Abstract
Purpose.
Gap junctions are present in all corneal cell types and have been shown to have a critical role in cell phenotype determination. Vitamin D has been shown to influence cell differentiation, and recent work demonstrates the presence of vitamin D in the ocular anterior segment. This study measured and compared gap junction diffusion coefficients among different cornea epithelium phenotypes and in keratocytes using a noninvasive technique, fluorescence recovery after photobleaching (FRAP), and examined the influence of vitamin D receptor (VDR) knockout on epithelial gap junction communication in intact corneas. Previous gap junction studies in cornea epithelium and keratocytes were performed using cultured cells or ex vivo invasive techniques. These invasive techniques were unable to measure diffusion coefficients and likely were disruptive to normal cell physiology.
Methods.
Corneas from VDR knockout and control mice were stained with 5(6)-carboxyfluorescein diacetate (CFDA). Gap junction diffusion coefficients of the corneal epithelium phenotypes and of keratocytes, residing in intact corneas, were detected using FRAP.
Results.
Diffusion coefficients equaled 18.7, 9.8, 5.6, and 4.2 μm2/s for superficial squamous cells, middle wing cells, basal cells, and keratocytes, respectively. Corneal thickness, superficial cell size, and the superficial squamous cell diffusion coefficient of 10-week-old VDR knockout mice were significantly lower than those of control mice (P < 0.01). The superficial cell diffusion coefficient of heterozygous mice was significantly lower than control mice (P < 0.05).
Conclusions.
Our results demonstrate differences in gap junction dye spread among the epithelial cell phenotypes, mirroring the epithelial developmental axis. The VDR knockout influences previously unreported cell-to-cell communication in superficial epithelium.
Keywords: corneal epithelium, vitamin D receptor, gap junction, FRAP, keratocyte
FRAP multi-photon microscopy was used to examine the influence of vitamin D on the function of corneal epithelial cell gap junctions. Our results demonstrate differences in gap junction dye spread among the epithelial cell phenotypes, mirroring the epithelial developmental axis.
Introduction
Gap junction communication has a pivotal role in tissue development and maintenance through homeostatic control of cooperative, interacting cells within tissues and organs.1 Gap junctions are formed by connexin proteins,2 and are correlated positively with cell proliferation and the rate of migration of immature cells.3–5 Gap junction dysfunction has been linked to a variety of human diseases, including cataracts, cardiovascular anomalies, peripheral neuropathies, deafness, diabetes, and skin disorders.6–8 Gap junctions influence cellular phenotypes in wound healing skin,9 and mediate the spread of cell injury and death during myocardial ischemia–reperfusion.10 Loss- or gain-of-function connexin mutations may change cellular phenotypes in the developmental axis, leading to diseases of the skin.9,11,12 Furthermore, previous studies have demonstrated that gap junction expression in the embryo is diverse, with specific cell types expressing different connexins, which may imply differences in the function of these intercellular channels in different loci and developmental stages.13,14
The functions of gap junctions in nonexcitable tissues are only recently starting to become understood. They likely have a role in coordinating cell division activity during embryonic tissue development. Studies also have linked them to the wound healing process. In addition, because the cornea is an avascular structure, gap junctions likely serve as a route for the distribution of nutrients and metabolites.15 Diffusion coefficients directly reflect gap junction permeability, and, thus, quantitatively define the functionality of the gap junctions under the stated conditions. The corneal epithelium has three major phenotypes that are influenced by their location along the vertical axis of the epithelium, and are defined as superficial squamous cells, middle wing cells, and inner basal cells. Thoft and Friend16 proposed that the basal cells of the epithelium are formed in the limbal region. As these cells divide, they migrate toward the surface to form the wing cells in the middle layers of the epithelium. As the outer surface layers slough off into the tear film, the wing cells flatten out and take their place as the new surface cells. This process appears to work for daily maintenance and wound healing.17
It is known that all cell layers of the corneal epithelium contain functional gap junctions.15,18,19 Previous studies have examined the diversity of gap junctions in the corneal epithelium cell using electron microscopy,20 immunostaining,21 and microinjection techniques.22 In recent years, previous studies have detected expression of connexins 26, 30, and 43 in mouse (Djalilian AR, et al. IOVS 2004;45:ARVO E-Abstract 3769) and human corneal epithelium using RT-PCR.23 Multiple gap junction genes and proteins have been described during development of lens, retina, embryo, and skin.24–28 Moreover, the combined use of electrophysiology, live-cell imaging, and molecular biology has brought new insights into gap junction function in the cells of the eye. Ex vivo gap junctions functioning in corneal epithelia, except for superficial squamous cell, were first observed by our group using microelectrode dye injection of 5,6-carboxyfluorescein.18 However, systematic quantification of corneal epithelial gap junction dye diffusion coefficients has not been reported, nor has the influence of genotype perturbation on in situ or ex vivo corneal epithelial gap junction function been reported.
The biological structure of the corneal stroma has been studied widely.29 In the corneal stroma, keratocytes reside between the collagen lamellae, and appear to decrease in density gradually from the anterior to posterior cornea in humans and rabbits.30,31 Keratocytes connect with neighboring keratocytes via gap junctions to form a cellular network.32 The shape and extent of the keratocyte network correlate with the pattern of collagen lamellae. Recent data demonstrated that keratocytes form a single contiguous 3-D network, rather than a series of independent parallel networks.33,34 Ex vivo gap junction communication between stromal keratocytes was observed and evaluated directly by our group using microelectrode dye injection in rabbit and human corneas.35 However, this model was restricted to examining only the posterior-most keratocytes directly under the endothelium, and had the problem of being significantly invasive to the cell being injected, which is the case in all microelectrode injection techniques.
