The authors performed a meta-analysis of 20 studies to more precisely evaluate the PTEN/PI3K/Akt signaling pathway’s clinical significance in patients with epithelial ovarian cancer. Analyses showed that high PI3K and pAkt expression was associated with poor overall survival and that only high pAkt expression was significantly associated with poor progression-free survival.
Keywords: PTEN/PI3K/Akt, Overall survival, Progression-free survival, Epithelial ovarian cancer, Meta-analysis
Abstract
Introduction.
The PTEN/PI3K/Akt signaling pathway, a key player in mediating apoptosis, metabolism, cell proliferation, and cell growth, is frequently dysregulated in many cancers. However, the pathway’s prognostic impact in epithelial ovarian cancer (EOC) is still inconsistent. We performed a meta-analysis based on individual study outcomes to more precisely evaluate its clinical significance in EOC patients.
Methods.
We searched all potentially relevant studies published between January 1, 1990, and March 1, 2013, that assessed the association between PTEN, PI3K, and Akt status and survival in EOC. Meta-analysis was performed using a fixed-effect or random-effects model as appropriate. We investigated the possibility of publication bias through a funnel plot and identified the heterogeneity by I2 statistics.
Results.
Eleven eligible studies were analyzed for PTEN, 5 for PI3K, and 11 for pAkt. High PI3K and pAkt expression was associated with poor overall survival (OS; pooled adjusted hazard ratio [HR] = 1.44, 95% CI, 1.08–1.91 for PI3K; HR = 1.60, 95% CI, 1.26–2.04 for pAkt). In addition, both the meta-analyses of univariate and multivariate estimates showed that only high pAkt expression was significantly associated with poor progression-free survival (PFS; pooled unadjusted HR = 1.24, 95% CI, 1.10–1.39; pooled adjusted HR = 1.65, 95% CI, 1.07–2.55).
Conclusion.
Published studies suggest that high pAkt expression is significantly associated with poor OS and PFS in EOC patients, but currently available evidence is insufficient to recommend that PTEN, PI3K, or Akt be used as prognostic predictors in EOC in clinical practice.
Abstract
摘要
简介 PTEN/PI3K/Akt 信号转导通路在介导凋亡、代谢、细胞增殖和细胞生长方面发挥着关键作用,在很多癌症中往往都存在失调。但是,关于这个通路对上皮性卵巢癌 (EOC) 的预后影响,目前仍无一致结论。我们对各个研究结局指标进行了一项荟萃分析,以便更确切地评估它在 EOC 患者中的临床意义。
方法 我们搜索了 1990 年 1 月 1 日至 2013 年 3 月 1 日期间发表的所有可能与此有关的研究,这些研究评估了 PTEN、PI3K 和 Akt 状态与 EOC 生存期之间的关联。荟萃分析的模型酌情采用了固定效应模型或随机效应模型。我们通过一个漏斗曲线评估了发表偏倚的概率,并通过 I2 统计值确定了异质性。
结果 我们为 PTEN 分析了 11 项合格研究,为 PI3K 分析了 5 项,为 pAkt 分析了 11 项。PI3K 和 pAkt 的高表达通常伴随较低的总生存期(OS;PI3K 的汇总校正后风险比 [HR] = 1.44, 95% CI, 1.08–1.91;pAkt 的 HR = 1.60, 95% CI, 1.26–2.04)。此外,单一变量和多变量估计值的荟萃分析均显示,只有 pAkt 的高表达与无进展生存期不佳存在显著关联(PFS;汇总非校正 HR = 1.24 CI, 95% CI, 1.10–1.39;汇总校正后 HR = 1.65, 95% CI, 1.07–2.55)。
结论 已发表研究表明,pAkt 的高表达与 EOC 患者的 OS 和 PFS 不佳存在显著关联,但现有证据不足以支持将 PTEN、PI3K 或 Akt 作为 EOC 的预后预测因子应用于临床。The Oncologist 2014;19:528–535
Implications for Practice:
We performed a meta-analysis based on individual study outcomes to evaluate the clinical significance of the PTEN/PI3K/Akt signaling pathway in epithelial ovarian cancer (EOC) patients. We found that high expression of activated Akt (pAkt) was significantly associated with poor survival in EOC patients, but currently available evidence is insufficient to recommend PTEN, PI3K, or Akt to be used as a prognostic predictor in EOC in clinical practice. This study is both important and immediately clinically relevant because currently, in spite of tremendous efforts in the field of biomarker discovery and development, for EOC, molecular assists in the clinicopathologic and prognosis toolbox are lacking. Furthermore, identification of more accurate and more effective detection techniques and unified evaluation criteria to mandate homogeneous data collection is urgently needed.
