Abstract
Background
Galectin-3 is a marker of myocardial fibrosis that has been implicated in the pathophysiologic pathway of fibrosis; its association with all-cause and cardiovascular disease (CVD) mortality in a community-based cohort free of baseline CVD has not been reported. Our aim was to determine the association between galectin-3 levels and all-cause and CVD mortality in community-dwelling older adults without known CVD.
Methods
We measured plasma galectin-3 levels in 1,393 Rancho Bernardo Study participants without CVD with a mean age of 70 years. Participants were followed up for a mean of 11 years for coronary heart disease, CVD mortality, and all-cause mortality.
Results
During follow-up, 436 participants died (169 from CVD). In models adjusted for traditional CVD risk factors and renal function, galectin-3 was a significant predictor of CVD mortality (hazard ratio [HR] per SD log increase 1.30, 95% CI 1.10-1.53) and all-cause mortality (HR 1.12, 1.01-1.24), but not coronary heart disease (HR 1.09, 0.92-1.30). After further adjusting for N-terminal pro B-type natriuretic peptide, galectin-3 remained an independent predictor (HR 1.24, 1.05-1.47) of CVD mortality. Galectin-3 improved the c statistic (0.847-0.851, P = .003) for prediction of CVD death. Net reclassification improvement (>0) with the addition of galectin-3 was 35% (P< .0001); the integrated discrimination index was also significant (P = .03). Participants with both galectin-3 and N-terminal pro B-type natriuretic peptide above the median had increased risk of CVD death vs those with higher levels of only 1 of these markers (HR 1.74, 1.24-2.43).
Conclusion
Higher levels of galectin-3 are independently associated with all-cause and CVD mortality among community-dwelling older adults with no known CVD at baseline. (Am Heart J 2014;0:1-9.e1.)
Galectin-3 (Gal-3) is a member of the β-galactoside– binding lectin family of proteins and plays an important role in inflammation and fibrosis. Galectin-3 is expressed in fibroblasts, endothelial cells, and inflammatory cells including macrophages.1-3 In the myocardium, Gal-3 is nearly undetectable in cardiomyocytes, but is expressed at higher levels in cardiac fibroblasts and appears to exert a profibrotic effect.1 Galectin-3 induces myocardial collagen deposition and remodeling when infused into the pericardium in hypertensive rats.1 It plays a role in cardiac dysfunction via cardiac fibroblast proliferation, collagen deposition, and ventricular dysfunction.1 Galectin-3 also appears to be a mediator of aldosterone-induced vascular fibrosis.4
In the setting of acute and chronic heart failure, higher Gal-3 levels are associated with increased morbidity and mortality.5-9 Elevated Gal-3 levels are also associated with a higher risk of incident heart failure among patients with acute coronary syndromes.10
In contrast to what is known about Gal-3 in patients with underlying cardiovascular disease (CVD), relatively little is known about Gal-3 in the general population. Only 2 previous studies have evaluated Gal-3 in the general population. The Prevention of Renal and Vascular End-Stage Disease (PREVEND) study and the Framingham Offspring Study reported that Gal-3 levels are associated with cardiovascular risk factors, as well as with all-cause mortality11 and incident heart failure.12 However, neither of these studies excluded individuals with known CVD at baseline. To our knowledge, no studies have reported on the association between Gal-3 levels and coronary heart disease (CHD) or mortality outcomes in apparently CVD-free individuals from the community. Because Gal-3 levels have previously been shown to be prognostic for individuals with underlying CVD, we sought to evaluate whether Gal-3 levels are independently associated with CVD and mortality among older, community-dwelling individuals from the Rancho Bernardo Study who were free of known CVD at baseline.
