 µg/ml), significantly inhibited LPS-induced TNF-α and IL-12 p40+p70 secretion. We also found that MBL at 20 µg/ml significantly decreased mRNA expression of TNF-α, IL-12p35 and IL-12p40 in THP-1 cells as indicated by RT-PCR analysis, indicating that MBL may also play a role in the cytokine network. Our data provide clear evidence demonstrating that MBL was able to bind to THP-1 cells in the presence physiological concentrations of calcium, and the binding was optimal at supraphysiological calcium concentrations. Additionally, at physiological calcium concentrations, activation of THP-1 cells by LPS treatment resulted in increased MBL binding. The binding of MBL to the surface of THP-1 cells attenuates the binding of LPS, resulting in less NF-κB activation. This, in turn, leads to a reduction in both mRNA synthesis and protein production. Together, these data suggest that regulation of MBL occurs at the level of ligand binding.
To further investigate the mechanisms underlying this process, we studied possible interactions between MBL and certain surface molecules on THP-1 cells, as well as signaling pathways initiated by these molecules. Because LPS-/TLR-signaling pathways play crucial roles in the regulation of early responses to inflammation, and TLR4 is critical for LPS recognition and signaling,21 it is important to investigate the interaction between MBL and TLR4. Recently, Shimizu et al.33 reported that recombinant MBL could bind to recombinant sTLR4 in a calcium-dependent manner, and that the oligosaccharide moieties of sTLR4 were important for the recognition by MBL. They could not, however, completely exclude the possibility that MBL was binding to peptide portions of sTLR4 because binding inhibition using excess mannose in solid-phase experiments was incomplete, and weak binding to deglycosylated sTLR4 was detected after long exposures using ligand blot analysis. Our data clearly demonstrate that MBL interacts with both glycosylated and deglycosylated sTLR4 proteins, and the concentration of calcium is crucial for the binding of MBL to TLR4 because the presence of EDTA instead of calcium resulted in significant inhibition of binding as seen in solid phase analysis. These results suggest that MBL may bind to both the peptide portion and the oligosaccharide moieties of sTLR4, further demonstrating the versatile ability of MBL to interact with cell surface molecules.
NF-κB is essential for LPS-induced inflammatory reactions, and several previous studies using the human monocytoid cell line THP-1 have demonstrated that LPS can interact with TLR4 and affect cytokine expression via NF-κB signaling pathways.45, 46, 47 NF-κB can be activated within minutes by various stimuli, including inflammatory cytokines such as TNF-α and IL-1. Our study demonstrated that MBL bound directly to TLR4 in a dose-dependent manner. Additionally, the interaction between MBL and TLR4 expressed on THP-1 cells attenuated cell surface binding of LPS and LPS-induced NF-κB translocation in THP-1 cells, suggesting that MBL and TLR may establish a large host defense network.
Monocytes and macrophages play important roles in host defense. Not only are they responsible for pathogen phagocytosis, but they are also involved in numerous additional functions related to immune regulation, such as activation of the complement system, promotion of lymphocyte division and differentiation, and the secretion of dozens of inflammatory factors. The majority of these effects are regulated by inflammatory mediators. Our data indicate that TLR4 expression was upregulated in LPS-activated THP-1 cells. Additionally, THP-1 cells exposed to inflammatory stimuli showed increased MBL binding, possibly due to the upregulated expression of TLR4 in these cells, further demonstrating the versatility of MBL under inflammatory conditions.
LPS-activated cells can induce microcirculatory dysfunction and inflammatory changes, resulting in various types of tissue damage, circulatory failure, and occasionally death.48, 49, 50 To prevent excessive and prolonged responses of host innate immune cells to LPS, the host may acquire a system for downregulation to ensure safe responses to LPS and/or unresponsiveness to a second stimulation with LPS, a process known as LPS tolerance.51 Several previous studies have demonstrated LPS tolerance in THP-1 cells.45, 46, 52 The observation that high concentrations of MBL can inhibit LPS-induced secretion of proinflammatory cytokines suggests that MBL may be responsible for such downregulation mechanisms under conditions of inflammation. Additionally, using the Limulus amebocyte lysate assay, it was determined that the MBL preparation used for this study was endotoxin-free, ruling out the possibility that LPS tolerance was induced by endotoxin contamination of the MBL preparation.
