Abstract
Anemia and immunological dysfunction (i.e. immunosenescence) are commonly found in older subjects and nutritional approaches are sought to counteract these phenomena. Spirulina is a filamentous and multicellular bule-green alga capable of reducing inflammation and also manifesting antioxidant effects. We hypothesized that Spirulina may ameliorate anemia and immunosenescence in senior citizens with a history of anemia. We enrolled 40 volunteers of both sexes with an age of 50 years or older who had no history of major chronic diseases. Participants took a Spirulina supplementation for 12 weeks and were administered comprehensive dietary questionnaires to determine their nutritional regimen during the study. Complete cell count (CCC) and indoleamine 2,3-dioxygenase (IDO) enzyme activity, as a sign of immune function, were determined at baseline and weeks 6 and 12 of supplementation. Thirty study participants completed the entire study and the data obtained were analyzed. Over the 12-week study period, there was a steady increase in average values of mean corpuscular hemoglobin in subjects of both sexes. In addition, mean corpuscular volume and mean corpuscular hemoglobin concentration also increased in male participants. Older women appeared to benefit more rapidly from Spirulina supplements. Similarly, the majority of subjects manifested increased IDO activity and white blood cell count at 6 and 12 weeks of Spirulina supplementation. Spirulina may ameliorate anemia and immunosenescence in older subjects. We encourage large human studies to determine whether this safe supplement could prove beneficial in randomized clinical trials.
Keywords: functional food, immunosenescence, red blood cell, IDO
Introduction
Aging is physiologically associated with a variable decline in several changes of the hematopoietic system, including anemia and immunosenescence. The latter is coined as the aging of the immune system and is characterized by a reduced activity against infections.1, 2, 3 Both innate and acquired arms of the immune system including immune specific cells, cell signaling, cytokine production and cell surface marker expression are affected,4 clinically relevant5 with enormous healthcare costs.6 Strategies to stimulate the immune system and improve the effectiveness of vaccines in senior citizens are of seminal importance7 as a more robust immune response could lead to lower rates of infection and infectious disease-associated morbidity and mortality in this susceptible and vulnerable population.6 Variable degrees of reduction of hemoglobin (HGB) levels (i.e. anemia) are also commonly encountered in senior populations,8 thus significantly affecting the quality of life of patients.9 An adequate nutritional intake is physiologically necessary for normal red cell production and even in the absence of clinically significant anemia, red cell defects may reflect a nutritionally at risk host related to poor nutritional decisions.
The study of nutritional influences on anemia and the immune system represents an area of growing interest to nutritionists, food scientists, hematologists and immunologists as the immune system cells within the digestive tract are an accessible and ideal target to stimulate immunity10, 11, 12 and possibly enhance red cell production and function.8 This is also based on the significant and dynamic interaction between luminal nutrients and the overall immune function.12, 13
Spirulina, a cyanobacterial blue-green algae commonly used as a dietary supplement, is amongst the most ‘nutritionally packed' dietary supplement available. With respect to immunity, it modulates the production of cytokines by human peripheral blood mononuclear cells, not surprising given the rich content of flavanoids and sulfalipids.14 Spirulina products contain bioactive proteins with the ability to stimulate the intestinal immune system15 to enhance the responsiveness to vaccines and improve allergic rhinitis.16, 17
Based on the available data, we hypothesized that dietary Spirulina supplementation can improve anemia as well as immune function in volunteers >50 years of age with a diagnosis of anemia who were supplemented with Spirulina for 12 weeks. Endpoints included red blood cell (RBC) indices within complete blood count (CBC) and indoleamine 2,3-dioxygenase (IDO) enzyme activity as a sign of immune function18 at baseline and weeks 6 and 12. Our data support the effects of Spirulina on several variables suggesting an amelioration of the anemia status and immune function, particularly in senior citizens.
Materials and methods
Subjects
Subjects were recruited from Sacramento, CA and the surrounding area by advertisements in local newspapers. Interested individuals were initially screened over the phone for eligibility criteria. Subjects were eligible if they had an age >50 years and a diagnosis of anemia (HGB levels, <12 g/dl for women and <13 g/dl for men) during the previous 12 months. Eligible subjects were then invited to undergo a clinical evaluation for further health history assessment. Exclusion criteria included: any underlying neoplasia or immunological disease; the use oral steroids or other immunosuppressive agents; ongoing prolonged use of Spirulina; food faddism or other non-traditional diet; known history of Spirulina allergy; chronic renal failure; chronic inflammatory diseases taking daily doses of anti-inflammatory drugs for longer than 4 weeks at the time of enrollment; and reduced physical activity (i.e. NYHA classes III–IV). Enrolled subjects completed an online dietary questionnaire (see below) and once included in the study were instructed to take 6 tablets of 500 mg Spirulina per day for a 12-week period. Blood samples were collected at 0, 6 and 12 weeks.
A total of 148 subjects responded to our advertisement and 81 indicated an interest to continue with the telephone screening procedure. Of these, 53 subjects were eligible and 40 agreed to undergo a clinical evaluation. Eligible subjects who declined to participate noted time restrictions or travel plans or inconvenience due to distance or refused to take the tablet. Thirty subjects (mean age 63 years) completed the entire study, including dietary questionnaires and 12 weeks of Spirulina supplement. Of these 30 subjects, 14 were between the ages of 50 and 60, 10 between 61 and 70, and 6 over 70 years of age.