Since gap junctions were discovered in the myocardium and in neurons,36,37 numerous methods have been developed to explore gap junction channels.38 In the cornea, patch-clamp techniques have been applied to study ion and dye movement across endothelial and epithelial gap junctions of several species.39–42 In addition, corneal gap junctions have been analyzed by measuring dye transfer using techniques, such as scrape loading.43 In cells from other tissues, gap junctions have been examined using electroporation,44 preloading assays, local activation of molecular fluorescent probes,45,46 and fluorescence recovery after photo bleaching (FRAP).47 In recent years, FRAP has been used widely to study molecule transport, diffusion, interactions, and immobilization in live cells. The FRAP experiments are based on photobleaching a fluorescent marker in a selected area, followed by measurement of dye return back to the original equilibrium state via gap junction transport in the photobleached cell.48
The vitamin D endocrine system controlling calcium homeostasis was discovered in 1970.49 Since that time, the role of vitamin D, working through the vitamin D nuclear receptor (VDR), has been investigated in a wide range of tissues. Physiologic and pathophysiologic processes, including autoimmune, infectious, and granuloma-forming diseases; cardiovascular disorders; and cancers, have been linked to the vitamin D/VDR system.50,51 Vitamin D deficiency is a global health problem, and one billion people are estimated to be vitamin D-deficient or -insufficient.52 At the same time, 10 million people worldwide are blind due to severe corneal disease. Epithelial gap junction communication is associated with healthy tissue. Vitamin D repletion in patients with low vitamin D could lead to increased gap junction communication and corneal epithelial health, including supporting the corneal epithelial phenotypes associated with epithelial regeneration.
Our previous studies determined that vitamin D can be produced in the cornea and can enhance corneal epithelial barrier function.53,54 Moreover, our previous work has demonstrated that elevated epithelial calcium concentrations stimulate gap junction connectivity in corneal epithelial cells.18 More recent work from our lab demonstrates that VDR knockout results in delayed epithelial wound healing and disruption of the epithelial tight junction network, and that supplementing VDR knockout mice with a diet rich in calcium reverses these problems (Watsky MA, et al. IOVS 2013;54:ARVO E-Abstract 2583). Previous work also has shown that VDR knockout mice are calcium-deficient.47,49 This leads us to hypothesize that, at least in part, calcium deficiency is involved in the observed VDR knockout corneal epithelial issues. We hypothesize that this same VDR-related calcium deficiency may result in a reduction of gap junction connectivity in VDR knockout mice, and that vitamin D, thus, is important for maintaining the normal connectivity of the corneal epithelium.
In this study, we described the use of FRAP to measure ex vivo gap junction diffusion rates noninvasively in the epithelium and keratocytes of intact mouse corneas. Furthermore, we investigated gap junction activity in the corneal epithelium of vitamin D receptor knockout mice.
Materials and Methods
Animals and Sample Preparation
A breeding pair of vitamin D receptor knockout mice (B6.129S4-Vdrtm1Mbd/J) was purchased from The Jackson Laboratories (Bar Harbor, ME, USA) and mice subsequently were bred for this study. Ten VDR−/−, 10 VDR+/−, and 10 VDR+/+ mice were used. Mice were euthanized at 4 or 10 weeks of age. They were housed and treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All studies were reviewed and approved by the Institutional Animal Care and Use Committee.
Euthanasia was carried out by cervical dislocation after deep anesthetization with isoflurane. The eyes were removed and dissected from the overlying sclera at room temperature (RT) into a NaCl Ringer's solution (100 mM NaCl, 0.5 mM KCl, 2.5 mM CaCl2, 5 mM glucose, 5 mM HEPES). Eyes were pinned epithelium side up to a Sylgard (Dow Corning, Midland, MI, USA) disc and placed in a custom-constructed acrylic chamber that served as the stage for an LSM710 confocal microscope (Carl Zeiss, Jena, Germany).
Dye Loading
The 5(6)-carboxyfluorescein diacetate (CFDA) was purchase from Sigma-Aldrich (St. Louis, MO, USA). A stock solution of CFDA was prepared in dimethyl sulfoxide (DMSO) at 13 mmol (6 mg/mL), which was protected from light. The CFDA stock solution was diluted to 1:200 vol/vol with PBS medium to obtain a final working concentration of 65 μmol. Diluted solutions were used immediately. Eyes used for FRAP analysis of superficial squamous cells were exposed to CFDA at 4°C for 1 hour, while all others were exposed to CFDA for 3 hours. For corneal epithelium FRAP experiments, whole eyes were used, while corneas were dissected with a scleral rim from the globes before dye exposure for keratocyte FRAP studies.
Thickness Measurements
Corneal images were acquired with a Zeiss LSM 710 confocal system (Carl Zeiss) using ZEN 2010 software and an x/y/z motorized scanning stage. Corneas were scanned from the epithelium to the endothelium and a Z-stack was created from which the corneal thickness was determined. To obtain the Z-stacks, 40 optical sections were acquired sequentially from the superficial squamous epithelium to the endothelium using a C-Apochromat 63×/1.20 W korr UV-VIS-IR M27 objective. The Z-value between epithelial and endothelial positions was considered the corneal thickness. Corneal thickness measurements were made from one cornea per mouse.
FRAP Measurements
The FRAP measurements were carried out with the LSM710 multiphoton confocal microscope and C-Apochromat 63×/1.20 W korr UV-VIS-IR M27 objective. Scan frame size was 512 × 512 μm, pixel dwell 1.58 μsec, and scan time 3.87 msec. The pinhole was set at 300 Airy Unit (1 AU = 1.082 micron section) and no line averaging was used. The CFDA was excited with the argon laser at 488 nm at 16% power to minimize bleaching of the sample during monitoring. The CFDA fluorescence emission was detected between 492 and 602 nm. Six bleach iterations, pixel dwell 25.21 μsec at 100% transmission were sufficient to bleach 95% to 100% of the intracellular CFDA. One prebleach image was taken to assess noise. An image size of 134.7 × 134.7 μm was chosen to allow for simultaneous monitoring of two neighboring cells to calculate the overall photobleaching and to evaluate focal plane drifts during postbleaching data acquisition. For each mouse, central corneal cells were preferred for FRAP measurements, and at least 20 cells were analyzed from both corneas. Some bias possibly was introduced into the FRAP measurements in that FRAP cells were chosen that had well-stained neighbors to ensure that dye could move into the bleached cell from all possible bordering cells.
The bleaching area for epithelial cells covered approximately 90% to 100% of the cell area. For keratocytes, the bleaching area covered approximately 80% to 90% of the cell body, but did not extend to the projections radiating from the cell body.
