Abstract
This study evaluated whether the combination of biodegradable β-tricalcium phosphate (β-TCP) scaffolds with recombinant human bone morphogenetic protein-2 (rhBMP-2) or platelet-rich plasma (PRP) could accelerate bone formation and increase bone height using a rabbit non-through cranial bone defect model. Four non-through cylindrical bone defects with a diameter of 8-mm were surgically created on the cranium of rabbits. β-TCP scaffolds in the presence and absence of impregnated rhBMP-2 or PRP were placed into the defects. At 8 and 16 weeks after implantation, samples were dissected and fixed for analysis by microcomputed tomography and histology. Only defects with rhBMP-2 impregnated β-TCP scaffolds showed significantly enhanced bone formation compared to non-impregnated β-TCP scaffolds (p<0.05). Although new bone was higher than adjacent bone at 8 weeks after implantation, vertical bone augmentation was not observed at 16 weeks after implantation, probably due to scaffold resorption occurring concurrently with new bone formation.
Keywords: rhBMP-2, PRP, calvarial defect, porous scaffolds, ceramic scaffolds, vertical augmentation
1 Introduction
In the United States alone, over 20 million people are totally edentulous [1]. Degenerative oral function due to tooth loss can be partially restored with titanium dental implants. However, in many cases, vertical ridge augmentation or bone regeneration in the vertical direction must be performed prior to implant placement. Vertical bone regeneration remains a significant clinical challenge in dentistry [2]. One approach to designing bone substitutes for ridge augmentation or repair of critical defects is mimicking the regenerative properties of autogenous bone. Calcium phosphate (CaP) ceramics, such as hydroxyapatite (HA) and beta-tricalcium phosphate (β-TCP) have been widely used in clinical practice for bone defect repair due to their biocompatibility, mechanical strength, and osteoconductive properties [3, 4]. To accelerate integration and repair of the affected site [5], interconnected porous structure and porosity are essential, as it allows cell penetration into the scaffold, and assures interactions between cells and diffusion of oxygen and nutrients to the interior of the scaffold [6, 7]. Though porous CaP scaffolds have been fabricated using a variety of techniques [8–11], we have designed a template casting technique to create a highly-interconnected porous β-TCP scaffold with high compressive mechanical strength [12, 13]. This interconnected porous, biodegradable CaP scaffold has been shown to allow the growth and spread of mesenchymal stem cells (MSCs) [13] and aid bone formation [14, 15].
However, by themselves, CaP ceramic scaffolds do not have the ability to induce differentiation of MSCs into osteoblasts, which is required for mineralization and regeneration of bone tissue. A number of differentiation factors have been investigated as facilitators of bone repair. Several bone morphogenetic proteins, which are part of the transforming growth factor-β superfamily, can activate the chemical signaling cascades for the differentiation of MSCs to osteogenic lineages. Among these, recombinant human bone morphogenetic protein-2 (rhBMP-2) is a potent osteogenic differentiation factor [16] used clinically for spinal fusion [17] and in bone repair [18–20]. Osteoinduction by rhBMP-2 has been studied for decades [21]. Its effectiveness is limited by body regulation [22], which might decrease its concentration in the area of interest while causing bone overgrowth away from the affected regions [23]. Therefore, controlled release of the protein in the area of interest is highly desired. CaP scaffolds with slow BMP-2 release have promoted long-term repair in critical size defects and significantly increased bone mineral content in surrounding areas [24, 25]. CaP and BMP-2 interact with each other, facilitating protein attachment and slowing release [26]. Slow release of BMP-2 into affected areas has been shown to promote mineralization more than a fast release [27].
In addition to differentiation factors, platelet-rich plasma (PRP) has also been used to repair bone defects [28, 29]. PRP contains proteins and growth factors that can promote differentiation of cells and may promote healing at the site of injury, possibly accelerating bone regrowth and repair [30]. Marx et al demonstrated in a study of 44 patients undergoing autogenous bone grafting that the addition of PRP at 3 to 4 times its normal concentration increased radiopacity 1.6 to 2.2 times and significantly increased bone tissue formation [31]. Anitua and coworkers reported that direct application of PRP in the extracted socket of 3 patients resulted in increased bone formation [32]. However, the role of PRP in enhancing osteogenic activity when used in combination with biomaterials remains uncertain.
The purpose of this study was to evaluate whether the combination of biodegradable β-TCP scaffolds with rhBMP-2 or PRP can accelerate bone formation and induce vertical augmentation of bone in a rabbit non-through cranial bone defect model. The non-through bone defect model was designated for vertical bone growth by keeping the inner cortex and only removing the outer cortex of the cranium. The scaffolds were also intended to have their middle and bottom portions embedded within bone, while the top portion sat higher than the adjacent bone. We hypothesized that BMP and PRP would enhance the osteoinductive potential of tissue engineering ceramic scaffolds in our model.
