Abstract
Aim:
To test whether carbachol can influence endothelial barrier dysfunction induced by tumor necrosis factor (TNF)-α and whether the alpha 7 nicotinic receptor can mediate this process.
Methods:
Rat cardiac microvascular endothelial cells were exposed to carbachol followed by TNF-α treatment in the presence or the absence of α-bungarotoxin (an antagonist of the alpha 7 nicotinic receptor). Permeability of endothelial cells cultured on Transwell filters was assayed using FITC-albumin. F-actin was stained with FITC- phalloidin. Expression of vascular endothelial cadherin, intercellular adhesion molecule 1 (ICAM-1), phosphor-ERK1/2 and phosphor-JNK was detected using Western blot.
Results:
Carbachol (2 μmol/L-2 mmol/L) prevented increase in endothelial cell permeability induced by TNF-α (500 ng/mL) in a dose-dependent manner. Further, it attenuated the down-regulation of vascular endothelial cadherin and the up-regulation of ICAM-1 induced by TNF-α. In addition, treatment of endothelial cells with carbachol decreased phosphor-ERK1/2 and phosphor-JNK. These effects of carbachol were blocked by α-bungarotoxin 3 μg/mL.
Conclusion:
These data suggest that the inhibitory effect of carbachol on TNF-α-induced endothelial barrier dysfunction mediated by the alpha 7 nicotinic receptor.
Keywords: carbachol, alpha 7 nicotinic receptors, endothelium, barrier function, tumor necrosis factor α, intercellular adhesion molecule 1, ERK1/2
Introduction
Systemic inflammatory response and vascular hyperpermeability are important for the pathophysiological basis of acute tissue injury induced by trauma, infection and shock. Proinflammatory molecules produced during trauma, infection and shock activate endothelial cells. Upon activation, endothelial cells interact with inflammatory cells. This interaction disrupts the integrity of vascular barrier function, resulting in an increase of vascular permeability1. Therefore, inhibition of the systemic inflammatory response and a reduction of vascular hyperpermeability are important for the prevention and treatment of acute tissue injury.
It has been documented that the cholinergic anti-inflammatory pathway is a physiological mechanism that modulates inflammatory responses. For example, nicotinic acetylcholine receptor (nAChR) agonists block tumor necrosis factor TNF-α production by lipopolysaccharide (LPS)-stimulated macrophages via alpha 7 nAChR2. Stimulation of the vagus nerve releases ACh, leading to suppression of (TNF)-α production in vivo via alpha 7 nAChR3. Unfortunately, the use of acetylcholine and nicotine as therapeutic agents is limited by their easy hydrolysis and toxicity, respectively4, 5.
Carbachol is an artificially synthesized cholinomimetic agonist6. Among its important features are stability to hydrolysis and low toxicity6, 7. We and others have shown that carbachol inhibits the release of TNF-α3 and reduces the levels of TNF-α and interleukin-6 (IL-6) released from rat peritoneal macrophages stimulated by LPS8. These effects of carbachol are mediated by alpha 7 nAChR. The data indicate that carbachol has the ability to inhibit the inflammatory response. However, it is not yet clear whether carbachol is able to inhibit vascular hyperpermeability.
The endothelium is the first barrier influencing vascular permeability9. Barrier function of the endothelium is associated with endothelial cell permeability, cytoskeletal reorganization and expression of adherent molecules10. In the present study, employing an in vitro endothelial cell model, we observed the effect of carbachol on endothelial permeability, the rearrangement of F-actin and the expression of vascular endothelial cadherin (VE-cadherin) and intercellular adhesion molecule 1 (ICAM-1). Also, we tested whether alpha 7 nAChR mediated these effects of carbachol.
Materials and methods
Animals and chemicals
Male Sprague-Dawley rats weighing 80–100 g (1 month old) were used for EC culture. All animals were from the Experimental Animal Center, PLA General Hospital, Beijing, China. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No 85-23, revised 1996) and approved by the local animal care and use committee. Carbachol, TNF-α, α-bungarotoxin, collagenase I, endothelial cell growth supplement (ECGS), β-glycerophosphate, sodium orthovanadate, leupeptin, DTT, FITC-phalloidin, FITC-albumin, EDTA and HEPES were purchased from Sigma (St Louis, MO, USA). Trypsin and M199 medium were from Difco (USA). Anti-VE-cadherin, anti-ICAM-1, anti-phosphor-ERK1/2, and anti-phosphor-JNK antibodies were from Santa Cruz Biotechnology (USA). Newborn calf serum was from Hong Zhou Biological Research Institute (China). Other chemicals were purchased from Sigma.