Introduction
Epithelial ovarian cancer (EOC) is the most frequent cause of death from gynecological malignancies among American women [1] and is characterized by a low rate of early diagnosis, high rate of metastasis, very poor prognosis, and lack of effective therapies. The current management of patients with advanced disease involves optimal surgical debulking followed by chemotherapy. Platinum-based regimens remain at the core of postoperative treatment. Overall tumor response rates initially associated with this treatment are relatively high and range from approximately 70% to 80% [2, 3]. However, long-term survival remains poor as a result of recurrence and resistance to chemotherapeutics. Current clinicopathological prognostic factors do not allow individualized prediction of disease outcome. Identification of molecular markers predicting the clinical outcomes would be of great value for adjustment of patients’ treatment [4].
PTEN (phosphatase and tensin homolog deleted on chromosome 10) is a dual protein/lipid phosphatase that dephosphorylates the phosphatidylinositol 3-kinase, the product of PI3K [5] (Fig. 1). PTEN can suppress human ovarian cancer cell growth and provoke an arrest in G1 of the cell cycle through negatively regulating the PI3K/Akt (protein kinase B) pathway [6–8]. PI3K is a phosphatidylinositol kinase, including a regulatory subunit p85 and catalytic subunit p110, which is encoded by the PIK3CA gene. Akt is a cytoplasmic serine-threonine protein kinase that promotes cell cycle progression and inhibits apoptosis when activated (phosphorylation of the protein at T308 and S473 [pAkt]) [9]. Several studies have shown that abnormal expression of the PTEN/PI3K/Akt pathway has been associated with poor prognosis in ovarian carcinomas [10–12]. However, the results from the previous studies are inconclusive. In order to more precisely evaluate the clinical significance of the PTEN/PI3K/Akt pathway in EOC patients, we performed a meta-analysis based on individual study outcomes.
Figure 1.
Overview of the PTEN/PI3K/Akt signaling pathway. PI3K is initially activated by membrane receptor (such as cytokine receptor, RTK, CD19, and integrin). An increase in PI3K function leads to the accumulation of PIP3, which subsequently activates PDK1 and Akt. PTEN regulates Akt by dephosphorylating PIP3, the product of PI3K. Increased Akt activity results in subsequent downstream functions.
Abbreviations: PDK1, pyruvate dehydrogenase kinase isozyme 1; PI3K, phosphoinositide 3-kinase; PIP2, phosphatidylinositol-4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-triphosphate; PTEN, phosphatase and tensin homolog deleted on chromosome 10; R, cell surface receptor.
Materials and Methods
We performed this meta-analysis according to the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines [13].