2 Materials and methods
2.1 Animals
Seven adult male New Zealand white rabbits weighing 3.3 to 3.5kg were used for the study. Before the experiment, the health of the rabbits was monitored for 4 weeks. They were kept in standard cages in an experimental animal room (24°C, 55% humidity, 1 atm, 12-hour light/dark cycle) and were fed a standard laboratory diet and water. This study was approved by the Animal Experimentation Committee at Chonnam National University.
2.2 β-TCP scaffold preparation
β-TCP scaffolds (porosity, 73%; pore size, 600 to 800 μm; diameter, 7.7mm; thickness, 3mm) were fabricated by a novel template-casting technique (Fig. 1) [33]. Scaffolds were sterilized with EO (ethylene oxide) gas before implantation.
Fig. 1.
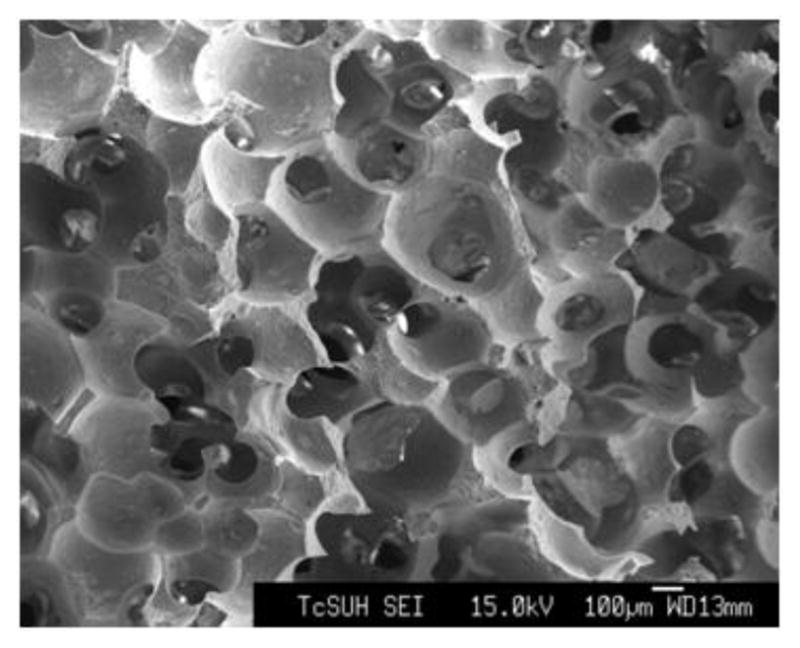
Scanning electron microscope image of a β-TCP scaffold.
2.3 Preparation of rhBMP-2
670μl distilled water was added to a tube containing 1mg of sterilized freeze-dried rhBMP-2 (Cowellmedi Co., Busan, Korea) following manufacturer’s instructions. The final concentration was 1.5 mg/mL. The experimental scaffold was soaked with 20 μL rhBMP-2 solution in a sterilized Petri dish and placed on a clean bench for 5 minutes. After verifying the dehydration of the protein solution on the scaffold, the process of adding the solution and drying was repeated. 67 μL of the rhBMP-2 stock solution was applied to each scaffold, so consequently 100 μg rhBMP-2 was added to each scaffold.
2.4 Preparation of PRP
PRP was prepared according to a method reported by Hokugo et al [34]. Briefly, 10 mL fresh blood were obtained from each rabbit, using a 20-mL sterilized syringe containing 1 mL citrate-dextrose A solution as an anticoagulant. The blood was centrifuged in a laboratory centrifugation apparatus (Placon™, Oscotec, Seoul, Korea) at 4 °C for 10 minutes at 2,400 rpm. Subsequently, the yellow plasma containing the platelet fraction was collected and centrifuged further at 4 °C for 15 minutes at 3,600 rpm to separate the platelets from the plasma. The approximate volume of PRP obtained was 0.8 mL.