Diffusion Coefficient Analysis
Bleach recovery data were normalized and then fit to a single exponential using the FRAP analysis module of the Zeiss ZEN software. The half-time for fluorescence recovery (t1/2) was determined as the time needed for the intensity at the center of the bleached area to reach 50% of the asymptotic recovery value. The t1/2 is solved by the software using the diffusion equation
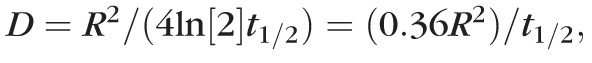 where D is the diffusion coefficient and R is radius of the bleached disc.55,56
where D is the diffusion coefficient and R is radius of the bleached disc.55,56
Image Processing and Statistical Analysis
All images were processed and analyzed using ImageJ software (National Institutes of Health [NIH]; available in the public domain at http://imagej.nih.gov/ij/). GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) was used for the statistical analysis. Diffusion coefficients of the different phenotypes and genotypes were compared using an unpaired Student's t-test, assuming unequal variance. For multiple comparisons a 2-way ANOVA test was used.
Results
Corneal Thickness and Epithelia Size of VDR−/− Mice
The body weight and superficial cell radius of 10-week-old VDR−/− mice were significantly lower than those of VDR+/− or VDR+/+ mice (P < 0.05 and P < 0.01, respectively; Table 1). The mean corneal thickness of 10-week-old VDR−/− mice was significantly thinner than those of VDR+/+ mice (P < 0.05). Although the body weight of 4-week-old VDR−/− mice was significantly lower than that of VDR+/− or VDR+/+ mice (P < 0.05), there were no differences in the corneal thickness or superficial squamous cell size among the three genotypes of 4-week-old mice (Table 2).
Table 1.
Body Weight, Corneal Thickness, and Superficial Cell Radius of 10-Week-Old VDR Mice
|
Genotype |
Body Weight, g |
Corneal Thickness, μm |
Superficial Cell Radius, μm |
| VDR+/+ | 21.38 ± 1.22 | 153.20 ± 17.28 | 14.32 ± 1.08 |
| VDR+/− | 23.05 ± 1.83 | 149.20 ± 17.12 | 13.71 ± 0.84 |
| VDR−/− | 17.28 ± 2.25* | 129.60 ± 32.47† | 10.32 ± 0.80‡ |
Data represent 102 cells from 5 corneas of 5 VDR+/+ mice, 73 cells from 10 corneas of 5 VDR+/− mice, and 110 cells from 10 corneas of 5 VDR−/− mice.
P < 0.05 body weight of VDR−/− versus VDR+/+ and VDR+/−.
P < 0.05 corneal thickness of VDR−/− versus VDR+/+.
P < 0.01 superficial cell radius of VDR−/− versus VDR+/+.
Table 2.
Body Weight, Corneal Thickness, and Superficial Cell Radius of 4-Week-Old VDR Mice
|
Genotype |
Body Weight, g |
Corneal Thickness, μm |
Superficial Cell Radius, μm |
| VDR+/+ | 20.07 ± 2.11 | 129.40 ± 9.04 | 13.56 ± 0.72 |
| VDR+/− | 21.13 ± 1.53 | 128.00 ± 13.04 | 12.34 ± 0.59 |
| VDR−/− | 16.78 ± 1.42* | 127.80 ± 10.51 | 13.25 ± 1.03 |
Data represent 93 cells from 5 corneas of 5 VDR+/+ mice, 119 cells from 10 corneas of 5 VDR+/− mice, and 88 cells from 10 corneas of 5 VDR−/− mice.
P < 0.05 body weight of VDR−/− versus VDR+/+ and VDR+/−.
Recovery Patterns of Corneal Epithelial Cells
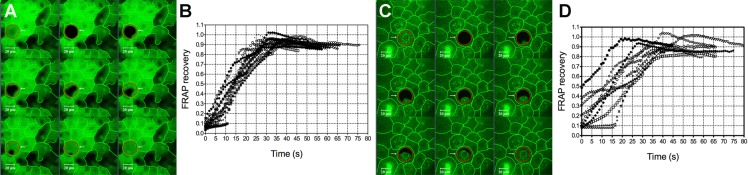
Figure 1 shows the different patterns of CFDA recovery into bleached superficial squamous cells from their surrounding cell(s). Three distinct patterns of CFDA recovery were observed; from a single neighboring cell (Fig. 1A), from two neighboring cells (Fig. 1B), and from three or more neighboring cells (Fig. 1C). Figure 1A is the typical recovery pattern of superficial cell of VDR−/− mice, while dye recovery typically was from more than one direction in the superficial cells of VDR+/+ and VDR+/− mice. The above described patterns also were observed in middle wing cells and inner basal cells.
Figure 1.
Different fluorescence recovery patterns observed in corneal epithelial cells. Recovery was seen moving from a either a single direction, typically as was observed in superficial cells from VDR−/− mice (A), or from two (B) or more (C) neighboring cells. Cell borders have been highlighted.
Gap Junctions in Superficial Squamous Cells
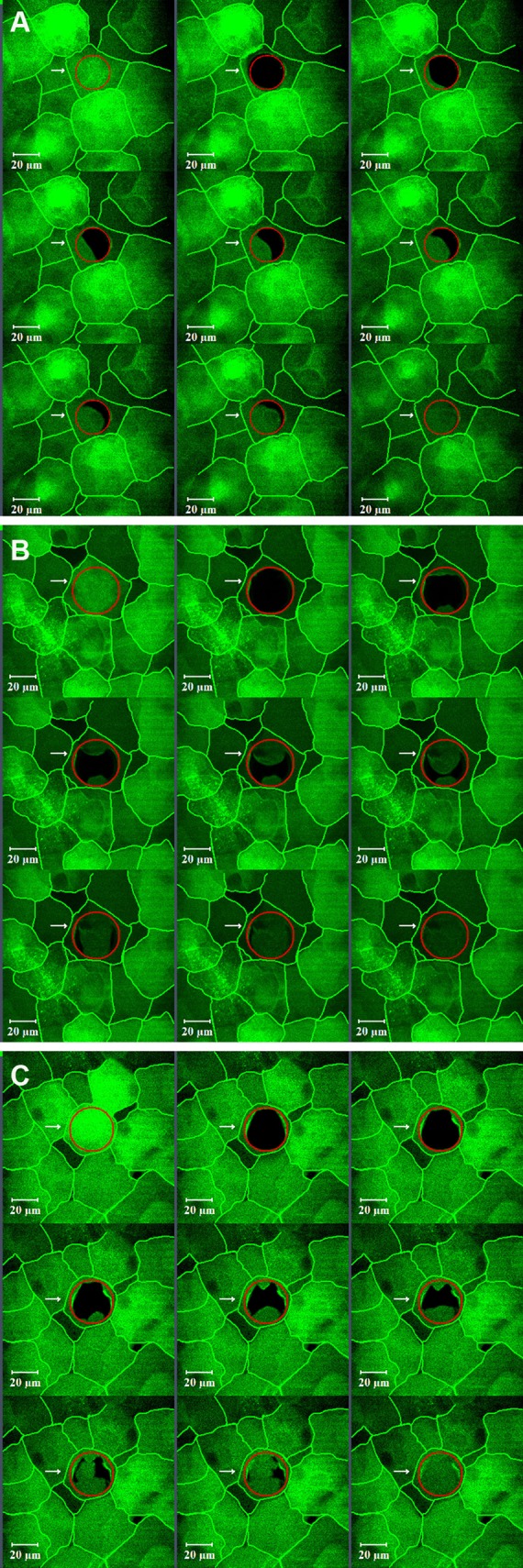
Figure 2 shows representative bleaching and recovery images, along with FRAP recovery curves, from superficial squamous cells of VDR+/+ (Figs. 2A, 2B) and VDR−/− (Figs. 2C, 2D) mice. Recovery curves shown in Figure 2 are from all squamous cells examined from the paired corneas of a single representative mouse. It can be seen from the curves that there is much more variability in the shape and slopes of the recovery curves of VDR−/− cells compared to VDR+/+ cells.