Isolation and culture of rat cardiac microvascular endothelial cells and experimental protocols
Rats were anesthetized with 20% urethane by abdominal injection (10 mL/kg). The left ventricles were fully minced and digested with 0.1% collagenase I for 6 min at 37 °C in a shaking water bath. Then, 0.1% trypsin was added and incubated for 4 min at 37 °C. The digested solution was filtered through 100-μm mesh filter, and the filtrates were collected and suspended in standard M199 medium containing 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 25% newborn calf serum, 40 U/mL heparin and 100 μg/mL ECGS. Then the suspension was cultured in a humidified chamber with 5% CO2 at 37 °C, and the medium was changed every 3 d. The cells were characterized based on their typical cobblestone morphology and on the presence of CD31, a surface marker of microvascular endothelial cells.
All studies were performed on cells between passages three and five. Endothelial cells were exposed to concentration gradients of carbachol (2 mmol/L, 0.2 mmol/L, 0.02 mmol/L, 2 μmol/L, 0.2 μmol/L) for 15 min. Then they were treated with TNF-α at a final concentration of 500 ng/mL for 24 h. Our preliminary results showed that 500 ng/mL TNF-α consistently induces a significant increase in the permeability of endothelial cells. For experiments using the specific antagonist of the alpha 7 nicotinic acetylcholine receptor, α-bungarotoxin (3 μg/mL) was added to the cultures 15 min before carbachol administration.
Characterization of the cultured cells by cDNA microarray analysis
Total RNA was prepared from the cultured cells using TRIzol (Invitrogen Life Technologies). cDNA probe synthesis and hybridization were made according to manufacturer's instructions (GEArrayTM Q serial endothelial cell biology gene array bulk kit, Bethesda, MD). The hybridization signals on the X-ray film were scanned, and the image was converted to grayscale to obtain digital number for its density. Each GEArrayTM Q Series membrane was spotted with negative controls (pUC18 DNA and blanks) and housekeeping genes, including β-actin, GAPDH, cyclophilin A and ribosomal protein L13a. All raw signal intensities were corrected for background by subtracting the minimum value to avoid the appearance of negative numbers. All signal intensities were normalized to that of a housekeeping gene. The corrected and normalized signals were then used to estimate the relative abundance of particular transcripts.
Permeability study of endothelial cells
Endothelial cells were seeded on Transwell filters (Corning Costar). After reaching confluence, cells were treated according to experimental protocols. Then, 100 μL FITC-albumin (1 mg/mL) were added gently to the upper chamber and incubated for 45 min in a humidified atmosphere with 5% CO2 at 37 °C. The medium in the lower chamber was removed, and the fluorescence intensity was measured with a fluorescence spectrophotometer (excitation: 490 nm; emission: 525 nm).
F-actin staining
Endothelial cells were grown to confluence on gelatin-coated glass cover slips. After exposure to experimental conditions, endothelial cells were washed five times with PBS and fixed with 2.5% glutaraldehyde for 30 min at room temperature. Samples were washed three times with PBS and permeabilized with 1% Triton X for 10 min at room temperature. Then, the cells were incubated for 1 h at room temperature with 0.33 μmol/L FITC-phalloidin. Cover slips were routinely screened by fluorescence microscopy.
Western blot
Cells were lysed at 4 °C in a lysis buffer containing (mmol/L) HEPES 20 (pH 7.7), MgC12 2.5, EDTA 0.1, β-glycerophosphate 20, DTT 0.5, sodium orthovanadate 0.1, NaCl 75, leupeptin 4 μg/mL, PMSF 20 μg/mL and Triton X-100 0.05% (v/v). Samples were subjected to 8% SDS-PAGE and transferred to nitrocellulose membranes (Millipore Corporation, Bedford). The blotted membranes were incubated with primary antibodies, followed by a peroxidase-conjugated secondary antibody. Antigen-antibody complexes were visualized by enhanced chemiluminescence.