2.5 Anesthesia and Surgery
Surgery was conducted on all rabbits (3 rabbits at 8 weeks and 4 rabbits at 16 weeks) under sterile conditions. General anesthesia was induced by intramuscular injection of 0.2 mg/kg xylazine HCl (Rompun®, Bayer Korea, Seoul, Korea) and 0.3 mg/kg ketamine HCl (Ketalar®, Yuhan Co., Seoul, Korea). Ongoing anesthesia was maintained with a maximum of 5 dosages of 0.3mg/kg ketamine HCl. The incision site was shaved and sterilized. An injection of 1.8 mL lidocaine HCl containing 1:80,000 epinephrine was used as a local anesthetic to reduce subcutaneous hemorrhage over the the calvarium. An incision was made along the midsagittal plane and the calvarium was exposed. Four non-through bone defects, approximately 2 mm in depth and going through cortical and cancellous layers, were formed on the cranium of each rabbit using an 8-mm trephine bur. Fibrin adhesive (Greenplast®, Green Cross Co., Yongin, Korea) was applied to the bone defect and then a scaffold, a scaffold with rhBMP-2, and a scaffold with PRP were placed on three of the defects. All scaffolds sat 1 mm higher than adjacent bone so that vertical bone augmentation could be evaluated (Fig. 2). The fourth bone defect was not filled with a scaffold and served as a control (Table 1, Fig. 3). After repeated application of fibrin adhesive, the periosteum was closed with 5.0 Vicryl (Johnson & Johnson/Ethicon, Summerville, NJ, USA) and the skin with surgical silk. Postoperatively, the rabbits received 25,000,000 units of penicillin G (Sigma-Aldrich, St. Louis, MO, USA) at a volume of 0.1 mL/kg, given as a single intramuscular injection.
Fig. 2.
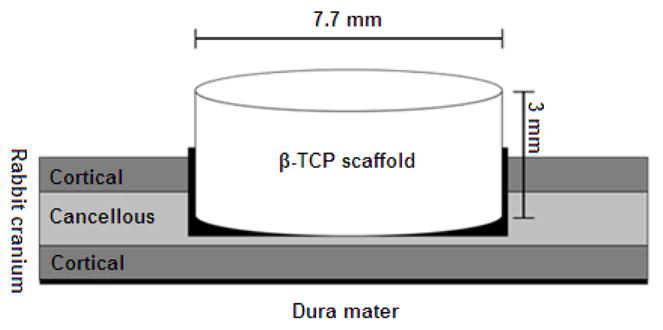
Schematic diagram of cranial defect and placement of a β-TCP scaffold.
Table 1.
Classification of groups in this study
| Group | β-TCP Scaffold | rhBMP-2 | PRP |
|---|---|---|---|
| S | O | X | X |
| SB | O | O | X |
| SP | O | X | O |
| Control | X | X | X |
Fig. 3.

An example of an experimental group. S: β-TCP scaffold; SB: β-TCP scaffold +rhBMP-2; SP: β-TCP scaffold +PRP; C: control.
2.6 Specimen preparation
After healing for 8 and 16 weeks, the animals were sacrificed with an overdose of KCl solution. A quadrilateral incision was made, and the calvarium was dissected and fixed in 4% formalin immediately.
2.7 Micro-CT analysis
Micro-CT (Skyscan-1172, SKYSCAN, Antwerpen, Belgium) scans were performed on the samples at 8 and 16 weeks. During the scanning process, specimens were maintained in humid state by keeping them in the formalin solution. For scanning, the voltage was 49 kVp, the current was 200 μA and 0.5 mm aluminium filtration was used. The shooting time was 295 ms and the pixel size was 11.96 μm. The cross-sectional images were generated using NRecon (SKYSCAN, Antwerpen, Belgium); then, image reconstruction was performed using DataViewer (SKYSCAN, Antwerpen, Belgium) to align the axis of the scaffold to the z-axis of the image. On the images, the β-TCP scaffold and bone tissue were separated from marrow and soft tissue using a global thresholding procedure, where a threshold greater than 68% of the maximal X-ray attenuation was used to identify calcium phosphate biomaterial, a threshold between 50% and 68% signified bone, and thresholds below 50% identified marrow and soft tissue properties [35]. 3D images were constructed with the reconstituted data using CT-AN™ and CT-Vol™ (SKYSCAN, Antwerpen, Belgium). The percentage of remaining β-TCP scaffold and new bone was calculated from the reconstituted data.
2.8 Histologic analysis
The calvarium bone containing the β-TCP scaffolds was fixed in 10% neutral buffered formalin. Samples were then dehydrated, decalcified in 5% nitric acid for 6 hours, neutralized and washed. Afterwards, they were embedded in paraffin, and processed into 4- to 5-μm thick hematoxylin and eosin sections. The sections were examined under a light microscope (BX-51T, Olympus, Tokyo, Japan) connected to a personal computer.
2.9 Statistical analysis
Means and standard deviations were calculated for the remaining β-TCP scaffold and mineralized bone. One-way ANOVA was used to analyze differences and Tukey’s test was used for post-hoc analysis. Differences with p<0.05 were considered significant. SPSS Version 19 (SPSS Inc., Chicago, IL, USA) was used for all statistical analysis.