Methods
Study population
The Rancho Bernardo Study is an ongoing, prospective, population-based study of the epidemiology of cardiovascular and other chronic diseases. The study began in 1972, when all adults between 30 and 80 years of age who resided in Rancho Bernardo, California, were invited to participate in a study of heart disease risk factors; 5,052 (82%) enrolled. Nearly all were white and middle to upper-middle class. At a follow-up study visit in 1992 to 1996, which served as the baseline visit for the present analyses, 1,781 of the surviving participants returned. Sufficient blood was available for measurement of Gal-3 in 98% (n = 1,742) of the participants, of whom 1,393 (80%) had no history of CVD and are the focus of these analyses. Prevalent CVD at baseline was defined as a history of coronary revascularization, physician-diagnosed myocardial infarction, transient ischemic attack, stroke, or peripheral arterial disease. Four participants who were lost to follow-up immediately after their study visit were excluded from outcomes analyses (Figure 1). Medical histories and information about physical activity (exercise at least times per week, yes/no), alcohol consumption (at least 1 drink per day vs less or none), and current smoking (yes/no) were obtained using standard questionnaires developed by the Rancho Bernardo Research Group. Blood pressure, height, and weight were measured, and body mass index (BMI; in kilograms pre meter squared) was calculated. Diabetes mellitus was defined as a morning fasting plasma glucose level ≥126 mg/dL, reported physician diagnosis, or use of diabetes-specific medication. Hypertension was defined as a resting blood pressure ≥140 mm Hg systolic or ≥90 mm Hg diastolic, reported physician diagnosis, or use of antihypertensive medication. Estimated glomerular filtration rate (GFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration method.13 Participants were followed up with periodic clinic visits and annual mailed questionnaires through July 30, 2009, an average of 11.0 ± 3.7 years. All participants provided written informed consent, and the study protocol was approved by the human research protection program at the University of California at San Diego.
Figure 1.
Flowchart showing which Rancho Bernardo Study participants were included in the present analyses.
Definition of end points
The primary outcomes for this study were all-cause mortality, CVD death, and CHD, defined as the first incidence of coronary revascularization (percutaneous coronary intervention or coronary artery bypass graft surgery) or fatal or nonfatal myocardial infarction. Nonfatal events were identified using standardized questionnaires at baseline, at clinic visits approximately every 4 years thereafter, and from periodic mailings. Myocardial infarction was diagnosed based on a history of physician-diagnosed myocardial infarction or evidence of myocardial infarction on resting 12-lead electrocardiogram using the Whitehall criteria as applied in the World Health Organization's Multinational Study of Diabetes and Vascular Disease (Minnesota codes 1.1-1.2 [major Q wave] or 7.1.1 [left bundle-branch block]).14,15 At another study visit, diagnosis of a myocardial infarction had been validated in 85% of the cases by a panel of cardiologists who reviewed the medical records of a 30% sample of this cohort. Death certificates were obtained for decedents and coded by a certified nosologist using the International Classification of Disease, Ninth Revision criteria. Cardiovascular disease death included deaths assigned codes 390-459.
Laboratory methods
Morning blood samples were obtained by venipuncture after a 12-hour fast. Serum and plasma were separated, and lipids, lipoproteins, and glucose levels were measured on fresh samples; the remaining samples were stored frozen at –70°C. Galectin-3 was measured in 2011 in EDTA plasma that had been collected at the 1992 to 1996 study visit and had been stored frozen at –70°C. The assay was performed using a Luminex platform as a competitive immunoassay (measureable range 5.0-86.6 ng/mL; Alere Inc, Waltham, MA). Intra-assay coefficient of variation was 9.3% and interassay coefficient of variation was 13.6% at a Gal-3 level of 12.5 ng/mL. Measurement of N-terminal pro B-type natriuretic peptide (NT-proBNP) has been previously described.16
Statistical analysis
Continuous variables are presented as means ± SD; most laboratory values were not normally distributed and are presented as medians (quartile 1–quartile 3). Dichotomous variables are presented as percentages. Galectin-3 and NT-proBNP were log10 transformed for incorporation in subsequent analyses. Galectin-3 levels were higher in women than in men, so analyses were initially performed using both overall and sex-specific logGal3 levels. Because this did not make a substantive difference, results are reported per SD of overall logGal3 levels, with analyses adjusted for sex. The only exception to this was in analyses based on quartiles of Gal-3, where sex-specific quartiles were used to facilitate comparison with other studies.12
Mann-Whitney U test was used to compare median Gal-3 levels between groups. Single-predictor associations between the clinical variables listed in Table I and logGal-3 levels were determined by linear regression analyses. Backward multivariable regression analysis including variables with significant individual associations was used to determine which covariates were independently associated with logGal-3 level.
Table I.