Dietary questionnaire
Enrolled subjects were asked to complete an online food frequency questionnaire (FFQ) to provide information on their regular eating habits. The FFQ was developed by Nutritionquest (Berkeley, CA, USA) and has been extensively used and validated for research purposes19, 20 to include over 50 essential and non-essential nutrients. Subjects were asked to self-administer two questionnaires during the duration of the study, one after the first clinic visit (FFQ1, baseline) and again after the second clinic visit (FFQ2, week 12). The questionnaire was modified to focus specifically on eating habits during the past month. Average intake values and differences from FFQ1 and FFQ2 for each nutrient of interest were used for analysis.
IDO activity and CBC variables
To determine the IDO enzyme activity, serum concentrations of tryptophan (µmol/l) and kynurenine (µmol/l) were measured by reverse-phase high-performance liquid chromatography,21, 22, 23 based on the principle that tryptophan is converted to kynurenine by the action of IDO. As a consequence, the tryptophan/kynurenine ratio will accurately reflect the IDO activity. The IDO activity was then calculated by determination of kynurenine/tryptophan (µmol/mmol) ratio. Blood samples collected from subjects were tested for CBCs using routine laboratory methods and gathered data included HGB, hematocrit (HCT), mean corpuscular HGB concentration (MCHC), mean corpuscular HGB (MCH), mean corpuscular volume (MCV) and white blood cells (WBCs).
Statistical analysis
Complete data sets from 30 subjects who finished the entire study including dietary questionnaire and 12 weeks of Spirulina supplement were used for analysis. Partial data on the other 10 enrolled subjects who did not finish the entire study were available but were not included. Regression analysis was used to determine significant correlations between average diet and immune and anemia endpoints. Student's t-test was used to determine whether there was a significant difference in average consumption of key nutrients during the duration of the study. For all tests P values of <0.05 were considered statistically significant and Intercooled Stata 8.0 for Macintosh (Stata Corporation, College Station, TX, USA) was used for all analyses.
Results
Nutrient intake
Nutritional variables included total energy, total protein, total fat, total carbohydrate, iron, glutathione, folate, vitamin E, zinc, vitamin A, vitamin B1, vitamin D, vitamin C and vitamin B12. No significant differences were observed for any of these indices between the two time-points (FFQ1 and FFQ2) while the average nutrient intakes are illustrated in Table 1.
Table 1. Daily dietary intake of nutrients at the two study time-points. P values were obtained using the Student's t-test.
| Nutrient | FFQ1 | FFQ2 | P value |
|---|---|---|---|
| Total energy (kCal) | 1746.81 | 1629.52 | ns |
| Total protein (g) | 70.49 | 66.13 | ns |
| Total fat (g) | 71.52 | 67.77 | ns |
| Total carbohydrate (g) | 205.89 | 183.99 | ns |
| Dietary iron (mg) | 14.72 | 13.38 | ns |
| Dietary glutathione | 42.97 | 42.31 | ns |
| Dietary folate | 338.95 | 345.04 | ns |
| Dietary vitamin E | 8.80 | 9.18 | ns |
| Dietary zinc (mg) | 11.20 | 9.50 | ns |
| Vitamin A (RAE) | 990.76 | 1028.52 | ns |
| Vitamin B1 (mcg) | 4.97 | 4.64 | ns |
| Vitamin D (IU) | 122.00 | 122.11 | ns |
Abbreviations: FFQ, food frequency questionnaire; ns, non significant; RAE, retinol activity equivalents.
CBC
CBC variables at baseline, week 6 and week 12 are illustrated in Table 2. Differences in the values of each variable between baseline, week 6 and week 12 were calculated and illustrated in Figure 1.
Table 2. Raw values of CBC variables and IDO activity in subgroups of study participants at the three time-points (weeks 0, 6 and 12). Values are expressed as mean and standard deviation.
| Baseline | Total(n=30) | Females(n=15) | Males(n=15) | Females 50–60 years (n=7) | Females 61–70 years (n=5) | Females ≥71 years (n=3) | Males 50–60 years (n=7) | Males 61–70 years (n=5) | Males ≥71 years (n=3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | |
| WBC (/mm3) | 5.89 | 1.42 | 5.973 | 1.676 | 5.8 | 1.169 | 6.43 | 2.12 | 5.8 | 1.24 | 5.2 | 1.56 | 5.4 | 0.65 | 7.42 | 1.53 | 4.95 | 0.64 |
| RBC (/mm3) | 4.26 | 0.68 | 4.135 | 0.432 | 4.375 | 0.865 | 4.26 | 0.43 | 4.14 | 0.39 | 3.95 | 0.65 | 4.81 | 0.86 | 4.25 | 0.77 | 3.96 | 0.19 |
| Hemoglobin (g/dl) | 12.1 | 1.52 | 11.21 | 1.279 | 12.91 | 1.266 | 11.5 | 1.31 | 11.5 | 0.56 | 10.2 | 2.33 | 13.4 | 0.92 | 12.92 | 1.31 | 12.9 | 0.07 |
| Hematocrit (%) | 36.8 | 4.19 | 34.72 | 3.269 | 38.87 | 4.052 | 35.9 | 2.96 | 35.4 | 1.61 | 31.1 | 5.73 | 40.4 | 3.18 | 39.04 | 4.23 | 38.1 | 1.06 |
| MCV (fl) | 87.6 | 8.5 | 84.7 | 6.642 | 90.49 | 9.362 | 85.5 | 7.18 | 85.9 | 7.07 | 78.4 | 1.56 | 85.5 | 10.1 | 92.96 | 7.93 | 96.3 | 1.98 |
| MCH (pg) | 28.8 | 3.32 | 27.43 | 2.562 | 30.11 | 3.524 | 27.7 | 2.83 | 28 | 2.95 | 25.6 | 1.56 | 28.3 | 3.97 | 30.82 | 2.84 | 32.6 | 1.34 |
| MCHC (%) | 32.7 | 1.01 | 32.36 | 0.969 | 33.09 | 0.943 | 32.4 | 1.14 | 32.6 | 0.79 | 32.7 | 1.34 | 32.8 | 1.17 | 33.12 | 0.54 | 33.8 | 0.71 |
| Platelets (/mm3) | 259 | 77.7 | 303 | 69.21 | 212.5 | 57.28 | 309 | 69.5 | 253 | 52.1 | 344 | 43.8 | 193 | 39.5 | 245 | 36.12 | 288 | 103 |
| IDO (K/T ratio) | 15.73 | 8.65 | 15.89 | 8.17 | 15.57 | 9.39 | 14.04 | 4.84 | 13 | 4.17 | 17.17 | 5.95 | 11.6 | 1.5 | 13.81 | 1.4 | 14.79 | 0.75 |
| Week 6 | ||||||||||||||||||
| WBC (/mm3) | 6.17 | 1.49 | 6.007 | 1.769 | 6.333 | 1.184 | 6.26 | 2.31 | 5.54 | 0.98 | 6.4 | 2.12 | 5.986 | 0.85 | 6.86 | 1.53 | 5.55 | 0.49 |
| RBC (/mm3) | 4.24 | 0.71 | 4.113 | 0.467 | 4.369 | 0.897 | 4.11 | 0.46 | 4.18 | 0.52 | 4.34 | 0.11 | 4.891 | 0.79 | 4.18 | 0.81 | 3.88 | 0.06 |
| Hemoglobin (g/dl) | 12.2 | 1.72 | 11.3 | 1.423 | 13.01 | 1.584 | 11.5 | 1.55 | 11.4 | 1.32 | 11.6 | 0.14 | 13.89 | 1.04 | 12.72 | 1.45 | 12.5 | 0.85 |
| Hematocrit (%) | 37 | 4.79 | 34.93 | 3.877 | 39.07 | 4.818 | 35.3 | 4.11 | 35.7 | 3.39 | 35.5 | 0.21 | 41.54 | 2.92 | 38.34 | 5.46 | 37.3 | 1.77 |
| MCV (fl) | 88.3 | 8.3 | 85.55 | 6.966 | 91.06 | 8.826 | 86 | 7.23 | 87.2 | 8.68 | 81.9 | 2.47 | 86.19 | 9.59 | 93 | 6.62 | 96 | 3.04 |
| MCH (pg) | 28.9 | 3.3 | 27.55 | 2.698 | 30.33 | 3.331 | 28.1 | 3.21 | 27.6 | 3.11 | 26.8 | 0.35 | 28.87 | 3.81 | 30.82 | 2.87 | 32.4 | 1.63 |
| MCHC (%) | 32.8 | 1.03 | 32.35 | 1.017 | 33.27 | 0.854 | 32.6 | 1.19 | 32.1 | 1.11 | 32.9 | 0.78 | 33.44 | 0.98 | 33.08 | 0.79 | 33.7 | 0.71 |
| Platelets (/mm3) | 246 | 67.2 | 277.5 | 62.95 | 213.9 | 56.81 | 290 | 57 | 243 | 58.9 | 325 | 64.3 | 212.4 | 49.09 | 210.8 | 72.6 | 259 | 69.3 |
| IDO (K/T ratio) | 16.14 | 8.75 | 17.97 | 11.19 | 14.32 | 5.11 | 13.91 | 6.41 | 18.51 | 14.15 | 18.76 | 1.31 | 12.18 | 3.63 | 13.73 | 4.16 | 13.05 | 0.04 |
| Week 12 | ||||||||||||||||||
| WBC (/mm3) | 6.07 | 1.63 | 6.007 | 1.912 | 6.14 | 1.355 | 6.2 | 2.48 | 5.72 | 1.26 | 6.5 | 2.12 | 5.89 | 0.97 | 5.74 | 3.66 | 5.1 | 0.42 |
| RBC (/mm3) | 4.22 | 0.61 | 4.111 | 0.276 | 4.327 | 0.817 | 4.1 | 0.18 | 4.22 | 0.29 | 4.18 | 0.44 | 4.78 | 0.73 | 3.35 | 2.01 | 3.91 | 0.08 |
| Hemoglobin (g/dl) | 12.1 | 1.55 | 11.25 | 1.189 | 12.93 | 1.431 | 11.3 | 1.48 | 11.9 | 1 | 11.3 | 1.2 | 13.6 | 0.92 | 10.3 | 5.93 | 12.8 | 0.92 |
| Hematocrit (%) | 36.7 | 4.07 | 34.75 | 2.859 | 38.69 | 4.231 | 35.2 | 3.32 | 35.6 | 2.08 | 34.9 | 5.02 | 40.8 | 2.69 | 31 | 17.8 | 36.7 | 1.91 |
| MCV (fl) | 87.8 | 8.27 | 84.68 | 6.731 | 90.85 | 8.708 | 85.8 | 7.76 | 84.7 | 6.86 | 83.2 | 3.39 | 86.6 | 9.83 | 74.6 | 42.3 | 93.8 | 2.83 |
| MCH (pg) | 28.9 | 3.44 | 27.43 | 2.932 | 30.39 | 3.337 | 27.7 | 3.47 | 28.2 | 3.11 | 26.9 | 0.14 | 28.8 | 3.97 | 25 | 14.2 | 32.6 | 1.56 |
| MCHC (%) | 32.9 | 1.2 | 32.32 | 1.183 | 33.41 | 0.962 | 32.2 | 1.28 | 33.3 | 1.01 | 32.4 | 1.2 | 33.2 | 0.96 | 26.8 | 15 | 34.8 | 0.64 |
| Platelets (/mm3) | 245 | 73.2 | 284.5 | 59.87 | 205.9 | 64.79 | 303 | 52.2 | 234 | 41.5 | 324 | 5.66 | 210 | 65.9 | 141 | 101 | 260 | 61.5 |
| IDO (K/T ratio) | 16.21 | 10.26 | 18.13 | 13.53 | 14.31 | 5.22 | 14.58 | 6.41 | 14.29 | 2.82 | 17.14 | 0.05 | 13.44 | 5.54 | 11.58 | 1.8 | 15 | 2.55 |
Abbreviations: CBC, complete blood count; IDO, indoleamine 2,3-dioxygenase; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; RBC, red blood cell; WBC, white blood cell.