3 Results
3.1 Micro-CT analysis
The percentages of remaining β-TCP scaffold and newly-formed bone are shown in Fig. 4. The β-TCP scaffold content decreased with time. At 8 weeks, the percentages of remaining β-TCP, β-TCP+rhBMP2, and β-TCP+PRP scaffolds were 13.6±2.7%, 13.1±3.6%, and 14.5±3.2%, respectively. At 16 weeks, these had decreased to 10.5±1.8%, 7.6±3.0%, and 8.9±1.7%. Although the amount of remaining β-TCP at 16 weeks was less than at 8 weeks, there were no statistical differences among the groups. As time progressed, the percentage of new bone formation increased in all groups. At 8 weeks, the mineral content for β-TCP, β-TCP+rhBMP2, and β-TCP+PRP groups were 24.2±2.9%, 33.2±3.9%, and 31.5±2.6%, respectively. Values increased to 33.9±4.9%, 42.9±2.6%, and 37.2±3.0% at 16 weeks. As the results indicate, both at 8 weeks and 16 weeks, the addition of rhBMP-2 on the β-TCP scaffolds significantly induced more bone formation compared to the β-TCP scaffold only (p<0.05).
Fig. 4.

Percentage of mineralized bone. One star (*) indicates SD at p<0.05.
Fig. 5 shows the 2D/3D micro CT images of specimens. After 8 weeks, the β-TCP scaffolds showed definitive radiopacity. The radiopacity of new bone was similar to adjacent bone. More radiopacity was observed for β-TCP scaffolds with rhBMP-2 and PRP than for β-TCP scaffolds only. No significant new bone formation was seen for the control group. After 16 weeks, the amount of remaining β-TCP scaffold was less than at 8 weeks. New bone formation was mostly observed in the β-TCP scaffold with rhBMP-2. It is interesting that the total amount of new bone increased over time, but the bone height was not kept over the duration of the study. Although newly-formed bone inside the top portion of the scaffold was higher than adjacent bone 8 weeks after implantation, vertical augmentation of bone was not observed 16 weeks after implantation.
Fig. 5.

Micro-CT images of specimens. 2D and 3D make reference to two- and three-dimensional scans.
3.2 Histologic analysis
Fig. 6 shows histologic images of specimens at 8 and 16 weeks. New bone formation was seen in groups with β-TCP scaffolds, but only adipose tissue was seen in the control groups. There was no inflammatory infiltration around the scaffolds in any group, and no connective tissue invasion from the cutaneous tissue was observed. The scaffold was clearly visible radiographically at 8 weeks, but interval resorption was evident in images obtained at 16 weeks. Thin fibroblast layers were seen at the top of the constructs at 8 weeks, with a larger layer for the control group at 16 weeks.
Figure 6.

Histology sections of specimens at 8 weeks and 16 weeks. Scale bars represent 200 microns.
Fig. 7 shows the sagittal histologic section of β-TCP scaffold with rhBMP-2 at 8 weeks. New bone formation, including Haversian canals, osteoblasts, bone marrow, and lamellar bone inside the scaffold, was observed. Fibroblast-like cells were observed in the upper part of the scaffold and new bone formation was higher than adjacent bone. Fig. 8 shows the sagittal histologic section of β-TCP scaffold with rhBMP-2 at 16 weeks. Mineralization up to upper margin of the defect was definitively observed. However, vertically-augmented bone seemed to be resorbed with the degradation of the scaffold, which is consistent with the observation in the MicroCT images in Fig. 5.
Fig. 7.
Sagittal histologic section of β-TCP scaffold with rhBMP-2 at 8 weeks. MB: Newly formed mineralized bone, FC: Fibroblast like cell. The scale bar of the top image is 200 microns; the scale bars for the bottom images represent 20 microns.
Fig. 8.
Sagittal histologic section of β-TCP scaffold with rhBMP-2 at 16 weeks. MB: Newly formed mineralized bone. The scale bar of the top image is 200 microns; the scale bars for the bottom images represent 20 microns.
4 Discussion
The purpose of this study was to determine if the combination of a β-TCP scaffold and rhBMP-2 or PRP would promote vertical bone growth in a rabbit cranial defect model. Interconnected porous β-TCP scaffolds were implanted in bone defects on the calvaria of rabbits, with the expectation that the addition of rhBMP-2 or PRP would enhance the formation of new bone. The scaffold sat higher than surrounding bone so that vertical bone augmentation could be induced. Our results confirm that β-TCP scaffolds aid bone healing. The addition of rhBMP-2 to the scaffold leads to a significant increase in bone formation while the addition of PRP did not. Our results on osteocondutive CaP scaffold aided bone healing in the absence and presence of BMP-2 are consistent with previous work [36–39], but the role of PRP in mineralization and bone augmentation remains uncertain. The rationale for the use of PRP is derived from its increased potential for injury healing and the high concentration of growth factors. Platelets have a lifespan of 8 to 10 days and secrete dense and α-granules when activated, and cytokines are released through this degranulation reaction [40], including growth factors such as platelet derived growth factor (PDGF), transforming growth factor-beta (TGF-β) 1 and 2, and insulin-like growth factor-1 (IGF-1). These growth factors increase cell mitosis and collagen synthesis. In a bone graft, increased platelet concentration has been associated with an increased concentration of growth factors and consequently, faster bone regeneration [30, 41, 42]. However, the osteogenic effects of PRP are controversial [31, 32]. While some studies suggest that PRP may promote osteogenesis, albeit to a limited extent [43–45], others have suggested that the combination of PRP and biomaterials has no effect on new bone formation [46, 47]. This is probably because PRP highly varies from donor to donor; and each PRP preparation can differ in the concentration of proteins and growth factors [48]. In addition, the cytokines in platelets are time-restricted and may break down prior to exerting any osteogenic effect.