Statistical analysis
The results are expressed as the mean±SEM. For the comparison between two groups, the Student's t-test was employed. A one-way ANOVA was used for multiple comparisons. A value of P<0.05 was considered significant.
Results
Characterization of the cultured cells by microvascular endothelial markers
cDNA microarray was used to identify the expression of microvascular endothelial markers including CD31, cadherin 5 and von Willebrand factor. As shown in Table 1, CD31, cadherin 5 and von Willebrand factor were highly expressed in the cultured cells. These data indicate that the cultured cells were microvascular endothelial cells.
Table 1. Expression of microvascular endothelial markers in the cultured cells.
| Markers | Full name | Accession number | Expression intensity |
|---|---|---|---|
| Cdh5 | Cadherin5 | NM009868 | +++ |
| CD31 | Platelet/endothelial cell adhesion molecule | NM008816 | +++ |
| Vwf | Von Willebrand factor homolog | NM011708 | +++ |
−: non-expressing genes; +: slightly expressing genes; ++: moderately expressing genes; +++: high expressing genes.
Carbachol inhibits TNF-α-induced increase in permeability of endothelial cells by alpha 7 nAChR
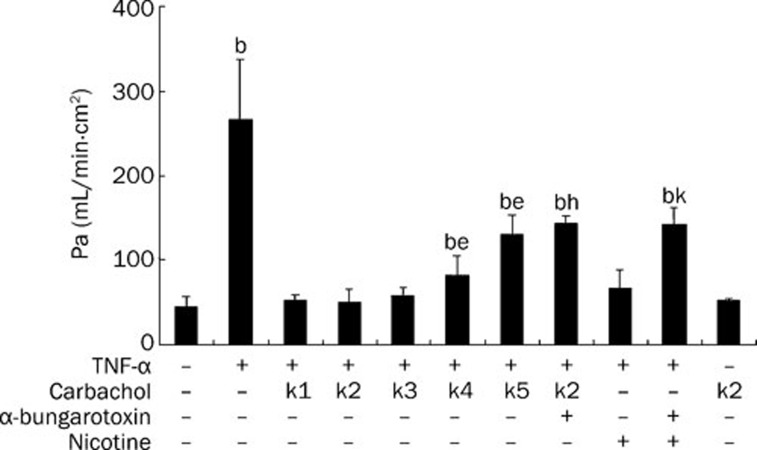
We first examined whether carbachol had a protective effect on endothelial monolayer permeability. As shown in Figure 1, in the control group, the permeablity index of endothelial cells was 44.43±12.39 (Pa×10−5). In TNF-α-challenged cells, the permeablity index was significantly increased (P<0.05, compared with the control group). However, when carbachol was added to the cells at concentrations of 2 μmol/L (K4 group) and 0.2 μmol/L (K5 group) followed by exposure to TNF-α, the endothelial cell-permeable indexes were lower than those of the TNF-α-challenged group (P<0.05), but still higher than those of the control group (P<0.05). When the cells were subjected to carbachol at concentrations of 2 mmol/L (K1 group), 0.2 mmol/L (K2 group) and 0.02 mmol/L (K3 group), respectively, before stimulation with TNF-α, there was no significant difference in permeability index between the carbachol-treated group and the control group (P>0.05). It is possible that α-bungarotoxin 3 μg/mL antagonizes the inhibitory effect of carbachol on TNF-α-challenged hyperpermeability of endothelial cells, indicating that alpha 7 nAChR may participate in mediating the protective effect of carbachol on endothelial monolayer permeability. In addition, we analyzed the effect of carbachol alone in the absence of TNF-α and found that carbachol alone had no effect on endothelial cell permeability (P>0.05, compared with control group). These data indicate that carbachol inhibits the hyperpermeability of endothelial cells induced by TNF-α and that this effect is mediated by alpha 7 nAChR.
Figure 1.