Search Strategy and Selection
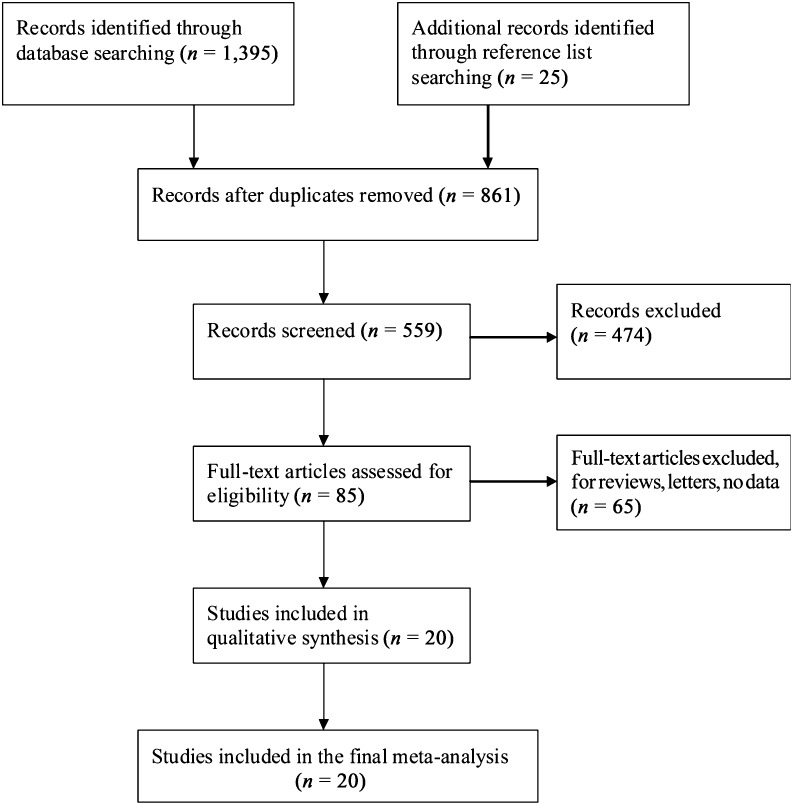
All potentially relevant papers published in English between January 1, 1990, and March 1, 2013, were identified by searching PubMed using “ovarian cancer,” “PTEN,” “PI3K,” and “pAkt” as the search terms (Fig. 2). The reference lists of relevant reviews and all eligible articles were hand-searched to identify additional studies.
Figure 2.
Process used for the trial selection.
The studies were included if they met the following criteria: (a) an original paper on primary EOC, (b) a clinical study (prospective, randomized, retrospective, etc.) that investigated the association of PTEN, PI3K, and pAkt expression status with overall survival (OS) or progression-free survival (PFS) in EOC patients, (c) articles that presented a hazard ratio (HR) and 95% confidence interval (CI), (d) articles with enough data to assess the HR and 95% CI from univariate survival analysis, and (e) articles with all pathology specimens (such as tumor tissues) obtained at initial surgery prior to any therapeutics. The studies published in languages other than English were excluded from this meta-analysis, as were nonoriginal research articles, reviews, abstracts, and letters on nonepithelial or recurrent ovarian cancer. Furthermore, duplication of a previous publication and specimens collected after chemotherapy or radiotherapy were also excluded.
Data Extraction
In order to ensure homogeneity of the data gathering and to preclude subjectivity in the data collection and entry, two researchers independently extracted the data. Cases of disagreement were settled by iteration, discussion, and consensus. The following information was collected for each study: authors’ names; year of publication; country; age of the patients at the time of diagnosis (mean, median, range); number of patients; study type (prospective, randomized, retrospective, etc.); time of follow-up (median, mean, minimum, and maximum); stage, tumor type, assay method, and scoring protocol used; treatment; number of biomarker-positive and -negative tumors; number of deaths (disease specific and overall); and results of univariate or multivariate survival analysis.
Assessment of Publication Bias and Statistical Analysis
The possibility of publication and selection bias was investigated using funnel plots and was tested by Egger’s test [14, 15].
The HR was used as a measure of the prognostic value, which was defined as the risk of death for patients with biomarker-positive tumors over patients with biomarker-negative tumors, so a HR >1 indicated an increased risk of death in cases with biomarker-positive tumors. Using the methods described by Parmar et al. [16], we extracted a log-HR and its 95% CI from a univariate/multivariate survival analysis for each study. Subsequently, we calculated the pooled HRs and 95% CIs for prognosis using the DerSimonian-Laird random-effects or the Mantel-Haenszel fixed-effects model [17, 18]. In this meta-analysis, statistical heterogeneity among studies was assessed by the I2 statistic [19], and an I2 >50% was considered as representing substantial heterogeneity between studies. Our meta-analyses were carried out by STATA, version 11 (Stata Corporation, College Station, TX, http://www.stata.com).