It is worth noting that no meaningful vertical bone augmentation was observed after completion of the study at 16 weeks, although mineralization inside scaffolds above surrounding bone was observed in the rhBMP-2 scaffold after 8 weeks post-surgery. The early bone augmentation within the biodegradable scaffolds is probably due to osteoconductivity of the scaffold and its capability of remaining volume. Araújo et al [49] reported that a Bio-Oss® bone graft substitute block implanted into the lateral ridge of dogs maintained its volume and formed a limited amount of new bone over a period of 6 months. Sartori [50] showed maxillary sinus augmentation at 8 months, 2 years and 10 years after implantation of Bio-Oss® in a case study, with progressive replacement of the bone void filler with lamellar bone. Over time, vertical bone seemed to be resorbed at the same rate as the scaffold degraded. It is possible that the newly-formed bone in the scaffolds did not experience the mechanical loading required for remodeling due to our use of a non-load-bearing cranial bone defect model. This has been seen in previous work. Schmid et al, for example, showed that deproteininzed bovine bone mineral fixed with a biodegradable PLA implanted in rabbit calvaria assisted in the initial stages of new bone development, while no differences could be noted in longer periods of time [51]. The role of the scaffold in providing mechanical support or protection for vertical bone development, with or without signaling cues, also remains unclear. For example, Okazaki reported that natural deproteinized bone mineral matrices could support bone formation over a period of time after implantation in the cortical bone of rabbit skulls, but control groups with blood clots also showed significant increase in bone formation [52]. In the future, we will evaluate the role of non-biodegradable and long-lasting biodegradable ceramic scaffolds in forming lasting vertically-augmented bone in vivo. Moreover, we will study the effect of combining both PRP and rhBMP-2 in scaffolds to evaluate if there is a synergistic effect of both agents in vertical bone augmentation.
5 Conclusions
In this study, we created a rabbit non-through bone defect model to evaluate β-TCP as a bone graft substitute and explore the roles of BMP-2 and PRP in accelerating in vivo bone formation. Within the limitations of this study, the β-TCP scaffold resulted in increased bone formation compared to control. Bone formation increased significantly with the addition of rhBMP-2 to the scaffold but not with the addition of PRP. Early bone augmentation within the biodegradable scaffolds was observed, but vertical bone growth was not maintained, probably because the newly-formed bone was resorbed during the biodegradation of the scaffolds. Further studies are needed to evaluate long-term results, the effect of other growth factors, and the effects of varying growth factor concentration and scaffold structure.
Supplementary Material
Acknowledgments
We would like to acknowledge the support of the Chonnam National University Hospital Research Institute of Clinical Medicine (Gwangju, Republic of Korea), the National Institutes of Health (NIH, United States), and the Department of Defense (DOD, United States) in the form of research grants CRI 11061-1, NIH AR057837 (NIAMS), NIH DE021468 (NIDCR), DOD W81XWH-10-1-0966 (PRORP), DOD W81XWH-10-200-10 (Airlift Research Foundation) and DOD W81XWH-11-2-0168-P4 (Alliance of NanoHealth).