Carbachol inhibits TNF-α-induced hyperpermeability of endothelial cells by alpha 7 nicotinic receptors. Endothelial cells were exposed to concentration gradients of carbachol (K1: 2 mmol/L; K2: 0.2 mmol/L; K3: 0.02 mmol/L; K4: 2 μmol/L; K5: 0.2 μmol/L) for 15 min. Then they were treated with TNF-α at a final concentration of 500 ng/mL for 24 h. For experiments using the antagonist of alpha 7 nicotinic receptor, α-bungarotoxin 3 μg/mL was added to the cultures 15 min before carbachol treatment. Nicotine (10 μmol/L) was used as a positive control. bP<0.05 vs control group; eP<0.05 vs TNF-α group; hP<0.05 vs K2+TNF-α group; kP<0.05 vs nicotine+TNF-α group.
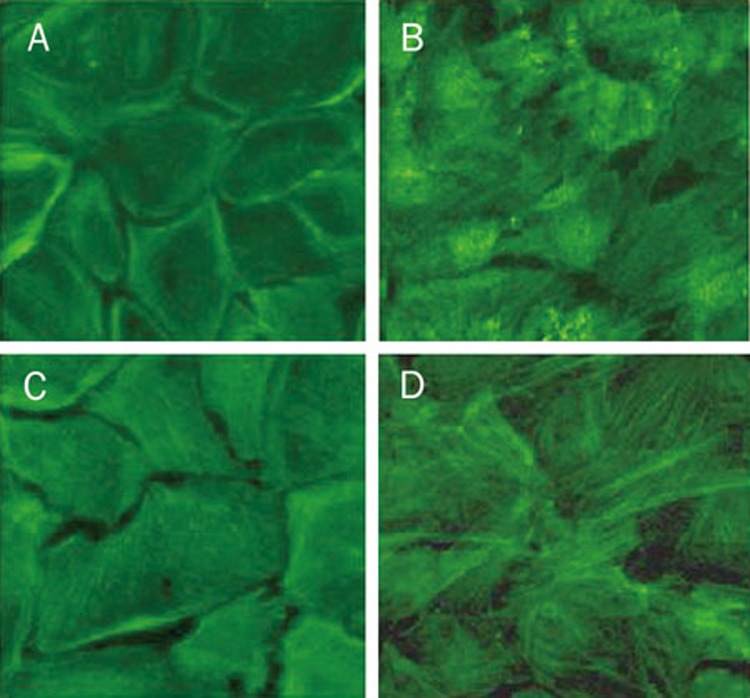
Carbachol prevents F-actin rearrangement of endothelial cells exposed to TNF-α via alpha 7 nAChR
It has been well documented that the cytoskeleton exerts a critical role in the regulation of endothelial monolayer permeability. To better understand the effects of carbachol on the cytoskeleton and the contribution of alpha 7 nAChR activation to these effects, we next investigated the roles of carbachol and alpha 7 nAChR in F-actin rearrangement of endothelial cells by analyzing the spatial distribution of F-actin using immunofluorescent microscopy. Under the control conditions, endothelial cells maintained their polygonal shape, with a prominent dense peripheral actin-containing cortical band (Figure 2A). TNF-α induced a significant decrease in circumferential actin staining in association with a dramatic increase in stress fiber formation, particularly in the central regions of the cells (Figure 2B). These changes were inhibited by carbachol (Figure 2C). However, blocking alpha 7 nAChR by α-bungarotoxin, the protective role of carbachol in F-actin was abolished (Figure 2D). These data suggest that carbachol prevents F-actin rearrangement of endothelial cells exposed to TNF-α via alpha 7 nAChR.
Figure 2.
Carbachol prevents F-actin rearrangement of endothelial cells exposed to TNF-α via alpha 7 nicotinic receptors. Cells were treated as described in Figure 1. F-actin was stained with FITC-phalloidin. (A) Control; (B) TNF-α (C) carbachol + TNF-α (D) α-bungarotoxin+carbachol+TNF-α.
