Abstract
Aim:
To investigate the effect of matrine on proliferation of vascular smooth muscle cells (VSMCs) and elucidate the underlying mechanisms.
Methods:
Rat aortic VSMCs were cultured in medium supplemented with 10% fetal bovine serum and treated with various concentrations (0, 5, 10, 15, and 20 mg/L) of matrine for 72 h. VSMCs proliferation and cell cycle profiling were assessed using a methylene blue incorporation assay and flow cytometry, respectively. The underlying protein signaling mechanisms were determined using Western blot analysis of the expression levels of cell cycle regulatory genes, including p53, p21, p27, cyclin D1, cyclin E, cyclin-dependent kinase 2 and 4 (cdk2, cdk4), and phosphorylated Rb. The involvement of p21 and p27 pathways was further determined using small interfering RNA (siRNA) knockdown.
Results:
Matrine inhibited VSMC proliferation in a dose-dependent manner by promoting G1 arrest. The G1 arrest was accompanied by up-regulation of p53 and p21 protein levels, and down-regulation of cyclin D1/cdk4, cyclin E/cdk2 and phosphorylated Rb protein levels. Matrine did not affect p27 expression. Furthermore, the anti-proliferative effect of matrine was abolished by silencing of p21, but not by silencing of p27.
Conclusion:
Our data indicate that matrine has an inhibitory effect on VSMC proliferation via up-regulation of the p53/p21 signaling pathway and modulation of other cell cycle regulatory genes.
Keywords: matrine; vascular smooth muscle cells; proliferation; cell cycle; p21; p27; cyclin D1, cyclin E; cdk 2; cdk 4
Introduction
Intimal hyperplasia following angioplasty and stenting remains a major obstacle limiting the success of clinical vascular interventions. It probably arises due to activation and tumor-like transformation of normally quiescent medial vascular smooth muscle cells (VSMCs). Transformed VSMCs migrate from the medium into the intima, where they proliferate and promote intimal hyperplasia1, 2. Therefore, identification of new therapies and pharmacological targets aimed at reducing VSMCs proliferation is of great clinical significance.
Matrine (C15H24N2O) is a major components of Radix Sophorae Flavescentis3, 4. It has a wide range of pharmacological applications including anti-hepatitis5, anti-cancer6, and anti-inflammatory properties, with no obvious toxicity or side effects. Matrine's anti-cancer effect has been observed in various cancer cells, including leukemia, cervical, gastric and lung cancers7, 8, 9, 10. The anti-cancer properties of matrine may stem from inhibitory effects on cell proliferation, as well as induction of apoptosis, perhaps via modulation of apoptosis and proliferation regulating genes, such as p53, c-myc, E2F-1, Apaf-1, Rb, and Bcl-2 family members11, 12, 13.
Matrine has also been shown to affect the cardiovascular system, with demonstrated effects on hypotension and bradycardia14. Matrine promotes G1 cell cycle arrest and induces apoptosis in cardiac fibroblasts4. However, whether and how matrine affects VSMCs are poorly understood. The p53, p21, and p27 cell cycle regulating proteins regulate VSMC proliferation. Previous studies demonstrated that statins cause G1 arrest in VSMCs by increasing p27 expression and reducing cyclin E expression15, 16. Alternatively, rapamycin inhibits VSMC proliferation by inducing a p21-mediated G1 arrest17. Cyclin-dependent kinase 2 and 4 (cdk2 and cdk4) and their respective partners, cyclin E and cyclin D, play pivotal roles in cell cycle progression18. Cyclin D1/cdk4 and cyclin E/cdk2 mediate phosphorylation of retinoblastoma (Rb) protein. Expression of cyclin D1 seems to be regulated by extracellular mitogens, while expression of cyclin E is controlled by autonomous mechanisms19, 20. However, cyclin E is thought to promote Rb phosphorylation by cyclin D-cdk4 complexes21. A study in cyclin E knockout mice demonstrates that it is essential for cell cycle re-entry22.
The aim of this study was to determine the effect of matrine on VSMC proliferation and cell cycle progression by modulating the expression of cell cycle regulatory genes.
Materials and methods
Materials
Matrine (purity, >99%) was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Rapamycin and all antibodies were purchased from Sigma (St Louis, MO, USA). Dulbecco's modified Eagle's medium (DMEM), heat-inactivated fetal bovine serum (FBS) and penicillin/streptomycin were obtained from Gibco Life Technologies Inc (Rockville, MD, USA). The siRNA construction kit was purchased from Ambion (Cambridgeshire, UK). The p27 siRNA was purchased from Santa Cruz Biotechnology Inc (Paso Robles, CA, USA). All experimental procedures were approved by the Guangdong Academy of Medical Sciences Research Committee and were in accordance with National Institutes of Health guidelines.
Cell culture
Rat aortic VSMCs were isolated from the intimal-medial layer of the aorta as described previously23, 24. Briefly, thoracic aortas from 2-month-old male Sprague-Dawley rats were used for explant cultures. Explants were cultured in DMEM supplemented with 10% (v/v) FBS and 1% (v/v) penicillin/streptomycin at 37 °C in 5% CO2. VSMCs purity was evaluated by morphological analysis and immunocytochemical staining with a monoclonal antibody against smooth muscle α-actin. Prior to treatment, cells were grown to ∼75% confluence and incubated in a serum-free medium for 24 h to induce quiescence. VSMCs from passages 4–6 were used in this study.