Results
Study Characteristics
Of 20 eligible articles in this meta-analysis, there were 11 for PTEN, 5 for PI3K, and 11 for pAkt. The characteristics of studies analyzed are shown in supplemental online Table 1. The number of participants ranged from 17 to 522, and, in total, 2,499 patients were identified. Study populations were of different races/ethnicities. The mean/median age in all studies was similar. The majority of patients had serous carcinoma; there were 2 studies focusing exclusively on patients with serous carcinoma [20, 21]. In addition, there were 4 studies focusing exclusively on patients with clear cell carcinoma [22–25]. Most studies used platinum-based chemotherapy, and there was no mention regarding chemotherapy regimens in the remaining 6 studies [10, 12, 20, 22, 26, 27]. The reported median/mean follow-up duration ranged from 22 to 72 months [12, 21, 23, 25, 26, 28–31]. All specimens were tumor tissues. Most studies used immunohistochemistry (IHC) analysis to determine biomarker expression status. Other methods used included immunocytochemistry and real-time quantitative polymerase chain reaction. There was only 1 prospective study [11], and the rest were all retrospective studies [10, 12, 20–36].
Publication Bias
The possibility of publication bias was investigated using a funnel plot. For all biomarkers, we did not find funnel plot asymmetry (Fig. 3). These results were verified by Egger’s test (Tables 1, 2).
Figure 3.
Funnel plot for publication bias showing the relationship between the effect size of individual studies and the precision of the study estimate for the PTEN/PI3K/Akt signaling pathway. (A): Hazard ratio for unadjusted overall survival. (B): Hazard ratio for adjusted overall survival. (C): Hazard ratio for unadjusted progression-free survival.
Abbreviations: PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homolog deleted on chromosome 10.
Table 1.
Meta-analysis estimates for overall survival

Table 2.
Meta-analysis estimates for progression-free survival

Meta-Analysis
The Relationship Between Biomarker Status and OS
PTEN
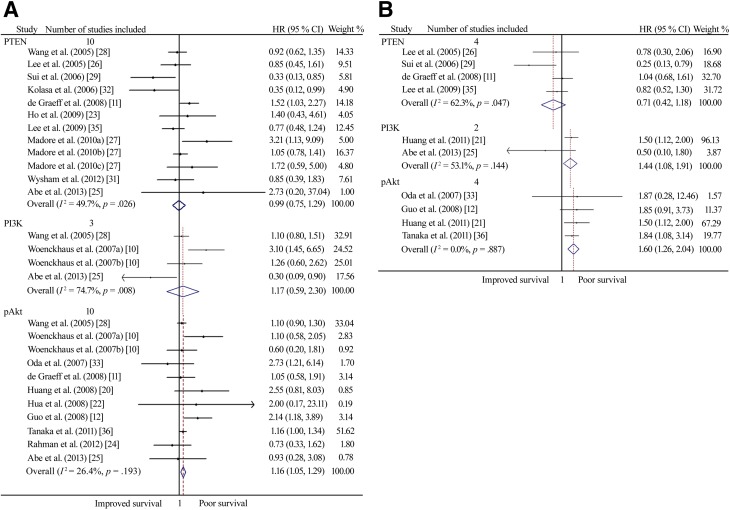
There were 10 studies that reported an association between PTEN expression and overall survival with univariate analysis, whereas with multivariate analysis, an association between its expression and OS was reported in 4 studies. Neither the meta-analysis of univariate nor of multivariate estimates provided evidence that its status had prognostic value for OS, and considerable heterogeneity existed (Fig. 4; Table 1). When restricted to studies using IHC staining with the 6H2.1 antibody (n = 2), patients with reduced PTEN expression were related to poor OS (pooled unadjusted HR = 1.50, 95% CI, 1.03–2.19). There was no heterogeneity between studies.
Figure 4.
A forest plot showing results of studies on the prognostic value of the PTEN/PI3K/Akt pathway expression. (A): Unadjusted hazard ratio estimates for OS in patients with these biomarker-positive tumors. (B): Adjusted hazard ratio estimates for OS in patients with these biomarker-positive tumors.
Abbreviations: HR, hazard ratio; OS, overall survival; PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homolog deleted on chromosome 10.