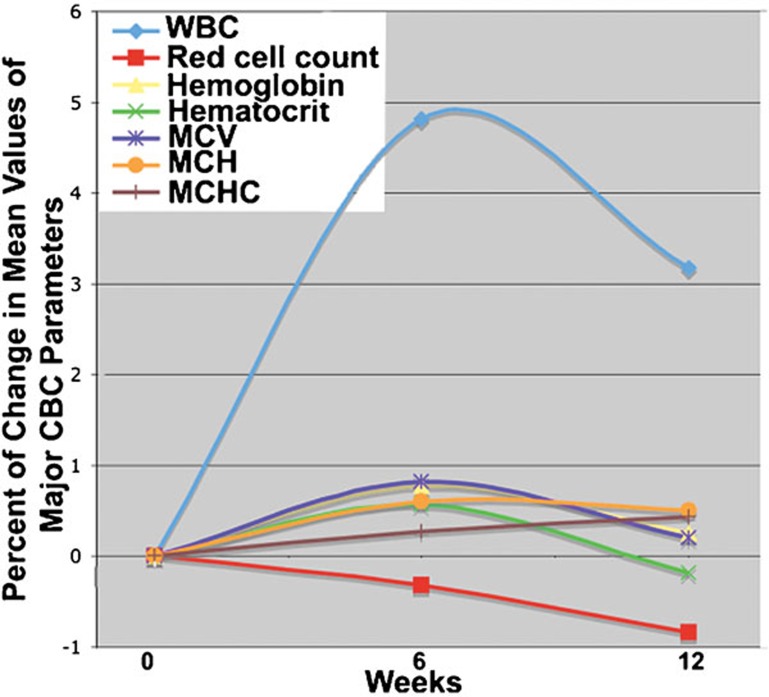
Figure 1.
Percentage of change between weeks 0, 6 and 12 in mean values of major CBC parameters of all subjects (n=30) who completed the Spirulina supplementation period. CBC, complete blood count; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; WBC, white blood cell.
We observed a significant increase in MCH, MCV and MCHC values between baseline and subsequent weeks in men while MCH was elevated in women during the supplementation. No significant changes were observed in HGB levels or the counts of WBCs and platelets in any subgroup. The percentage of subjects manifesting an increase in each CBC variable is illustrated in Figure 2. Of note, over half of the subjects had increased values of HCT, MCV and MCH after 6 weeks of Spirulina supplementation and MCHC after 12 weeks. When subjects were arrayed according to sex, over 50% of enrolled women had increased HCT at 6 weeks and MCHC at 12 weeks. Over half of the male study participants had increased HGB, HCT, MCV and MCH at week 6 and all these changes were found also at 12 weeks with the exception of HCT.