Rapamycin was used as a positive control because it is known to exert an antiproliferative effect on VSMCs, and its effects on the expression of cell cycle genes are well documented17, 24, 25, 26.
Cell proliferation analysis
VSMC proliferation was assessed by using methylene blue incorporation assay in 96-well microculture plates, as previously described27. Briefly, VSMC monolayer cells were suspended in 0.02% EDTA/0.02% trypsin, calcium- and magnesium-free phosphate-buffered saline (PBS), spun down and resuspended in DMEM medium. Cells (6×103/100 μL/well) were transferred into 96-well culture plates and cultured in DMEM medium supplemented with 10% FBS and then treated with various concentrations (0, 5, 10, 15, and 20 mg/L) of matrine or rapamycin (100 ng/L, with rapamycin acting as positive control) for 72 h. Cells were then fixed in 10% formaldehyde saline for 30 min, and then incubated with 1 % (w/v) methylene blue in 0.01 mol/L borate buffer (pH 8.5) for 30 min. The remaining dye was washed off by serially dipping the plate into each of four tanks of 0.01 mol/L borate buffer (pH 8.5). To elute the dye, 100 μL of 1:1 (v/v) ethanol and 0.1 mol/L HCl were added to each well. The plates were then gently shaken and absorbance measurements at 650 nm (A650) were collected for each well using a microplate photometer (Flow Laboratories Ltd, Irvine, Scotland). The photometer was blanked on the first column of control wells containing elution solvent alone. The percent changes in A650 were calculated and compared.
Cell cycle analysis
VSMCs were cultured and treated as described for proliferation assays. Following 72 h of treatment with matrine (0, 5, 10, 15, and 20 mg/L) or rapamycin (100 ng/L, as positive control), VSMCs were harvested with 0.02% EDTA/0.02% trypsin in calcium- and magnesium-free PBS, fixed in 70 % ethanol for 1 h, and treated with 100 μg/mL RNase A for 1 h at 37 °C. Then, cells (5×105 cells/100 μL) were stained with 25 μg/mL propidium iodide (PI) (Jingmei Biotech Co Ltd, Shenzhen, China) for 15 min at room temperature in the dark. Cell cycle distribution was analyzed by flow cytometry (FACS Calibur system, Becton& Dickinson; San Jose, CA, USA) as previously described28. The number of cells in G0/G1, S, and G2/M phases was expressed as a percentage of total cells.
Western blotting
VSMCs that were cultured and treated with matrine as described above were used for Western blot analysis. Protein concentrations in the cell lysates were determined using a Bicinchoninic acid kit (Sigma). Proteins (30 μg/lane) were fractionated via 10%–12% (w/v) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Milan, Italy). The membranes were incubated overnight with primary antibodies (1:400 dilutions) for p53, p21, p27, cyclin D1, cyclin E, cdk2, cdk4, phosphorylated Rb, or anti-β-actin (1:1000 dilutions) for 1 h. After washing with PBS containing 0.1% (v/v) Tween 20, membranes were incubated with corresponding horseradish peroxidase conjugated secondary antibodies (1:1000 dilutions) for 1 h, followed by exposure using enhanced chemiluminescence detection reagents (Roche Applied Science). Western blots were scanned and quantified using Quantity One software (Bio-Rad Laboratories). Protein levels were normalized relative to β-actin protein levels. Fold changes in protein levels were calculated and compared.
Transfection of p21 or p27 siRNA
The siRNA for p21 (5′-GACCAUGUGGACCUGUCAC-3′, antisense) was synthesized in vitro using the siRNA construction kit (Ambion, Cambridgeshire, UK) as previously reported29. Cells were plated at 60% confluency in six-well culture dishes 1 day prior to transfection. Immediately before transfection, cells were washed with PBS. Transfection was carried out using Genesilencer (PeqLab, Germany) according to the manufacturer's protocol. Briefly, 1000 ng of p21 or p27 target siRNA (Santa Cruz Biotechnology Inc, Paso Robles, USA) was diluted in a mixture of 25 μL siRNA diluent and 15 μL serum-free medium, and incubated for 5 min at room temperature. The siRNA solution was added to the Genesilencer and incubated for 5 min at room temperature before the entire solution was added to the cells. Twelve hrs after transfection, the cells were treated with matrine (20 mg/L) for 72 h, and subjected to proliferation and western blot analyses, as described above.
Statistical analysis
The data were expressed as means±standard error of the mean (SEM) and subjected to statistical analysis using the software package SPSS 12.0 (SPSS Inc, Chicago, IL). One-way or two-way ANOVA was used for the analysis of variance and followed by the Spearman method to evaluate differences between groups. Differences were significant when P<0.05.