References
- 1.Wayne DB, Trajtenberg CP, Hyman DJ. Tooth and Periodontal Disease: A Review for the Primary-Care Physician. South Med J. 2001;94:925–932. [PubMed] [Google Scholar]
- 2.Yang Y, Oh N, Liu Y, Chen W, Oh S, Appleford M, Kim S, Kim K, Park S, Bumgardner J, Haggard W, Ong J. Enhancing osseointegration using surface-modified titanium implants. JOM-J Min Met Mat Soc. 2006;58:71–76. [Google Scholar]
- 3.Chow LC. Calcium phosphate cements. Monogr Oral Sci. 2001;18:148–163. doi: 10.1159/000061653. [DOI] [PubMed] [Google Scholar]
- 4.LeGeros RZ. Calcium phosphates in oral biology and medicine. Monogr Oral Sci. 1991;15:1–201. [PubMed] [Google Scholar]
- 5.Story BJ, Wagner WR, Gaisser DM, Cook SD, Rust-Dawicki AM. In Vivo Performance of a Modified CSTi Dental Implant Coating. Int J Oral Maxillofac Implants. 1998;13:749–757. [PubMed] [Google Scholar]
- 6.Karande TS, Ong JL, Agrawal CM. Diffusion in Musculoskeletal Tissue Engineering Scaffolds: Design Issues Related to Porosity, Permeability, Architecture, and Nutrient Mixing. Ann Biomed Eng. 2004;32:1728–1743. doi: 10.1007/s10439-004-7825-2. [DOI] [PubMed] [Google Scholar]
- 7.Kim K, Yeatts A, Dean D, Fisher JP. Stereolithographic Bone Scaffold Design Parameters: Osteogenic Differentiation and Signal Expression. Tissue Eng B. 2010;16:523–539. doi: 10.1089/ten.teb.2010.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Impens S, Schelstraete R, Luyten J, Mullens S, Thijs I, van Humbeeck J, Schrooten J. Production and characterisation of porous calcium phosphate structures with controllable hydroxyapatite/b-tricalcium phosphate ratios. Adv Appl Ceram. 2009;108:494–500. [Google Scholar]
- 9.Li X, Li D, Lu B, Tang Y. Design and fabrication of CAP scaffolds by indirect solid free form fabrication. Rapid Prototyping J. 2005;11:312–318. [Google Scholar]
- 10.Mondrinos MJ, Dembzynski R, Lu L, Byrapogu VKC, Wootton DM, Lelkes PI, Zhou J. Porogen-based solid freeform fabrication of polycaprolactone-calcium phosphate scaffolds for tissue engineering. Biomaterials. 2006;27:4399–4408. doi: 10.1016/j.biomaterials.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 11.Vivanco J, Aiyangar A, Araneda A, Ploeg H-L. Mechanical characterization of injection-molded macro porous bioceramic bone scaffolds. J Mech Behav Biomed Mater. 2012;9:137–152. doi: 10.1016/j.jmbbm.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Kang Y, Kim S, Khademhosseini A, Yang Y. Creation of bony microenvironment with CaP and cell-derived ECM to enhance human bone-marrow MSC behavior and delivery of BMP-2. Biomaterials. 2011;32:6119–30. doi: 10.1016/j.biomaterials.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang Y, Scully A, Young DA, Kim S, Tsao H, Sen M, Yang Y. Enhanced mechanical performance and biological evaluation of a PLGA coated b-TCP composite scaffold for load-bearing applications. Eur Polym J. 2011;47:1569–1577. doi: 10.1016/j.eurpolymj.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen LH, Annabi N, Nikkhah M, Bae H, Binan L, Park S, Kang Y, Yang Y, Khademhosseini A. Vascularized Bone Tissue Engineering: Approaches for Potential Improvement. Tissue Eng C. 2012 doi: 10.1089/ten.teb.2012.0012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Kang Y, Sen M, Park S. Bioceramics in Tissue Engineering. In: Burdick J, Mauck R, editors. Biomaterials for Tissue Engineering Applications: A Review of the Past and Future Trends. New York: Springer Wien; 2011. pp. 179–208. [Google Scholar]
- 16.Wozney JM. The potential role of bone morphogenetic proteins in periodontal reconstruction. J Periodontol. 1995;66:506–10. doi: 10.1902/jop.1995.66.6.506. [DOI] [PubMed] [Google Scholar]
- 17.Alden TD, Pittman DD, Beres EJ, Hankins GR, Kallmes DF, Wisotsky BM, Kerns KM, Helm GA. Percutaneous spinal fusion using bone morphogenetic protein-2 gene therapy. J Neurosurg-Spine. 1999;90:109–114. doi: 10.3171/spi.1999.90.1.0109. [DOI] [PubMed] [Google Scholar]
- 18.Baltzer AW, Lattermann C, Whalen JD, Wooley P, Weiss K, Grimm M, Ghivizzani SC, Robbins PD, Evans CH. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000;7:734–9. doi: 10.1038/sj.gt.3301166. [DOI] [PubMed] [Google Scholar]
