Abstract
Objectives
To determine the relation between height, FOXO3 genotype and age of death in humans.
Methods
Observational study of 8,003 American men of Japanese ancestry from the Honolulu Heart Program/Honolulu-Asia Aging Study (HHP/HAAS), a genetically and culturally homogeneous cohort followed for over 40 years. A Cox regression model with age as the time scale, stratified by year of birth, was used to estimate the effect of baseline height on mortality during follow-up. An analysis of height and longevity-associated variants of the key regulatory gene in the insulin/IGF-1 signaling (IIS) pathway, FOXO3, was performed in a HHP-HAAS subpopulation. A study of fasting insulin level and height was conducted in another HHP-HAAS subpopulation.
Results
A positive association was found between baseline height and all-cause mortality (RR = 1.007; 95% CI 1.003–1.011; P = 0.002) over the follow-up period. Adjustments for possible confounding variables reduced this association only slightly (RR = 1.006; 95% CI 1.002–1.010; P = 0.007). In addition, height was positively associated with all cancer mortality and mortality from cancer unrelated to smoking. A Cox regression model with time-dependent covariates showed that relative risk for baseline height on mortality increased as the population aged. Comparison of genotypes of a longevity-associated single nucleotide polymorphism in FOXO3 showed that the longevity allele was inversely associated with height. This finding was consistent with prior findings in model organisms of aging. Height was also positively associated with fasting blood insulin level, a risk factor for mortality. Regression analysis of fasting insulin level (mIU/L) on height (cm) adjusting for the age both data were collected yielded a regression coefficient of 0.26 (95% CI 0.10–0.42; P = 0.001).
Conclusion
Height in mid-life is positively associated with mortality, with shorter stature predicting longer lifespan. Height was, moreover, associated with fasting insulin level and the longevity genotype of FOXO3, consistent with a mechanistic role for the IIS pathway.
Introduction
Several studies have found an inverse association between height and mortality, especially for deaths arising from respiratory diseases, coronary heart disease (CHD) and stroke [1]–[8]. In contrast, other studies found a direct relationship between height and mortality [9]–[13], one caveat being those infants born underweight who undergo post-partum compensatory growth and have increased risk of cardiovascular disease [14], [15]. The higher caloric intake of taller people may predispose them to increased risk of cancer and thus premature death [2], [10], [16]. In support, a study of 1,915 healthy nonsmoking Japanese American men from the Honolulu Heart Program (HHP), followed for 36 years, found lower mortality in those whose energy consumption was 15% below average [17].
The sex difference in lifespan has been attributed in part to the average shorter stature of women [18]. Reduced insulin/insulin-like growth factor-1 (IGF-1) signaling is associated with increased lifespan and shorter height in women [11]. These associations were most pronounced for genetic variants of the human growth hormone (GH1), IGF1 and insulin receptor substrate-1 (IRS1) genes [11].
Studies to date suffer from confounding factors such as population stratification artifact and from racial, ethnic, dietary, socio-economic and other confounders, as well as inadequate length of follow-up. These may be contributing to the lack of consensus about whether height affects lifespan positively or negatively. In the present study, we examined this question using data from the HHP/Honolulu-Asia Aging Study (HAAS), a population-based study of genetically homogeneous Japanese-American men, who have been followed-up for more than 40 years. Given that smaller body size is a phenotype that is strongly associated with increased lifespan in model organisms of aging, and may be mediated through energy-sensing pathways, particularly the insulin/IGF-1 (IIS) signaling pathway, we hypothesized that shorter height in humans would be associated with increased longevity and genotype of FOXO3, a key IIS regulatory gene associated with human longevity [19] and reduced insulin signaling. The present study therefore assessed whether height was associated with longevity, FOXO3 genotype and fasting insulin level, a marker of insulin sensitivity.