Results
VSMCs were isolated from rat aortas
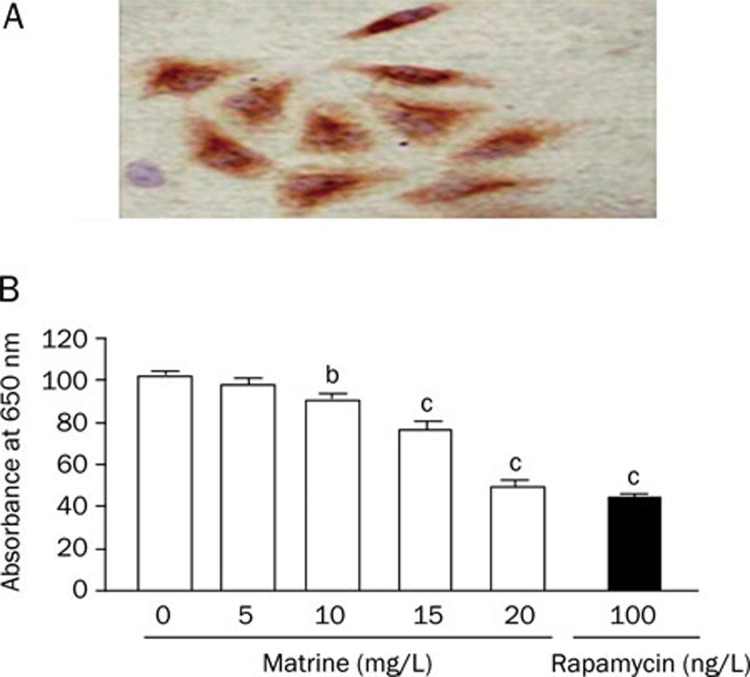
Rat aortic VSMCs were isolated from aortic explants. The purity of VSMCs was assessed via immunostaining for smooth muscle α-actin (Figure 1A). Primary cultures consisting of <95% VSMCs were discarded. Cellular viability was determined by trypan blue exclusion.
Figure 1.
(A) Characterization of cultured rat aortic VSMCs (3th passage, 400×) by immunohistochemical staining with anti-α-smooth muscle actin monoclonal antibody; (B) Effect of matrine on VSMC proliferation in microwell plates. VSMCs were treated with various concentrations of matrine (0, 5, 10, 15, and 20 mg/L) for 72 h. Rapamycin (100 ng/L) was as a positive control. Cell growth was determined by colorimetric assay of methylene blue incorporation. The changes in percentage of control (0 mg/L matrine) were calculated. Data are expressed as mean±SEM. n=5/group, One-way ANOVA. bP<0.05, cP<0.01 vs control.
Matrine inhibits VSMCs proliferation
VSMCs proliferation was assessed using the colorimetric assay of methylene blue incorporation. We found that treatment with matrine inhibited VSMCs proliferation in a concentration-dependent manner (Figure 1B). Cell number was significantly lower in 10 mg/L of matrine, and was further decreased in 20 mg/L of matrine, similar to the proliferation inhibition observed following treatment with 100 ng/L of rapamycin. The variation within a plate was 3.5%. The coefficient of variation between plates was 2.5%.
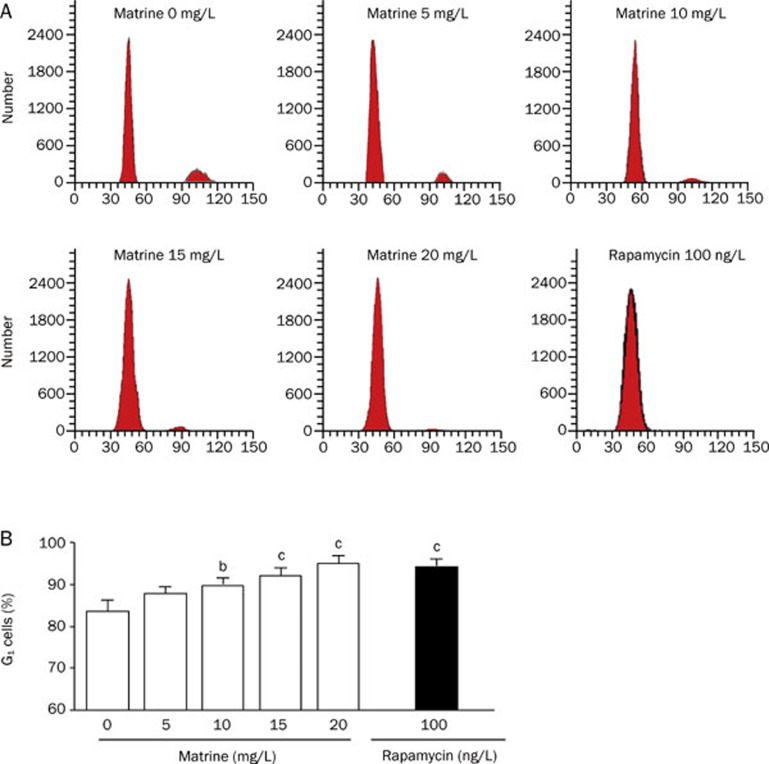
Matrine treatment leads to G1 arrest in VSMCs
Cultured VSMCs were treated with matrine and PI stained for flow-cytometric analysis. The DNA content histograms revealed that matrine led to a G1 phase halt in a dose- dependent manner (Figure 2A). Figure 2B summarizes the effect of matrine on the proportion of G1 phase VSMCs at different concentrations. After treatment with 20 mg/L of matrine, the proportion of G1 phase cells was increased from 84.8%±1.3% to 96.6%±0.8% (n =5/group, P<0.05) and the proportion of S phase cells decreased from 9.9%±1.2% to 1.5%±0.9% (n=5/group, P<0.05). Treatment with matrine at a 20 mg/L concetration induced G1 cell arrest similar to that induced by treatment of 100 ng/L rapamycin.
Figure 2.