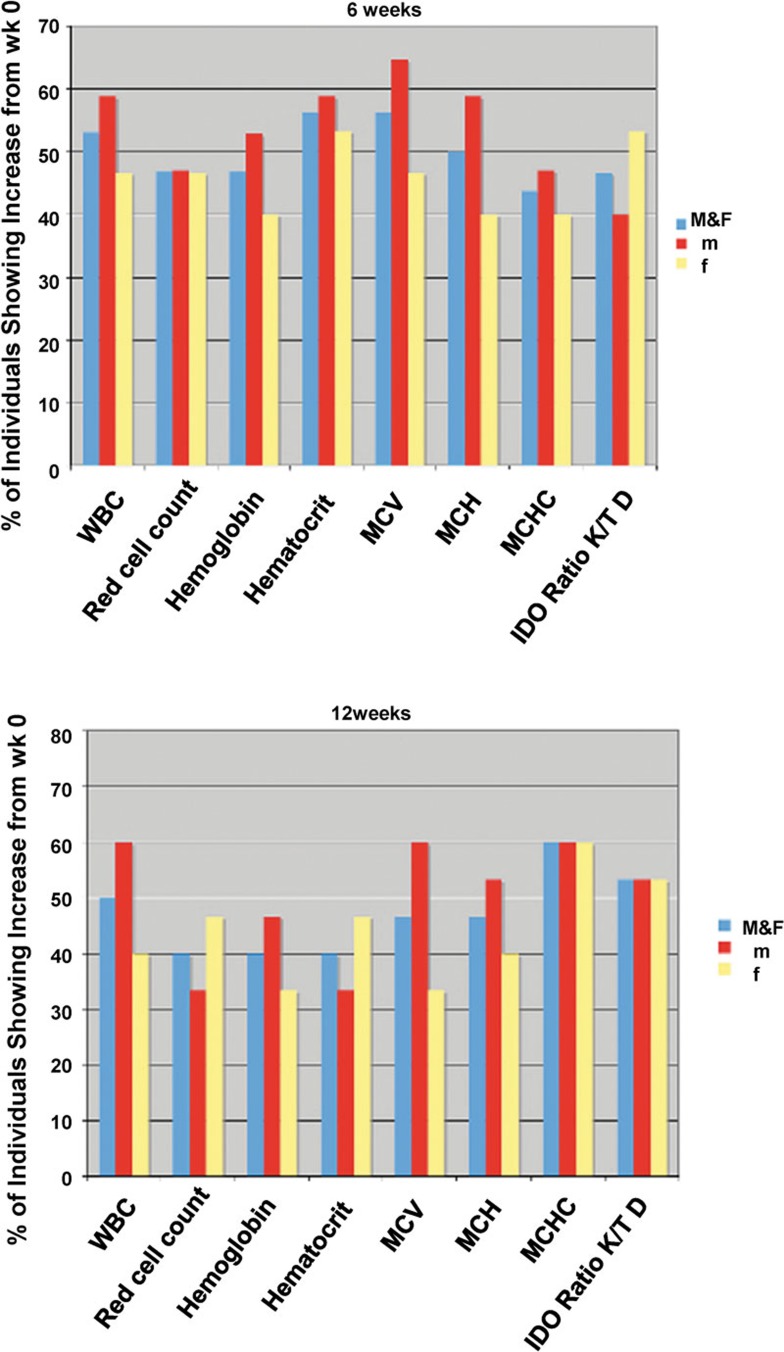
Figure 2.
Percentage of participants in which an increase in CBC variables and IDO activity between 0 and 6 (upper panel) or 12 (lower panel) weeks. CBC, complete blood count; IDO, indoleamine 2,3-dioxygenase; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; WBC, white blood cell.
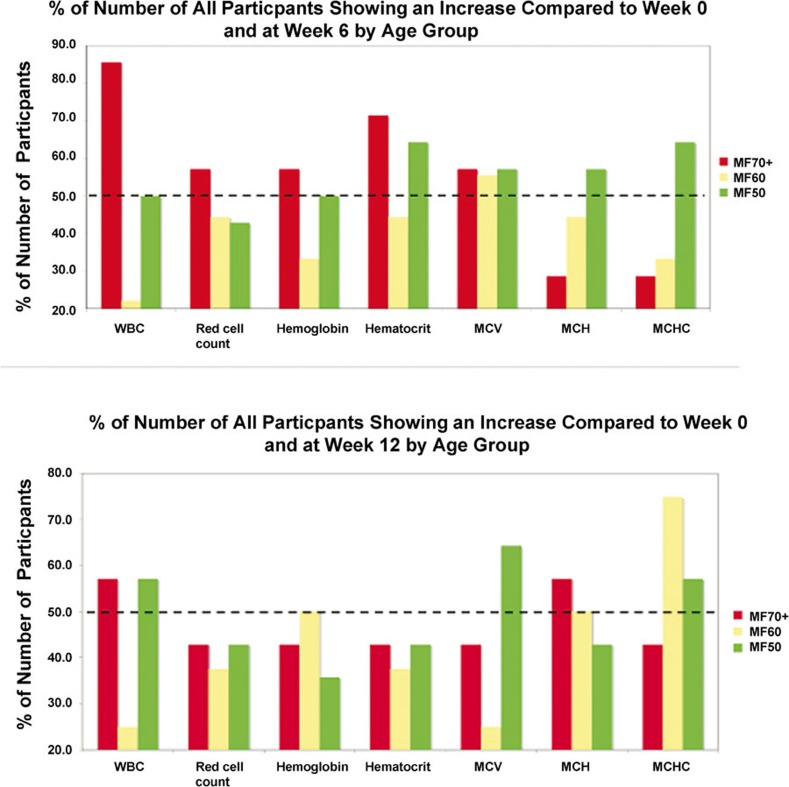
Subjects were then subdivided according to age ranges (50–60, 61–70 and ≥71 years) (Figure 3). At 6 weeks the oldest subject population had increased RBC, HGB, HCT and MCV, while younger subjects had positive changes in MCV (61–70 years group) or HCT, MCV, MCH and MCHC (50–60 years group). At week 12 an increase in WBCs and MCH was observed in over 50% of the oldest subjects, MCH and MCHC in subjects between the ages of 61 and 70 years while the youngest group had increased MCV and MCHC.
Figure 3.
Percentage of all subjects at age 70 and above, 60–70 years old and 50–60 years old showing an increase in WBC, red cell count, hemogloblin, hematocrit, MCV, MCH and MCHC from 0 to 6 weeks and 6–12 weeks. MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; WBC, white blood cell.
IDO activity and WBC count
IDO activity was compared between baseline and weeks 6 and 12. Over 50% of subjects taking Spirulina had increased IDO activity at both time-points (Figure 2) while this proportion was striking in men with over 75% of subjects manifesting such phenomenon.