PI3K
Three studies evaluated an association between PI3K expression and OS with univariate analysis and two studies evaluated this association through multivariate analysis. Only the meta-analysis of multivariate estimates provided evidence that high PI3K expression was significantly related to poor OS in EOC patients (HR obtained from Mantel–Haenszel fixed-effects model: 1.44, 95% CI, 1.08–1.91), although there was moderate heterogeneity (I2 = 53.1%, p = .144).
pAkt
There were 10 studies that reported an association between pAkt expression and OS with univariate analysis and 4 studies with multivariate analysis. Both the meta-analyses of univariate and multivariate estimates showed that high pAkt expression was significantly associated with poor OS (pooled unadjusted HR = 1.16, 95% CI, 1.05–1.29; pooled adjusted HR = 1.60, 95% CI, 1.26–2.04), and there was no heterogeneity between studies.
The Relationship Between Biomarker Status and PFS
A meta-analysis of univariate estimates showed that only high pAkt expression was associated with PFS. In fact, patients with high pAkt were related to poor PFS (pooled HR = 1.24, 95% CI, 1.10–1.39; Fig. 5A; Table 2). There was no heterogeneity between studies.
Figure 5.
A forest plot showing results of studies on the prognostic value of the PTEN/PI3K/Akt pathway expression. (A): Unadjusted hazard ratio estimates for PFS in patients with these biomarker-positive tumors. (B): Adjusted hazard ratio estimates for PFS in patients with these biomarker-positive tumors.
Abbreviations: HR, hazard ratio; PFS, progression-free survival; PTEN, phosphatase and tensin homolog deleted on chromosome 10.
Five studies were assayed for PFS by multivariate analysis. The results of the meta-analysis showed that patients with high PI3K and pAkt expression were related to poor PFS (HR = 3.35, 95% CI, 1.14–9.82 for PI3K; pooled HR = 1.65, 95% CI, 1.07–2.55 for pAkt; Fig. 5B). There was no heterogeneity between studies. However, there was only one study that reported an association between PI3K expression and PFS with multivariate analysis [34].
Discussion
The PTEN/PI3K/Akt signaling pathway is frequently disrupted in many human cancers, and it is a key player in mediating tumor cell survival and escape from apoptosis [37–39]. The goal of this meta-analysis was to provide a comprehensive, reliable, and current summary of the prognostic and predictive value of the PTEN/PI3K/Akt pathway in EOC patients and to provide a reference for clinical practice. For each biomarker, we gathered data according to OS and PFS, including univariate and multivariate survival analyses.
Earlier studies have shown that PTEN is a tumor suppressor gene. Although deletion or mutation of PTEN has been reported in several types of human tumors, few studies have reported on the relation of PTEN protein level and survival in ovarian carcinomas [40, 41]. In the present study, neither the meta-analysis of univariate nor of multivariate estimates proved to be of significant prognostic value for OS or PFS in EOC patients, and considerable heterogeneity existed. In this meta-analysis, most studies used IHC staining to assess PTEN expression. Although IHC analysis is simple and cost-effective to perform, tremendous variation exists in the experimental procedures, which may influence the results and in part the observed heterogeneity. For example, when restricted to studies using IHC staining with the 6H2.1 antibody, patients with reduced PTEN expression were related to poor OS and PFS and the heterogeneity between studies was reduced.
Although deletion or mutation of PTEN has been reported in several different types of human tumors, few studies have reported on the relation of PTEN protein level and survival in ovarian carcinomas. In the present study, neither the meta-analysis of univariate nor of multivariate estimates proved to be of significant prognostic value for OS or PFS in EOC patients, and considerable heterogeneity existed.
The PI3K/Akt signaling pathway controls many cellular processes, such as the cell proliferation, apoptosis, and motility [42, 43]. A previous study [44] showed that activation scores for PI3K were associated with survival in advanced serous ovarian tumors, which is consistent with our data estimated by the multivariate survival analysis. But for univariate analysis, no significant correlation between PI3K protein expression and survival was found in EOC patients, and considerable heterogeneity existed. This analysis was affected by the small number of eligible studies, partly as a result of the imperfections in our search strategy. We restricted our meta-analysis to articles published in English and did not search the unpublished data, which would have likely increased the proportions of null views. In addition, there were several studies excluded based on the language restriction. The limitations in our search strategy may have resulted in publication or language bias and may have led to an overestimation of the effect sizes [14].