Figure 2.
Fluorescence recovery and FRAP recovery curves obtained from superficial squamous cells of VDR+/+ (A, B) and VDR−/− (C, D). Variability in the shape and slopes was observed in the recovery curves of VDR−/− cells. Cell borders have been highlighted.
Gap Junctions of Middle Wing Cells and Inner Basal Cells
Recovery curves were not different for middle wing cells or inner basal cells of either genotype.
Diffusion Coefficient Comparisons Between Cell Types
Table 3 (across rows) compares diffusion coefficients of the different cell types within genotypes of 4-week-old mice. Significant differences in diffusion coefficients were found between superficial squamous cells and middle wing cells (P < 0.05), and between superficial squamous cells and inner basal cells (P < 0.01) within all three genotypes of 4-week-old mice. In addition, the middle wing cell diffusion coefficients were significantly different compared to inner basal cells (P < 0.05) within all three 4-week-old genotypes.
Table 3.
Cornea Cell Diffusion Coefficients: 4-Week-Old Mice
|
Genotype |
Cell Type |
||
|
Superficial Squamous Cell |
Middle Wing Cell |
Inner Basal Cell |
|
| VDR+/+ | 14.21 ± 1.18 | 9.05 ± 3.72* (P < 0.05) | 6.98 ± 2.29† (P < 0.01) |
| VDR+/− | 12.99 ± 1.36 | 9.87 ± 1.58* (P < 0.05) | 7.93 ± 2.54† (P < 0.01) |
| VDR−/− | 14.32 ± 2.61 | 8.91 ± 2.23* (P < 0.05) | 5.82 ± 0.81† (P < 0.01) |
Data represent 93 superficial squamous cells, 64 middle wing cells, and 101 inner basal cells from 10 corneas of 5 VDR+/+ mice; 119 superficial squamous cells, 44 middle wing cells, and 111 inner basal cells from 10 corneas of 5 VDR+/− mice; and 88 superficial squamous cells, 74 middle wing cells, and 76 inner basal cells from 10 corneas of 5 VDR−/− mice.
P < 0.05 middle wing cell versus superficial squamous cell for all genotypes.
P < 0.01 inner basal cell versus superficial squamous cell for all genotypes.
Within 10-week-old control mice and VDR+/− mice, diffusion coefficients of superficial squamous and middle wing cells, and between superficial squamous and inner basal cells also were significantly different (P < 0.01; Table 4, across rows). For 10-week-old VDR−/− mice, the superficial squamous cell versus inner basal cell, and middle wing cell versus inner basal cell diffusion coefficients were significantly different (P < 0.01). There were no significant differences between superficial squamous cells and middle wing cells. Comparing diffusion coefficients between the same cell types of 4- vs. 10-week-old VDR+/+ mice, the only significant difference was a lower coefficient for the squamous cells of 4-week-old mice (P < 0.05).
Table 4.
Cornea Cell Diffusion Coefficients: 10-Week-Old Mice
|
Genotype |
Cell Type |
||
|
Superficial Squamous Cell |
Middle Wing Cell |
Inner Basal Cell |
|
| VDR+/+ | 18.71 ± 1.98 | 9.81 ± 2.86 | 5.60 ± 0.66 |
| VDR+/− | 14.89 ± 2.5* (P < 0.05) | 7.85 ± 0.97 | 6.88 ± 1.58 |
| VDR−/− | 10.90 ± 2.20† (P < 0.01) | 9.55 ± 1.43 | 6.72 ± 1.75 |
Data represent 102 superficial squamous cells, 56 middle wing cells, and 85 inner basal cells from 10 corneas of 5 VDR+/+ mice; 73 superficial squamous cells, 86 middle wing cells, and 66 inner basal cells from 10 corneas of 5 VDR+/− mice; and 110 superficial squamous cells, 67 middle wing cells, and 96 inner basal cells from 10 corneas of 5 VDR−/− mice.
P < 0.05 VDR+/− versus VDR+/+ superficial squamous cells.
P < 0.01 VDR−/− versus VDR+/+ superficial squamous cells.
Diffusion Coefficient Comparisons Between Genotypes
Comparing diffusion coefficients of the same cell types between genotypes in 4-week-old mice (Table 3, down columns), there were no significant differences between any cell types. However, in 10-week-old mice the diffusion coefficients of squamous cells were significantly different between VDR+/+ and VDR+/− mice (P < 0.05), VDR+/+ and VDR−/− mice (P < 0.01), and between VDR+/− mice and VDR−/− mice (P < 0.05; Table 4, down columns). There were no significant diffusion coefficient differences between middle wing cells or inner basal cells of 10-week-old VDR+/+, VDR+/−, or VDR−/− mice.
Keratocyte Gap Junctions
Figure 3 shows representative CFDA dye spread between a bleached keratocyte and its surrounding keratocytes within the cornea of a VDR+/+ mouse, along with FRAP recovery curves. Keratocyte CFDA diffusion coefficients ranged from 2.84 to 7.03 μm2/s for three VDR+/+ mice. Mean keratocyte diffusion coefficients equaled 4.26 ± 1.37 μm2/s (mean ± SD, 30 stroma cells from 6 corneas of 3 VDR+/+ mice).