Materials and Methods
Study population
The study utilized the HHP/HAAS dataset. The HHP is a population-based, prospective cohort comprising 8,006 Japanese-American men who were born between 1900 and 1919 and recruited to study cardiovascular disease. All were residents of the island of Oahu, Hawaii, at the time of study enrolment (1965–1968), and were aged 45 to 68 years (mean 54 years). Approximately 12% were born in Japan and the rest were mainly second generation Japanese-Americans. Approximately 12% of the latter went to live in Japan as children at an average age of 5 years. Most of this subset continued to reside in Japan until age 17–25 years before returning to Oahu. Recruitment, design, subjects and procedures have been described elsewhere [20]–[23]. The HAAS was developed from within the HHP in 1991 for the purpose of studying dementia and conditions related to aging [24]. The HHP/HAAS has a surveillance system to continuously track morbidity from major cardiovascular diseases and to determine the mortality status of participants. Information on incident CHD and stroke, as well as mortality from all causes, were obtained through monitoring obituaries in local newspapers (in both English and Japanese), surveillance of hospital discharge records and state health department records of death certificates. A follow-up survey in the 1991–1993 examination identified mortality information for all but 5 men [25]. The original complete HHP cohort has been re-examined 11 times up until 2012. As of July 2008, 7,063 subjects had a death date recorded in our surveillance system and most of the remaining 943 participants were still alive, with age ranging from 88 to 106 years.
Data collection: measurement of height and other parameters
HHP participants received a baseline physical examination when recruited in 1965–1968. Standing height (was measured in inches, and then converted into cm in the present analysis) and other demographic and epidemiological variables were recorded. The latter included weight, blood pressure (BP), smoking status, blood sample random serum glucose and uric acid, medical history, alcohol consumption and dietary nutrition intake based on a questionnaire of 24 hour dietary recall, amongst other variables. The procedures used, together with a document for participants explaining the research project and a consent form, were each approved in writing by the Kuakini Medical Center institutional ethical review committee. The study participants read the explanation and then signed the consent form [22].
In the HHP follow-up Exam 4 in 1991–1993 (also known as HAAS Exam 1), 3,741 of the original cohort participated. This reduced number was a consequence of over half of the original cohort having died. The parameters above were re-recorded. Fasting insulin was measured in 3,458 of these. In this examination, standing height was measured (in cm) [23].
Subjects for FOXO3 genetic association study
Later (in 2007), a subset of 614 subjects who participated in Exam 4 were chosen for a genetic association study of single nucleotide polymorphisms (SNPs) of FOXO3 and longevity, as reported previously [19]. We used genotype data for 587 of those subjects for whom we had height data and examined the relationship between the FOXO3 SNP (rs 2802292) most strongly associated with longevity in our prior study [19] and height.
Data analysis
Cox’s proportional hazard model was used to analyze the survival data [26] in order to investigate the relation between baseline (mid-life) height and mortality during the follow-up period. For men without mortality information, either because they were still alive or had been lost to follow-up, their age (as censored) at latest contact was used in the model. Due to the rapid change of human mortality rate from middle to later life, as well as a large age range in the study participants, age was used as the time scale in the analysis. We were aware of the possibility of a cohort effect on height due to nutritional status pre-partum and during pediatric development. Therefore, stratification by year of birth was used in the Cox model, which assumes each stratum has its own baseline risk function (or risk curve) and the relative risk (RR) for each of the experimental variables is the same across strata. More specifically, a Cox regression model with age as the time scale and stratified by year of birth was used in all survival analyses for this study.
We also investigated the potential confounding effects of other risk factors on the relation between longevity and baseline height. For alcohol usage, non-drinkers and heavy drinkers (>480 g/month) were compared with moderate drinkers (≤480 g/month). Heavy smokers (>48 pack-year) and moderate smokers (≤48 pack-year) were compared to non-smokers. For other continuous variables, including systolic and diastolic blood pressure, random serum glucose and serum uric acid, quartiles were used in the analysis, and the high quartile and the middle two quartiles were compared to the low quartile. In our analysis, body mass index (BMI) showed a U-shaped relation to mortality. Therefore the comparisons used here are the low quartile vs. the middle two quartiles, and the high quartile vs. the middle two quartiles. Because our analysis showed that the group of participants who had lived in Japan in their early life (either born in Japan, or born in Hawaii and sent back to Japan for a period when they were young) had an advantage in survival compared to those who had never lived in Japan when young, an indicator variable was created for this subgroup of participants, and was included in our analysis as a potential confounder.
Subsequently, we analyzed all possible confounding variables individually in separate models with baseline height and baseline age. Then all variables that showed a significant association with all-cause mortality in the models with baseline height and baseline age only, were adjusted in the final model, to assess the association between height and mortality.
FOXO3 genotype and fasting insulin are two important variables related to mortality and both are related to insulin signaling. Longevity-associated FOXO3 genotypes are associated with reduced insulin-signaling, as are lower fasting insulin levels [19]. Therefore, we examined (i) the relation between height and FOXO3 genotype in the case-control study subset described above and (ii) the relation between fasting insulin level and height, using data from HHP Exam 4. Pearson’s X2 test was used for contingency tables analysis and ANOVA was used for analysis of the association of height with genotype.