As a further measurement of immune function, WBC count from CBC was compared at different time-points (Figure 2). WBCs manifested an increase in over 50% of subjects receiving Spirulina for 6 weeks, particularly in men in which the increase was observed also at week 12, although values remained within the physiological upper value. Both older study populations (61–70 and ≥71 years old) had increased WBCs at weeks 6 and 12.
Discussion
Managing anemia and immunological dysfunctions that commonly characterize aging is a clinical challenge. Similarly, providing nutritional approaches through functional foods to counteract these phenomena is a research priority. The data obtained from our open-label uncontrolled study suggest that Spirulina could prove beneficial for both conditions. Anemia is responsible for a large number of hospitalizations and the cause of enormous healthcare costs,24 particularly due to a high prevalence among senior citizens.25 Anemia can be secondary to a variety of factors, including problems with RBC production and destruction or blood loss. In all cases, and even including in normal individuals, an adequate nutrition intake is necessary for normal red cell production. Among individuals who may not manifest clinically overt signs and symptoms of anemia, there may be defects in red cell indices that reflect a nutritional at risk host. This may be particularly a problem due to the enormous use of fast foods and poor health nutrition decisions by the older population.26
Aging is physiologically associated with a variable decline in several features of the immune function. In fact, immunosenescence or aging of the immune system, is characterized by a reduced ability to fight infection and mount an adequate immune response once a novel infection is introduced. Both the innate and acquired immune systems can be affected with immune specific cells, cell signaling, cytokine production and cell surface marker expression being all subject to age-related changes.4 These may ultimately be clinically relevant, particularly to the susceptibility to infections and the decreased reactivity to vaccination against microorganisms.5 The immune system cells within the digestive tract are an accessible and ideal target to stimulate immunity10, 11, 12 and diet-based nutritional interventions represent a non-invasive, relatively low-cost and effective method of stimulating the immune function, thus ultimately improving the antibody production in subjects at risk for immunosenescence. This is also based on the significant and dynamic interaction between luminal nutrients and the overall immune function.12 The study of nutritional influences on the immune system represents an area of growing interest to nutritionists, food scientists and immunologists. In general terms, dietary components have the potential to be an accessible and effective immune stimulant given the antigen presenting capacity of the intestine.13
Spirulina is an multicellular cyanobacterium within the Oscillatoriaceae algae family27 which is commonly used as a food additive rich in proteins, carotenoids, vitamins and minerals.15 Spirulina is not a new drug as its story as a nutritional element is quite old, dating back to the Aztecs.28 Inhibitory effects of Spirulina on atherosclerosis were reported29 possibly via the inhibition of leptin secretion and improvement of leptin resistance. Previous studies support the beneficial effects of Spirulina against fatty liver,30, 31 oxidative stress,32 hyperglicemia,33, 34 hypercholesterolemia,35 and arterial hypertension.36 We hypothesized that Spirulina may provide a unique source of nutraceuticals that will enhance the homeostatic hematologic and immunological systems.
Our data cumulatively suggest that Spirulina daily supplementation, regardless of other dietary intake, may prove beneficial for major variables associated with anemia. Indeed, this applies to RBC number, size and HGB content and is particularly relevant in advanced age and male subjects. Over the 12-week study period, there was a steady increase in average values of MCH in both male and female subjects. In addition, MCV and MCHC were also steadily increasing in male subjects. Females at more advanced ages appears to benefit quickly from Spirulina supplements. At 6 weeks of Spirulina supplementation, the percentage of female subjects showing an increase in the number of parameters appears to increase with age, with the highest improvement in the ≥71 years group. Prolonged supplementation, at 12 weeks of Spirulina supplementation, appears to provide the highest benefit to those between 60 and 70 years old among female subjects. Conversely, 50–60 years old male subjects appear to benefit from Spirulina supplementation. In fact, at 6 and 12 weeks of Spirulina supplementation the youngest male group (50–60 years old) had the highest incidence of increase in numerous CBC variables. At 6 and 12 weeks of supplementation, over 50% of male subjects at 50–60 years had increased values in all and 5/7 CBC parameters, respectively. Most importantly, during the study period, there were no significant changes in average dietary intake with respect to total energy, total protein, total fat, total carbohydrate, iron, glutathione, folate, vitamin E, zinc, vitamin A, vitamin B1 and vitamin D.