Baseline characteristics of the study population (n = 1393)
| Age (y) | 70 ± 11 |
| % Male | 35.8 |
| Vital signs | |
| Heart rate (beats/min) | 65 ± 11 |
| Systolic BP (mm Hg) | 135 ± 22 |
| Diastolic BP (mm Hg) | 76 ± 9 |
| Cardiovascular risk factors | |
| Hypertension (%) | 49.0 |
| Current smoking (%) | 7.7 |
| Ever smoked (%) | 55.1 |
| Diabetes (%) | 12.4 |
| Medication use | |
| Aspirin (%) | 26.1 |
| Lipid lowering (%) | 9.6 |
| Nutrition and activity | |
| BMI (kg/m2) | 25.5 ± 4.0 |
| Waist-hip ratio (cm/cm) | 0.83 ± 0.09 |
| Exercise >3×/wk (%) | 71.0 |
| Alcohol >3×/wk (%) | 45.2 |
| Laboratory values | |
| NT-proBNP* (pg/mL) | 112 (56-211) |
| GFR (mL/min) | 66 ± 15 |
| Fasting glucose* (mg/dL) | 94 (88-100) |
| Total cholesterol* (mg/dL) | 209 (187-234) |
| Triglycerides* (mg/dL) | 103 (74-146) |
| HDL* (mg/dL) | 57 (46-70) |
| LDL* (mg/dL) | 127 (106-148) |
Abbreviations: BP, blood pressure; LDL, low-density lipoprotein.
Median (quartile 1–quartile 3).
Kaplan-Meier cumulative incidence curves were used to compare all-cause and CVD mortality based on quartiles of Gal-3. The log-rank test was used to compare survival across groups. Multivariable Cox proportional hazard regression models were used to determine the association of logGal-3 with each end point. Missing data points (<0.01% of data) were mean substituted. Model 1 was adjusted for age and sex. Model 2 was additionally adjusted for traditional cardiovascular risk factors including categorically defined diabetes, hypertension, and current smoking, plus continuously defined systolic blood pressure, total cholesterol, high-density lipoprotein (HDL), kidney function, and BMI. Model 3 was additionally adjusted for logNT-proNBP. For exploratory purposes, a Forest plot was constructed based on hazard ratios (HRs) from multivariable Cox proportional hazard models, to compare outcomes among various subgroups of participants.
To further understand the incremental benefit of Gal-3 when combined with NT-proBNP, we divided participants into 3 groups based on the number of markers (0, 1, or 2) above the median. Multivariable Cox proportional hazard models were used to compare groups after adjusting for Model 2 covariates.
c Statistics were calculated using a method adapted for survival models,17 to evaluate the incremental improvement in discrimination with logGal-3 added to Model 2, for each outcome. Cox model increment tests were used to assess whether prediction improved with the addition of logGal-3. Model calibration was assessed using a Hosmer-Lemeshow test modified for use with Cox proportional hazards models.18 Integrated discrimination improvement (IDI), relative IDI, and net reclassification improvement (NRI) for the addition of logGal-3 to model 2 were calculated according to the methods of Pencina et al.19-21 Because NRI calculations are highly sensitive to chosen cut-points and because there are no prespecified cut-points for long-term follow-up in elderly individuals with these specific outcomes, the category-free NRI (NRI > 0) was used. The NRI > 0 also facilitates comparison with other studies. Both the “event NRI” and the “nonevent NRI” were also calculated.20 For reclassification analyses, we estimated risk at 10 years. These metrics were also calculated to evaluate the incremental benefits of adding logGal-3 to a baseline model that included NT-proBNP in addition to traditional cardiovascular risk factors.
A 2-tailed P < .05 was considered statistically significant. Data were analyzed using SPSS 19.0 (Chicago, IL).
Results
Baseline characteristics
Baseline characteristics of the 1,393 participants included in the present analyses are shown in Table I. Among these, 36% were men, and the mean age was 70 ± 11 years. Twelve percent of participants had diabetes, and approximately half had hypertension. Overall, participants were healthy at baseline, with 71% exercising at least 3 times per week, only 8% currently smoking cigarettes, and a small percentage taking aspirin or lipid-lowering medication.
The median Gal-3 level was 14.8 ng/mL (11.5-18.9 ng/ mL), and levels were higher in women than in men (15.3 ng/mL [12.1-19.8 ng/mL] vs 13.7 ng/mL [10.7-17.4 ng/ mL], P < .0001). All subsequent correlation and regression analyses were therefore repeated using sex-standardized logGal3 levels, and because this did not make a substantive difference, results are reported using unadjusted logGal3 levels, with analyses adjusted for sex.
