Abstract
The role of vitamin D as an immune modulator has been emphasized in recent years, and low levels of the hormone were observed in several autoimmune diseases including multiple sclerosis and systemic lupus erythematosus. Vitamin D mediates its effect though binding to vitamin D receptor (VDR), and activation of VDR-responsive genes. While VDR gene polymorphism was found to associate with autoimmune thyroid diseases (AITDs), few studies examined levels of vitamin D in these patients and those that did yielded conflicting results. We therefore undertook to evaluate the levels of vitamin D in patients with AITDs compared to patients with non-AITDs and healthy controls. Serum vitamin D (25-OH) levels were measured in 50 patients with AITDs, 42 patients with non-AITDs and 98 healthy subjects, utilizing the LIAISON chemiluminescence immunoassay (DiaSorin, Saluggia, Italy). Vitamin D deficiency was designated at levels lower than 10 ng/ml. Antithyroid antibodies, thyroid functions and demographic parameters were evaluated in all patients. The prevalence of vitamin D deficiency was significantly higher in patients with AITDs compared with healthy individuals (72% versus 30.6% P<0.001), as well as in patients with Hashimoto's thyroiditis compared to patients with non-AITDs (79% versus 52% P<0.05). Vitamin D deficiency also correlated to the presence of antithyroid antibodies (P=0.01) and abnormal thyroid function tests (P=0.059). Significantly low levels of vitamin D were documented in patients with AITDs that were related to the presence of anti thyroid antibodies and abnormal thyroid function tests, suggesting the involvement of vitamin D in the pathogenesis of AITDs and the advisability of supplementation.
Keywords: autoantibodies, autoimmune thyroid disease, Graves' disease, Hashimoto's thyroiditis, vitamin D
Introduction
Vitamin D deficiency has become a common problem in the general population, and has been linked to an increase in cardiovascular diseases,1 cancer2 and infections.3 The low levels of vitamin D have been attributed to reduced sun exposure and physical activity as well as obesity. Vitamin D supplement appears to reduce the incidence of these morbidities and may reduce all-cause mortality.4, 5 Deficiency of vitamin D was also found to correlate with an increased incidence of autoimmune diseases, including type I diabetes mellitus (T1DM),6 rheumatoid arthritis7 and systemic lupus erythematosus.8, 9, 10 This observation was linked to the latitude differences found in several autoimmune diseases.11, 12 For example, the geographic distribution of multiple sclerosis (MS) was inversely correlated with vitamin D status and areas of sunny weather;13 the incidence of T1DM was positively correlated with north–south latitude, less sunshine exposure and season of birth;14 and vitamin D supplements in early life was inversely correlated with risk of T1DM later on.15
In animal models of autoimmune diseases, vitamin D was shown to reduce severity of symptoms and decrease the Th1 response in experimental autoimmune encephalomyelitis and collagen-induced arthritis, and prevent clinical diabetes and pancreatic lesions in the non-obese diabetic mice model.16 Treatment with vitamin D proved beneficial in the management of autoimmune disorders in humans, by diminishing exacerbations of MS, reducing pain and C-reactive protein levels in rheumatoid arthritis and psoriatic arthritis, and preventing the development of MS or T1DM when given prophylactically.6, 7, 17, 18, 19, 20, 21 Animal models have also demonstrated a role for vitamin D in autoimmune thyroid diseases (AITDs): vitamin D supplementation, in addition to cyclosporine, effectively prevented the induction of experimental autoimmune thyroiditis,22 and vitamin D-deficient BALB/c mice developed persistent hyperthyroidism.23
Few studies have examined the impact of vitamin D deficiency on the incidence of AITDs in humans and those that did yielded conflicting results. Vitamin D levels were found to be lower in patients with AITDs than in healthy volunteers in one study,24 and in Graves' disease patients when compared to patients with non-AITDs (e.g., toxic nodular goiter).25 In contrast, a recent study from India found only a weak connection between low vitamin D levels and AITDs.26. In view of these conflicting results and the dearth of published studies, we examined levels of vitamin D among patients with thyroid diseases, and the correlation between vitamin D deficiency and AITDs (Hashimoto's thyroiditis and Graves' disease), thyroid function, thyroid autoantibodies and demographic characteristics.