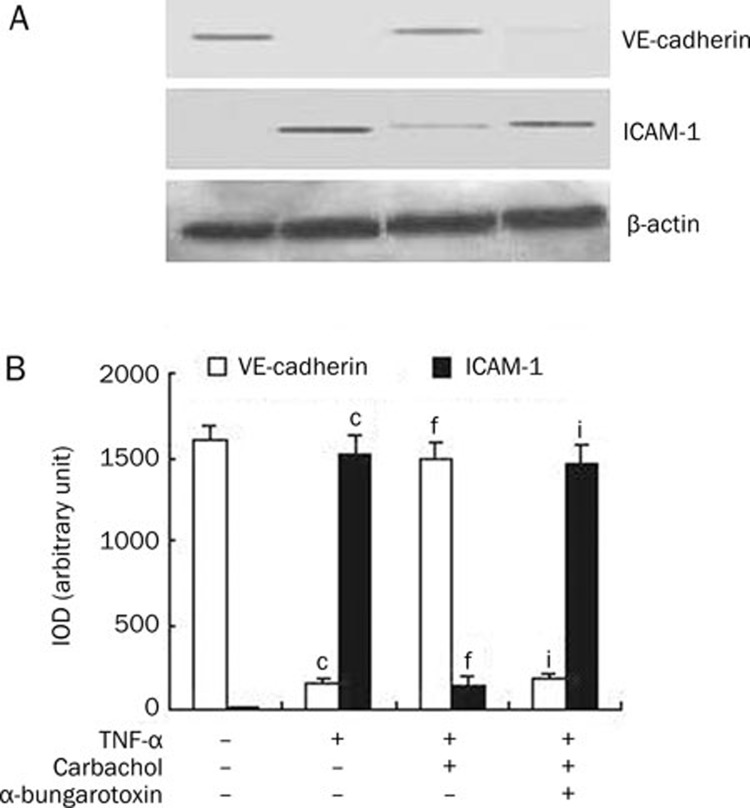
Carbachol regulates the expression of VE-cadherin and ICAM-1 in endothelial cells by activation of alpha 7 nAChR
The effects of carbachol on the changes of TNF-α-induced VE-cadherin and ICAM-1 expression by endothelial cells were studied using Western blot. As shown in Figure 3A, VE-cadherin, but not ICAM-1, was expressed in the control group of endothelial cells. When endothelial cells were exposed to TNF-α, the expression of VE-cadherin was down-regulated and the expression of ICAM-1 was up-regulated. Treatment of endothelial cells with carbachol attenuated the VE-cadherin down-regulation and ICAM-1 up-regulation induced by TNF-α. Furthermore, the effect of carbachol on the expression of VE-cadherin and ICAM-1 was decreased when endothelial cells were subjected to α-bungarotoxin. To quantify the expression levels of VE-cadherin and ICAM-1, we used Image-Pro Plus software to analyze the integrated optical density (IOD) of VE-cadherin and ICAM-1 bands. As shown in Figure 3B, carbachol induced an increase in VE-cadherin expression (P<0.001 vs TNF-α group) and a reduction in ICAM-1 expression (P<0.001 vs TNF-α group) in endothelial cells. However, α-bungarotoxin significantly blocked the increase of VE-cadherin expression (P<0.001 vs carbachol+TNF-α group) and the reduction of ICAM-1 expression (P<0.001 vs carbachol+TNF-α group) induced by carbachol. These data indicate that carbachol regulates the expression of VE-cadherin and ICAM-1 in endothelial cells induced by TNF-α through activation of alpha 7 nAChR.
Figure 3.
Carbachol 0.2 mmol/L regulates the expression of VE-cadherin and ICAM-1 in endothelial cells by activation of alpha 7 nicotinic receptors. (A) The expression of VE-cadherin and ICAM-1 was assessed by Western blot using protein lysates extracted from cells exposed to carbachol followed by TNF-α 500 ng/mL in the presence or the absence of α-bungarotoxin 3 μg/mL. β-Actin was used as a normalization control. (B) Integrated optical density (IOD) of VE-cadherin and ICAM-1 expression bands was analyzed by Image-Pro Plus software. IOD=area×average density. cP<0.01 vs control, fP<0.01 vs TNF-α group, iP<0.01 vs carbachol+TNF-α group.