Covariates of Gal-3 levels
Factors most strongly associated with logGal-3 levels on univariable analyses included renal function (inverse association with GFR), age, NT-proBNP level, female sex, and systolic blood pressure, although several other factors had weaker yet significant associations as well (Table II). On multivariable analysis, poorer renal function, higher NT-proBNP level, consumption of less than 3 alcoholic drinks per week, higher heart rate, and female sex were all significantly associated with higher Gal-3 levels. However these 5 covariates together accounted for only 8% of Gal-3 variability.
Table II.
Individual and multivariable covariates of logGal-3 levels
| Individual |
Multivariable* |
|||
|---|---|---|---|---|
| Variable | r | P | β | P |
| Demographics | ||||
| Age | 0.18 | <.001 | ||
| Female Sex | 0.12 | <.001 | 0.06 | .02 |
| Vital signs | ||||
| Heart rate | 0.06 | .02 | 0.07 | .009 |
| SBP | 0.11 | <.001 | ||
| DBP | –0.01 | NS | ||
| Cardiovascular risk factors | ||||
| Hypertension | 0.09 | <.01 | ||
| Current smoking | 0.01 | NS | ||
| Diabetes | –0.001 | NS | ||
| Nutrition and activity | ||||
| Waist-hip ratio | –0.07 | <.01 | ||
| BMI | 0.01 | NS | ||
| Exercise 3×/wk | –0.01 | NS | ||
| Alcohol 3+ drinks/wk | –0.09 | <.001 | –0.08 | .003 |
| Medications | ||||
| Aspirin | –0.01 | NS | ||
| Lipid lowering | 0.001 | NS | ||
| Laboratory values | ||||
| GFR | –0.20 | <.001 | –0.15 | <.001 |
| Log fasting glucose | –0.04 | NS | ||
| Log triglycerides | –0.01 | NS | ||
| Log total cholesterol | –0.02 | NS | ||
| Log HDL | 0.03 | NS | ||
| Log LDL | –0.04 | NS | ||
| Log NT-proBNP | 0.19 | <.001 | 0.13 | <.001 |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL, low-density lipoprotein; NS, not significant. β, standardized regression coefficient.
R2 = 0.08.
Association of Gal-3 levels with mortality and incident CHD outcomes
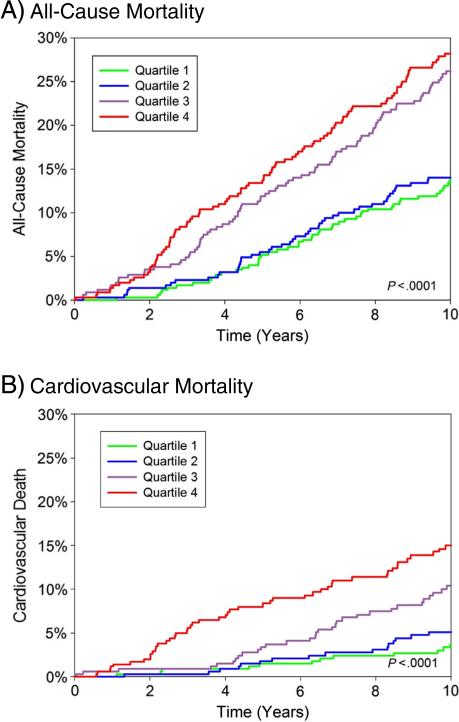
Participants were followed up for a mean of 11.0 ± 3.7 years and a maximum of 16.2 years. Over this time, there were 436 deaths including 169 cardiovascular deaths, and 151 individuals developed incident CHD. As shown in the cumulative incidence graphs in Figure 2, increasing quartiles of Gal-3 were associated with increased risk of all-cause and cardiovascular mortality (P < .0001 for both). Participants with Gal-3 levels in the highest quartile had a 16% increased risk of all-cause mortality and a 90% increased risk of CVD mortality, compared with individuals with levels in the lowest quartile, in age- and sex-adjusted analyses.
Figure 2.
Cumulative incidence of all-cause (A) and cardiovascular (B) mortality by sex-specific quartile of Gal-3. Cut-points for men were as follows: ≤10.6 ng/mL, 10.7 to 13.7 ng/mL, 13.8 to 17.4 ng/mL, and ≥17.5 ng/mL. Cut-points for women were as follows: ≤12.0 ng/ mL, 12.1 to 15.2 ng/mL, 15.3 to 19.8 ng/mL, and ≥19.9 ng/mL.
