Abstract
The flagellin component FliC of Salmonella typhimurium is capable of activating the innate immune system via specific interactions with TLR5 and can also act as a carrier of foreign antigen to elicit antigen-specific immune responses. Thus, we constructed an attenuated Salmonella strain SL5928(fliC/esat) expressing chimeric flagellin that contained the ESAT-6 antigen coding sequence of Mycobacterium tuberculosis inserted into the highly variable region of the Salmonella flagellin coding gene fliCi. The chimeric flagellin functioned normally, as demonstrated using a flagella swarming assay and electron microscopy. To analyze the effects of chimeric flagellin, the cell-mediated immune response and cytotoxic T lymphocyte (CTL) effects specific for ESAT-6 antigen were tested after intranasal immunization of mice with flagellated Salmonella SL5928(fliC/esat). The results showed that SL5928(fliC/esat) intranasal immunization can strongly elicit an ESAT-6-specific T helper (Th) 1-type immune response in mucosal lymphoid tissues, such as nasopharynx-associated lymph nodes, lung and Peyer's patches, and a Th1/Th2 response was elicited in spleen and mesenteric lymph nodes. Furthermore, intranasal immunization of SL5928(fliC/esat) produced efficient CTL effects, as demonstrated using a 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE) assay. Thus, our study revealed that Salmonella flagellin acts as a carrier for foreign antigen and triggers strong Th1 and CTL responses during intranasal immunization. Chimeric flagellin is potentially an effective strategy for the development of novel vaccines against tuberculosis in humans and animals.
Keywords: cellular immune response, ESAT-6, flagellin, intranasal immunization, Mycobacterium tuberculosis
Introduction
Tuberculosis (TB) remains a major health problem for both humans and animals worldwide. Although the Bacille Calmette–Guérin (BCG) vaccine is widely administered to prevent this disease, its efficacy remains unpredictable.1, 2 Therefore, there is an urgent need to develop more effective vaccines and vaccination strategies against TB. Cell-mediated immunity is thought to be essential for protection against TB,3 with critical involvement of CD4+ Th1 lymphocytes.4 Furthermore, there is evidence that CD8+ T cells,5, 6 as well as unconventional T cells, contribute to optimal protection.4, 7
Mycobacterium tuberculosis (MTB) infection often occurs through the upper respiratory tract. Intranasal (i.n.) immunization with either live BCG or killed-BCG formulated in an adjuvant or recombinant adenovirus-based vaccines has been shown to provide protection against MTB.8 In addition, i.n. immunization of mice with an adenoviral-based vaccine expressing Ag85A9 or microparticles encapsulated with ESAT-6 elicited antigen-specific CD4+ and CD8+ T cells capable of interferon (IFN)-γ production and cytotoxic activity in the airways, lungs and mediastinal lymph nodes.10 Furthermore, a previous study indicated that recombinant flagellin containing foreign antigens elicited protective immune responses in the absence of additional adjuvant.11 Effects of flagellin on CD4+ T-cell proliferative and cytokine responses have also been reported.12 Salmonella flagellins are flexible adjuvants and induce adaptive immune responses when administered by different routes or in vaccine formulations.13 Therefore, the aim of the present study was to assess whether efficient cell-mediated immune responses and specific CTL effects could be stimulated by i.n. administration of flagellated Salmonella containing the ESAT-6 antigen coding sequences of MTB inserted in the highly variable region of Salmonella flagellin gene fliCi.
Materials and methods
Bacterial strains and plasmid
Escherichia coli strains DH5α and BL21 (DE3) and Salmonella typhimurium (Stm) (LT2) were from our laboratory stocks. Salmonella dublin SL1438 (1,9,12:−:−) and SL5928 (1,9,12:−:− Amp−, Tet+, fliCi::Tn10) were kindly provided by Dr Bruce AD Stocker (Stanford University, School of Medicine, Stanford, CA, USA). Phage P22 Ht int and Stm LB5000 (1,4,12:−:− r−m−flaA66, Amp−) were kindly provided by Dr AD Harshey (University of Texas, Austin, TX, USA). pYUB412 plasmid was provided by Dr. Claude Leclerc (Pasteur Institute, Paris).