Figure 3.
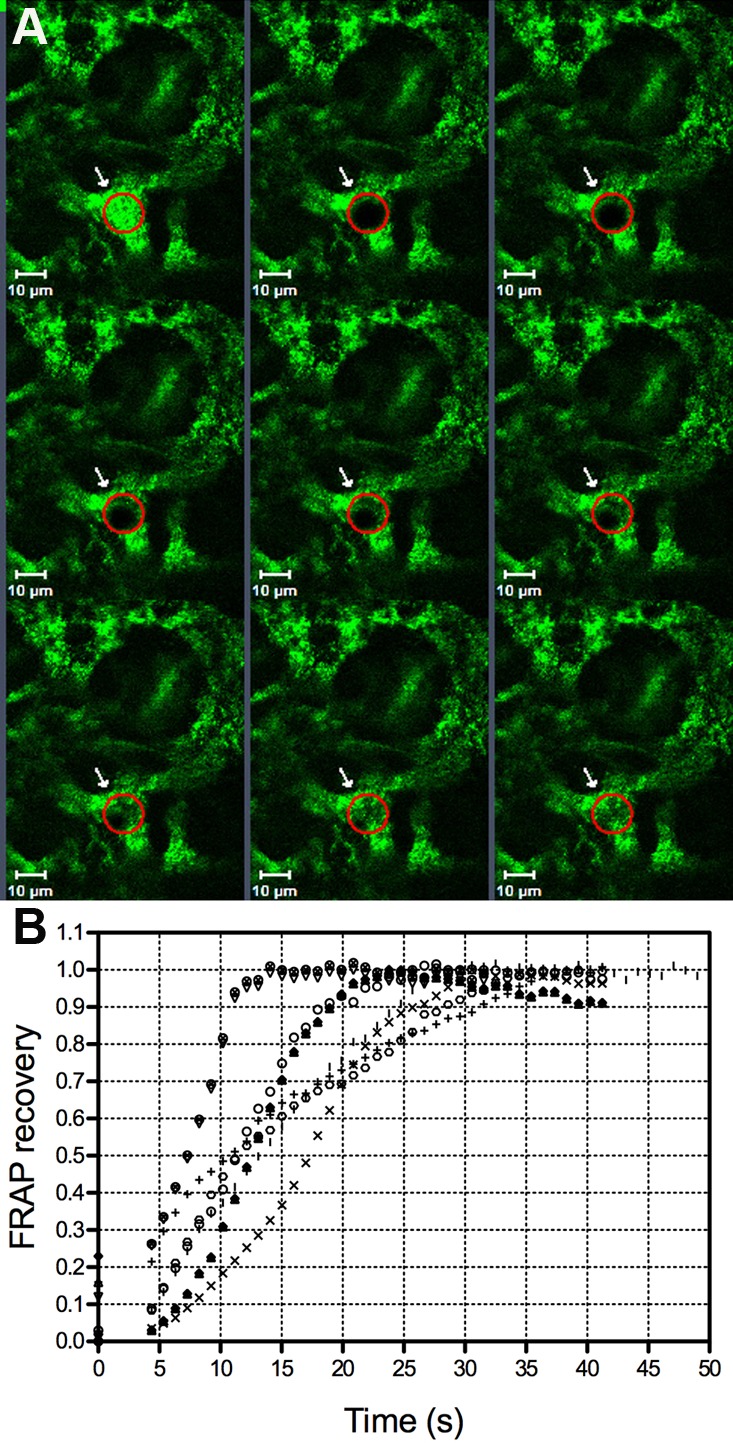
Fluorescence recovery and FRAP recovery curves obtained from keratocytes of VDR+/+ mice (A, B). Variability in the shape and slopes was observed in the recovery curves.
Discussion
Gap junctions are important for all aspects of multicellular life, including cell proliferation and cell migration in many tissues. Cornea gap junctions have been studied previously, and the gap junction diffusion coefficient for Lucifer yellow has been reported for corneal endothelium.11,13,57,58 Cornea epithelium and keratocyte gap junction diffusion coefficients have never been reported to our knowledge, although previous studies have detected gap junction proteins, connexins, and/or dye spread in corneal epithelial cells and keratocytes in isolated corneas and in monolayer cell culture.18,59 While previous microelectrode studies from our laboratory examined ex vivo dye spread in the different layers of the corneal epithelium,15,18,19 it was not possible to quantify dye diffusion coefficients for the different epithelial phenotypes. In monolayer cell culture gap junction studies, it also is not possible to distinguish between different corneal epithelium phenotypes. In the current study, we used FRAP methodology to measure cornea epithelial gap junction dye diffusion coefficients in superficial squamous cells, middle wing cells, and inner basal cells, as well as keratocytes, in intact corneas.
The diffusion coefficient of superficial squamous cells was significantly greater than that of middle wing cells and inner basal cells. These findings are significant in that there was no evidence for dye spread in superficial squamous cells in previous microelectrode studies.15,18 These previous results likely are due to the invasive nature of microelectrodes combined with the thin, flat morphology of superficial cornea epithelial cells. It also is possible that there are species differences given that the previous studies were performed in human and rabbit corneas, while the current study was performed with mouse corneas. The current data showed that the gap junction diffusion coefficient value changed along the developmental axis of corneal epithelium, increasing from basal cells to wing cells to squamous cells, following the centripetal migration pattern and vertical movement of cells from the stroma to the surface.16,60,61 It is not clear why the superficial cells have the highest diffusion coefficient. It is possible that these superficial cells contain an increased number of connexin proteins or possibly have an increased connexin channel open probability. Previous studies determined that at least three connexin proteins are expressed in mouse corneal epithelium, with connexin 43 restricted predominantly to the basal cells that are involved in the control of cell migration,62–64 while connexins 26 and 30 were present in all cell phenotypes (Djalilian AR, et al. IOVS 2004;45:ARVO E-Abstract 3769 and Ref. 14).