The serum concentration of MBL normally ranges from 0.01 to 10 µg/ml,32, 53 and as an acute-phase protein, MBL can increase up to threefold under inflammatory conditions.54 MBL is also present in synovial fluid and amniotic fluid and in lung and vaginal lavages,55, 56, 57, 58, 59 where it behaves as an acute-phase reactant in individuals with ‘sufficient' (wild-type) MBL genotypes.54 We therefore presume that binding of MBL to the autologous cells most likely occurs at inflammatory loci, where a local elevation of extravascular MBL and a concomitant influx of immune cells are to be expected. The data presented in this paper demonstrate an increased binding of MBL to THP-1 cells under inflammatory conditions and a significant effect of MBL on cytokine expressions at higher concentrations (10–20 µg/ml) that was not observed at lower concentrations (1–5 µg/ml). As such, further investigations may provide evidence that during the acute phase, high concentrations of MBL are excreted to affect cytokine-mediated immune regulation in the serum and in some extravascular local tissues.
It would seem counterintuitive that a protein that suppresses monocytoid cell function would arise acutely just at the time when vigorous immune stimulation is most needed (one to several days post infection). Normally, this is a desirable homeostatic mechanism that would occur after sterilizing immunity had been achieved. Additionally, it is interesting to note that Ip et al.60, 61 recently demonstrated that MBL treatment enhances TLR2 and TLR6 signaling. The finding that MBL inhibits TLR4 function suggests that innate immune receptors may be more discriminating in the signaling systems that they influence than was traditionally thought, and it suggests that they may achieve selective responsiveness to ligands through combinatorial arrangements of receptors.
In summary, we have confirmed that MBL binds to THP-1 cells in a Ca2+-dependent manner under basic and inflammatory culture conditions, and that MBL affects cytokine expression by modulating LPS-/TLR-signaling pathways. This study identifies an important role for MBL in immune regulation and the signaling pathways involved in the cytokine network.
Acknowledgments
This work was supported by the Natural Science Foundation of China (30972679). Special thanks are given to Professors Tianyun Wang, Wenming Yong and Weiren Dong for their dedicated revision of the paper.
References
- Super M, Thiel S, Lu J, Levinsky RJ, Turner MW. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989;2:1236–1239. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- Lu J, Wiedemann H, Timpl R, Reid KB. Similarity in structure between C1q and the collectins as judged by electron microscopy. Behring Inst Mitt. 1993;93:6–16. [PubMed] [Google Scholar]
- Palaniyar N, Zhang L, Kuzmenko A, Ikegami M, Wan S, Wu H, et al. The role of pulmonary collectin N-terminal domains in surfactant structure, function, and homeostasis in vivo. . J Biol Chem. 2002;277:26971–26979. doi: 10.1074/jbc.M110080200. [DOI] [PubMed] [Google Scholar]
- Schweinle JE, Nishiyasu M, Ding TQ, Sastry K, Gillies SD, Ezekowitz RA. Truncated forms of mannose-binding protein multimerize and bind to mannose-rich Salmonella montevideo but fail to activate complement in vitro. . J Biol Chem. 1993;268:364–370. [PubMed] [Google Scholar]
- Ikegami M, Elhalwagi BM, Palaniyar N, Dienger K, Korfhagen T, Whitsett JA, et al. The collagen-like region of surfactant protein A (SP-A) is required for correction of surfactant structural and functional defects in the SP-A null mouse. J Biol Chem. 2001;276:38542–38548. doi: 10.1074/jbc.M102054200. [DOI] [PubMed] [Google Scholar]
- Sheriff S, Chang CY, Ezekowitz RA. Human mannose-binding protein carbohydrate recognition domain trimerizes through a triple alpha-helical coiled-coil. Nat Struct Biol. 1994;1:789–794. doi: 10.1038/nsb1194-789. [DOI] [PubMed] [Google Scholar]