Mice
Six- to eight-week-old female C57BL/6 mice were purchased from the Comparative Medical Center of Yangzhou University (Yangzhou, China) and housed at the Animal Biosafety facilities. Animal experiments were conducted in strict accordance with guidelines provided by the Animal Care and Ethics Committee of Yangzhou University.
Construction and cloning of chimeric flagellin gene fliC/esat
For cloning of the ESAT-6 encoding sequence, the esxA gene was amplified from the pYUB412 plasmid using esat-1 (5′-AAT AGT ACT ATG ACA GAG CAG CAG TGG-3′) and esat-2 (5′-C GTA ATA TTT TGC GA A CAT CCC AGT GAC-3′) primers. PCR products were purified and used in the following experiments. The fliCi gene encoding the phase I flagellin was amplified by PCR from Stm (LT2) genomic DNA using fliC-1 (5′-ATA GAA TTC AAG GAA AAG ATC ATG GCA CAA-3′) and fliC-2 (5′-TCA AAG CTT AAC GCA GTA AAG AGA G-3′) primers. The fliCi gene product was purified and amplified using an overlap PCR and further used to clone the chimeric flagellin gene fliC/esat. The coding sequence corresponding to the N-terminal domain of flagellin was amplified using fliC-1 and F-E-2 (5′-CCA CTG CTG CTC TGT CAT AGT ACT ATT GTC TAA A-3′) primers, and the C-terminal domain of flagellin was amplified using F-E-1 (5′-GTC ACT GGG ATG TTC GCA AAA TAT TAC GCC AAA-3′) and fliC-2 primers. The two PCR products were fliC-N and fliC-C, respectively. The fliC/esat-1 fragments were cloned by overlap PCR using fliC-1 and esat-2 primers to amplify the fliC-N and ESAT-6 purified PCR products. The fliC/esat-2 fragments were cloned by overlap PCR using esat-1 and fliC-2 primers to amplify the fliC-C and ESAT-6 purified PCR products. A chimeric fragment of fliC/esat was amplified from fliC/esat-1 and fliC/esat-2 purified PCR products by overlap PCR using fliC-1 and fliC-2 primers. The chimeric fragment carried the incomplete fliC coding sequence, in which the coding region for amino-acid residues 205–232 of flagellin had been replaced with the entire ESAT-6 coding sequence (Figure 1). The chimeric fliC/esat gene PCR products were purified, digested with EcoRI and HindIII and ligated into a pET30(a) expression vector to form the pET-fliC/esat plasmid.
Figure 1.
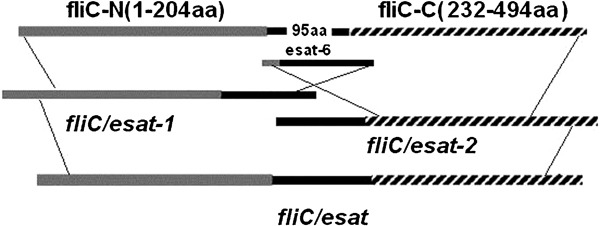
Diagram showing construction of the chimeric fliC/esat fragment.
P22 phage transduction
The pET-fliC/esat plasmid was electrotransformed into Stm LB5000. The recombinant bacteria, LB5000 (pET-fliC/esat) (donor strain), were grown in LB broth overnight. Ten milliliters of P22 broth were added to 200 µl of the LB culture and incubated at 37 °C on a shaker for 10 h. The donor strain/phage mixture was centrifuged for 5 min (8000 g) to pellet the cell debris, and the supernatant was collected. Several drops of CHCl3 were added to the supernatant before being vortexed and stored at 4 °C. The recipient strain Salmonella dublin SL5928 was grown overnight in 1-ml LB. Twenty microliters of the phage lysate was added to 200 µl of the overnight SL5928 culture and incubated at 37 °C on a shaker for 1 h. Finally, the mixture was spread onto LB agar containing 50 µg/ml of kanamycin.