IDO, an enzyme degrading tryptophan to kynurenine, has emerged as an important regulator of the immune system. IDO is expressed in a variety of immune cell types including B cells, macrophages, eosinophils and dendritic cells. Accumulating evidence has implicated the role of IDO in aging and immune competence. For example, IDO activity was found to be significantly higher in nonagenarians than in control subjects (median age=45 years) and that increased IDO activity might be a mechanism involved in the decline of T-cell responses in immunosenescence.18 Using an in vitro system consisting of IDO-expressing fibroblasts with bystander human CD4+ and CD8+ T cells, Forouzandeh et al. showed that IDO-induced low tryptophan environment differentially influences the viability and proliferation of both activated CD4+ and CD8+ T cells; this effect was greater in CD8+ cells compared to CD4+ T cells. This differential effect seems to be due, at least in part, to differences in the activation of GCN2 kinase pathway.37 Another study has shown that enhanced IDO activity was associated with reduced cognitive performance in patients with dementia.38 Furthermore, it has also been shown that IDO-dependent immunosuppressive mechanisms are activated in patients with systemic lupus erythematosus22 and primary biliary cirrhosis.39 In addition, increased activity of IDO induced by the activation of macrophage may play a role in the etiology of neurological symptoms in familial erythrophagocytic lymphohistiocytosis.40 Thus, IDO activity is becoming a recognized and relevant marker of immune status and is an ideal candidate to be used as quantifiable marker to define the effect of dietary bioactive compounds on the immune system. Our data obtained at three time-points during Spirulina supplementation clearly demonstrate that over half of supplemented subjects manifest an increase in IDO activity which is more prominent among male participants. This is paralleled by an increase in WBC count while further WBC subpopulation analysis is poorly feasible considering the wide variability of measurements, the small number of subjects and the number of potential confounding factors.41
In conclusion, while our study has limitations, we note that the solid nutritional evaluation included in the study design minimized the impact of confounding factors that burden similar studies.42 Our data suggest that Spirulina may counteract anemia and immunosenescence. Further mechanistic studies are needed. Considering the optimal safety profile of Spirulina, we encourage the design of larger clinical studies with solid endpoints and sufficiently long follow-up with appropriate randomization.
References
- Weng NP. Aging of the immune system: how much can the adaptive immune system adapt. Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology. 2008;23:64–74. doi: 10.1152/physiol.00040.2007. [DOI] [PubMed] [Google Scholar]
- Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Vaccines: biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008;46:1078–1084. doi: 10.1086/529197. [DOI] [PubMed] [Google Scholar]
- Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine. 2007;25:3066–3069. doi: 10.1016/j.vaccine.2007.01.025. [DOI] [PubMed] [Google Scholar]
- McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- Gnann JW., Jr Vaccination to prevent herpes zoster in older adults. J Pain. 2008;9:S31–S36. doi: 10.1016/j.jpain.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Long BR, Michaelsson J, Loo CP, Ballan WM, Vu BA, Hecht FM, et al. Elevated frequency of gamma interferon-producing NK cells in healthy adults vaccinated against influenza virus. Clin Vaccine Immunol. 2008;15:120–130. doi: 10.1128/CVI.00357-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel R. Nutritional anemias and the elderly. Semin Hematol. 2008;45:225–234. doi: 10.1053/j.seminhematol.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Thomas DR. Anemia: it's all about quality of life. J Am Med Dir Assoc. 2007;8:80–82. doi: 10.1016/j.jamda.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Clavel T, Haller D. Molecular interactions between bacteria, the epithelium, and the mucosal immune system in the intestinal tract: implications for chronic inflammation. Curr Issues Intest Microbiol. 2007;8:25–43. [PubMed] [Google Scholar]
- Mehandru S, Dandekar S. Role of the gastrointestinal tract in establishing infection in primates and humans. Curr Opin HIV AIDS. 2008;3:22–27. doi: 10.1097/COH.0b013e3282f331b0. [DOI] [PubMed] [Google Scholar]
- Wershil BK, Furuta GT. Gastrointestinal mucosal immunity. J Allergy Clin Immunol. 2008;121:S380–S383. doi: 10.1016/j.jaci.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Abrahamson DR, Powers A, Rodewald R. Intestinal absorption of immune complexes by neonatal rats: a route of antigen transfer from mother to young. Science. 1979;206:567–569. doi: 10.1126/science.493961. [DOI] [PubMed] [Google Scholar]
- Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Khan Z, Bhadouria P, Bisen PS. Nutritional and therapeutic potential of Spirulina. Curr Pharm Biotechnol. 2005;6:373–379. doi: 10.2174/138920105774370607. [DOI] [PubMed] [Google Scholar]
- Mao TK, van de Water J, Gershwin ME. Effects of a Spirulina-based dietary supplement on cytokine production from allergic rhinitis patients. J Med Food. 2005;8:27–30. doi: 10.1089/jmf.2005.8.27. [DOI] [PubMed] [Google Scholar]
- Cingi C, Conk-Dalay M, Cakli H, Bal C. The effects of Spirulina on allergic rhinitis. Eur Arch Otorhinolaryngol. 2008;65:1219–1223. doi: 10.1007/s00405-008-0642-8. [DOI] [PubMed] [Google Scholar]