Effect of matrine on cell cycle progress in cultured VSMCs. VSMCs were treated with various doses of matrine (0, 5, 10, 15, and 20 mg/L) for 72 h. Rapamycin (100 ng/L) was as a positive control. Cell cycle progress was determined by flow cytometry. Panel A shows representative histograms of flow cytometric analysis; Panel B is the summarized data of G1 phase VSMCs after treatments. The percentage of G1 phase cells in total events was calculated. Data are expressed as mean±SEM. n=5/group, One-way ANOVA. bP<0.05, cP<0.01 vs treatment control (0 mg/L matrine).
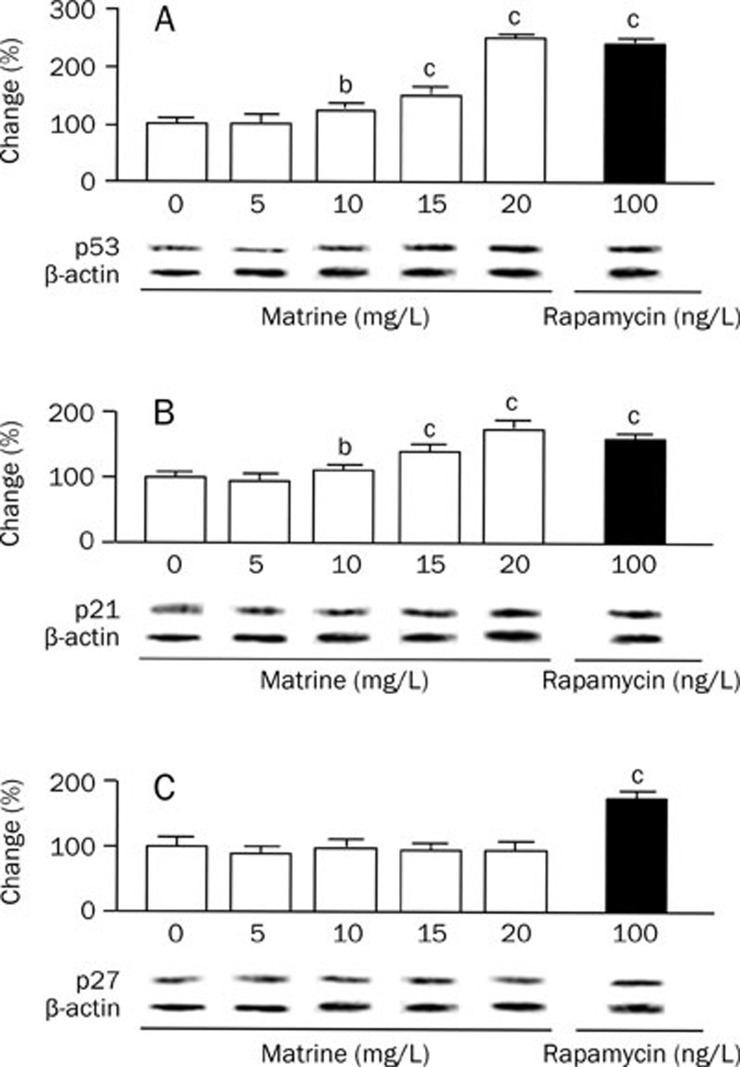
Matrine upregulates p53 and p21 protein levels, but does not affect p27 protein level
To explore the mechanism by which matrine inhibites VSMCs proliferation, we examined protein levels of p53, p21, and p27 using Western blot analysis. As seen in Figure 3, matrine led to a dose-dependent increase in the expression of p53 and p21. At 20 mg/L, matrine induced increases in p53 and p21 proteins similar to those observed following 100 ng/L rapamycin treatment. Unlike rapamecin, matrine (5–20 mg/L) did not significantly alter the level of p27 protein in VSMCs.
Figure 3.
Effect of matrine on the expression of p53, p21, and p27 proteins in cultured VSMCs examined with Western blot. The levels of cell cycle-related proteins in cultured rat aortic VSMCs were investigated after treatments with various doses of matrine (0, 5, 10, 15, and 20 mg/L) for 72 h. Rapamycin (100 ng/L) was as a positive control. (A) p53; (B) p21; (C) p27. Results were normalized by the respective level of β-actin. The changes in percentage of treatment control (0 mg/L matrine) were calculated. Data are expressed as mean±SEM. n=5/group, One-way ANOVA. bP<0.05, cP<0.01 vs treatment control.