In multivariable Cox proportional hazard analyses adjusted for age and sex (model 1), increased logGal-3 levels were associated with an increased risk of all-cause mortality (HR per 1-SD increase in logGal-3 1.14, 95% CI 1.03-1.27, P = .01) and cardiovascular mortality (HR 1.34, 95% CI 1.14-1.57, P < .001), but were not related to incident CHD (Table III). In models further adjusted for traditional cardiovascular risk factors (model 2), logGal-3 was still significantly associated with both all-cause (HR 1.12, 95% CI 1.01-1.24, P = .03) and cardiovascular (HR 1.30, 95% CI 1.10-1.53, P ≤ .002) mortality. After additionally adjusting for logNT-proBNP levels, the association of Gal-3 with all-cause mortality was attenuated, but the association with cardiovascular mortality persisted (HR 1.24, 95% CI 1.05-1.47, P = .01).
Table III.
Multivariable Cox proportional hazard models for risk of death or CVD per 1-SD increase in logGal-3 and logNT-proBNP
| All-cause mortality |
Cardiovascular mortality |
CHD |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| No. of deaths/events | 436 | 169 | 151 | |||
| Unadjusted | 1.22 (1.12-1.33)* | <.001 | 1.39 (1.22-1.58)* | <.001 | 1.15 (0.99-1.34) | .06 |
| Model 1 | 1.14 (1.03-1.27)† | .01 | 1.34 (1.14-1.57)* | <.001 | 1.12 (0.95-1.32) | .17 |
| Model 2 | 1.12 (1.01-1.24) | .03 | 1.30 (1.10-1.53)† | .002 | 1.11 (0.94-1.32) | .21 |
| Model 3 | ||||||
| Gal-3 | 1.09 (0.98-1.21) | .10 | 1.24 (1.05-1.47) | .01 | 1.09 (0.92-1.30) | .31 |
| NT-proBNP | 1.47 (1.29-1.68)* | <.001 | 1.87 (1.51-2.30)* | <.001 | 1.38 (1.10-1.73)* | .005 |
Model 1, adjusted for age and sex; model 2, adjusted for model 1 + diabetes, hypertension, and current smoking (dichotomous variables) and systolic blood pressure, total cholesterol, HDL, estimated GFR, and BMI; model 3, adjusted for model 2 + log10NT-proBNP.
Values in bold font are significant at P < .05.
Values are significant at P < .001.
Walues are significant at P < .01.
For exploratory purposes, we compared the association between Gal-3 and mortality in various subgroups (Figure 3). Interaction terms were all nonsignificant, but the trend suggested that Gal-3 levels were more strongly associated with mortality in men than in women, in participants 70 years and older vs those younger than 70 years, in participants with impaired renal function vs those with GFR ≥60 mL/min, and in those with NT-proBNP levels ≥100 pg/mL vs those with levels under 100 pg/mL. Patterns were similar for both all-cause mortality and cardiovascular mortality.
Figure 3.
Forest plot of adjusted HR and 95% CI for the risk of death per 1-SD increase in log10 units of Gal-3. All HRs are adjusted for age, sex (except for the sex subgroup analysis), current smoking, hypertension, systolic blood pressure, total cholesterol, HDL, GFR (except for the GFR subgroup analysis), and BMI.
In fully adjusted models, individuals with both Gal-3 and NT-proBNP above the median were at significantly increased risk for CVD death compared with individuals with only 1 marker elevated (HR 1.74, 95% CI 1.24-2.43, P = .001) or with neither marker elevated (HR 3.07, 95% CI 1.64-5.73, P < .001). Those participants with both markers above the median were also at significantly increased risk for all-cause mortality compared with individuals with only 1 marker elevated (HR 1.37, 95% CI 1.11-1.70, P = .003) or with neither marker elevated (HR 1.71, 95% CI 1.25-2.35, P = .0001).
Reclassification and discrimination with Gal-3
Addition of Gal-3 to the baseline model that included traditional cardiovascular risk factors (model 2) significantly improved discrimination as measured by the c statistic for cardiovascular mortality (0.847 vs 0.851, P = .003); there was a slight but significant improvement for all-cause mortality as well (0.800 vs 0.801, P = .04) (Table IV). Galectin-3 also significantly improved the IDI for prediction of cardiovascular mortality. Assessment with the Hosmer-Lemeshow test indicated good calibration for the addition of logGal-3 to the adjusted model 2, for both cardiovascular and all-cause mortality (P > .09 for both).
Table IV.