- Hakansson K, Lim NK, Hoppe HJ, Reid KB. Crystal structure of the trimeric alpha-helical coiled-coil and the three lectin domains of human lung surfactant protein D. Structure. 1999;7:255–264. doi: 10.1016/s0969-2126(99)80036-7. [DOI] [PubMed] [Google Scholar]
- Weis WI, Kahn R, Fourme R, Drickamer K, Hendrickson WA. Structure of the calcium-dependent lectin domain from a rat mannose-binding protein determined by MAD phasing. Science. 1991;254:1608–1615. doi: 10.1126/science.1721241. [DOI] [PubMed] [Google Scholar]
- Wallis R, Drickamer K. Asymmetry adjacent to the collagen-like domain in rat liver mannose-binding protein. Biochem J. 1997;325:391–400. doi: 10.1042/bj3250391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Arai T, Kawasaki T. Oligomeric structures required for complement activation of serum mannan-binding proteins. J Biochem. 1995;117:414–419. doi: 10.1093/jb/117.2.414. [DOI] [PubMed] [Google Scholar]
- Fraser IP, Koziel H, Ezekowitz RA. The serum mannose-binding protein and the macrophage mannose receptor are pattern recognition molecules that link innate and adaptive immunity. Semin Immunol. 1998;10:363–372. doi: 10.1006/smim.1998.0141. [DOI] [PubMed] [Google Scholar]
- Zuo DM, Zhang LY, Lu X, Liu Y, Chen ZL. Protective role of mouse MBL-C on intestinal mucosa during Shigella flexneri invasion. Int Immunol. 2009;21:1125–1134. doi: 10.1093/intimm/dxp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. Protein proves to be a key link in innate immunity. Science. 1995;269:301–302. doi: 10.1126/science.7618098. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol. 2004;5:971–974. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54 Pt 1:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6:1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Feng GJ, Goodridge HS, Harnett MM, Wei XQ, Nikolaev AV, Higson AP, et al. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol. 1999;163:6403–6412. [PubMed] [Google Scholar]
- Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Macdonald SL, Downing I, Atkinson AP, Gallagher RC, Turner ML, Kilpatrick DC. Dendritic cells previously exposed to mannan-binding lectin (MBL) enhance cytokine production in allogeneic mononuclear cell cultures. Hum Immunol. 2010;71:1077–1083. doi: 10.1016/j.humimm.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Chaka W, Verheul AF, Vaishnav VV, Cherniak R, Scharringa J, Verhoef J, et al. Induction of TNF-alpha in human peripheral blood mononuclear cells by the mannoprotein of Cryptococcus neoformans involves human mannose binding protein. J Immunol. 1997;159:2979–2985. [PubMed] [Google Scholar]
- Soell M, Diab M, Haan-Archipoff G, Beretz A, Herbelin C, Poutrel B, et al. Capsular polysaccharide types 5 and 8 of Staphylococcus aureus bind specifically to human epithelial (KB) cells, endothelial cells, and monocytes and induce release of cytokines. Infect Immun. 1995;63:1380–1386. doi: 10.1128/iai.63.4.1380-1386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi MC, Raponi G, Angeletti S, Mancini C. Serum-mediated enhancement of TNF-alpha release by human monocytes stimulated with the yeast form of Candida albicans. . J Infect Dis. 1998;178:1743–1749. doi: 10.1086/314484. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Gordon J, Liu H, Sastry KN, Epstein JE, Motwani M, et al. Lack of mannose-binding lectin-A enhances survival in a mouse model of acute septic peritonitis. Microbes Infect. 2002;4:773–784. doi: 10.1016/s1286-4579(02)01597-6. [DOI] [PubMed] [Google Scholar]
- Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J Immunol. 1997;158:4525–4528. [PubMed] [Google Scholar]
- Navratil JS, Watkins SC, Wisnieski JJ, Ahearn JM. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J Immunol. 2001;166:3231–3239. doi: 10.4049/jimmunol.166.5.3231. [DOI] [PubMed] [Google Scholar]
- Downing I, MacDonald SL, Turner ML, Kilpatrick DC. Detection of an autologous ligand for mannan-binding lectin on human B lymphocytes. Scand J Immunol. 2005;62:507–514. doi: 10.1111/j.1365-3083.2005.01693.x. [DOI] [PubMed] [Google Scholar]