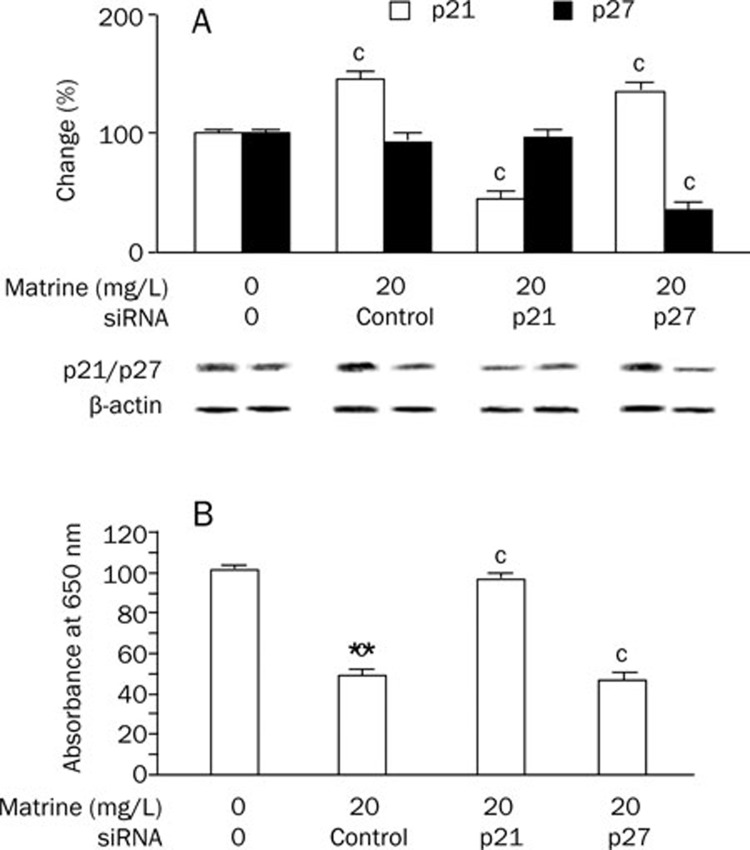
Inhibition of p21, but not p27, rescued matrine-induced proliferation in VSMCs
To determine the roles of p21 and p27 in mediating the anti-proliferation effect of matrine on VSMCs, we transfected the cells with p21 or p27 siRNA 12 h before the cells were treated with matrine (20 mg/L). As seen in Figure 4A, both p21 and p27 siRNAs specifically down-regulated target gene expression in VSMCs by 65%–75%. Additionally, siRNA-mediated down-regulation of p21 abolished the inhibitory effect of matrine on VSMC proliferation. Silencing p27 had no significant influence on the inhibitory effect of matrine (Figure 4B).
Figure 4.
Role of p21 and p27 pathways in the inhibitory effect of matrine on VSMC proliferation. (A) siRNA treatment on p21 and p27 expression. Expression of p21 or p27 proteins in cultured rat aortic VSMCs were determined by Western blot after siRNAs transfection followed by matrine (20 mg/L) treatment for 72 h. Results were normalized by the respective level of β-actin. The changes in percentage of basal (0 mg/L matrine and 0 ng siRNA) were calculated; (B) Effect of silencing p21 or p27 on matrine's anti-proliferative effect on cultured VSMCs. Cell growth was determined by colorimetric assay of methylene blue incorporation after respective siRNAs transfection followed with matrine (20 mg/L) for 72 h. The changes in percentage of basal (0 mg/L matrine and 0 ng siRNA) were calculated. Data are expressed as mean±SEM. n= 5/group, two-way ANOVA. cP<0.01 vs basal or treatment control (siRNA control).
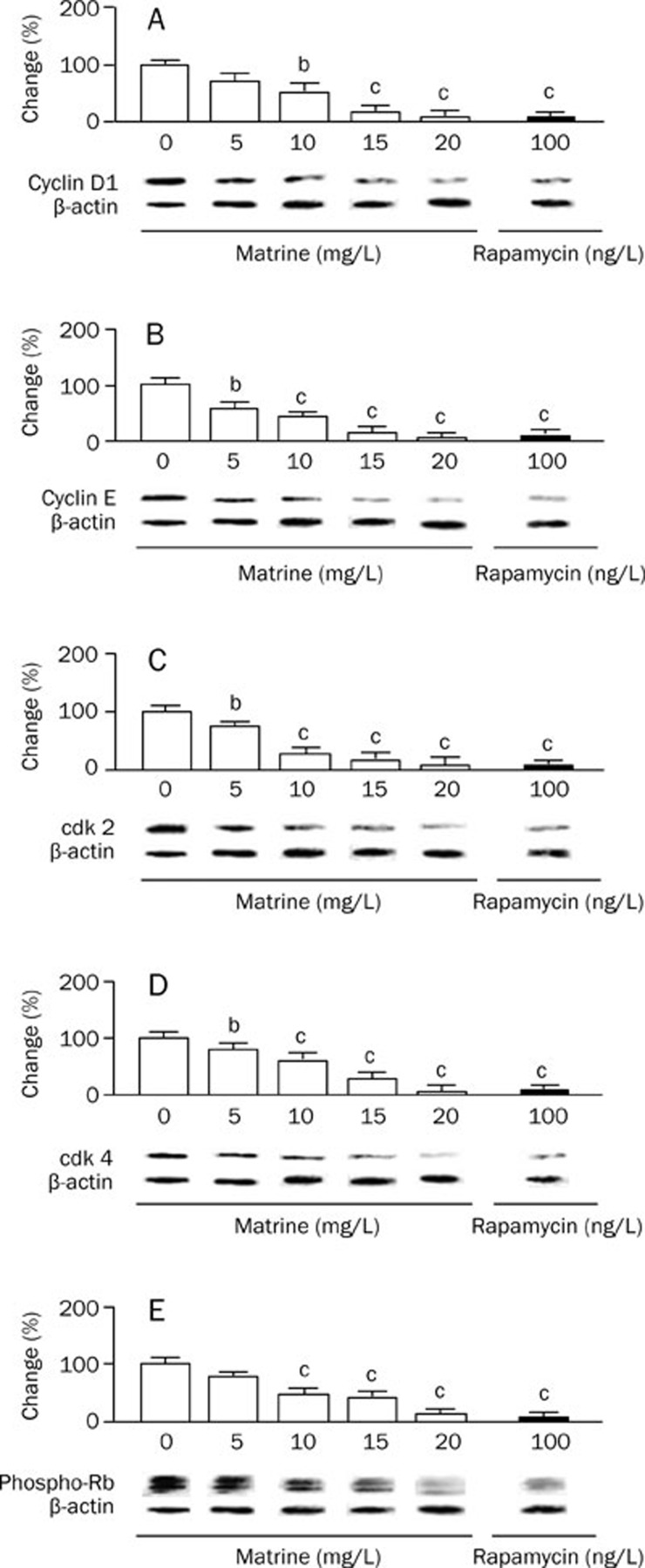
Effect of matrine on protein expression levels of cyclin D1, cyclin E, cdk2, cd4, and phosphorylated Rb
To further investigate the mechanism by which matrine leads to G1 arrest in VSMCs, we examined levels of cell cycle regulatory proteins, including cyclin D1, cyclin E, cdk2, cd4, and phosphorylated Rb. As seen in Figure 5, matrine decreased levels of cyclin D1, cyclin E, cdk2, and cdk4 and phosphorylated Rb in a dose-dependent fashion. Matrine (20 mg/L) treatment induced increases in protein levels similar to those observed following rapamycin (100 ng/L) treatment.