A previous study showed that activation scores for PI3K were associated with survival in advanced serous ovarian tumors, which is consistent with our data estimated by the multivariate survival analysis. But for univariate analysis, no significant correlation between PI3K protein expression and survival was found in EOC patients, and considerable heterogeneity existed.
Akt is a cytoplasmic serine-threonine protein kinase, whose activation stimulates cell cycle progression, survival, metabolism, and migration through phosphorylation of many physiological substrates [9, 45]. Previous studies of Akt in EOC have focused mainly on screening expression levels at the protein or mRNA level. Furthermore, the relation between pAkt status and clinical outcome of EOC patients has not been fully investigated. Although our results show that high pAkt expression was significantly associated with poor OS and PFS, the clinical application for this finding is not recommended because the statistical power of this analysis is limited by a small sample size and the type of studies (mainly retrospective).
In this meta-analysis, almost all studies used a single method to assess the status of biomarkers, which could have made the data imprecise. In addition, EOC is a heterogeneous tumor that can be classified according to the histology in high-grade serous, endometrioid, clear cell, mucinous, and low-grade serous tumors, and these variants are different in epidemiology, genetic risk factors, molecular events, premalignant lesions, patterns of spread, response to chemotherapy, and prognosis [46]. Unfortunately, there were very few studies included in the present study focusing exclusively on serous (n = 2) or clear cell carcinoma (n = 4) in which distinct biomarkers were evaluated, so that an analysis regarding a specific histological subtype was not feasible.
Because the current quality assessment scale was mainly designed for the randomized controlled studies and there is no suitable tool for examining prognostic and predictive biomarker studies, we did not assess the quality of the primary studies [47].
Conclusion
Although high pAkt expression is significantly related to poor OS and PFS in EOC patients, this marker alone is unlikely to be used as a predictor for prognosis in clinical practice. Studies using multiple methods to evaluate the expression and activity of Akt and more detailed subanalyses, especially according to histopathological types, would be helpful in validating the value of pAkt as a prognostic predictor in patients with EOC.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This study was supported by grants 81202062 and 81072134 from the National Natural Science Foundation of China. Jing Cai and Linjuan Xu contributed equally to this work.
Footnotes
For Further Reading: Daniela Fischerova, Michal Zikan, Pavel Dundr et al. Diagnosis, Treatment, and Follow-Up of Borderline Ovarian Tumors. The Oncologist 2012;17:1515–1533.
Abstract Borderline ovarian tumors represent a heterogeneous group of noninvasive tumors of uncertain malignant potential with characteristic histology. They occur in younger women, are present at an early stage, and have a favorable prognosis, but symptomatic recurrence and death may be found as long as 20 years after therapy in some patients. The molecular changes in borderline ovarian tumors indicate linkage of this disease to type I ovarian tumors (low-grade ovarian carcinomas). The pathological stage of disease and subclassification of extraovarian disease into invasive and noninvasive implants, together with the presence of postoperative macroscopic residual disease, appear to be the major predictor of recurrence and survival. However, it should be emphasized that the most important negative prognostic factor for recurrence is just the use of conservative surgery, but without any impact on patient survival because most recurrent diseases are of the borderline type—easily curable and with an excellent prognosis. Borderline tumors are difficult masses to correctly preoperatively diagnose using imaging methods because their macroscopic features may overlap with invasive and benign ovarian tumors. Over the past several decades, surgical therapy has shifted from a radical approach to more conservative treatment; however, oncologic safety must always be balanced. Follow-up is essential using routine ultrasound imaging, with special attention paid to the remaining ovary in conservatively treated patients. Current literature on this topic leads to a number of controversies that will be discussed thoroughly in this article, with the aim to provide recommendations for the clinical management of these patients.
Author Contributions
Conception/design: Zehua Wang, Jing Cai
Provision of study material or patients: Huijuan Tang, Xiaoqing Yi
Collection and/or assembly of data: Linjuan Xu, Huijuan Tang, Qiang Yang, Yan Fang
Data analysis and interpretation: Zehua Wang, Linjuan Xu, Ying Zhu
Manuscript writing: Jing Cai, Linjuan Xu
Final approval of manuscript: Zehua Wang
Disclosures
The authors indicated no financial relationships.
