Abstract
There is considerable interest in whether non-nutritive sweeteners are sensed in the gastrointestinal tract to modulate appetitive or absorptive responses to ingested carbohydrate. We determined the effect of a panel of non-nutritive sweeteners, aspartame, saccharin and acesulfame-K, delivered in doses that would be consumed in normal usage. Each was given in combination with glucose, assessing their effect on glycemic responses and appetite in ten healthy human subjects. There was no additional effect of aspartame or saccharin on the blood glucose response to oral glucose at any time point, although acesulfame-K exerted a small effect. However, none had an effect on perceptions of hunger or fullness. We conclude that there is no consistent evidence that non-nutrient sweeteners, when acutely consumed with glucose in dietetically relevant doses, have a class effect in modulating blood glucose in healthy human subjects. However, acesulfame-K may require further exploration.
Keywords: Non-nutritive sweeteners, glucose, glucose metabolism, appetite
Introduction
Non-nutritive sweetener (NNS) consumption has increased considerably (1). However, consumption of NNS in isolation is uncommon (except in diet beverages) due to the substantial variations in the diet. Following reports that NNS could enhance sweet receptor activation in the rodent gut concerns were raised about the synergistic effect of NNS and caloric sugars, potentially increasing small intestinal glucose absorption via upregulation and insertion of transporters (2, 3). There is also a lack of clarity about the effects of NNS on appetite (4) when given in combination with caloric sugars (5). What is completely unknown is whether they exert any effect in humans if ingested together at dietetically relevant doses. The aim of this pilot study was therefore to examine the effect of a panel of commonly used NNS on glycemic and appetitive responses, when given in combination with glucose but at commercial doses used in real life. We hypothesise that, if a caloric sugar and NNS have a synergistic effect and enhance glucose absorption large enough to be dietetically relevant, then the addition of a NNS to a glucose solution would increase blood glucose more than glucose alone.
Subjects and methods
Ten healthy fasted subjects (6 male, 4 female, mean age 21 (SD 2.4; range 18-24) years, mean BMI 21.8 (SD 1.8; range 19-24.7) kg/m2) were studied on four separate days, with at least 3 days in between each visit, receiving (randomised order), 1) 45 g glucose (Sigma-Aldrich), 2) 45g glucose and 150mg aspartame (Fuerst Day Lawson LTD, UK), 3) 45g glucose and 20 mg saccharin (Hermesetas, UK) or 4) 45g glucose and 85mg of ace-K (Fuerst Day Lawson LTD, UK) in 250ml water. Blood glucose (BG) was determined immediately (intravenous cannula) using HemoCue Glucose 201+ Analyser (HemoCue, Angelholm, Sweden) and ratings of hunger and fullness were measured by validated visual analogue scales (VAS) at baseline (~5 mins prior ingestion), immediately following ingestion (0 mins), and regular intervals thereafter up to 60 minutes (6). These studies were approved by the National Health Service North West Research Ethics Committee and written informed consent was obtained from all subjects.
Analyses used SPSS software (version 20.0; SPSS Inc). Results are presented as means ± SEM. BG and VAS data were analysed using repeated measures ANOVA with time and condition as factors. Where there were significant main effects, post-hoc analysis using Bonferonni correction for multiple comparisons were performed. BG data were also analysed using incremental area under the curve (iAUC) using the trapezoidal rule. Statistical significance was accepted at P<0.05.
Results
All subjects tolerated the study well. There was no difference in fasting BG between conditions. Following glucose alone, BG peaked at 15 minutes (7.6 ± 0.3 mmol/L). The mean BG also peaked identically 15 minutes after the consumption of the glucose + aspartame test drink (7.6 ± 0.3 mmol/L); greater after glucose + ace-k 8.3 ± 0.4 mmol/L. The mean BG concentrations peaked 30 minutes after the consumption of the glucose + saccharin test drink (7.9 ± 0.5 mmol/L). There was a main effect of time (F(7, 63) = 38.7 p = 0.0) and time × condition interaction (F(4.8, 43.6) = 2.7 p = 0.03) indicating BG differed over time between the four conditions (Figure 1). However post-hoc analysis revealed no differences between the glucose and NNS conditions at any time point. Analysis of iAUC demonstrated a main effect of condition (F(2.6, 23.4) =3.7 p= 0.03) but post-hoc analysis revealed no differences between the glucose and NNS conditions. Examining iAUC showed a 17.4% larger integrated glycemic response following ace-K compared to glucose alone (147.1 ± 15.9 and 125.3±16.2) but a smaller response (17.8%) following aspartame (102.9 ± 12.1). Hunger decreased and fullness increased (figure 2) following consumption of all four test drinks, with no main effect of NNS on perceptions of hunger and fullness (p =0.51; p =0.09).