- 19.Forslund C. BMP treatment for improving tendon repair. Acta Orthop. 2003;74:1–30. doi: 10.1080/000164702760300006. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman JR, Daluiski A, Stevenson S, Wu L, Mcallister P, Lee YP, Kabo JM, Finerman GAM, Berk AJ, Witte ON. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg. 1999;81:905–917. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Urist MR. Bone: Formation by Autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 22.Balemans W, Van Hul W. Extracellular Regulation of BMP Signaling in Vertebrates: A Cocktail of Modulators. Dev Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- 23.Perri B, Cooper M, Lauryssen C, Anand N. Adverse swelling associated with use of rh-BMP-2 in anterior cervical discectomy and fusion: a case study. Spine J. 2007;7:235–239. doi: 10.1016/j.spinee.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Tazaki J, Murata M, Akazawa T, Yamamoto M, Ito K, Arisue M, Shibata T, Tabata Y. BMP-2 release and dose-response studies in hydroxyapatite and β-tricalcium phosphate. Biomed Mater Eng. 2009;19:141–146. doi: 10.3233/BME-2009-0573. [DOI] [PubMed] [Google Scholar]
- 25.Xu XL, Lou J, Tang T, Ng KW, Zhang J, Yu C, Dai K. Evaluation of different scaffolds for BMP-2 genetic orthopedic tissue engineering. J Biomed Mater Res B. 2005;75B:289–303. doi: 10.1002/jbm.b.30299. [DOI] [PubMed] [Google Scholar]
- 26.Ruhé PQ, Boerman OC, Russel FGM, Spauwen PHM, Mikos AG, Jansen JA. Controlled release of rhBMP-2 loaded poly(dl-lactic-co-glycolic acid)/calcium phosphate cement composites in vivo. J Control Release. 2005;106:162–171. doi: 10.1016/j.jconrel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Jeon O, Song SJ, Yang HS, Bhang S-H, Kang S-W, Sung MA, Lee JH, Kim B-S. Long-term delivery enhances in vivo osteogenic efficacy of bone morphogenetic protein-2 compared to short-term delivery. Biochem Biophys Res Commun. 2008;369:774–780. doi: 10.1016/j.bbrc.2008.02.099. [DOI] [PubMed] [Google Scholar]
- 28.Gumieiro EH, Abrahão M, Jahn RS, Segretto H, Alves MTdS, Nannmark U, Granström G, Dib LL. Platelet-rich plasma in bone repair of irradiated tibiae of Wistar rats. Acta Cir Bras. 2010;25:257–263. doi: 10.1590/s0102-86502010000300007. [DOI] [PubMed] [Google Scholar]
- 29.Yamada Y, Ueda M, Hibi H, Nagasaka T. Translational Research for Injectable Tissue-Engineered Bone Regeneration Using Mesenchymal Stem Cells and Platelet-Rich Plasma: From Basic Research to Clinical Case Study. Cell Transplant. 2004;13:343–355. doi: 10.3727/000000004783983909. [DOI] [PubMed] [Google Scholar]
- 30.Marx RE. Platelet-Rich Plasma (PRP): What Is PRP and What Is Not PRP? Implant Dent. 2001;10:225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol. 1998;85:638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 32.Anitua E. The use of plasma-rich growth factors (PRGF) in oral surgery. Pract Proced Aesthet Dent. 2001;13:487–93. quiz 487–93. [PubMed] [Google Scholar]
- 33.Liu Y, Kim J-H, Young D, Kim S, Nishimoto SK, Yang Y. Novel template-casting technique for fabricating β-tricalcium phosphate scaffolds with high interconnectivity and mechanical strength and in vitro cell responses. J Biomed Mater Res A. 2009;92A:997–1006. doi: 10.1002/jbm.a.32443. [DOI] [PubMed] [Google Scholar]
- 34.Hokugo A, Ozeki M, Kawakami O, Sugimoto K, Mushimoto K, Morita S, Tabata Y. Augmented bone regeneration activity of platelet-rich plasma by biodegradable gelatin hydrogel. Tissue Eng. 2005;11:1224–1233. doi: 10.1089/ten.2005.11.1224. [DOI] [PubMed] [Google Scholar]
- 35.Müller R, Rüegsegger P. Micro-tomographic imaging for the nondestructive evaluation of trabecular bone architecture. Stud Health Technol Inf. 1997;40:61–79. [PubMed] [Google Scholar]
- 36.Lan Levengood SK, Polak SJ, Poellmann MJ, Hoelzle DJ, Maki AJ, Clark SG, Wheeler MB, Wagoner Johnson AJ. The effect of BMP-2 on micro- and macroscale osteointegration of biphasic calcium phosphate scaffolds with multiscale porosity. Acta Biomater. 2010;6:3283–3291. doi: 10.1016/j.actbio.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 37.Murai M, Sato S, Fukase Y, Yamada Y, Komiyama K, Ito K. Effects of Different Sizes of β-tricalcium Phosphate Particles on Bone Augmentation within a Titanium Cap in Rabbit Calvarium. Dent Mater J. 2006;25:87–96. doi: 10.4012/dmj.25.87. [DOI] [PubMed] [Google Scholar]