Figure 5.
Effect of matrine on the expression of cell cycle regulating proteins in cultured VSMCs examined with Western blot. The levels of cell cycle-related proteins in cultured rat aortic VSMCs were investigated after treatment with various doses of matrine (0, 5, 10, 15 and 20 mg/L) for 72 h. Rapamycin (100 ng/L) was as a positive control. (A) cyclin D1; (B) cyclin E; (C) cdk2; (D) cdk4; (E) Phos-Rb. Results were normalized by the respective level of β-actin. The changes in percentage of treatment control (0 mg/L matrine) were calculated. Data are expressed as mean±SEM. n=5/group, One-way ANOVA. bP<0.05, cP<0.01 vs treatment control.
Discussion
To investigate the role of matrine in VSMC proliferation and determine underlying mechanisms, we studied the effect of matrine on 10% FBS-stimulated VSMC proliferation, cell cycle progression, and expression of cell cycle regulatory genes. We found that matrine inhibited VSMC proliferation and reduced cell cycle progression by promoting a G1 phase block. These effects are accompanied by increased levels of p53 and p21, and down-regulation of cyclin D1, cyclin E, cdk 2, cdk4, and phosphorylated Rb protein.
VSMCs cultured in 10% FBS medium are commonly used as an in vitro model to study neointimal proliferation. Under this culture condition, VSMCs undergo tumor-like transformation and proliferation. In previous studies7, 8, 9, 10, 11, several tumor cell lines, such as leukemia K562 and hepatocellular carcinoma H22 cells, were incubated with matrine (100–900 mg/L) for 48 h to 4 days. In these cell lines, matrine was able to inhibit cell growth and increase p53 and p21 mRNA expression levels. We treated cells with lower doses (10–20 mg/L) of matrine because we reasoned that VSMCs would not be as malignant as tumor cells and would therefore be more sensitive to treatment. As we expected, our results show that matrine inhibits the proliferation in transformed VSMCs at these relatively low doses. We also found that matrine elicits its anti-proliferative effect on VSMCs by inducing G1 phase arrest. In this study, rapamycin was used as a positive control because it has demonstrated anti-proliferative properties in a variety of cell types. Rapamycin inhibits a multifunctional serine-threonine kinase, the mammalian target of rapamycin (mTOR)24, 25. Furthermore, our data showed that matrine (20 mg/L) treatment was similar to rapamycin (100 ng/L) treatment because both inhibited VSMC proliferation and induced G1 arrest17. These observations demonstrate that matrine is effective in inhibiting VSMC proliferation.
Previous studies demonstrated that statins promote G1 arrest in VSMCs by increasing cellular p27 levels and reducing cyclin E expression15, 16. In contrast, rapamycin inhibits VSMC proliferation by halting cells in G1 by up-regulation of p21 protein17. To explore the mechanism by which matrine inhibits proliferation and causes G1 arrest in VSMCs, we first examined protein levels of p53, p21, and p27 in VSMCs following treatment with various concentrations of matrine (0–20 mg/L). Then, we determined the role of p21 and p27 pathways using siRNA knockdown to decrease p21 and p27 expression. We found that matrine dose-dependently up-regulated p53 and p21 expression in VSMCs, but it did not affect the expression of p2715, 28. SiRNA transfection targeting p21 abolished the inhibitory effect of matrine on VSMC proliferation, whereas, down-regulation of p27 had no influence on matrine's effect. Expression of p21 can be regulated by both p53 dependent and independent mechanisms30, 31, 32. In this study, we found that matrine treatment lead to increased p21 protein levels along with increased p53 levels in VSMCs. Therefore, the effect of matrine on p21 expression in VSMCs could be downstream of p53/p21 signaling. As mentioned previously, matrine has effects on multiple cellular functions, including anti-infection, anti-inflammation, anti-cancer activities. Matrine's anti-proliferative effect on VSMCs deserves further attention.