References
- 1.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 3.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 4.Crijns AP, Duiker EW, de Jong S, et al. Molecular prognostic markers in ovarian cancer: Toward patient-tailored therapy. Int J Gynecol Cancer. 2006;16(Suppl 1):152–165. doi: 10.1111/j.1525-1438.2006.00503.x. [DOI] [PubMed] [Google Scholar]
- 5.Stambolic V, Suzuki A, de la Pompa JL, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 6.Minaguchi T, Mori T, Kanamori Y, et al. Growth suppression of human ovarian cancer cells by adenovirus-mediated transfer of the PTEN gene. Cancer Res. 1999;59:6063–6067. [PubMed] [Google Scholar]
- 7.Weng LP, Smith WM, Dahia PL, et al. PTEN suppresses breast cancer cell growth by phosphatase activity-dependent G1 arrest followed by cell death. Cancer Res. 1999;59:5808–5814. [PubMed] [Google Scholar]
- 8.Choi Y, Zhang J, Murga C, et al. PTEN, but not SHIP and SHIP2, suppresses the PI3K/Akt pathway and induces growth inhibition and apoptosis of myeloma cells. Oncogene. 2002;21:5289–5300. doi: 10.1038/sj.onc.1205650. [DOI] [PubMed] [Google Scholar]
- 9.Bellacosa A, Chan TO, Ahmed NN, et al. Akt activation by growth factors is a multiple-step process: The role of the PH domain. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 10.Woenckhaus J, Steger K, Sturm K, et al. Prognostic value of PIK3CA and phosphorylated AKT expression in ovarian cancer. Virchows Arch. 2007;450:387–395. doi: 10.1007/s00428-006-0358-3. [DOI] [PubMed] [Google Scholar]
- 11.de Graeff P, Crijns APG, Ten Hoor KA, et al. The ErbB signalling pathway: Protein expression and prognostic value in epithelial ovarian cancer. Br J Cancer. 2008;99:341–349. doi: 10.1038/sj.bjc.6604471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo RX, Qiao YH, Zhou Y, et al. Increased staining for phosphorylated AKT and nuclear factor-kappaB p65 and their relationship with prognosis in epithelial ovarian cancer. Pathol Int. 2008;58:749–756. doi: 10.1111/j.1440-1827.2008.02306.x. [DOI] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne JAC, Egger M, Moher D. Addressing reporting biases. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons Ltd, 2008:297–334. [Google Scholar]
- 16.Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, Hua K, Zhou X, et al. Activation of the PI3K/AKT pathway mediates FSH-stimulated VEGF expression in ovarian serous cystadenocarcinoma. Cell Res. 2008;18:780–791. doi: 10.1038/cr.2008.70. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Zhang L, Greshock J, et al. Frequent genetic abnormalities of the PI3K/AKT pathway in primary ovarian cancer predict patient outcome. Genes Chromosomes Cancer. 2011;50:606–618. doi: 10.1002/gcc.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hua K, Feng W, Cao Q, et al. Estrogen and progestin regulate metastasis through the PI3K/AKT pathway in human ovarian cancer. Int J Oncol. 2008;33:959–967. [PubMed] [Google Scholar]
- 23.Ho CM, Lin MC, Huang SH, et al. PTEN promoter methylation and LOH of 10q22-23 locus in PTEN expression of ovarian clear cell adenocarcinomas. Gynecol Oncol. 2009;112:307–313. doi: 10.1016/j.ygyno.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 24.Rahman M, Nakayama K, Rahman MT, et al. Clinicopathologic and biological analysis of PIK3CA mutation in ovarian clear cell carcinoma. Hum Pathol. 2012;43:2197–2206. doi: 10.1016/j.humpath.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Abe A, Minaguchi T, Ochi H, et al. PIK3CA overexpression is a possible prognostic factor for favorable survival in ovarian clear cell carcinoma. Hum Pathol. 2013;44:199–207. doi: 10.1016/j.humpath.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Lee JS, Choi YD, Choi C, et al. Expression of PTEN in ovarian epithelial tumors and its relation to tumor behavior and growth. Anal Quant Cytol Histol. 2005;27:202–210. [PubMed] [Google Scholar]