To assess the relative risk of baseline height in different stages of life, a Cox regression model with time-dependent variables was used to examine the change in relative risk of baseline height on mortality at different life stages. First, index variables were created for different life stages (age ≤70, 71–80, 81–85 and >95 years). These variables were ascribed a value of 1 in each of the corresponding life stage, and 0 outside that life stage. To estimate the relative risk of height at each of these life stages, the products of baseline height and each of the index variables were entered into the Cox regression model together with confounding factors. In this manner, the estimated relative risk of height for each of the life stages could be obtained in one model and the relation between the relative risks of baseline height between different life stages could be assessed. (See Appendix S1 for more detailed information about the models used to compare the relative risks between baseline height at different life stages and mortality.)
Results
The HHP study began in 1965 and initially identified 12,261 Japanese-American men born between 1900 and 1919 and having a residential address on Oahu. Of these, 8,006 participated and were followed for more than 40 years or until death. Since height data at baseline were not available for 3 subjects, only 8,003 were used in the present analysis.
The baseline characteristics of the study population are presented in Table 1. Baseline height by age group is presented in Table 2. Individuals in the oldest age group (65–68 years old) were on average 4.6 cm shorter than those in the youngest age group (45–49 years old). We compared survival curves between participants who were 165 cm or taller in height, those who were 158 cm or shorter, and those whose height was between 158 cm and 165 cm. We found no significant difference between the groups for survival prior to the age of 80 years. Survival curves in the follow-up for these three groups of people differed significantly between age 80 and 95 years (Wilcoxon test for difference: X2 2 d.f. = 17.9; P = 0.0001; Figure 1).
Table 1. Distribution of demographic and epidemiological variables at baseline.
| Variable name | n | Mean ± SD | Range | Low quartile | High quartile |
| Age at baseline (years) | 8006 | 54.9±5.6 | 45.8–68.5 | 50.2 | 59.2 |
| Height at baseline (cm) | 8003 | 162.8±5.8 | 140–188 | 160 | 168 |
| Systolic BP (mmHg) | 8006 | 134.1±21.0 | 79.6–264 | 119.3 | 146.0 |
| Diastolic BP (mmHg) | 8006 | 82.2±11.6 | 40.0–172 | 74.7 | 89.3 |
| Body mass index (kg/m2) | 8001 | 23.8±3.1 | 14.3–44.6 | 21.7 | 25.8 |
| Random serum glucose (mg/dl) | 7977 | 162±58 | 40–671 | 122 | 189 |
| Serum uric acid (mg/dL) | 7971 | 59.9±15.1 | 7–148 | 50 | 69 |
| Alcohol use (g/month)* | 7988 | 388±691 | 0–10320 | 0 | 482 |
| Smoking (pack-year)† | 7918 | 31.6±29.8 | 0–192 | 0 | 48 |
| Major disease at baseline§ | 8006 | 1501 (18.7%) with major disease | |||
| Married at baseline | 8000 | 7103 (88.8%) married at baseline | |||
| Lived in Japan when young¶ | 8006 | 2288 (28.6%) had lived in Japan |
* 2,985 (37.4%) of subjects were non-drinkers.
2,242 (28.3%) had never smoked.
CHD, CVD, cancer, diabetes, liver, renal disease, lung disease or other chronic diseases at baseline.
Subjects either born in Japan or born in Oahu and then sent back to Japan, where they lived during early life.
Table 2. Height by age group at baseline (Honolulu Heart Program Exam 1).
| Age at Exam 1 | n | Height (cm) * | Range |
| 46–49 | 1524 | 164±5.6 | 142–188 |
| 50–54 | 2897 | 164±5.6 | 145–183 |
| 55–59 | 1688 | 163±5.8 | 140–183 |
| 60–64 | 1348 | 161±5.8 | 145–180 |
| 65–68 | 546 | 160±5.3 | 142–175 |
*Mean ± SD. Height was measured in inches and converted to cm before analysis.
Figure 1. Survival curves for groups differing in baseline height between age 80 and 95 years.