The use of VDR knockout mice is a common model to study vitamin D deficiency because of its specificity for 1,25-dihydroxyvitamin D, as well as the general acceptance that the vitamin D responses, particularly chronic responses, are mediated through VDR. Corneal epithelial cell gap junction diffusion coefficients also were compared between the different VDR mouse genotypes. The diffusion coefficient of superficial squamous cells of VDR+/+ mice was significantly greater than that of VDR−/− mice. This appeared to be due in part to the recovery pattern of the different genotypes, with dye recovery originating from several neighboring cells in VDR+/+ mice and typically only one neighbor in VDR−/− mice. No differences were found in the diffusion coefficients or recovery pattern of middle wing cells or inner basal cells among the different mouse genotypes. Interestingly, vitamin D and VDR have been shown to regulate gap junctional communication in human skin fibroblasts.65
The differences in the recovery pattern of the VDR−/− versus the VDR+/+ mice likely are related to the pattern of connectivity of the superficial cells between the two genotypes. We observed that the superficial cells of VDR+/+ mice typically are tightly abutted to their neighboring cells, with the epithelium appearing regular and fully intact. The surface cells of VDR−/− mice, on the other hand, appeared to be more irregular and patchy (Fig. 4). This morphology supported previous work from our laboratory indicating vitamin D is involved in corneal epithelial tight junction control, and points to a possible role of vitamin D and VDR in corneal epithelial regeneration. This is supported further by our recent work demonstrating that vitamin D is involved in corneal epithelial wound healing.53
Figure 4.
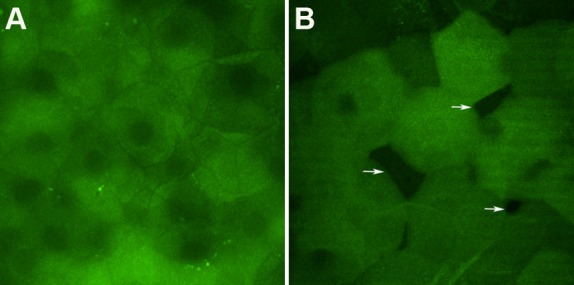
Confocal images obtained from corneal surface of VDR+/+ (A) and VDR−/− mice (B). Superficial squamous cells of VDR−/− mice partly abutted to their neighboring cells. Arrows indicate spaces between cells.
The VDR+/+ mouse corneal thickness was larger than that of VDR−/− mice. The VDR−/− mouse had a smaller superficial squamous cell size, which implies greater cell density. Interestingly, connexin 31.1 has been shown to regulate the differentiation and thickness of human and mouse corneal epithelia.66 It is possible that the difference in diffusion coefficients is due to a reduced number of functional gap junctions, a change in the distribution of connexin protein species, or a defect in the function of gap junctions themselves. Additional studies are needed to quantify the effects of vitamin D and VDR on the expression of Cx31.1 and other connexins in the corneal epithelium.
The FRAP methodology presented in this study also was used to measure gap junction diffusion coefficients in intact keratocytes. The results supported previous work showing that keratocytes form an intercommunicating network within the corneal stroma. The current study recorded a recovery time for mouse keratocytes of approximately 18 seconds. This is notably faster than that of carboxyfluorescein (CF) spread in rabbit and human keratocytes measured by dye microinjection, in which dye was seen spreading to two or three cells surrounding the source cell 4.5 minutes after the microinjection.22,35
Significant variability was observed in the diffusion coefficient among keratocytes, which appears to be related to the keratocyte phenotype and location within the stroma. It has been shown that keratocyte density is significantly higher in the anterior stroma than that in the posterior stroma,67 which would allow for increased connectivity in the anterior stroma. In addition, morphologically, keratocytes have been found to be a heterogeneous cell population in rabbits and humans.68 Furthermore, keratocytes isolated from perilimbal and central locations of human corneas show different characteristics, including their proliferative capacity.69
In summary, to our knowledge this study is the first to demonstrate gap junction dye spread between superficial corneal epithelial squamous cells. The data demonstrated a significant difference in gap junction dye spread among the different epithelial cell phenotypes that mirrors the developmental axis of the epithelium. Interestingly, the cells that are the most differentiated, surface squamous cells, had the highest diffusion coefficient. In addition, it was found that the squamous cells of VDR−/− mice had lower diffusion coefficients than those of VDR+/+ mice. The VDR−/− mice also had thinner corneas and smaller cell sizes. Thus, VDR and likely vitamin D modulation of gap junctions may be involved in the regeneration and development of corneal epithelium.
Acknowledgments
The authors thank Zhaohong Yin and Sandeep Pallikkuth from University of Tennessee Health Science Center (Memphis, TN, USA), for their assistance with animal administration and microscopy technique, respectively.
Supported by National Institutes of Health Grant R01EY021747.
Disclosure: X. Lu, None; M.A. Watsky, None
References
- 1. Holder JW, Elmore E, Barrett JC. Gap junction function and cancer. Cancer Res. 1993; 53: 3475–3485 [PubMed] [Google Scholar]
- 2. Hanner F, Sorensen CM, Holstein-Rathlou NH, Peti-Peterdi J. Connexins and the kidney. Am J Physiol Regul Integr Comp Physiol. 2010; 298: R1143–R1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ewart JL, Cohen MF, Meyer RA, et al. Heart and neural tube defects in transgenic mice overexpressing the Cx43 gap junction gene. Development. 1997; 124: 1281–1292 [DOI] [PubMed] [Google Scholar]
- 4. Pearson RA, Luneborg NL, Becker DL, Mobbs P. Gap junctions modulate interkinetic nuclear movement in retinal progenitor cells. J Neurosci. 2005; 25: 10803–10814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thevenin AF, Kowal TJ, Fong JT, Kells RM, Fisher CG, Falk MM. Proteins and mechanisms regulating gap-junction assembly, internalization, and degradation. Physiology. 2013; 28: 93–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wei CJ, Xu X, Lo CW. Connexins and cell signaling in development and disease. Annu Rev Cell Dev Biol. 2004; 20: 811–838 [DOI] [PubMed] [Google Scholar]