Materials and methods
Study population
Serum samples were collected from 92 patients followed by the outpatient endocrinology clinic of a medical center in Debrecen, Hungary, for thyroid disorders. Blood was drawn during the first 2 weeks of March 2006. Fifty patients were found to have AITDs (evidenced by autoimmune features or elevated antithyroid peroxidase or antithyroglobulin antibodies) and 42 showed no evidence of autoimmunity (non-AITDs). Gender, age and thyroid function tests and specific autoantibodies associated with AITDs were determined for each patient on the day the blood was drawn. Sera from 98 age-matched matched healthy donors served as controls. The study was approved by the local ethics committee, and complied with the guidelines of the most recent Helsinki declaration (Edinburgh, 2000).
Laboratory tests
Antithyroid peroxidase and antithyroglobulin antibodies were tested by ELISA (Hycor Autostat II; Hycor Biomedical Inc., Garden Grove, CA, USA). Antithyroid stimulating hormone receptor antibodies were measured with a commercial ELISA kit (Medizym T.R.A.; Medipan Diagnostica, Berlin, Germany). Thyroid function tests were evaluated by routine endocrine laboratory investigations.
Vitamin-D measurement
Serum concentrations of 25-OH vitamin D in patients and controls were measured by the commercial kit LIAISON 25-OH vitamin D assay (DiaSorin, Saluggia, Italy). The quantitative determination of 25-OH vitamin D was carried out by a direct, competitive chemiluminescence immunoassay. Magnetic particles (solid phase) are coated with a specific antibody to vitamin D, and vitamin D is linked to an isoluminol derivative. During the incubation, 25-OH vitamin D is dissociated from its binding protein and competes with labeled vitamin D for binding sites on the antibody. After the incubation, the unbound material is removed with a wash cycle, the starter reagents are added, and a flash chemiluminescent reaction is initiated. The light signal is measured by a photomultiplier as relative light units and is inversely proportional to the concentration of 25-OH vitamin D present in calibrators, controls or samples. Vitamin D deficiency in our study was defined, in accordance with the manufacturer, as levels of 25-OH vitamin D below 10 ng/ml.
Statistical analysis
The statistical program SPSS 13.0 (SPSS, Chicago, IL, USA) was used for all analyses. Comparison of categorical variables between groups was performed by chi-square test and Fisher's exact test (two-tailed) as appropriate. Continuous variables are expressed as mean±standard deviation (s.d.). The non-parametric Mann–Whitney U test was performed for comparison of levels between groups. P values <0.05 were considered statistically significant for all tests.
Results
Vitamin D deficiency in patients compared to healthy controls
Vitamin D deficiency was diagnosed in 63% (58/92) of the patients with thyroid diseases compared to 30% (30/98) of the healthy controls (P<0.001) (Figure 1). The prevalence of vitamin D deficiency was even higher in patients with AITDs, 72% (36/50) (P<0.001), particularly in those with Hashimoto's thyroiditis, 79% (22/28) (P<0.001), but also in Graves' disease, 64% (14/22) (P<0.01).
Figure 1.
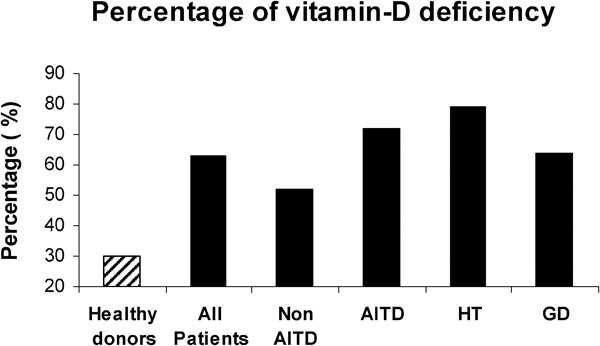
Prevalence of vitamin D deficiency among healthy subjects and patients with thyroid disease. AITD, autoimmune thyroid disease (including HT and GD); GD, Graves' disease; HT, Hashimoto's thyroiditis.