NRI, IDI, and changes in the c statistic with the addition of Gal-3 to the traditional risk factor model
| All-cause mortality |
Cardiovascular mortality |
CHD mortality |
||||
|---|---|---|---|---|---|---|
| Metric | P | P | P | |||
| c Statistic | ||||||
| Risk factors alone (model 2) | 0.800 | 0.847 | 0.705 | |||
| Risk factors + Gal-3 | 0.801 | .04* | 0.851 | .003* | 0.706 | .21* |
| IDI | 0.002 | .20 | 0.006 | .03 | 0.001 | .50 |
| Relative IDI | 0.005 | 0.178 | 0.011 | |||
| NRI | ||||||
| NRI (>0) | 16.1% | .005 | 35.0% | <.0001 | 15.9% | .04 |
| Event NRI | 7.0% | 26.8% | 7.1% | |||
| Nonevent NRI | 9.1% | 8.3% | 8.8% | |||
P-value is for the comparison with the model above.
We assessed reclassification using the category-free NRI (NRI > 0), and it was highly significant for both cardiovascular (35.0%, P > .0001) and all-cause (16.1%, P = .005) mortality. The improved reclassification was the result of both correct upward reclassification of individuals who went on to have events (event NRI), and correct downward reclassification of those who did not have events (nonevent NRI) (Table IV).
When NT-proBNP was included in the baseline model along with traditional cardiovascular risk factors, the addition of Gal-3 still significantly improved the c statistic for cardiovascular mortality (P = .01), but not for all-cause mortality (Supplementary Table). The NRI > 0 remained significant for all 3 outcomes, whereas the IDI was no longer significant.
Discussion
This is the first study to demonstrate that in community-dwelling older individuals without known CVD at baseline, Gal-3 levels improve prediction of cardiovascular mortality and, to a lesser degree, all-cause mortality, independent of traditional cardiovascular risk factors. Furthermore, Gal-3 levels improved discrimination and reclassification when used in addition to traditional risk factors and renal function.
The strongest association of Gal-3 was with cardiovascular mortality, which is consistent with the known pathophysiology of Gal-3, a potent marker of myocar-dial fibrosis. As such, diseases such as heart failure and cardiomyopathy, which are captured by the CVD but not the CHD outcome, likely mediate much of the association and probably explain, in part, why there was no significant association between Gal-3 levels and incident CHD. In addition, CHD that ultimately leads to a cardiovascular death is, by definition, more severe than nonfatal CHD and thus is more likely to be associated with myocardial fibrosis and elevated Gal-3 levels than is nonfatal CHD, which includes events such as elective revascularizations and smaller myocar-dial infarctions. Thus, the association between Gal-3 and CHD was probably also attenuated by including these nonfatal events along with fatal events in the CHD outcome measure.
When added to natriuretic peptides, Gal-3 levels were still independently associated with CVD mortality. In addition, individuals with both NT-proBNP and Gal-3 levels above the median had a significantly higher risk of death and of CVD death than did individuals with only 1 or no markers above the median. Each marker reflects a distinct pathophysiologic pathway, and these findings suggest that their clinical value may be additive.
In the PREVEND study of 7,968 Dutch individuals with an average age of 50 years drawn from the general population but with increased urinary albumin excretion, Gal-3 levels were associated with all-cause mortality, with HRs remarkably similar to ours (HR 1.09 per 1-SD increase in logGal-3 in the PREVEND model adjusted for classical risk factors).11 In contrast to our findings, Gal-3 levels were not significantly associated with CVD mortality in PREVEND. The difference may be due to the younger age of that population and to the fact that only 2% of PREVEND subjects died of cardiovascular causes vs 12% in the Rancho Bernardo study. Also, the enrichment of the PREVEND study population with individuals with albuminuria may have skewed their outcomes and affected results.
The only other study to date that has evaluated Gal-3 in the general population is the Framingham Offspring Study.12 In this study of 3,353 individuals with an average age of 59 years, Gal-3 levels were associated with both all-cause and CVD mortality, and both outcomes had HRs very similar to what we report here for the Rancho Bernardo population (HR 1.15 per 1-SD increase in logGal-3 levels for all-cause mortality and HR 1.25 for CVD mortality, in Framingham Study models adjusted for clinical covariates). The Framingham study also found that Gal-3 levels were strongly associated with incident heart failure. Thus, the Rancho Bernardo study extends these findings for the first time to an older population and to individuals without known CVD at baseline.