- 38.Ruhé PQ, Kroese-Deutman HC, Wolke JGC, Spauwen PHM, Jansen JA. Bone inductive properties of rhBMP-2 loaded porous calcium phosphate cement implants in cranial defects in rabbits. Biomaterials. 2004;25:2123–2132. doi: 10.1016/j.biomaterials.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Wada T, Hara K, Ozawa H. Ultrastructural and histochemical study of β-tricalcium phosphate resorbing cells in periodontium of dogs. J Periodontal Res. 1989;24:391–401. doi: 10.1111/j.1600-0765.1989.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 40.Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18:93–103. [PubMed] [Google Scholar]
- 41.Plachokova AS, Van Den Dolder J, Stoelinga PJ, Jansen JA. The bone regenerative effect of platelet-rich plasma in combination with an osteoconductive material in rat cranial defects. Clin Oral Implants Res. 2006;17:305–311. doi: 10.1111/j.1600-0501.2005.01208.x. [DOI] [PubMed] [Google Scholar]
- 42.Plachokova AS, Van Den Dolder J, Stoelinga PJ, Jansen JA. Early effect of platelet-rich plasma on bone healing in combination with an osteoconductive material in rat cranial defects. Clin Oral Implants Res. 2007;18:244–251. doi: 10.1111/j.1600-0501.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- 43.Kovács K, Velich N, Huszár T, Szabó G, Semjén G, Reiczigel J, Suba Z. Comparative study of β-tricalcium phosphate mixed with platelet-rich plasma versus β-tricalcium phosphate, a bone substitute material in dentistry. Acta Vet Hung. 2003;51:475–484. doi: 10.1556/AVet.51.2003.4.5. [DOI] [PubMed] [Google Scholar]
- 44.Poeschl PW, Ziya-Ghazvini F, Schicho K, Buchta C, Moser D, Seemann R, Ewers R, Schopper C. Application of Platelet-Rich Plasma for Enhanced Bone Regeneration in Grafted Sinus. J Oral Maxillofac Surg. 2012;70:657–664. doi: 10.1016/j.joms.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 45.Sammartino G, Tia M, Marenzi G, di Lauro AE, D’Agostino E, Claudio PP. Use of autologous platelet-rich plasma (PRP) in periodontal defect treatment after extraction of impacted mandibular third molars. J Oral Maxillofac Surg. 2005;63:766–70. doi: 10.1016/j.joms.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Cabbar F, Güler N, Kürkcü M, Işeri U, Şençift K. The Effect of Bovine Bone Graft With or Without Platelet-Rich Plasma on Maxillary Sinus Floor Augmentation. J Oral Maxillofac Surg. 2011;69:2537–2547. doi: 10.1016/j.joms.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 47.Thorwarth M, Wehrhan F, Schultze-Mosgau S, Wiltfang J, Schlegel KA. PRP modulates expression of bone matrix proteins in vivo without long-term effects on bone formation. Bone. 2006;38:30–40. doi: 10.1016/j.bone.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 48.Boyan BD, Schwartz Z, Patterson TE, Muschler G. Clinical use of platelet-rich plasma in orthopaedics. Am Acad Orthop Surg Now. 2007;1:44–46. [Google Scholar]
- 49.Araújo MG, Sonohara M, Hayacibara R, Cardaropoli G, Lindhe J. Lateral ridge augmentation by the use of grafts comprised of autologous bone or a biomaterial. An experiment in the dog. J Clin Periodontol. 2002;29:1122–1131. doi: 10.1034/j.1600-051x.2002.291213.x. [DOI] [PubMed] [Google Scholar]
- 50.Sartori S, Silvestri M, Forni F, Icaro Cornaglia A, Tesei P, Cattaneo V. Ten-year follow-up in a maxillary sinus augmentation using anorganic bovine bone (Bio-Oss). A case report with histomorphometric evaluation. Clin Oral Implants Res. 2003;14:369–372. doi: 10.1034/j.1600-0501.2003.140316.x. [DOI] [PubMed] [Google Scholar]
- 51.Schmid J, Hämmerle CHF, Flückiger L, Winkler JR, Olah AJ, Gogolewskiz S, Lang NP. Blood-filled spaces with and without filler materials in guided bone regeneration. A comparative experimental study in the rabbit using bioresorbable membranes. Clin Oral Implants Res. 1997;8:75–81. doi: 10.1034/j.1600-0501.1997.080201.x. [DOI] [PubMed] [Google Scholar]
- 52.Okazaki K, Shimizu Y, Xu H, Ooya K. Blood-filled spaces with and without deproteinized bone grafts in guided bone regeneration. Clin Oral Implants Res. 2005;16:236–243. doi: 10.1111/j.1600-0501.2004.01095.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