- Downing I, Koch C, Kilpatrick DC. Immature dendritic cells possess a sugar-sensitive receptor for human mannan-binding lectin. Immunology. 2003;109:360–364. doi: 10.1046/j.1365-2567.2003.01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser DA, Bohlson SS, Jasinskiene N, Rawal N, Palmarini G, Ruiz S, et al. C1q and MBL, components of the innate immune system, influence monocyte cytokine expression. J Leukoc Biol. 2006;80:107–116. doi: 10.1189/jlb.1105683. [DOI] [PubMed] [Google Scholar]
- Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol Immunol. 2007;44:33–43. doi: 10.1016/j.molimm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Nishitani C, Mitsuzawa H, Ariki S, Takahashi M, Ohtani K, et al. Mannose binding lectin and lung collectins interact with Toll-like receptor 4 and MD-2 by different mechanisms. Biochim Biophys Acta. 2009;1790:1705–1710. doi: 10.1016/j.bbagen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Chiba H, Sano H, Iwaki D, Murakami S, Mitsuzawa H, Takahashi T, et al. Rat mannose-binding protein a binds CD14. Infect Immun. 2001;69:1587–1592. doi: 10.1128/IAI.69.3.1587-1592.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringhuis SI, Dunnen den J, Litjens M, Het Hof van B, Kooyk van Y, Geijtenbeek TB. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Tan SM, Chung MC, Kon OL, Thiel S, Lee SH, Lu J. Improvements on the purification of mannan-binding lectin and demonstration of its Ca2+-independent association with a C1s-like serine protease. Biochem J. 1996;19 Pt 2:329–332. doi: 10.1042/bj3190329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumestre-Perard C, Ponard D, Arlaud GJ, Monnier N, Sim RB, Colomb MG. Evaluation and clinical interest of mannan binding lectin function in human plasma. Mol Immunol. 2002;39:465–473. doi: 10.1016/s0161-5890(02)00119-0. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DC, Fujita T, Matsushita M. P35, an opsonic lectin of the ficolin family, in human blood from neonates, normal adults, and recurrent miscarriage patients. Immunol Lett. 1999;67:109–112. doi: 10.1016/s0165-2478(98)00147-3. [DOI] [PubMed] [Google Scholar]
- Perona-Wright G, Anderton SM, Howie SE, Gray D. IL-10 permits transient activation of dendritic cells to tolerize T cells and protect from central nervous system autoimmune disease. Int Immunol. 2007;18 9:1033–1034. doi: 10.1093/intimm/dxm084. [DOI] [PubMed] [Google Scholar]
- Joyner JL, Augustine NH, Taylor KA, La Pine TR, Hill HR. Effects of group B streptococci on cord and adult mononuclear cell interleukin-12 and interferon-gamma mRNA accumulation and protein secretion. J Infect Dis. 2000;182:974–977. doi: 10.1086/315796. [DOI] [PubMed] [Google Scholar]
- Ma W, Gee K, Lim W, Chambers K, Angel JB, Kozlowski M, et al. Dexamethasone inhibits IL-12p40 production in lipopolysaccharide-stimulated human monocytic cells by down-regulating the activity of c-Jun N-terminal kinase, the activation protein-1, and NF-kappa B transcription factors. J Immunol. 2004;172:318–330. doi: 10.4049/jimmunol.172.1.318. [DOI] [PubMed] [Google Scholar]
- Faure E, Equils O, Sieling PA, Thomas L, Zhang FX, Kirschning CJ, et al. Bacterial lipopolysaccharide activates NF-kappaB through Toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- Hyakushima N, Mitsuzawa H, Nishitani C, Sano H, Kuronuma K, Konishi M, et al. Interaction of soluble form of recombinant extracellular TLR4 domain with MD-2 enables lipopolysaccharide binding and attenuates TLR4-mediated signaling J Immunol2004; 1736949–6954. [DOI] [PubMed] [Google Scholar]
- Murakami S, Iwaki D, Mitsuzawa H, Sano H, Takahashi H, Voelker DR, et al. Surfactant protein A inhibits peptidoglycan-induced tumor necrosis factor-alpha secretion in U937 cells and alveolar macrophages by direct interaction with Toll-like receptor 2. J Biol Chem. 2002;277:6830–6837. doi: 10.1074/jbc.M106671200. [DOI] [PubMed] [Google Scholar]