Swarming assays
Non-motile S. dublin SL5928, flagellated SL5928(fliC/esat) and SL5928(fliC) colonies were punctured using a needle and placed into 0.4% semisolid medium tubes (100 ml: tryptone 10 g, NaCl 5 g, agar 0.4 g) that were incubated at 37 °C for 6–8 h.
Electron microscopy
Negative staining of bacteria for electron microscopy was performed with 2% phosphotungstic acid as described by Hayat and Miller.14
Western blot assay
Flagellin of SL5928(fliC) and chimeric flagellin of SL5928(fliC/esat) were extracted by acid treatment followed by ammonium sulfate precipitation.15 Under these experimental conditions, the extracted fraction was represented mainly by polymeric flagellin. The purified flagellin extracts were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and western blot analysis16 using a murine anti-ESAT-6 monoclonal antibody (mAb) HYB 076–08 (Abcam, Cambridge, Massachusetts, USA).
Immunization of mice
Flagellated Salmonella SL5928(fliC/esat) or non-flagellated Salmonella SL5928 were harvested at an optical density of 0.8. Each bacterial pellet was then resuspended in phosphate-buffered saline (PBS) with a concentration of 5×108 colony-forming units/ml. The immunization of C57BL/6 mice (6–8 weeks old) was performed as described previously.17 Briefly, the mice were anesthetized by intraperitoneal injection with 0.1 ml of 1% pentobarbital sodium and immunized i.n. with 1×107 colony-forming units SL5928(fliC/esat) and SL5928 on days 0 and 18, respectively. The negative control group was immunized i.n. with PBS.
Cell isolation
Cells were prepared from the spleens, lungs, Peyer's patches (PPs), mesenteric lymph nodes (MLNs) and nasopharynx-associated lymph nodes (NALTs) of immunized mice. Single cells were obtained from each of these tissues by pressing the organs through a stainless steel mesh and centrifuging at 200 g for 10 min at 4 °C. Lung and spleen cell suspensions were prepared through a murine Ez-sep (Dakewe Biotech Company, Shenzhen, China) density–gradient centrifugation (800 g for 25 min at 4 °C). Total cell yields were calculated using a hemocytometer and 0.4% trypan blue to assess the viability of cells kept in RPMI 1640 (Invitrogen, Carlsbad, CA, USA) medium with 10% fetal bovine serum (Hyclone, Logan, UT, USA).
ELISPOT assay
The ELISPOT technique was performed as described previously.17 Briefly, spleen, lung, PP, MLN and NALT cells were incubated with 10 µg/ml of ESAT-6 peptide (1–20 aa) in a 96-well plate coated with rat anti-mouse IFN-γ and IL-4 mAbs (BD Biosciences, San Diego, CA, USA) or ConA (5 µg/ml; Sigma-Aldrich, St Louis, MO, USA) as a positive control for 36 h. Cytokine production was detected using biotin-labeled rat anti-mouse IFN-γ and IL-4 mAb (BD Biosciences) and probed with alkaline phosphatase-conjugated streptavidin (BD Biosciences). The enzyme reaction was developed with 5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium (Sigma-Aldrich), and the results were expressed as the number of immunospots per 106 cells.
CTL assay in vivo
The 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE) assay used in this study demonstrated ESAT-6-specific cytotoxicity T lymphocyte effector function in vivo by flow cytometry. In vivo cytotoxic activity initiated by SL5928(fliC/esat) was established using a murine model. The donor cells were prepared from the spleen of naïve mice, washed three times with PBS and divided into two populations. One population (as target cells), CFSEhigh cells, was pulsed with 10 µM ESAT-6-specific peptide (1–20 aa), incubated at 37 °C for about 45 min and then labeled with a high concentration of the fluorescent dyes CFSE (5.0 µM). The second control population, dyestuff CFSElow cells, was left without peptide and labeled with a low concentration of CFSE (0.25 µM). The CFSElow and CFSEhigh cells were mixed together (a total of 2×107 cells) at a 2∶1 ratio from each population and injected into each immunized mouse (7 days after the second immunization) via the tail vein. Splenocytes were isolated from the spleens of sacrificed mice 15 h later and analyzed by flow cytometry. The following formulas were used:
Ratio=percentage CFSElow/percentage CFSEhigh
Percentage specific lysis=1−(ratio unprimed/ratio primed)×100%.