All 3 studies have demonstrated that Gal-3 levels increase with age and are higher in women than in men and higher in individuals with worse renal function. Galectin-3 levels were modestly correlated with natriuretic peptide levels as well. Despite these considerations, clinical covariates accounted for only 8% of the variation in Gal-3 levels in the present study and only 15% to 16% in the other 2 studies.11,12
Our findings that Gal-3 is associated with mortality from CVD even in the absence of known CVD at baseline have potentially important implications for cardiovascular prevention. Because Gal-3 is a mediator of cardiac fibrosis,1,22 individuals with elevated levels of Gal-3 may represent a specific phenotype who benefit from closer monitoring and more aggressive prevention efforts. More specifically, based on the pathophysiology of Gal-3, high-risk individuals with elevated Gal-3 levels may benefit from preferential treatment with aldosterone inhibitors.4 This strategy has yet to be formally studied in humans, although it is scientifically attractive based on findings in this and previous studies.4
One advantage to using Gal-3 as a gauge for assessing cardiovascular risk is that Gal-3 levels tend to be more stable over time with less intraindividual variability as compared with natriuretic peptides or C-reactive protein. For instance, in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) study of 1,329 chronic heart failure patients who had serial Gal-3 measurements, Gal-3 levels changed less than 15% over a 3 month period for most subjects.23 Galectin-3 levels are also less sensitive than natriuretic peptide levels to acute changes in volume status.24 In addition, several inhibitors of Gal-3, including modified citrus pectin, have been described.25 Modified citrus pectin is a naturally derived inhibitor of Gal-3 obtained from the peel and pulp of citrus fruits, and the present results open the door to future studies evaluating whether increased consumption of citrus in the general population is associated with reduced CVD.
This study has several strengths including the high rate of long-term follow-up and well-characterized study population and our ability to exclude those with known CVD. There are also some weaknesses. The Rancho Bernardo Study population is largely white and middle to upper-middle class. Because of this relative homogeneity, these results may not be generalizable to other populations. On the other hand, this can also be viewed as a strength insofar as it limits confounding by socioeconomic status and access to health care. Another limitation is the long-term storage of samples prior to measurement of Gal-3 levels. Prior studies have demonstrated the stability of Gal-3 at –70°C, although for shorter periods than the 14 to 18 years that samples were stored here.26 Nonetheless, any decay would be more likely to lead to a type II than a type I error. Finally, the absence of echocardiographic or other cardiac imaging data limited our ability to identify subclinical CVD and to learn more about the influences of cardiac structural abnormalities on Gal-3 levels.
Conclusion
In a population of older, community-based individuals without CVD at baseline, higher Gal-3 levels are independently associated with all-cause and CVD mortality and add prognostic information beyond that provided by natriuretic peptides. Future studies should investigate whether early identification of at-risk individuals and treatment with aldosterone- or Gal-3 inhibitors can lead to improved outcomes.
Relationships with industry
L.B.D. has served as a consultant for Alere, GenWay, and ThermoFisher; has served on an advisory board for Singulex; has received speaking fees from Critical Diagnostics; and has received research supplies from BG Medicine and Critical Diagnostics. A.S.M. has served as a consultant for Alere and BG Medicine and has received research support from Abbott, Alere, BG Medicine, and Siemens.
Supplementary Material
Acknowledgments
Sources of funding
The Rancho Bernardo Study was funded by research Grants AG07181 and AG028507 from the National Institute on Aging, and Grant DK31801 from the National Institute of Diabetes and Digestive and Kidney Diseases. This work was also supported by grants from the American Heart Association (L.B.D. and G.A.L.) and the Sandra Daugherty Foundation (G.A.L.). Alere Inc performed the measurement of Gal-3 levels for this study, but the authors alone were responsible for all other aspects of the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
References
- 1.Sharma UC, Pokharel S, van Brakel TJ, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110(19):3121–8. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 2.Thijssen VL, Hulsmans S, Griffioen AW. The galectin profile of the endothelium: altered expression and localization in activated and tumor endothelial cells. Am J Pathol. 2008;172(2):545–53. doi: 10.2353/ajpath.2008.070938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papaspyridonos M, McNeill E, de Bono JP, et al. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler Thromb Vasc Biol. 2008;28(3):433–40. doi: 10.1161/ATVBAHA.107.159160. [DOI] [PubMed] [Google Scholar]