- Wang JH, Doyle M, Manning BJ, Wu Di Q, Blankson S, Redmond HP. Induction of bacterial lipoprotein tolerance is associated with suppression of Toll-like receptor 2 expression. J Biol Chem. 2002;277:36068–36075. doi: 10.1074/jbc.M205584200. [DOI] [PubMed] [Google Scholar]
- Li CH, Wang JH, Redmond HP. Bacterial lipoprotein-induced self-tolerance and cross-tolerance to LPS are associated with reduced IRAK-1 expression and MyD88-IRAK complex formation. J Leukoc Biol. 2006;79:867–875. doi: 10.1189/jlb.0905505. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Martin M, Schifferle RE, Genco RJ. Counteracting interactions between lipopolysaccharide molecules with differential activation of Toll-like receptors. Infect Immun. 2002;70:6658–6664. doi: 10.1128/IAI.70.12.6658-6664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg MA, Keppler D, Galanos C. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect Immun. 1986;51:891–895. doi: 10.1128/iai.51.3.891-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek SM, Moore RN, McGhee JR, Rosenstreich DL, Mergenhagen SE. The primary role of lymphoreticular cells in the mediation of host responses to bacterial endotoxim. J Infect Dis. 1980;141:55–63. doi: 10.1093/infdis/141.1.55. [DOI] [PubMed] [Google Scholar]
- Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW, Wedel A, Schraut W, Strobel M, Wendelgass P, Sternsdorf T, et al. Tolerance to lipopolysaccharide involves mobilization of nuclear factor kappa B with predominance of p50 homodimers. J Biol Chem. 1994;269:17001–17004. [PubMed] [Google Scholar]
- Mizel SB, Snipes JA. Gram-negative flagellin-induced self-tolerance is associated with a block in interleukin-1 receptor-associated kinase release from Toll-like receptor 5. J Biol Chem. 2002;277:22414–22420. doi: 10.1074/jbc.M201762200. [DOI] [PubMed] [Google Scholar]
- Turner MW, Hamvas RM. Mannose-binding lectin: structure, function, genetics and disease associations. Rev Immunogenet. 2000;2:305–322. [PubMed] [Google Scholar]
- Thiel S, Holmskov U, Hviid L, Laursen SB, Jensenius JC. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin Exp Immunol. 1992;90:31–35. doi: 10.1111/j.1365-2249.1992.tb05827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med. 1995;1:237–243. doi: 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- Malhotra R, Willis AC, Lopez Bernal A, Thiel S, Sim RB. Mannan-binding protein levels in human amniotic fluid during gestation and its interaction with collectin receptor from amnion cells. Immunology. 1994;82:439–444. [PMC free article] [PubMed] [Google Scholar]
- Gomi K, Tokue Y, Kobayashi T, Takahashi H, Watanabe, Fujita T, et al. Mannose-binding lectin gene polymorphism is a modulating factor in repeated respiratory infections. Chest. 2004;126:95–99. doi: 10.1378/chest.126.1.95. [DOI] [PubMed] [Google Scholar]
- Babula O, Lazdane G, Kroica J, Ledger WJ, Witkin SS. Relation between recurrent vulvovaginal candidiasis, vaginal concentrations of mannose-binding lectin, and a mannose-binding lectin gene polymorphism in Latvian women. Clin Infect Dis. 2003;37:733–737. doi: 10.1086/377234. [DOI] [PubMed] [Google Scholar]
- Pellis V, de Seta F, Crovella S, Bossi F, Bulla R, Guaschino S, et al. Mannose binding lectin and C3 act as recognition molecules for infectious agents in the vagina. Clin Exp Immunol. 2005;139:120–126. doi: 10.1111/j.1365-2249.2005.02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip WK, Takahashi K, Moore KJ, Stuart LM, Ezekowitz RA. Mannose-binding lectin enhances Toll-like receptors 2 and 6 signaling from the phagosome. J Exp Med. 2008;205:169–181. doi: 10.1084/jem.20071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip WK, Takahashi K, Ezekowitz RA, Stuart LM. Mannose-binding lectin and innate immunity. Immunol Rev. 2009;230:9–21. doi: 10.1111/j.1600-065X.2009.00789.x. [DOI] [PubMed] [Google Scholar]