Statistical methods
Data obtained were tested by SPSS 13.0. Differences between means were assessed for statistical significance using Student's t-test. A value of P<0.05 was considered to be significant.
Results
Construction and identification of the chimeric flagellin specifying ESAT-6 antigen
Specific primers were designed based on the Stm flagellin fliCi gene sequence to amplify the fliCi gene. Similarly, the ESAT-6 coding sequence was also amplified by PCR using specific primers. Overlap PCR was further used to synthesize the chimeric flagellin gene, and the ESAT-6 coding fragment was inserted into the unique hypervariable region of fliC. The recombinant plasmid carrying the chimeric flagellin gene was sequenced and named pET-fliC/esat. Salmonella SL5928(fliC/esat) were transduced with the chimeric flagellin, and the inserted ESAT-6 antigen was identified by western blot analysis. Western blot analysis using the anti-ESAT-6 mAb HYB 076–08 confirmed that the 52-kDa band corresponded to the chimeric fliC/esat protein (Figure 2).
Figure 2.
Western blot analysis. Flagellin of SL5928(fliC) and chimeric flagellin of SL5928(fliC/esat) were extracted by acid treatment followed by ammonium sulfate precipitation. (a) The crude extract of fliC protein and chimeric flagellin fliC/esat were subjected to SDS–PAGE (M, standard molecular weight markers in kDa). Lane 1, crude extract of flagellin of SL5928(fliC); lane 2, crude extract of chimeric flagellin of SL5928(fliC/esat). (b) The crude extract of fliC protein and chimeric flagellin fliC/esat were transferred onto nitrocellulose and treated with anti-ESAT-6 antibodies (mAb HYB 076–08). A prominent 52 kDa band was observed (lane 4). Lane 3, crude extract of flagellin of SL5928(fliC); lane 4, crude extract of chimeric flagellin of SL5928(fliC/esat). mAb, monoclonal antibody; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Swarming and electron microscopy of transduced cells
Swarming was observed within 6–8 h. Flagellated SL5928(fliC/esat) produced smooth swarming when compared with the SL5928 control (Figure 3). Electron microscopy revealed that flagellated SL5928(fliC/esat) expressed flagella similar to the wide-type control strain SL1438 (Figure 4).
Figure 3.

Observed motility phenotypes for the transduced cells: (a) SL5928(fliC); (b) SL5928(fliC/esat); (c) SL5928.
Figure 4.
Electron microscopy of transduced cells: (a) SL5928(fliC/esat); (b) SL1438.
Cytokine production following i.n. immunization with flagellated Salmonella SL5928(fliC/esat)
To measure the cellular immune responses elicited by i.n. administration of SL5928(fliC/esat), cytokine production of IFN-γ and IL-4 levels were quantified using an ELISPOT assay. The mean numbers of IFN-γ-producing cells (spots/106 cells) in the lung (216.67±55.08), PPs (52.5±10.02) and NALT (65.0±5.0) were significantly increased (P<0.01) in SL5928(fliC/esat)-immunized mice compared to the SL5928-immunized control group (Figure 5). The difference between the SL5928(fliC/esat)-immunized group and PBS control was also significant (P<0.01). However, the numbers of IL-4-producing cells from lungs, PPs and NALTs were significantly lower compared to those of IFN-γ-producing cells, independent of treatment group. The numbers of ESAT-6-specific IFN-γ-producing and IL-4-producing cells from spleen and MLNs were higher in the SL5928(fliC/esat)-immunized mice than in the SL5928-immunized group and the control group (Figure 5). These results suggest that mice receiving SL5928(fliC/esat) developed cellular immune responses against the ESAT-6 protein.
Figure 5.