- 7. Hamelin R, Allagnat F, Haefliger JA, Meda P. Connexins, diabetes and the metabolic syndrome. Curr Protein Pept Sci. 2009; 10: 18–29 [DOI] [PubMed] [Google Scholar]
- 8. Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003; 83: 1359–1400 [DOI] [PubMed] [Google Scholar]
- 9. Churko JM, Laird DW. Gap junction remodeling in skin repair following wounding and disease. Physiology. 2013; 28: 190–198 [DOI] [PubMed] [Google Scholar]
- 10. Garcia-Dorado D, Rodriguez-Sinovas A, Ruiz-Meana M. Gap junction-mediated spread of cell injury and death during myocardial ischemia-reperfusion. Cardiovasc Res. 2004; 61: 386–401 [DOI] [PubMed] [Google Scholar]
- 11. Djalilian AR, McGaughey D, Patel S, et al. Connexin 26 regulates epidermal barrier and wound remodeling and promotes psoriasiform response. J Clin Invest. 2006; 116: 1243–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coutinho P, Qiu C, Frank S, Tamber K, Becker D. Dynamic changes in connexin expression correlate with key events in the wound healing process. Cell Biol Int. 2003; 27: 525–541 [DOI] [PubMed] [Google Scholar]
- 13. Dermietzel R, Traub O, Hwang TK, et al. Differential expression of three gap junction proteins in developing and mature brain tissues. Proc Natl Acad Sci U S A. 1989; 86: 10148–10152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nagy JI, Patel D, Ochalski PA, Stelmack GL. Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience. 1999; 88: 447–468 [DOI] [PubMed] [Google Scholar]
- 15. Williams K, Watsky M. Gap junctional communication in the human corneal endothelium and epithelium. Curr Eye Res. 2002; 25: 29–36 [DOI] [PubMed] [Google Scholar]
- 16. Thoft RA, Friend J. The X, Y, Z Hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983; 24: 1442–1443 [PubMed] [Google Scholar]
- 17. Watsky MA. Loss of fenamate-activated K+ current from epithelial cells during corneal wound healing. Invest Ophthalmol Vis Sci. 1999; 40: 1356–1363 [PubMed] [Google Scholar]
- 18. Williams KK, Watsky MA. Dye spread through gap junctions in the corneal epithelium of the rabbit. Curr Eye Res. 1997; 16: 445–452 [DOI] [PubMed] [Google Scholar]
- 19. Williams KK, Watsky MA. Bicarbonate promotes dye coupling in the epithelium and endothelium of the rabbit cornea. Curr Eye Res. 2004; 28: 109–120 [DOI] [PubMed] [Google Scholar]
- 20. Hasty DL, Hay ED. Freeze-fracture studies of the developing cell surface. I. The plasmalemma of the corneal fibroblast. J Cell Biol. 1977; 72: 667–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beyer EC, Kistler J, Paul DL, Goodenough DA. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989; 108: 595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watsky MA. Loss of keratocyte ion channels during wound healing in the rabbit cornea. Invest Ophthalmol Vis Sci. 1995; 36: 1095–1099 [PubMed] [Google Scholar]
- 23. Shurman DL, Glazewski L, Gumpert A, Zieske JD, Richard G. In vivo and in vitro expression of connexins in the human corneal epithelium. Invest Ophthalmol Vis Sci. 2005; 46: 1957–1965 [DOI] [PubMed] [Google Scholar]
- 24. Risek B, Klier FG, Gilula NB. Multiple gap junction genes are utilized during rat skin and hair development. Development. 1992; 116: 639–651 [DOI] [PubMed] [Google Scholar]
- 25. Dahl E, Winterhager E, Reuss B, Traub O, Butterweck A, Willecke K. Expression of the gap junction proteins connexin31 and connexin43 correlates with communication compartments in extraembryonic tissues and in the gastrulating mouse embryo, respectively. J Cell Sci. 1996; 109: 191–197 [DOI] [PubMed] [Google Scholar]
- 26. Cook JE, Becker DL. Gap-junction proteins in retinal development: new roles for the “nexus.” Physiology. 2009; 24: 219–230 [DOI] [PubMed] [Google Scholar]
- 27. Mathias RT, White TW, Gong X. Lens gap junctions in growth, differentiation, and homeostasis. Physiol Rev. 2010; 90: 179–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bloomfield SA, Volgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci. 2009; 10: 495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petrescu MS, Larry CL, Bowden RA, et al. Neutrophil interactions with keratocytes during corneal epithelial wound healing: a role for CD18 integrins. Invest Ophthalmol Vis Sci. 2007; 48: 5023–5029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel SV, McLaren JW, Camp JJ, Nelson LR, Bourne WM. Automated quantification of keratocyte density by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 1999; 40: 320–326 [PubMed] [Google Scholar]
- 31. Labbe A, Liang H, Martin C, Brignole-Baudouin F, Warnet JM, Baudouin C. Comparative anatomy of laboratory animal corneas with a new-generation high-resolution in vivo confocal microscope. Curr Eye Res. 2006; 31: 501–509 [DOI] [PubMed] [Google Scholar]
- 32. Poole CA, Brookes NH, Clover GM. Confocal imaging of the human keratocyte network using the vital dye 5-chloromethylfluorescein diacetate. Clin Exp Ophthalmol. 2003; 31: 147–154 [DOI] [PubMed] [Google Scholar]
- 33. Olesen NE, Hofgaard JP, Holstein-Rathlou NH, Nielsen MS, Jacobsen JC. Estimation of the effective intercellular diffusion coefficient in cell monolayers coupled by gap junctions. Eur J Pharm Sci. 2012; 46: 222–232 [DOI] [PubMed] [Google Scholar]
- 34. Dhar A, Ebbinghaus S, Shen Z, Mishra T, Gruebele M. The diffusion coefficient for PGK folding in eukaryotic cells. Biophys J. 2010; 99: L69–L71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Watsky MA. Keratocyte gap junctional communication in normal and wounded rabbit corneas and human corneas. Invest Ophthalmol Vis Sci. 1995; 36: 2568–2576 [PubMed] [Google Scholar]
- 36. Weidmann S. The electrical constants of Purkinje fibres. J Physiol. 1952; 118: 348–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Furshpan EJ, Potter DD. Mechanism of nerve-impulse transmission at a crayfish synapse. Nature. 1957; 180: 342–343 [DOI] [PubMed] [Google Scholar]
- 38. Spray DC. Illuminating gap junctions. Nat Methods. 2005; 2: 12–14 [DOI] [PubMed] [Google Scholar]