Figure 1. Graph showing blood glucose responses following the ingestion of glucose alone or glucose and a non-nutrient sweetener (aspartame, saccharin, ace-K).
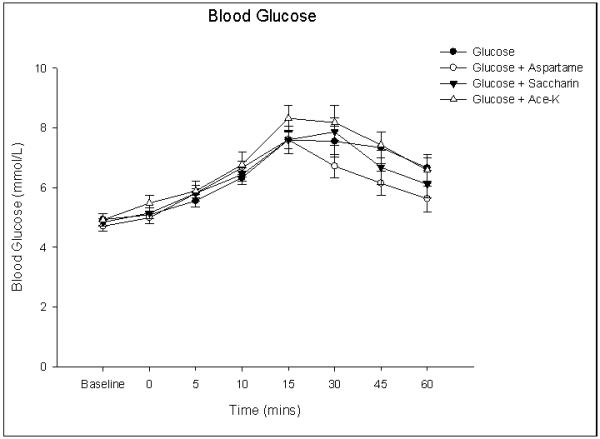
Values represent mean ± SEM (n=10).
Figure 2. Subjective appetite ratings for hunger and fullness following the ingestion of glucose or glucose and a non-nutrient sweetener (aspartame, saccharin, ace-K).
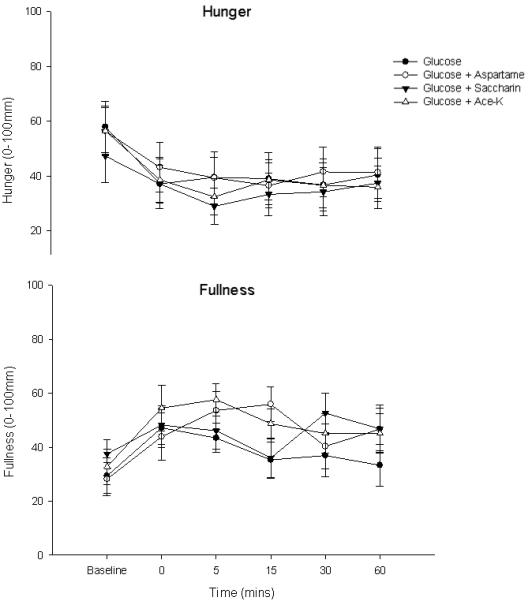
Values represent mean ± SEM (n=10).
Discussion
This pilot study is the first to directly evaluate the glycemic and appetitive responses to a panel of NNS ingested in combination with a caloric hexose sugar to determine whether this combination could exert a readily detectable synergistic effect in healthy humans.
Prior studies failed to support any metabolic activity following consumption of NNS alone (7, 8). However, concerns about synergistic effects of NNS and caloric sugars were raised following reports that NNS could activate enteroendocrine sweet taste receptors in the rat or mouse gut, increasing glucose absorption via upregulation and insertion of enterocyte transporters (2, 3). Hence, human diets, which increasingly involve the co-consumption of NNS with carbohydrates, might enhance postprandial glycaemia, arguably even more so in type 2 diabetics who already display glucose transporter overexpression (9).