- 27.Madore J, Ren F, Filali-Mouhim A, et al. Characterization of the molecular differences between ovarian endometrioid carcinoma and ovarian serous carcinoma. J Pathol. 2010;220:392–400. doi: 10.1002/path.2659. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Kristensen GB, Helland A, et al. Protein expression and prognostic value of genes in the erb-b signaling pathway in advanced ovarian carcinomas. Am J Clin Pathol. 2005;124:392–401. doi: 10.1309/BL7E-MW66-LQX6-GFRP. [DOI] [PubMed] [Google Scholar]
- 29.Sui L, Dong Y, Watanabe Y, et al. Alteration and clinical relevance of PTEN expression and its correlation with survivin expression in epithelial ovarian tumors. Oncol Rep. 2006;15:773–778. [PubMed] [Google Scholar]
- 30.Skírnisdóttir I, Seidal T. Prognostic impact of concomitant p53 and PTEN on outcome in early stage (FIGO I-II) epithelial ovarian cancer. Int J Gynecol Cancer. 2011;21:1024–1031. doi: 10.1097/IGC.0b013e31821dc906. [DOI] [PubMed] [Google Scholar]
- 31.Wysham WZ, Mhawech-Fauceglia P, Li H, et al. BRCAness profile of sporadic ovarian cancer predicts disease recurrence. PLoS One. 2012;7:e30042. doi: 10.1371/journal.pone.0030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolasa IK, Rembiszewska A, Janiec-Jankowska A, et al. PTEN mutation, expression and LOH at its locus in ovarian carcinomas. Relation to TP53, K-RAS and BRCA1 mutations. Gynecol Oncol. 2006;103:692–697. doi: 10.1016/j.ygyno.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Oda Y, Ohishi Y, Basaki Y, et al. Prognostic implications of the nuclear localization of Y-box-binding protein-1 and CXCR4 expression in ovarian cancer: Their correlation with activated Akt, LRP/MVP and P-glycoprotein expression. Cancer Sci. 2007;98:1020–1026. doi: 10.1111/j.1349-7006.2007.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolasa IK, Rembiszewska A, Felisiak A, et al. PIK3CA amplification associates with resistance to chemotherapy in ovarian cancer patients. Cancer Biol Ther. 2009;8:21–26. doi: 10.4161/cbt.8.1.7209. [DOI] [PubMed] [Google Scholar]
- 35.Lee YK, Park NH. Prognostic value and clinicopathological significance of p53 and PTEN in epithelial ovarian cancers. Gynecol Oncol. 2009;112:475–480. doi: 10.1016/j.ygyno.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka Y, Terai Y, Tanabe A, et al. Prognostic effect of epidermal growth factor receptor gene mutations and the aberrant phosphorylation of Akt and ERK in ovarian cancer. Cancer Biol Ther. 2011;11:50–57. doi: 10.4161/cbt.11.1.13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 38.Gutierrez A, Sanda T, Grebliunaite R, et al. High frequency of PTEN, PI3K, and AKT abnormalities in T-cell acute lymphoblastic leukemia. Blood. 2009;114:647–650. doi: 10.1182/blood-2009-02-206722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dahia PL. PTEN, a unique tumor suppressor gene. Endocr Relat Cancer. 2000;7:115–129. doi: 10.1677/erc.0.0070115. [DOI] [PubMed] [Google Scholar]
- 41.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 42.Fresno Vara JA, Casado E, de Castro J, et al. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 44.Trinh XB, Tjalma WA, Dirix LY, et al. Microarray-based oncogenic pathway profiling in advanced serous papillary ovarian carcinoma. PLoS One. 2011;6:e22469. doi: 10.1371/journal.pone.0022469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Prat J. Ovarian carcinomas: Five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012;460:237–249. doi: 10.1007/s00428-012-1203-5. [DOI] [PubMed] [Google Scholar]
- 47.Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427–437. doi: 10.7326/0003-4819-144-6-200603210-00010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.