- 4.Calvier L, Miana M, Reboul P, et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol. 2013;33(1):67–75. doi: 10.1161/ATVBAHA.112.300569. [DOI] [PubMed] [Google Scholar]
- 5.van Kimmenade RR, Januzzi JL, Jr, Ellinor PT, et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006;48(6):1217–24. doi: 10.1016/j.jacc.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 6.de Boer RA, Lok DJ, Jaarsma T, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011;43(1):60–8. doi: 10.3109/07853890.2010.538080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lok DJ, Van Der Meer P, de la Porte PW, et al. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol. 2010;99(5):323–8. doi: 10.1007/s00392-010-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah RV, Chen-Tournoux AA, Picard MH, et al. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12(8):826–32. doi: 10.1093/eurjhf/hfq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felker GM, Fiuzat M, Shaw LK, et al. Galectin-3 in ambulatory patients with heart failure: results from the HF-ACTION study. Circ Heart Fail. 2012;5(1):72–8. doi: 10.1161/CIRCHEARTFAILURE.111.963637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grandin EW, Jarolim P, Murphy SA, et al. Galectin-3 and the development of heart failure after acute coronary syndrome: pilot experience from PROVE IT-TIMI 22. Clin Chem. 2012;58(1):267–73. doi: 10.1373/clinchem.2011.174359. [DOI] [PubMed] [Google Scholar]
- 11.de Boer RA, van Veldhuisen DJ, Gansevoort RT, et al. The fibrosis marker galectin-3 and outcome in the general population. J Intern Med. 2012;272(1):55–64. doi: 10.1111/j.1365-2796.2011.02476.x. [DOI] [PubMed] [Google Scholar]
- 12.Ho JE, Liu C, Lyass A, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60(14):1249–56. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diabetes Drafting Group Prevalence of small vessel and large vessel disease in diabetic patients from 14 centres. The World Health Organisation Multinational Study of Vascular Disease in Diabetics. Diabetes Drafting Group. Diabetologia. 1985;(28 Suppl):615–40. doi: 10.1007/BF00290267. [DOI] [PubMed] [Google Scholar]
- 15.Reid DD, Hamilton PJ, McCartney P, et al. Smoking and other risk factors for coronary heart-disease in British civil servants. Lancet. 1976;2(7993):979–84. doi: 10.1016/s0140-6736(76)90830-8. [DOI] [PubMed] [Google Scholar]
- 16.Daniels LB, Barrett-Connor E, Clopton P, et al. Plasma neutrophil gelatinase–associated lipocalin is independently associated with cardiovascular disease and mortality in community-dwelling older adults: The Rancho Bernardo Study. J Am Coll Cardiol. 2012;59(12):1101–9. doi: 10.1016/j.jacc.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kremers WK. Concordance for survival time data: fixed and time-dependent covariates and possible ties in predictor and time. Mayo Clinic; Rochester, MN: 2007. [Google Scholar]
- 18.May S, Hosmer DW. A simplified method of calculating an overall goodness-of-fit test for the Cox proportional hazards model. Lifetime Data Anal. 1998;4(2):109–20. doi: 10.1023/a:1009612305785. [DOI] [PubMed] [Google Scholar]
- 19.Pencina MJ, D'Agostino Sr RB, D'Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, D'Agostino Sr RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pencina MJ, D'Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. 2010;48(12):1703–11. doi: 10.1515/CCLM.2010.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu YH, D'Ambrosio M, Liao TD, et al. N-acetyl-seryl-aspartyl-lysylproline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol. 2009;296(2):H404–12. doi: 10.1152/ajpheart.00747.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van der Velde AR, Gullestad L, Ueland T, et al. Prognostic value of changes in galectin-3 levels over time in patients with heart failure: data from CORONA and COACH. Circ Heart Fail. 2013;6(2):219–26. doi: 10.1161/CIRCHEARTFAILURE.112.000129. [DOI] [PubMed] [Google Scholar]
- 24.Milting H, Ellinghaus P, Seewald M, et al. Plasma biomarkers of myocardial fibrosis and remodeling in terminal heart failure patients supported by mechanical circulatory support devices. J Heart Lung Transplant. 2008;27(6):589–96. doi: 10.1016/j.healun.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Glinsky VV, Raz A. Modified citrus pectin anti-metastatic properties: one bullet, multiple targets. Carbohydr Res. 2009;344(14):1788–91. doi: 10.1016/j.carres.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christenson RH, Duh SH, Wu AH, et al. Multi-center determination of galectin-3 assay performance characteristics: anatomy of a novel assay for use in heart failure. Clin Biochem. 2010;43(7–8):683–90. doi: 10.1016/j.clinbiochem.2010.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.