Cytokine secretion of flagellated Salmonella SL5928(fliC/esat) through intranasal immunization. C57BL/6 mice were immunized intranasally with SL5928(fliC/esat), SL5928(fliC) or PBS. IFN-γ-producing and IL-4-producing cells in the lung, PPs, NALTs, spleen and MLNs were evaluated by ELISPOT assay with ESAT-6 peptide as the stimulus. Results were expressed as the mean number of spots per 106 cells (six mice per group). **P<0.01 for the SL5928(fliC)-immunized and PBS-immunized mice using a Student's t-test. IFN, interferon; MLN, mesenteric lymph node; NALT, nasopharynx-associated lymph node; PBS, phosphate-buffered saline; PP, Peyer's patch.
CTL effects in vivo
In this study, we provide evidence that ESAT-6-specific memory CD8+ T cells exert substantial Ag-specific cytotoxicity in vivo. We used CFSE to evaluate ESAT-specific cytotoxic T lymphocyte effector function in vivo by flow cytometry. As target cells, spleen cells from uninfected mice were pulsed with ESAT-6 peptide or left untreated. Each group was then labeled with different levels of CFSE and co-administered to animals that had been previously immunized with SL5928(fliC/esat) or SL5928(fliC). The frequency of the pulsed donor cell population of CD8+ T cells in the spleens of recipient mice was compared with that of the donor cell population that had not been pulsed with ESAT-6 peptide. At 15 h post-transfer into mice infected by SL5928(fliC/esat) or SL5928(fliC), >75% of ESAT-6 peptide-pulsed donor cells were cleared from the spleen (Figure 6). In spleens of mice infected by SL5928(fliC), the frequencies of ESAT-6 peptide-pulsed and untreated donor cells were equal to the initial ratio (Figure 6). The marked efficiency in eliminating ESAT-6 peptide-pulsed target cells demonstrated the CTL activity of memory CD8+ T cells in SL5928(fliC/esat) immunized mice.
Figure 6.

Characterization of ESAT-6-specific CTL activity in vivo in immunized SL5928(fliC/esat) mice. The histogram shows CFSElow untreated and CFSEhigh ESAT-6 peptide-pulsed target cells in the spleens of SL5928(fliC) and SL5928(fliC/esat) mice infected 15 h post-transfer, respectively. Results were representative of three experiments, each with five mice per group. CFSE, carboxyfluorescein diacetate succinimidyl ester; CTL, cytotoxic T lymphocyte.
Discussion
Recently, over 170 TB vaccine candidates have been tested in animal TB models18 using a variety of strategies, including DNA vaccines, subunits, recombinant BCGs and live attenuated vaccines. However, inconsistent results have been reported from these experimental models. Because protective immunity against TB requires the elicitation of cell-mediated immunity, it is necessary for candidate vaccines to elicit an effective cellular immune response or a CTL response. ESAT-6, which was originally isolated from a low-molecular-mass fraction of an MTB culture filtrate, is encoded by the RD1 region of MTB. ESAT-6 is a potent T-cell antigen and a diagnostic target for MTB and Mycobacterium bovis infection.
Bacterial flagellins have been studied for decades due to the importance of these proteins in bacterial motility and their adjuvant characteristics in vaccine studies. Flagellin is a highly conserved bacterial protein that elicits TLR-5-dependent inflammatory responses in both animal and plants.19 Previous studies have investigated the ability of flagellin to elicit antigen-specific T-cell responses when delivered as live attenuated vaccines.20, 21 In addition, Salmonella flagellin has previously been used as an adjuvant and carrier for a synthetic peptide and to potentiate antibody production against a cholera toxin epitope.22, 23 Chimeric flagellin products are functional unless the 5′ and 3′ coding frames, which encode the regions important for secretion and polymerization of flagellin, are changed. In the Salmonella fliCi gene, the 5′ and 3′ conserved regions are represented by 519 bp (encoding the N-terminal 173 amino-acid residues) and 270 bp (C-terminal 90 residues) sequences, respectively, separated by a highly variable region.24 We therefore generated a flagellated Salmonella in which the coding region for amino-acid resides 205–232 of the flagellin was replaced with the entire coding sequence for ESAT-6 of MTB. This ‘hypervariable' sequence region varies greatly in size and composition for different strains and species. However, our study demonstrated that flagellin function did not change after the foreign antigen was inserted. Furthermore, ESAT-6 expression was verified by western blotting using a specific anti-ESAT-6 monoclonal antibody HYB 076–08 (data not shown).