Vitamin D levels in all patients with thyroid disease
There was no significant correlation between vitamin D deficiency and age or gender in the 92 patients referred to the endocrinology clinic (Table 1). The presence of antithyroid antibodies was significantly more common in patients with vitamin D deficiency than in those with higher vitamin D levels (43% versus 17%, respectively; P=0.01). A non-significant association was also observed between vitamin D deficiency and abnormal thyroid function: patients with levels of vitamin D<10 ng/ml exhibited more abnormal thyroid function than patients with vitamin D>10 mg/ml (39% versus 18% P=0.059) (Table 1).
Table 1. The 92 patients with thyroid disease by vitamin D level, age and gender.
| Vitamin D (>10 ng/ml) | Vitamin D deficiency (≤10 ng/ml) | P | |
|---|---|---|---|
| No. of patients | 34 | 58 | |
| Vitamin D level (ng/ml)mean±s.d. (range) | 17±6(10–42) | 7.4±1(7–10) | <0.005 |
| Age (years)(mean±s.d.) | 46±15 | 50±16 | NS |
| Female gender (%) | 25 (73 %) | 46 (79%) | NS |
| Antithyroid antibodies | 6 (17%) | 25 (43%) | 0.01 |
| Abnormal thyroid function test | 6 (18%) | 22 (39%) | 0.059 |
Abbreviation: NS, not significant; s.d., standard deviation.
Vitamin D levels in AITD patients
A comparison of AITD and non-AITD patients is summarized in Table 2. Fifty of the total 92 patients were diagnosed with AITDs. Patients with AITDs were younger (mean age: 45 years versus 52 years; P=0.05), and included more females (88% versus 64% P=0.01). Vitamin D deficiency was more prevalent in patients with AITDs, 72% (36/50), than in those without AITDs, 52% (22/42) (P=0.08). This difference was most significant among patients with Hashimoto's thyroiditis, 79% (P<0.05), and non-significant in those with Graves' disease, 64% (P=0.4) (Figure 1). In the AITD patients, abnormal thyroid function (non-euthyroid) was documented in 53% of the patients with low levels of vitamin D compared with only 22% of patients with levels above 10 ng/ml (P=0.06).
Table 2. Comparison of AITD and non-AITD patients.
| Non-AITD | AITD | P | |
|---|---|---|---|
| No. of patients | 42 | 50 | |
| Age (years) (mean±s.d.) | 52±16 | 45±16 | 0.05 |
| Female gender (%) | 27 (64%) | 44 (88%) | 0.01 |
| Prevalence of vitamin D deficiency (≤10 ng/ml) | 22 (52%) | 36 (72%) | 0.08 |
| Abnormal thyroid function test | 7 (15%) | 22 (44%) | 0.003 |
| Antithyroid antibodies | 0 (0%) | 31 (62%) | <0.005 |
Abbreviation: AITD, autoimmune thyroid disease.
Thyroid-stimulating hormone (TSH) levels tended to have a direct relation to vitamin D status. TSH levels in Hashimoto's thyroiditis were 4.9 mlU/l when vitamin D was <10 ng/ml and 1.8 mlU/l when vitamin D was >10 ng/ml; and in Graves' disease patients, 0.58 mlU/l and 3.89 mlU/l among those with vitamin D<10 ng/ml and those with vitamin D>10 ng/ml, respectively. TSH levels did not correlate with vitamin D deficiency in non-AITD patients. Thyroid hormone levels (T3 and T4) did not correlate with vitamin D levels in any of the groups.
Discussion
Thyroid diseases are among the most common endocrine abnormalities, and AITDs are perhaps the most prevalent autoimmune diseases.11, 27 The prevalence of AITDs, including Hashimoto's thyroiditis, Graves' disease and postpartum thyroiditis, is estimated to be as high as 5% of the general population.11 Furthermore, abnormal thyroid function varies within 7–9% in females and 1–2% in males across different populations,28 and a significant proportion of these patients will eventually be diagnosed with non-autoimmune diseases due to lack of autoantibodies detection, and/or diagnosis of toxic nodular and multinodular goiter, iodine deficiency, radiation exposure or idiopathic cases.