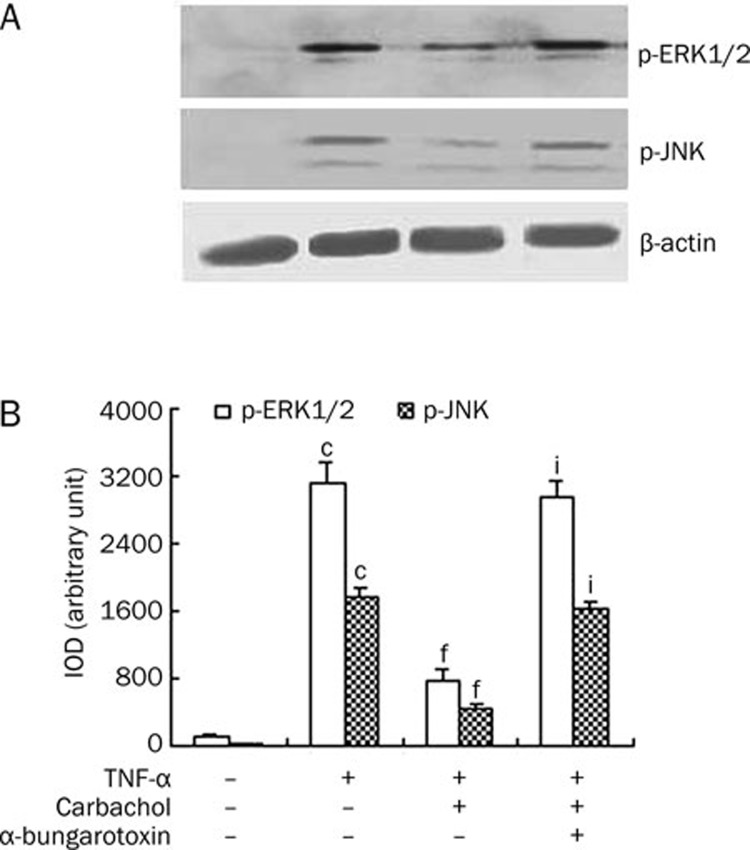
Alpha 7 nAChR mediates the inhibitory effects of carbachol on the phosphorylation of ERK1/2 and JNK in endothelial cells induced by TNF-α
TNF-α can activate mitogen-activated protein kinases (MAPKs) in the signaling pathway leading to changes of VE-cadherin and ICAM-1 expression7, 8. To examine whether carbachol regulates MAPK activation induced by TNF-α, we employed Western blot to analyze the expression of phosphor-ERK1/2 and phosphor-JNK in endothelial cells. As shown in Figure 4, TNF-α stimulated the expression of phosphor-ERK1/2 and phosphor-JNK (P<0.001 vs control group). In contrast, treatment of endothelial cells with carbachol inhibited the levels of phosphor-ERK1/2 and phosphor-JNK induced by TNF-α (P<0.001 vs TNF-α group). The presence of α-bungarotoxin blocked the inhibitory effect of carbachol on the levels of phosphor-ERK1/2 and phosphor-JNK (P<0.001 vs carbachol+TNF-α group). These results suggest that alpha 7 nAChR mediates the inhibitory effects of carbachol on the phosphorylation of ERK1/2 and JNK in endothelial cells induced by TNF-α.
Figure 4.
Alpha 7 nicotinic receptors mediate the inhibitory effects of carbachol 0.2 mmol/L on the phosphorylation of ERK1/2 and JNK in endothelial cells induced by TNF-α 500 ng/mL. (A) The levels of phosphor-ERK1/2 and JNK were assessed by Western blot using protein lysates extracted from cells exposed to carbachol followed by TNF-α in the presence or the absence of α-bungarotoxin 3 μg/mL. β-Actin was used as a normalization control. (B) Integrated optical density (IOD) of ERK1/2 and JNK levels was analyzed by Image-Pro Plus software. IOD=area×average density. cP<0.01 vs control, fP<0.01 vs TNF-α group, iP<0.01 vs carbachol+TNF-α group.
Discussion
In the present study, we showed that carbachol can inhibit the hyperpermeability and F-actin rearrangement of endothelial cells induced by TNF-α. Also, carbachol can regulate the expression of VE-cadherin and ICAM. These effects of carbachol are mediated by alpha 7 nAChR.