- 39. Rae JL, Watsky MA. Ionic channels in corneal endothelium. Am J Physiol. 1996; 270: C975–C989 [DOI] [PubMed] [Google Scholar]
- 40. Abbaci M, Barberi-Heyob M, Stines JR, et al. Gap junctional intercellular communication capacity by gap-FRAP technique: a comparative study. Biotechnol J. 2007; 2: 50–61 [DOI] [PubMed] [Google Scholar]
- 41. Nakatani K, Chen C, Koutalos Y. Calcium diffusion coefficient in rod photoreceptor outer segments. Biophys J. 2002; 82: 728–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caveney S. The role of gap junctions in development. Annu Rev Physiol. 1985; 47: 319–335 [DOI] [PubMed] [Google Scholar]
- 43. Spray DC, Bennett MV. Physiology and pharmacology of gap junctions. Annu Rev Physiol. 1985; 47: 281–303 [DOI] [PubMed] [Google Scholar]
- 44. Raptis LH, Brownell HL, Firth KL, Mackenzie LW. A novel technique for the study of intercellular, junctional communication: electroporation of adherent cells on a partly conductive slide. DNA Cell Biol. 1994; 13: 963–975 [DOI] [PubMed] [Google Scholar]
- 45. Abbaci M, Barberi-Heyob M, Blondel W, Guillemin F, Didelon J. Advantages and limitations of commonly used methods to assay the molecular permeability of gap junctional intercellular communication. Biotechniques. 2008; 45: 33–52 [DOI] [PubMed] [Google Scholar]
- 46. Peters R, Peters J, Tews KH, Bahr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974; 367: 282–294 [DOI] [PubMed] [Google Scholar]
- 47. Kato S, Takeyama K, Kitanaka S, Murayama A, Sekine K, Yoshizawa T. In vivo function of VDR in gene expression-VDR knock-out mice. J Steroid Biochem Mol Biol. 1999; 69: 247–251 [DOI] [PubMed] [Google Scholar]
- 48. Wade MH, Trosko JE, Schindler MA. Fluorescence photobleaching assay of gap junction-mediated communication between human-cells. Science. 1986; 232: 525–528 [DOI] [PubMed] [Google Scholar]
- 49. Panda DK, Miao D, Tremblay ML, et al. Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001; 98: 7498–7503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010; 9: 941–955 [DOI] [PubMed] [Google Scholar]
- 51. Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science. 1997; 277: 1827–1830 [DOI] [PubMed] [Google Scholar]
- 52. Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357: 266–281 [DOI] [PubMed] [Google Scholar]
- 53. Yin Z, Pintea V, Lin Y, Hammock BD, Watsky MA. Vitamin D enhances corneal epithelial barrier function. Invest Ophthalmol Vis Sci. 2011; 52: 7359–7364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lin Y, Ubels JL, Schotanus MP, et al. Enhancement of vitamin D metabolites in the eye following vitamin D3 supplementation and UV-B irradiation. Curr Eye Res. 2012; 37: 871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kramer EM, Frazer NL, Baskin TI. Measurement of diffusion within the cell wall in living roots of Arabidopsis thaliana. J Exp Bot. 2007; 58: 3005–3015 [DOI] [PubMed] [Google Scholar]
- 56. Crank J. The Mathematics of Diffusion. 2nd ed. Oxford, UK: Clarendon Press; 1985. [Google Scholar]
- 57. Watsky MA, McCartney MD, McLaughlin BJ, Edelhauser HF. Corneal endothelial junctions and the effect of ouabain. Invest Ophthalmol Vis Sci. 1990; 31: 933–941 [PubMed] [Google Scholar]
- 58. Wolosin JM, Candia OA, Peterson-Yantorno K, Civan MM, Shi XP. Effect of heptanol on the short circuit currents of cornea and ciliary body demonstrates rate limiting role of heterocellular gap junctions in active ciliary body transport. Exp Eye Res. 1997; 64: 945–952 [DOI] [PubMed] [Google Scholar]
- 59. Grupcheva CN, Laux WT, Rupenthal ID, McGhee J, McGhee CNJ, Green CR. Improved corneal wound healing through modulation of gap junction communication using connexin43-specific antisense oligodeoxynucleotides. Invest Ophthalmol Vis Sci. 2012; 53: 1130–1138 [DOI] [PubMed] [Google Scholar]
- 60. Sharma A, Coles WH. Kinetics of corneal epithelial maintenance and graft loss. A population balance model. Invest Ophthalmol Vis Sci. 1989; 30: 1962–1971 [PubMed] [Google Scholar]
- 61. Buck RC. Measurement of centripetal migration of normal corneal epithelial cells in the mouse. Invest Ophthalmol Vis Sci. 1985; 26: 1296–1299 [PubMed] [Google Scholar]
- 62. Behrens J, Kameritsch P, Wallner S, Pohl U, Pogoda K. The carboxyl tail of Cx43 augments p38 mediated cell migration in a gap junction-independent manner. Eur J Cell Biol. 2010; 89: 828–838 [DOI] [PubMed] [Google Scholar]
- 63. Huang GY, Cooper ES, Waldo K, Kirby ML, Gilula NB, Lo CW. Gap junction-mediated cell-cell communication modulates mouse neural crest migration. J Cell Biol. 1998; 143: 1725–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kwak BR, Pepper MS, Gros DB, Meda P. Inhibition of endothelial wound repair by dominant negative connexin inhibitors. Mol Biol Cell. 2001; 12: 831–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Clairmont A, Tessmann D, Stock A, Nicolai S, Stahl W, Sies H. Induction of gap junctional intercellular communication by vitamin D in human skin fibroblasts is dependent on the nuclear vitamin D receptor. Carcinogenesis. 1996; 17: 1389–1391 [DOI] [PubMed] [Google Scholar]
- 66. Chang CY, Laux-Fenton WT, Law LY, Becker DL, Sherwin T, Green CR. Antisense down regulation of connexin31.1 reduces apoptosis and increases thickness of human and animal corneal epithelia. Cell Biol Int. 2009; 33: 376–385 [DOI] [PubMed] [Google Scholar]
- 67. Reichard M, Hovakimyan M, Wree A, et al. Comparative in vivo confocal microscopical study of the cornea anatomy of different laboratory animals. Cur Eye Res. 2010; 35: 1072–1080 [DOI] [PubMed] [Google Scholar]
- 68. Ojeda JL, Ventosa JA, Piedra S. The three-dimensional microanatomy of the rabbit and human cornea. A chemical and mechanical microdissection-SEM approach. J Anat. 2001; 199: 567–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Builles N, Bechetoille N, Justin V, Andre V, Burillon C, Damour O. Variations in the characteristics of keratocytes in culture in relation to their location in human cornea. Biomed Mater Eng. 2008; 18: S87–S98 [PubMed] [Google Scholar]