The pathogenesis of AITDs, like other autoimmune diseases, is multifactorial, combining genetic, immune, environmental and hormonal influences such as vitamin D.29, 30, 31, 32, 33 Although distinct in manner, AITDs share similar autoimmune pathologies and autoantibodies; for example, both Hashimoto's thyroiditis and Graves' disease are characterized by a dominant T-lymphocyte infiltration of the thyroid gland. In the present study we demonstrate an increased prevalence of vitamin D deficiency among patients with AITDs, particularly Hashimoto's thyroiditis. In addition, we report, for the first time, to the best of our knowledge, a link between vitamin D deficiency and the presence of antithyroid antibodies. An association was also found between vitamin D deficiency and TSH levels, although not significant. The lack of statistical significance may be due to the relatively small group of patients, especially after subgroup analysis. Taken together, these data suggest a pathogenic relationship between vitamin D status and AITDs.
The biologically active form of vitamin D, a secosteroid hormone essential for bone and mineral homeostasis, has been shown to have immunoregulatory and anti-inflammatory properties.34, 35 Most of the known biological effects of vitamin D are mediated through the vitamin D3 receptor (VDR) 36 , and can be regulated by the vitamin D-binding protein37 and the CYP27B1 hydroxylase.38 The immune modulator properties of vitamin D are ascribed to its effect on cells of the innate and adaptive systems, including macrophages, dendritic cells, and T and B lymphocytes, all of which harbor VDRs. Dendritic cells are a primary target for the immunomodulatoric activity of vitamin D.39, 40 Vitamin D has been shown to inhibit dendritic cell-dependent T-cell activation, and promote tolerogenic properties that favor the induction of regulatory rather that effector T cells. In addition, in vitro studies showed that activation of CD4 T cells expressing VDR by vitamin D promotes a Th2 phenotype (with IL-4 and IL-5 production) while suppressing Th1 activity (with interferon-gamma and IL-2 production).41
Currently, there is no consensus regarding the optimal serum levels of vitamin D among healthy subjects, in addition to which the clinical significance of different vitamin D levels may vary between different populations and be dependent on body mass index, gender, age and seasonal variance.42 Nevertheless, at the present time vitamin D levels above 30 ng/ml are considered sufficient and confer protection from bone disease, whereas lower levels (vitamin D insufficiency) induce elevation of parathyroid hormone and are associated with other hazardous systemic effects.43 Very low levels of this hormone, defined as ‘vitamin D deficiency', were recently suggested to be related to even worse outcomes. Thus, we are able to estimate the impact of vitamin D deficiency on the pathogenesis of different diseases in a relative rather than quantitative manner by comparing levels of vitamin D in healthy and diseased subjects.
In the current study we set vitamin D deficiency at levels below 10 ng/ml—the value that defined the lower third of a normal population examined at the same time and by the same method. Our data indicate an association between vitamin D deficiency and thyroid dysfunction, specifically when caused by an autoimmune disease. The explanation for these findings is not clear: low levels of vitamin D may be a primary phenomenon involved in the pathogenesis of the disease, or they may simply represent a consequence of the disease. Low vitamin D levels in other autoimmune diseases can be explained by malabsorption (e.g., inflammatory bowel diseases or systemic sclerosis), or by lack of sun exposure due to skin involvement or photosensitivity (e.g., SLE and dermatomyositis). Furthermore, in diseases that cause disability, such as rheumatoid arthritis and MS, reduced outdoor activity and chronic corticosteroid treatment per se might induce low vitamin D levels. However, most patients with thyroid disease generally do not suffer from significant skin disease, malabsorption or reduced outdoor activity. Another plausible explanation is that accelerated bone turnover in patients with hyperthyroidism may lead to high calcium levels and a negative feedback on parathyroid hormone and 1,25(OH)2D3 synthesis.44 Hyperthyroidism had no association in our patients with lower vitamin D levels when compared to hypothyroidism. Therefore, it seems that a primary role for vitamin D deficiency in the pathogenesis of thyroid disease should be considered.