Characterization of the cytokine response following SL5928(fliC/esat) or SL5928 immunization was carried out by ELISPOT and identified lung, PP, NALT, spleen and MLN cells that produced IFN-γ and IL-4 in response to ESAT-6 peptide stimulation. These experiments demonstrated that in mucosal-associated lymphoid tissues, such as lungs, PPs and NALTs, an anti-ESAT-6 response was Th1-associated based on the significant elevation of the Th1-associated cytokine IFN-γ. In contrast, cells harvested from spleen and MLN produced a mixed Th1/Th2-type response based on the elevated levels of both IFN-γ and IL-4. Our study therefore demonstrated that a Th1-associated immune response following i.n. vaccination with flagellated Salmonella SL5928(fliC/esat) was observed in the lungs, PPs and NALTs. Previous studies have also demonstrated that flagellated Salmonella is an effective delivery system. One study reported that the product of the gene Rv2108 of MTB expressed in flagellin also produced the strongest Th1-like immune response.25 Manocha et al. further demonstrated that intranasal immunization of mice with Fla-91—a recombinant flagellin containing the influenza B-cell epitope HA91-108—led to partial protection of mice from viral challenge.26 High-level T-cell responses and increased IL-2 or IFN-γ cytokine production from PPs have also been detected following i.n. immunization.27 However, the mechanisms producing such specific immune responses in PPs remain unclear.
The induction of a Th1-like immune response to flagellin was demonstrated by i.n. immunization. Various routes of immunization have been investigated, including intramuscular (i.m.), i.n. and subcutaneous vaccination, as well as numerous methods of antigen delivery, such as co-encapsulation in poly-lactic acid microspheres,28, 29 expression by Salmonella30, 31, 32 and DNA vaccination.33, 34 However, our experiments suggest that i.n. immunization may be the most useful method for preventing infectious respiratory diseases.
In addition, CTL responses based on CFSE in vivo were tested using flow cytometry to demonstrate whether chimeric flagellated Salmonella can induce ESAT-6-specific CTL effects. The results showed that memory T cells in the spleen of recipient mice immunized with SL5928(fliC/esat) play a substantial part in clearing the ESAT-6 peptide-pulsed target cells. In other words, ESAT-6-specific memory CD8+ T cells exert substantial Ag-specific cytotoxicity in vivo. Similarly, Braga et al. also demonstrated that recombinant S. dublin flagella expressing the target CS (280–288) (the 9-mer (SYVPSAEQI) synthetic H2(d)-restricted CD8+ T cell-specific epitope) peptide fused at the central hypervariable domain were able to prime cytotoxic CD8+ T cells.13 Therefore, flagellated Salmonella may also offer protection by inducing efficient CTL responses.
Our study using flagellated Salmonella administered i.n has demonstrated that flagella represent a microbial surface display system that can function as a delivery system for foreign antigen in vivo. Live, attenuated and flagellated Salmonella may therefore be a cost-effective prophylactic measure against mucosal disease.