Mitogen stimulated cell cycle progression in mammalian cells requires the expression and activation of cyclin and cdk complexes33. These events are initiated by induction of cyclin D1, followed by induction of cyclin E, activation of cdk 4 and cdk2, and phosphorylation of Rb gene products. Mitogenic activation of cyclin D1/cdk4 and cyclin E/cdk2 during G1 results in phosphorylation of the Rb protein34. Phosphorylated Rb functions as a gatekeeper for the G1-S transition by binding and sequestering E2F, a transcription factor that induces the expression of a battery of genes that are necessary for S phase (DNA synthesis). In its unphosphorylated state, Rb binds and sequesters E2F to prevent transcriptional activation of target genes. In this study, we found that matrine decreased levels of cylin D1, cyclinE , cdk2, cdk4 and phosphorylated Rb in VSMCs in a pronounced manner. These findings are similar to findings from positive controls (rapamycin) and are consistent with previous reports17, including a study showing that up-regulation of cyclin E is absolutely required for cell cycle progression in VSMCs cultured in 10% FBS22. Since p53/p21 is a negative regulator of cyclins and cdks35, our data showing that matrine decreases cyclin D1 and E levels indicate that p53 and p21 may act downstream of matrine to affect cyclins and cdks. P27 is also a negative regulator that inhibits cyclins/cdks and Rb phosphorylation, and consequently results in G1 arrest36. Since p27 level did not change following matrine treatment, we do not expect that p27 participate in matrine's anti-proliferative effect in VSMCs.
In summary, we demonstrated that matrine was a potent inhibitor of VSMC proliferation. Matrine leads to increases in p53 and p21 protein levels, and decreases in cdk2, cdk4, cyclin D1, and cyclin E protein levels. However, whether matrine inhibits intimal hyperplasia must still be investigated in in vivo. Additionally, our data do not exclude the possibility that the matrine may exert its effects on VSMCs via affecting other cell cycle proteins such as E2F-137, or other mechanisms that regulate VSMC proliferation.
Author contribution
Ping ZHU, Yan-fang CHEN, and Jian ZHUANG designed research; Ping ZHU, Cheng ZHANG, Shao-yi ZHENG, Zhi-ling ZHOU, Rui-xin FAN, and Xiao-ping FAN performed research; Shu-zhen CHEN and Ji CHEN contributed new analytical tools and reagents; Ping ZHU, Ji-mei CHEN, and Guang LONG analyzed data; Ping ZHU, Yan-fang CHEN, and Jian ZHUANG wrote the paper.
Acknowledgments
This work was supported by the Major International (Regional) Joint Research Project (2008DFA31140, PZ) and American Diabetes Association (1-10-BS-25, YC). We thank Ms Cathy Graham in the Biomedical PhD Program at Wright State University for her kind help in proof-reading this manuscript.
References
- Welt FG, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol. 2002;22:1769–76. doi: 10.1161/01.atv.0000037100.44766.5b. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Shan H, Qiao G, Sui X, Lu Y, Yang B. Inotropic effects and mechanisms of matrine, a main alkaloid from Sophora flavescens AIT. Biol Pharm Bull. 2008;31:2057–62. doi: 10.1248/bpb.31.2057. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang B, Zhou C, Bi Y. Matrine induces apoptosis in angiotensin II-stimulated hyperplasia of cardiac fibroblasts: effects on Bcl-2/Bax expression and caspase-3 activation. Basic Clin Pharmacol Toxicol. 2007;101:1–8. doi: 10.1111/j.1742-7843.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang Y, Fang L, Zhang Z, Jin J, Zhang K. Anti-hepatitis activities in the broth of Ganoderma lucidum supplemented with a Chinese herbal medicine. Am J Chin Med. 2006;34:341–9. doi: 10.1142/S0192415X06003874. [DOI] [PubMed] [Google Scholar]
- Ma L, Wen S, Zhan Y, He Y, Liu X, Jiang J. Anticancer effects of the Chinese medicine matrine on murine hepatocellular carcinoma cells. Planta Med. 2008;74:245–51. doi: 10.1055/s-2008-1034304. [DOI] [PubMed] [Google Scholar]
- Liu XS, Jiang J. Molecular mechanism of matrine-induced apoptosis in leukemia K562 cells. Am J Chin Med. 2006;34:1095–103. doi: 10.1142/S0192415X06004557. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang T, Wen X, Wei Y, Peng X, Li H, et al. Effect of matrine on HeLa cell adhesion and migration. Eur J Pharmacol. 2007;563:69–76. doi: 10.1016/j.ejphar.2007.01.073. [DOI] [PubMed] [Google Scholar]
- Dai ZJ, Gao J, Ji ZZ, Wang XJ, Ren HT, Liu XX, et al. Matrine induces apoptosis in gastric carcinoma cells via alteration of Fas/FasL and activation of caspase-3. J Ethnopharmacol. 2009;123:91–6. doi: 10.1016/j.jep.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Jiang ZH, Lu YW, Guo Y, Gan JJ. Matrine and anti-tumor drugs in inhibiting the growth of human lung cancer cell line. Zhong Xi Yi Jie He Xue Bao. 2008;6:163–5. doi: 10.3736/jcim20080211. [DOI] [PubMed] [Google Scholar]
- Zhang LP, Jiang JK, Tam JW, Zhang Y, Liu XS, Xu XR, et al. Effects of matrine on proliferation and differentiation in K-562 cells. Leuk Res. 2001;25:793–800. doi: 10.1016/s0145-2126(00)00145-4. [DOI] [PubMed] [Google Scholar]