Allelic variations within the VDR gene have been implicated in mediating susceptibility to several endocrine autoimmune disorders.45, 46 Recently, several genetic studies demonstrated an association between AITDs and polymorphism of the VDR, as well as other proteins and enzymes associated with vitamin D functions. Horst-Sikorska et al.47 linked VDR polymorphism to thyroid autoimmunity susceptibility by demonstrating a correlation between F allele carriers of the VDR-FokI polymorphism and Graves' disease. Stefanic et al.48 reported an association between VDR-common haplotypic variants and Hashimoto's thyroiditis. In addition, several studies demonstrated genetic polymorphism of the vitamin D-binding protein among Polish49 and Japanese50 patients with AITDs, whereas the CYP27B1 hydroxylase polymorphism was found to predispose to Hashimoto's thyroiditis and Graves' disease in German subjects.51 Of note, some of these associations could not be confirmed in other studies.52
The present study has several limitations, including the small number of patients, the heterogeneity of the study population, and lack of information on nutrition, social behavior (e.g., outdoor activity) and concomitant diseases. Nevertheless, it seems that the evidence links vitamin D deficiency to AITDs either via gene polymorphism or via lack of environmental uptake, supporting the notion that the effects of vitamin D on the immune system may play a role in the pathogenesis of AITDs.
Conclusion
Significantly lower levels of vitamin D were documented in patients with AITDs. Deficiency of vitamin D was linked to the presence of antithyroid antibodies and abnormal thyroid functions. Our data and those of others pointing to the involvement of vitamin D in the pathogenesis of AITDs argue for screening for vitamin D levels in patients with thyroid diseases. Moreover, as treatment with vitamin D is inexpensive and carries minimal side effects, vitamin D supplements may be recommended for AITD patients. Further research is needed to evaluate the beneficial effects of such treatment as well as the optimal doses, including a high-dose regimen of vitamin D recently found to be effective and safe, and to increase compliance in these patients.53 Additional studies are required on VDR agonists and other reagents that may target tissue-specific agents and further increase the therapeutic range.
References
- Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr. 2005;94:483–492. doi: 10.1079/bjn20051544. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- Shapira Y, Agmon-Levin N, Shoenfeld Y. Mycobacterium tuberculosis, autoimmunity, and vitamin D. Clin Rev Allergy Immunol. 2009;39:3147–3159. doi: 10.1007/s12016-009-8150-1. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E. Epidemiological evidence for vitamin D and colorectal cancer. J Bone Miner Res. 2007;22 Suppl 2:V81–V85. doi: 10.1359/jbmr.07s206. [DOI] [PubMed] [Google Scholar]
- Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum. 2004;50:72–77. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- Huisman AM, White KP, Algra A, Harth M, Vieth R, Jacobs JW, et al. Vitamin D levels in women with systemic lupus erythematosus and fibromyalgia. J Rheumatol. 2001;28:2535–2539. [PubMed] [Google Scholar]
- Kamen DL, Cooper GS, Bouali H, Shaftman SR, Hollis BW, Gilkeson GS. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev. 2006;5:114–117. doi: 10.1016/j.autrev.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi I, Aranow C, Mackay M, Stanevsky A, Kamen DL, Marinescu LM, et al. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PLoS One. 2010;5:e9193. doi: 10.1371/journal.pone.0009193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira Y, Agmon-Levin N, Shoenfeld Y. Geoepidemiology of autoimmune diseases. Autoimmunity. 2010;8:468–476. doi: 10.1038/nrrheum.2010.86. [DOI] [PubMed] [Google Scholar]