The permeability of monolayer endothelial cells is controlled by the equilibrium between the endothelial cell contraction force and the intercellular contact force. This equilibrium is regulated by the cytoskeleton11. It has been reported that cytokines can destroy the endothelial cell cytoskeleton, cause gaps between the cells and increase transendothelial permeability12. Our results show that exposure of endothelial cells to TNF-α led to the increase of endothelial cell permeability accompanied by F-actin rearrangement and stress fiber formation in endothelial cells. Intriguingly, pre-treatment of endothelial cells with carbachol prevents hyperpermeability, cytoskeletal rearrangement and the formation of stress fibers in endothelial cells. Therefore, these data suggest that carbachol suppresses the increase of endothelial permeability by protecting the endothelial cell cytoskeleton.
It has been well documented that VE-cadherin and ICAM-1, as adhesion proteins, play a pivotal regulatory role in the permeability of endothelial cells. Inhibition of VE-cadherin induces a reorganization of the actin cytoskeleton, reduces cell-cell adhesion and increases the permeability of endothelial cells13. Also, overexpression of ICAM-1 causes alteration of the actin cytoskeleton–endothelial junction, thereby inducing the hyperpermeability of endothelial cells14. It has been reported that TNF-α induces an increase in the permeability of endothelial cells by decreasing VE-cadherin protein expression and stimulating ICAM-1 protein expression13, 15. In the present study, we found that TNF-α down-regulates VE-cadherin expression and up-regulates ICAM-1 expression. Our observation is consistent with previously reported results. Further, we showed that carbachol inhibits the effect of TNF-α on the expression of VE-cadherin and ICAM-1. It seems that carbachol inhibits the hyperpermeability of endothelial cells by regulating the expression of VE-cadherin and ICAM-1.
MAPKs are highly conserved serine/threonine kinases that are activated in response to a wide variety of stimuli. TNF-α inhibits VE-cadherin expression and stimulates ICAM-1 expression through the activation of ERK1/2 and JNK and, subsequently, increases the permeability of endothelial cells13, 16. In the present study, we showed that carbachol inhibits ERK1/2 and JNK phosphorylation induced by TNF-α. It is possible that carbachol exerts its function by ERK1/2 and JNK pathways. However, it is necessary to determine the kinase phosphorylation sites and whether the inhibitors of ERK1/2 and JNK block the effects of carbachol.
We have previously shown that alpha 7 nAChR mediates the anti-inflammatory effect of carbachol8. Also, others have reported that administration of acetylcholine inhibits LPS-induced TNF-α release by a reduction in phosphorylation of MAPKs. This inhibitory effect of acetylcholine is blocked by α-bungarotoxin, a specific antagonist of alpha 7 nAChR17. Our present study indicates that α-bungarotoxin blocks the effect of carbachol on ERK1/2 and JNK activation and the expression of VE-cadherin and ICAM-1 in endothelial cells. Additionally, α-bungarotoxin blocks the inhibitory effect of carbachol on the permeablity index of endothelial cells and F-actin rearrangement induced by TNF-α. Our findings suggest that, similar to anti-inflammatory effects, the inhibitory effect of carbachol on endothelial barrier dysfunction is also mediated by alpha 7 nAChR.
The present study provides evidence that carbachol improves endothelial barrier dysfunction induced by TNF-α and that alpha 7 nAChR mediates the process. Because endothelial barrier dysfunction is crucial for the pathophysiological basis of acute tissue injury, our study implies that carbachol may have therapeutic potential for acute tissue injury.
Author contribution
Xiu-hua LIU, Sen HU, and Zhi-yong SHENG designed research; Yu-zhen LI and Fei RONG performed research; Yu-zhen LI analyzed data and wrote the paper.
Acknowledgments
This work was supported by the Special Foundation of the 11th Five-Year Plan for Military Medical Project (No 06Z055).