The current study was designed to use doses of NNS that are used in commercial products. Aspartame, saccharin or ace-K in combination with glucose all had similar effects on BG and perceptions of hunger and fullness to glucose alone. No significant differences were present at any time point. There was however a small enhancement in BG iAUC following Ace-K which may merit further exploration. The significance of this small increase is unclear, and the response to Ace-K was more variable. Broadly speaking the data argue against a class effect of NNS when acutely consumed with glucose although a larger sample may be required to make a more definitive conclusion. These data are also consistent with reports that failed to show any effect of pre-supplementation with NNS on BG in humans (10), but we modelled the study differently since pre-consumption would generally not reflect real-life behaviour. Similarly Brown et al (5) found no change in BG following the consumption of a diet soda containing both sucralose and ace-K prior to a glucose load.
Although the study was arguably short at only 60 minutes, any dietetically meaningful effect would be expected to occur within this early phase. Similarly, a follow on study assessing biological measures such as gut peptides was not considered purposeful given the lack of an appetitive effect requiring mechanistic explanation.
Unlike cell line and animal models in which effects have been demonstrated, the concentrations of NNS used herein were pragmatically based on actual amounts used in representative commercial products that contain them (diet soft drinks, sweetener tablets) and, although it is not certain whether these concentrations are too high/low in terms of sweet receptor biology, the study was designed to be relevant to real life NNS doses. The addition of aspartame, saccharin and ace-k to glucose had no additional effect on appetite compared with glucose alone. Again the negative data cannot support substantive conclusions as large within and between subject variability can result in larger sample sizes, beyond the remit of this proof of concept study, being required to detect differences. However these data are consistent with the existing literature that NNS do not have any direct effects on appetite (11, 12). However, any potential effects on appetite warrant further investigation using a larger study sample and more robust methods to measure appetite and food intake both acutely and long term. In summary, there is no consistent evidence that NNS as a class affect the glycemic responses to ingested glucose in healthy humans. Additional studies are needed to elucidate the effect of NNS intake on glucose absorption and appetite, in particular ace-K may require separate attention as it may have different properties.
Acknowledgements
Supported by UK Biotechnology and Biological Research Council Diet and Health Research Industry Club (DRINC) grant BB/G005591/1) and PhD studentship (BB/G530076/1).
Footnotes
The authors have no conflicts of interest.
References
- 1.Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. European Journal of Clinical Nutrition. 2007;61:691–700. doi: 10.1038/sj.ejcn.1602649. [DOI] [PubMed] [Google Scholar]
- 2.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. Journal of Physiology. 2007;582:379–392. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattes RD, Popkin MB. Non-nutritive sweetener consumption in humans: effect on appetite and food intake and their putative mechanisms. American Journal of Clinical Nutrition. 2009;89:1–14. doi: 10.3945/ajcn.2008.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown RJ, Walter M, Rother KI. Ingestion of Diet Soda Before a Glucose Load Augments Glucagon-Like Peptide-1 Secretion. Diabetes Care. 2009;32:2184–2186. doi: 10.2337/dc09-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, et al. Appetite control: methodological aspects of the evaluation of foods. Obesity Reviews. 2010;11:251–270. doi: 10.1111/j.1467-789X.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma J, Bellon M, Wishart JM, Young R, Blackshaw LA, Jones KL, et al. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2009;296:G735–739. doi: 10.1152/ajpgi.90708.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinert RE, Frey F, Töpfer A, Drewe J, Beglinger C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. British Journal of Nutrition. 2011;105:1320–1328. doi: 10.1017/S000711451000512X. [DOI] [PubMed] [Google Scholar]
- 9.Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2002;282:G241–248. doi: 10.1152/ajpgi.00310.2001. [DOI] [PubMed] [Google Scholar]
- 10.Ma J, Chang J, Checklin HL, Young RL, Jones KL, Horowitz M, et al. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. British Journal of Nutrition. 2010;104:803–806. doi: 10.1017/S0007114510001327. [DOI] [PubMed] [Google Scholar]
- 11.Anton SD, Martin CK, Han H, Coulon S, Cefalu WT, Geiselman P. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite. 2010;55:37–43. doi: 10.1016/j.appet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blundell J, Green SM. Effect of sucrose and sweeteners on appetite and energy intake. International Journal of Obesity and Related Metabolic Disorder. 1996;20:S12–7. [PubMed] [Google Scholar]