- Shoenfeld N, Amital H, Shoenfeld Y. The effect of melanism and vitamin D synthesis on the incidence of autoimmune disease. Nat Clin Pract Rheumatol. 2009;5:99–105. doi: 10.1038/ncprheum0989. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol. 2007;61:504–513. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- Dahlquist G, Mustonen L. Childhood onset diabetes—time trends and climatological factors. Int J Epidemiol. 1994;23:1234–1241. doi: 10.1093/ije/23.6.1234. [DOI] [PubMed] [Google Scholar]
- The EURODIAB Substudy 2 Study Group Vitamin D supplement in early childhood and risk for Type I (insulin-dependent) diabetes mellitus. Diabetologia. 1999;42:51–54. doi: 10.1007/s001250051112. [DOI] [PubMed] [Google Scholar]
- Mathieu C, Laureys J, Sobis H, Vandeputte M, Waer M, Bouillon R. 1,25-Dihydroxyvitamin D3 prevents insulitis in NOD mice. Diabetes. 1992;41:1491–1495. doi: 10.2337/diab.41.11.1491. [DOI] [PubMed] [Google Scholar]
- Andjelkovic Z, Vojinovic J, Pejnovic N, Popovic M, Dujic A, Mitrovic D, et al. Disease modifying and immunomodulatory effects of high dose 1 alpha (OH) D3 in rheumatoid arthritis patients. Clin Exp Rheumatol. 1999;17:453–456. [PubMed] [Google Scholar]
- Myhr KM. Vitamin D treatment in multiple sclerosis. J Neurol Sci. 2009;286:104–108. doi: 10.1016/j.jns.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Munger KL, Zhang SM, O'Reilly E, Hernán MA, Olek MJ, Willett WC, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- Huckins D, Felson DT, Holick M. Treatment of psoriatic arthritis with oral 1,25-dihydroxyvitamin D3: a pilot study. Arthritis Rheum. 1990;33:1723–1727. doi: 10.1002/art.1780331117. [DOI] [PubMed] [Google Scholar]
- Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- Chen W, Lin H, Wang M. Immune intervention effects on the induction of experimental autoimmune thyroiditis. J Huazhong Univ Sci Technolog Med Sci. 2002;22:343–345, 354. doi: 10.1007/BF02896782. [DOI] [PubMed] [Google Scholar]
- Misharin A, Hewison M, Chen CR, Lagishetty V, Aliesky HA, Mizutori Y, et al. Vitamin D deficiency modulates Graves' hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinology. 2009;150:1051–1060. doi: 10.1210/en.2008-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach H, Shoenfeld Y. Vaccination infection and autoimmunity: myth and reality VIAMR 2005-10-26-28, Beau-Rivage Palace Hotel, Lausanne, Switzerland. Autoimmun Rev. 2007;6:261–266. doi: 10.1016/j.autrev.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Czernobilsky H, Scharla S, Schmidt-Gayk H, Ziegler R. Enhanced suppression of 1,25(OH)2D3 and intact parathyroid hormone in Graves' disease as compared to toxic nodular goiter. Calcif Tissue Int. 1988;42:5–12. doi: 10.1007/BF02555832. [DOI] [PubMed] [Google Scholar]
- Goswami R, Marwaha RK, Gupta N, Tandon N, Sreenivas V, Tomar N, et al. Prevalence of vitamin D deficiency and its relationship with thyroid autoimmunity in Asian Indians: a community-based survey. Br J Nutr. 2009;102:382–386. doi: 10.1017/S0007114509220824. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y, Selmi C, Zimlichman E, Gershwin ME. The autoimmunologist: geoepidemiology, a new center of gravity, and prime time for autoimmunity. J Autoimmun. 2008;31:325–330. doi: 10.1016/j.jaut.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y, Blank M, Abu-Shakra M, Amital H, Barzilai O, Berkun Y, et al. The mosaic of autoimmunity: prediction, autoantibodies, and therapy in autoimmune diseases—2008. Isr Med Assoc J. 2008;10:13–19. [PubMed] [Google Scholar]
- Shoenfeld Y, Gilburd B, Abu-Shakra M, Amital H, Barzilai O, Berkun Y, et al. The mosaic of autoimmunity: genetic factors involved in autoimmune diseases—2008. Isr Med Assoc J. 2008;10:3–7. [PubMed] [Google Scholar]
- Shoenfeld Y, Zandman-Goddard G, Stojanovich L, Cutolo M, Amital H, Levy Y, et al. The mosaic of autoimmunity: hormonal and environmental factors involved in autoimmune diseases—2008. Isr Med Assoc J. 2008;10:8–12. [PubMed] [Google Scholar]