- Pertovaara AR, Lehtimaki T, Karhunen PJ, Oja SS, Jylha M, Hervonen A, et al. Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech Ageing Dev. 2006;127:497–499. doi: 10.1016/j.mad.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Boeckner LS, Pullen CH, Walker SN, Abbott GW, Block T. Use and reliability of the world wide web version of the block health habits and history questionnaire with older rural women. J Nutr Educ Behav. 2002;34:S20–S24. doi: 10.1016/s1499-4046(06)60307-2. [DOI] [PubMed] [Google Scholar]
- Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006;9:84–93. doi: 10.1079/phn2005763. [DOI] [PubMed] [Google Scholar]
- Laich A, Neurauter G, Widner B, Fuchs D. More rapid method for simultaneous measurement of tryptophan and kynurenine by HPLC. Clin Chem. 2002;48:579–581. [PubMed] [Google Scholar]
- Pertovaara M, Hasan T, Raitala A, Oja SS, Yli-Kerttula U, Korpela M, et al. Indoleamine 2,3-dioxygenase activity is increased in patients with systemic lupus erythematosus and predicts disease activation in the sunny season. Clin Exp Immunol. 2007;150:274–278. doi: 10.1111/j.1365-2249.2007.03480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widner B, Werner ER, Schennach H, Fuchs D. An HPLC method to determine tryptophan and kynurenine in serum simultaneously. Adv Exp Med Biol. 1999;467:827–832. doi: 10.1007/978-1-4615-4709-9_105. [DOI] [PubMed] [Google Scholar]
- Spence RK. Medical and economic impact of anemia in hospitalized patients. Am J Health Syst Pharm. 2007;64:S3–S10. doi: 10.2146/ajhp070244. [DOI] [PubMed] [Google Scholar]
- Gaskell H, Derry S, Andrew Moore R, McQuay HJ. Prevalence of anaemia in older persons: systematic review. BMC Geriatr. 2008;8:1. doi: 10.1186/1471-2318-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bross MH, Soch K, Smith-Knuppel T. Anemia in older persons. Am Fam Physician. 2010;82:480–487. [PubMed] [Google Scholar]
- Ciferri O. Spirulina, the edible microorganism. Microbiol Rev. 1983;47:551–578. doi: 10.1128/mr.47.4.551-578.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar WV. Tecuitlatl: a glimpse of Aztec food technology. Nature. 1966;211:341–342. [Google Scholar]
- Cheong SH, Kim MY, Sok DE, Hwang SY, Kim JH, Kim HR, et al. Spirulina prevents atherosclerosis by reducing hypercholesterolemia in rabbits fed a high-cholesterol diet. J Nutr Sci Vitaminol (Tokyo) 2010;56:34–40. doi: 10.3177/jnsv.56.34. [DOI] [PubMed] [Google Scholar]
- Torres-Duran PV, Miranda-Zamora R, Paredes-Carbajal MC, Mascher D, Diaz-Zagoya JC, Juarez-Oropeza MA. Spirulina maxima prevents induction of fatty liver by carbon tetrachloride in the rat. Biochem Mol Biol Int. 1998;44:787–793. doi: 10.1080/15216549800201832. [DOI] [PubMed] [Google Scholar]
- Vadiraja BB, Gaikwad NW, Madyastha KM. Hepatoprotective effect of C-phycocyanin: protection for carbon tetrachloride and R-(+)-pulegone-mediated hepatotoxicty in rats. Biochem Biophys Res Commun. 1998;249:428–431. doi: 10.1006/bbrc.1998.9149. [DOI] [PubMed] [Google Scholar]
- Bhat VB, Madyastha KM. C-phycocyanin: a potent peroxyl radical scavenger in vivo and in vitro. . Biochem Biophys Res Commun. 2000;275:20–25. doi: 10.1006/bbrc.2000.3270. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Hernandez A, Ble-Castillo JL, Juarez-Oropeza MA, Diaz-Zagoya JC. Spirulina maxima prevents fatty liver formation in CD-1 male and female mice with experimental diabetes. Life Sci. 2001;69:1029–1037. doi: 10.1016/s0024-3205(01)01185-7. [DOI] [PubMed] [Google Scholar]
- Parikh P, Mani U, Iyer U. Role of Spirulina in the control of glycemia and lipidemia in type 2 diabetes mellitus. J Med Food. 2001;4:193–199. doi: 10.1089/10966200152744463. [DOI] [PubMed] [Google Scholar]
- Ble-Castillo JL, Rodriguez-Hernandez A, Miranda-Zamora R, Juarez-Oropeza MA, Diaz-Zagoya JC. Arthrospira maxima prevents the acute fatty liver induced by the administration of simvastatin, ethanol and a hypercholesterolemic diet to mice. Life Sci. 2002;70:2665–2673. doi: 10.1016/s0024-3205(02)01512-6. [DOI] [PubMed] [Google Scholar]
- Torres-Duran PV, Ferreira-Hermosillo A, Juarez-Oropeza MA. Antihyperlipemic and antihypertensive effects of Spirulina maxima in an open sample of Mexican population: a preliminary report. Lipids Health Dis. 2007;6:33. doi: 10.1186/1476-511X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouzandeh F, Jalili RB, Germain M, Duronio V, Ghahary A. Differential immunosuppressive effect of indoleamine 2,3-dioxygenase (IDO) on primary human CD4+ and CD8+ T cells. Mol Cell Biochem. 2008;309:1–7. doi: 10.1007/s11010-007-9635-y. [DOI] [PubMed] [Google Scholar]
- Widner B, Ledochowski M, Fuchs D. Interferon-gamma-induced tryptophan degradation: neuropsychiatric and immunological consequences. Curr Drug Metab. 2000;1:193–204. doi: 10.2174/1389200003339063. [DOI] [PubMed] [Google Scholar]
- Oertelt-Prigione S, Mao TK, Selmi C, Tsuneyama K, Ansari AA, Coppel RL, et al. Impaired indoleamine 2,3-dioxygenase production contributes to the development of autoimmunity in primary biliary cirrhosis. Autoimmunity. 2008;41:92–99. doi: 10.1080/08916930701619730. [DOI] [PubMed] [Google Scholar]
- Howells DW, Hyland K, Smith I, Strobel S. Tryptophan and serotonin metabolism in familial erythrophagocytic lymphohistiocytosis. J Inherit Metab Dis. 1992;15:891–897. doi: 10.1007/BF01800228. [DOI] [PubMed] [Google Scholar]
- Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, et al. The number of human peripheral blood CD4+CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140:540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L. Mendelian randomization in nutritional epidemiology. Nutr Rev. 2009;67:439–450. doi: 10.1111/j.1753-4887.2009.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]