Acknowledgments
This work was supported by grants from the Major State Basic Research Development Program of China (973 Program), the National S T Major Project (no. 2008 ZX10003-010), the National Department Public Benefit Research Foundation (200903027), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;12:186–191. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- Martin C.Tuberculosis vaccines: past, present and future Curr Opin Pulm Med20061997497–15. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH. Recent findings in immunology give tuberculosis vaccines a new boost. Trends Immunol. 2005;26:660–667. doi: 10.1016/j.it.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng CG, Bean AG, Hooi H, Briscoe H, Britton WJ. Increase in gamma interferon-secreting CD8+, as well as CD4+, T cells in lungs following aerosol infection with Mycobacterium tuberculosis. . Infect Immun. 1999;67:3242–3247. doi: 10.1128/iai.67.7.3242-3247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladel CH, Blum C, Dreher A, Reifenberg K, Kaufmann SH. Protective role of gamma/delta T cells and alpha/beta T cells in tuberculosis. Eur J Immunol. 1995;25:2877–2881. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- Wang J, Thorson L, Stokes RW, Santosuosso M, Huygen K, Zganiacz A, et al. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J Immunol. 2004;173:6357–6365. doi: 10.4049/jimmunol.173.10.6357. [DOI] [PubMed] [Google Scholar]
- Santosuosso M, Zhang X, McCormick S, Wang J, Hitt M, Xing Z. Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J Immunol. 2005;174:7986–7994. doi: 10.4049/jimmunol.174.12.7986. [DOI] [PubMed] [Google Scholar]
- Carpenter ZK, Williamson ED, Eyles JE. Mucosal delivery of microparticle encapsulated ESAT-6 induces robust cell-mediated responses in the lung milieu. J Control Release. 2005;104:67–77. doi: 10.1016/j.jconrel.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Jeon SH, Ben-Yedidia T, Arnon R. Intranasal immunization with synthetic recombinant vaccine containing multiple epitopes of influenza virus. Vaccine. 2002;20:2772–2780. doi: 10.1016/s0264-410x(02)00187-1. [DOI] [PubMed] [Google Scholar]
- MeSorley SJ, Ehst BD, Y Yu, Gewirtz AT. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. . J Immunol. 2002;169:3914–3919. doi: 10.4049/jimmunol.169.7.3914. [DOI] [PubMed] [Google Scholar]
- Braga CJ, Massis LM, Sbrogio-Almeida ME, Alencar BC, Bargieri DY, Boscardin SB, et al. CD8+ T cell adjuvant effects of Salmonella FliCd flagellin in live vaccine vectors or as purified protein. Vaccine. 2010;28:1373–1382. doi: 10.1016/j.vaccine.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Hayat MA, Miller SE. New York; McGraw-Hill Publishing Co.; 1990. Laboratory for advanced electron and light optical methods. Negative Staining; p. 216. [Google Scholar]
- lbrahim GF, Fleet GH, Lyons MJ, Walker RA. Method for the isolation of highly purified Salmonella flagellins. J Clin Microbiol. 1985;22:1040–1044. doi: 10.1128/jcm.22.6.1040-1044.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. New York; Cold Spring Harbor Laboratory Press; 2001. Molecular Cloning:A Laboratory Manual.3rd ed. [Google Scholar]
- Zhang H, Wen K, Shen J, Geng S, Huang J, Pan Z, et al. Characterization of immune responses following intranasal immunization with the Mycobacterium bovis CFP-10 protein expressed by attenuated Salmonella typhimurium. . Scand J Immunol. 2010;72:277–283. doi: 10.1111/j.1365-3083.2010.02421.x. [DOI] [PubMed] [Google Scholar]
- Reed S, Lobet Y. Tuberculosis vaccine development: from mouse to man. Microbes Infect. 2005;7:922–931. doi: 10.1016/j.micinf.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Skountzou I, Martin M, Wang B, Ye L, Koutsonanos D, Weldon W, et al. Salmonella flagellins are potent adjuvants for intranasally administered whole inactivated influenza vaccine. Vaccine. 2009;28:4103–4112. doi: 10.1016/j.vaccine.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma NK, Ziegler HK, Stocker BA, Schoolnik GK. Induction of a cellular immune response to a defined T-cell epitope as an insert in the flagellin of a live vaccine strain of Salmonella. . Vaccine. 1995;13:235–244. doi: 10.1016/0264-410x(95)93308-v. [DOI] [PubMed] [Google Scholar]