References
- Norman MU, Lister KJ, Yang YH, Issekutz A, Hickey MJ. TNF regulates leukocyte-endothelial cell interactions and microvascular dysfunction during immune complex-mediated inflammation. Br J Pharmacol. 2005;144:265–74. doi: 10.1038/sj.bjp.0706081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Bourne Y, Radic Z, Sulzenbacher G, Kim E, Taylor P, Marchot P. Substrate and product trafficking through the active center gorge of acetylcholinesterase analyzed by crystallography and equilibrium binding. J Bio Chem. 2006;281:29256–67. doi: 10.1074/jbc.M603018200. [DOI] [PubMed] [Google Scholar]
- Benowitz NL.The human pharmacology of nicotineIn: Capell HD, Glaser FB, Israel Y, Kalant H, Schmidt W, Sellers EM, et al, editors. Research advances in alcohol and drug problemsV 9. New York: Plenum Press; 1986. p1–52.
- Taggart DP, Dipp M, Mussa S, Nye PC. Phenoxybenzamine prevents spasm in radial artery conduits for coronary artery bypass grafting. Thorac Cardiovasc Surg. 2000;120:815–7. doi: 10.1067/mtc.2000.108167. [DOI] [PubMed] [Google Scholar]
- Béla H, Petra O, Zoltán B. Endothelial NOS-mediated relaxations of isolated thoracic aorta of the C57BL/6J mouse: a methodological study. J Cardiovasc Pharmacol. 2005;45:225–31. doi: 10.1097/01.fjc.0000154377.90069.b9. [DOI] [PubMed] [Google Scholar]
- Hu S, Zhou GY, Lü Y, Song Q, Zou XF, Sheng ZY. Effects of carbachol on inflammatory cytokine releases from rat peritoneal macrophages induced by lipopolysaccharide and its receptor. Chin Pharmacol Bull. 2007;23:1161–5. [Google Scholar]
- Vestweber D, Broermann A, Schulte D. Control of endothelial barrier function by regulating vascular endothelial-cadherin. Curr Opin Hematol. 2010;17:230–6. doi: 10.1097/MOH.0b013e328338664b. [DOI] [PubMed] [Google Scholar]
- Zeiller C, Mebarek S, Jaafar R, Pirola L, Lagarde M, Prigent AF, et al. Phospholipase D2 regulates endothelial permeability through cytoskeleton reorganization and occludin downregulation. Biochim Biophys Acta. 2009;1793:1236–49. doi: 10.1016/j.bbamcr.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Benz PM, Blume C, Moebius J, Oschatz C, Schuh K, Sickmann A, et al. Cytoskeleton assembly at endothelial cell-cell contacts is regulated by alphaII-spectrin-VASP complexes. J Cell Biol. 2008;180:205–19. doi: 10.1083/jcb.200709181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seynhaeve AL, Vermeulen CE, Eggermont AM, ten Hagen TL. Cytokines and vascular permeability: an in vitro study on human endothelial cells in relation to tumor necrosis factor-alpha-primed peripheral blood mononuclear cells. Cell Biochem Biophys. 2006;44:157–69. doi: 10.1385/CBB:44:1:157. [DOI] [PubMed] [Google Scholar]
- Nwariaku FE, Liu Z, Zhu X, Nahari D, Ingle C, Wu RF, et al. NADPH oxidase mediates vascular endothelial cadherin phosphorylation and endothelial dysfunction. Blood. 2004;104:3214–20. doi: 10.1182/blood-2004-05-1868. [DOI] [PubMed] [Google Scholar]
- Sumagin R, Lomakina E, Sarelius IH. Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling. Am J Physiol Heart Circ Physiol. 2008;295:H969–H977. doi: 10.1152/ajpheart.00400.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaid K, Rahman A, Anwar KN, Frey RS, Minshall RD, Malik AB. Tumor necrosis factor-α induces early-onset endothelial adhesivity by protein kinase Cζ–dependent activation of intercellular adhesion molecule-1. Circ Res. 2003;92:1089–97. doi: 10.1161/01.RES.0000072971.88704.CB. [DOI] [PubMed] [Google Scholar]
- Nwariaku FE, Chang J, Zhu X, Liu Z, Duffy SL, Halaihel NH, et al. The role of p38 map kinase in tumor necrosis factor-induced redistribution of vascular endothelial cadherin and increased endothelial permeability. Shock. 2002;18:82–5. doi: 10.1097/00024382-200207000-00015. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, et al. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337–43. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]