- Tozzoli R, Barzilai O, Ram M, Villalta D, Bizzaro N, Sherer Y, et al. Infections and autoimmune thyroid diseases: parallel detection of antibodies against pathogens with proteomic technology. Autoimmun Rev. 2008;8:112–115. doi: 10.1016/j.autrev.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Gershwin ME. The mosaic of autoimmunity. Autoimmun Rev. 2008;7:161–163. doi: 10.1016/j.autrev.2007.11.021. [DOI] [PubMed] [Google Scholar]
- van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66:1137–1142. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63:954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- Henry HL. Vitamin D hydroxylases. J Cell Biochem. 1992;49:4–9. doi: 10.1002/jcb.240490103. [DOI] [PubMed] [Google Scholar]
- Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- van Halteren AG, van Etten E, de Jong EC, Bouillon R, Roep BO, Mathieu C. Redirection of human autoreactive T-cells upon interaction with dendritic cells modulated by TX527, an analog of 1,25 dihydroxyvitamin D3. . Diabetes. 2002;51:2119–2125. doi: 10.2337/diabetes.51.7.2119. [DOI] [PubMed] [Google Scholar]
- Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009;29:3713–3720. [PubMed] [Google Scholar]
- Gomez-Alonso C, Naves-Diaz ML, Fernandez-Martin JL, Diaz-Lopez JB, Fernandez-Coto MT, Cannata-Andia JB. Vitamin D status and secondary hyperparathyroidism: the importance of 25-hydroxyvitamin D cut-off levels. Kidney Int Suppl. 2003. pp. S44–S48. [DOI] [PubMed]
- Iqbal AA, Burgess EH, Gallina DL, Nanes MS, Cook CB. Hypercalcemia in hyperthyroidism: patterns of serum calcium, parathyroid hormone, and 1,25-dihydroxyvitamin D3 levels during management of thyrotoxicosis. Endocr Pract. 2003;9:517–521. doi: 10.4158/EP.9.6.517. [DOI] [PubMed] [Google Scholar]
- Pani MA, Knapp M, Donner H, Braun J, Baur MP, Usadel KH, et al. Vitamin D receptor allele combinations influence genetic susceptibility to type 1 diabetes in Germans. Diabetes. 2000;49:504–507. doi: 10.2337/diabetes.49.3.504. [DOI] [PubMed] [Google Scholar]
- Pani MA, Seissler J, Usadel KH, Badenhoop K. Vitamin D receptor genotype is associated with Addison's disease. Eur J Endocrinol. 2002;147:635–640. doi: 10.1530/eje.0.1470635. [DOI] [PubMed] [Google Scholar]
- Horst-Sikorska W, Ignaszak-Szczepaniak M, Marcinkowska M, Kaczmarek M, Stajgis M, Slomski R. Association analysis of vitamin D receptor gene polymorphisms with bone mineral density in young women with Graves' disease. Acta Biochim Pol. 2008;55:371–380. [PubMed] [Google Scholar]
- Stefanic M, Papic S, Suver M, Glavas-Obrovac L, Karner I. Association of vitamin D receptor gene 3′-variants with Hashimoto's thyroiditis in the Croatian population. Int J Immunogenet. 2008;35:125–131. doi: 10.1111/j.1744-313X.2008.00748.x. [DOI] [PubMed] [Google Scholar]
- Kurylowicz A, Ramos-Lopez E, Bednarczuk T, Badenhoop K. Vitamin D-binding protein (DBP) gene polymorphism is associated with Graves' disease and the vitamin D status in a Polish population study. Exp Clin Endocrinol Diabetes. 2006;114:329–335. doi: 10.1055/s-2006-924256. [DOI] [PubMed] [Google Scholar]
- Ban Y, Ban Y, Taniyama M, Katagiri T. Vitamin D receptor initiation codon polymorphism in Japanese patients with Graves' disease. Thyroid. 2000;10:475–480. doi: 10.1089/thy.2000.10.475. [DOI] [PubMed] [Google Scholar]
- Miller AE, Morgante LA, Buchwald LY, Nutile SM, Coyle PK, Krupp LB, et al. A multicenter, randomized, double-blind, placebo-controlled trial of influenza immunization in multiple sclerosis. Neurology. 1997;48:312–314. doi: 10.1212/wnl.48.2.312. [DOI] [PubMed] [Google Scholar]
- Collins JE, Heward JM, Nithiyananthan R, Nejentsev S, Todd JA, Franklyn JA, et al. Lack of association of the vitamin D receptor gene with Graves' disease in UK Caucasians. Clin Endocrinol (Oxf) 2004;60:618–624. doi: 10.1111/j.1365-2265.2004.02015.x. [DOI] [PubMed] [Google Scholar]
- von Restorff C, Bischoff-Ferrari HA, Theiler R. High-dose oral vitamin D3 supplementation in rheumatology patients with severe vitamin D3 deficiency. Bone. 2009;45:747–749. doi: 10.1016/j.bone.2009.06.012. [DOI] [PubMed] [Google Scholar]