- Newton SMC, Joys TM, Anderson SA, Kennedy RC, Hovi ME, Stocker BA. Expression and immunogenicity of an 18-residue epitope of HIV1 gp41 inserted in the flagellar protein of a Salmonella live vaccine. Res Microbiol. 1995;146:203–216. doi: 10.1016/0923-2508(96)80276-2. [DOI] [PubMed] [Google Scholar]
- Chauhan N, Kumar R, Badhai J, Preet A, Yadava PK. Immunogenitity of cholera toxin B epitope inserted in Salmonella flagellin expressed on bacteria and administered as DNA vaccine. Mol Cell Biochem. 2005;276:1–6. doi: 10.1007/s11010-005-2240-z. [DOI] [PubMed] [Google Scholar]
- Newton SMC, Jacob OC, Stocker BAD. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989;244:70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- Okazaki N, Matsuo S, Saito K, Tominaga A, Enomoto M. Conversion of the Salmonella phase 1 flagellin gene fliC to the phase 2 gene fljB on the Escherichia coli K-12 chromosome. J Bacteriol. 1993;175:758–766. doi: 10.1128/jb.175.3.758-766.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le-Moigne V, Robreau G, Mahana W. Flagellin as a good carrier and potent adjuvant for Th1 responses: study of mice immune response to the p27 (Rv2108) Mycobacterium tuberculosis antigen. Mol Immunol. 2008;45:2499–2507. doi: 10.1016/j.molimm.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Manocha M, Pal PC, Chitralekha KT, Thomas BE, Tripathi V, Gupta SD, et al. Enhanced mucosal and systemic immune response with intranasal immunization of mice with HIV peptides entrapped in PLG microparticles in combination with Ulex Europaeus-I lectin as M cell target. Vaccine. 2005;23:5599–5617. doi: 10.1016/j.vaccine.2005.06.031. [DOI] [PubMed] [Google Scholar]
- McEwen J, Levi R, Horwitz RJ, Arnon R. Synthetic recombinant vaccine expressing influenza haemagglutinin epitope in Salmonella flagellin leads to partial protection in mice. Vaccine. 1992;10:405–411. doi: 10.1016/0264-410x(92)90071-q. [DOI] [PubMed] [Google Scholar]
- Eyles JE, Sharp GJ, Williamson ED, Spiers ID, Alpar HO. Intranasal administration of poly-lactic acid microsphere co-encapsulated Yersinia pestis subunits confers protection from pneumonic plague in the mouse. Vaccine. 1998;16:698–707. doi: 10.1016/s0264-410x(97)00249-1. [DOI] [PubMed] [Google Scholar]
- Eyles JE, Spiers ID, Williamson ED, Alpa HO. Analysis of local and systemic immunological responses after intra-tracheal, intranasal and intra-muscular administration of microsphere co-encapsulated Yersinia pestis sub-unit vaccines. Vaccine. 1998;16:2000–2009. doi: 10.1016/s0264-410x(98)00089-9. [DOI] [PubMed] [Google Scholar]
- Garmory HS, Griffin F, Brown KA, Titball RW. Oral immunization with live aroA attenuated Salmonella enterica serovar Typhimurium expressing the Yersinia pestis V antigen protects mice against plague. Vaccine. 2003;21:3051–3057. doi: 10.1016/s0264-410x(03)00112-9. [DOI] [PubMed] [Google Scholar]
- Leary SE, Griffin KF, Garmory HS, Williamson ED, Titball RW. Expression of an F1/V fusion protein in attenuated Salmonella typhimurium and protection of mice against plague. Microb Pathog. 1997;23:167–179. doi: 10.1006/mpat.1997.0141. [DOI] [PubMed] [Google Scholar]
- Morton M, Garmory HS, Perkins SD, O'Dowd AM, Griffin KF, Turner AK, et al. A Salmonella enterica serovar Typhi vaccine expressing Yersinia pestis F1 antigen on its surface provides protection against plague in mice. Vaccine. 2004;22:2524–2532. doi: 10.1016/j.vaccine.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Grosfeld H, Cohen S, Bino T, Flashner Y, Ber R, Mamroud E, et al. Effective protective immunity to Yersinia pestis infection conferred by DNA vaccine coding for derivatives of the F1 capsular antigen. Infect Immun. 2003;71:374–383. doi: 10.1128/IAI.71.1.374-383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Heilman D, Liu F, Giehl T, Joshi S, Huang X, et al. A DNA vaccine producing LcrV antigen in oligomers is effective in protecting mice from lethal mucosal challenge of plague. Vaccine. 2004;22:3348–3357. doi: 10.1016/j.vaccine.2004.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]