- Jiang H, Hou C, Zhang S, Xie H, Zhou W, Jin Q, et al. Matrine upregulates the cell cycle protein E2F-1 and triggers apoptosis via the mitochondrial pathway in K562 cells. Eur J Pharmacol. 2007;559:98–108. doi: 10.1016/j.ejphar.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97:425–34. [PubMed] [Google Scholar]
- Wei JW, Liao JF, Chuang CY, Chen CF, Han PW. Cardiovascular effects of matrine isolated from the Chinese herb Shan-dou-gen. Proc Natl Sci Counc Repub China B. 1985;9:215–9. [PubMed] [Google Scholar]
- Fouty BW, Rodman DM. Mevastatin can cause G1 arrest and induce apoptosis in pulmonary artery smooth muscle cells through a p27Kip1-independent pathway. Circ Res. 2003;92:501–9. doi: 10.1161/01.RES.0000061180.03813.0F. [DOI] [PubMed] [Google Scholar]
- Rao S, Porter DC, Chen X, Herliczek T, Lowe M, Keyomarsi K. Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase. Proc Natl Acad Sci USA. 1999;96:7797–802. doi: 10.1073/pnas.96.14.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun-Dullaeus RC, Mann MJ, Seay U, Zhang L, von Der Leyen HE, Morris RE, et al. Cell cycle protein expression in vascular smooth muscle cells in vitro and in vivo is regulated through phosphatidylinositol 3-kinase and mammalian target of rapamycin. Arterioscler Thromb Vasc Biol. 2001;21:1152–8. doi: 10.1161/hq0701.092104. [DOI] [PubMed] [Google Scholar]
- Adams PD. Regulation of the retinoblastoma tumor suppressor protein by cyclin/cdks. Biochim Biophys Acta. 2001;1471:M123–M133. doi: 10.1016/s0304-419x(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Ekholm SV, Reed SI. Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol. 2000;12:676–84. doi: 10.1016/s0955-0674(00)00151-4. [DOI] [PubMed] [Google Scholar]
- Aleem E, Kiyokawa H, Kaldis P. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat Cell Biol. 2005;7:831–6. doi: 10.1038/ncb1284. [DOI] [PubMed] [Google Scholar]
- Stacey DW. Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr Opin Cell Biol. 2003;15:158–63. doi: 10.1016/s0955-0674(03)00008-5. [DOI] [PubMed] [Google Scholar]
- Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, et al. Cyclin E ablation in the mouse. Cell. 2003;114:431–43. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Corsini A, Verri D, Raiteri M, Quarato P, Paoletti R, Fumagalli R. Effects of 26-aminocholesterol, 27-hydroxycholesterol, and 25-hydroxycholesterol on proliferation and cholesterol homeostasis in arterial myocytes. Arterioscler Thromb Vasc Biol. 1995;15:420–8. doi: 10.1161/01.atv.15.3.420. [DOI] [PubMed] [Google Scholar]
- Thyberg J, Palmberg L, Nilsson J, Ksiazek T, Sjolund M.Phenotype modulation in primary cultures of arterial smooth muscle cells. On the role of platelet-derived growth factor Differentiation 198325156–67. [DOI] [PubMed] [Google Scholar]
- Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–23. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- Schofer J, Schluter M, Gershlick AH, Wijns W, Garcia E, Schampaert E, et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS) Lancet. 2003;362:1093–9. doi: 10.1016/S0140-6736(03)14462-5. [DOI] [PubMed] [Google Scholar]
- Oliver MH, Harrison NK, Bishop JE, Cole PJ, Laurent GJ. A rapid and convenient assay for counting cells cultured in microwell plates: application for assessment of growth factors. J Cell Sci. 1989;92 (Pt 3:513–8. doi: 10.1242/jcs.92.3.513. [DOI] [PubMed] [Google Scholar]
- Ferri N, Granata A, Pirola C, Torti F, Pfister PJ, Dorent R, et al. Fluvastatin synergistically improves the antiproliferative effect of everolimus on rat smooth muscle cells by altering p27Kip1/cyclin E expression. Mol Pharmacol. 2008;74:144–53. doi: 10.1124/mol.108.046045. [DOI] [PubMed] [Google Scholar]
- Endesfelder S, Kliche A, Lochmuller H, von MA, Speer A. Antisense oligonucleotides and short interfering RNAs silencing the cyclin-dependent kinase inhibitor p21 improve proliferation of Duchenne muscular dystrophy patients' primary skeletal myoblasts. J Mol Med. 2005;83:64–71. doi: 10.1007/s00109-004-0607-3. [DOI] [PubMed] [Google Scholar]
- Macleod KF, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, et al. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995;9:935–44. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Taniguchi T, Ishikawa Y, Yokoyama M. Tranilast inhibits vascular smooth muscle cell growth and intimal hyperplasia by induction of p21(waf1/cip1/sdi1) and p53. Circ Res. 1999;84:543–50. doi: 10.1161/01.res.84.5.543. [DOI] [PubMed] [Google Scholar]
- Dulic V, Kaufmann WK, Wilson SJ, Tlsty TD, Lees E, Harper JW, et al. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–23. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–7. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–4. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–4. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Jiang H, Hou C, Zhang S, Xie H, Zhou W, Jin Q, et al. Matrine upregulates the cell cycle protein E2F-1 and triggers apoptosis via the mitochondrial pathway in K562 cells. Eur J Pharmacol. 2007;559:98–108. doi: 10.1016/j.ejphar.2006.12.017. [DOI] [PubMed] [Google Scholar]