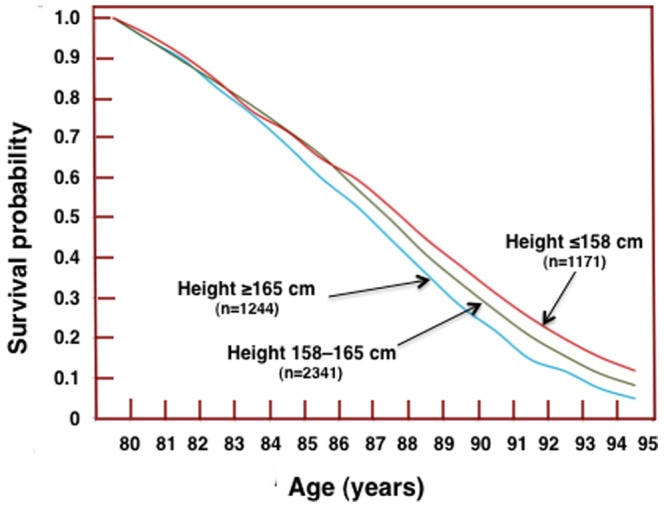
Wilcoxon test for difference between the three groups: P = 0.0001.
Using a Cox regression model with age as the time scale, stratified by birth year and adjusting for baseline age only, the RR for mortality associated with every 1 cm increase in baseline height was 1.007 (95% CI 1.003–1.011; P = 0.002). When additional confounding risk factors for mortality at baseline (BMI, existing major chronic conditions such as CHD, stroke, cancer, diabetes, liver and renal diseases, chronic obstructive lung diseases and other chronic diseases at baseline, systolic blood pressure, alcohol use, smoking status, serum glucose, cholesterol and uric acid, marital status at baseline and ever lived in Japan) were included in the model, the RR of baseline height for mortality decreased slightly to 1.006 for every 1 cm increase in baseline height (95% CI 1.002–1.010; P = 0.007). Table 3 shows results obtained for the final multivariate model.
Table 3. Results of multivariate Cox’s proportional hazard model for mortality.
| Variable name | Comparison | Relative risk (95% CI) | P |
| Age at baseline* | 0.973 (0.947–0.999) | 0.042 | |
| Height (per cm increase) | 1.006 (1.002–1.010) | 0.007 | |
| Major disease at baseline* | Yes vs. no | 1.311 (1.234–1.393) | <0.001 |
| Systolic blood pressure | High vs. low | 1.426 (1.328–1.531) | <0.001 |
| Medium vs. low | 1.112 (1.046–1.181) | <0.001 | |
| Random serum glucose | High vs. low | 1.272 (1.188–1.362) | <0.001 |
| Medium vs. low | 1.034 (0.975–1.097) | 0.27 | |
| Serum uric acid | High vs. low | 1.209 (1.1266–1.297) | <0.001 |
| Medium vs. low | 1.055 (0.995–1.118) | 0.07 | |
| Alcohol use | None vs. moderate | 1.110 (1.050–1.174) | <0.001 |
| Heavy vs. moderate | 1.126 (1.057–1.199) | <0.001 | |
| Married at Exam 1 | Yes vs. no | 0.856 (0.795–0.922) | <0.001 |
| Smoking | Moderate. vs. none | 1.309 (1.234–1.388) | <0.001 |
| Heavy vs. none | 1.640 (1.534–1.754) | <0.001 | |
| BMI at baseline | Low vs. median | 1.126 (1.061–1.195) | <0.001 |
| High vs. median | 1.062 (1.001–1.126) | 0.047 | |
| Lived in Japan when young | Yes vs. No | 0.919 (0.868–0.972) | 0.003 |
*For units of each measurement see Table 1.
Cause-specific mortality data for the HHP/HAAS cohort until the end of 1999 were available and were used to examine the relation between height and cause-specific mortality (Table 4). Cancer mortality, especially non-smoking related cancer, was significantly associated with greater baseline height, while smoking-related cancer was not. CHD and respiratory disease mortality were not significantly associated with baseline height.
Table 4. Relative risk for height (per cm increase) for various conditions using data up to the end of 1999.
| Type of mortality | No. of events | Age adjusted | RF adjusted for age ** | ||||
| RR | 95% CI | P value | RR | 95% CI | P | ||
| Total mortality | 5154 | 1.006 | 1.002–1.011 | 0.010 | 1.005 | 1.000–1.010 | 0.076 |
| All cancer | 1584 | 1.013 | 1.004–1.022 | 0.004 | 1.010 | 1.001–1.019 | 0.029 |
| • smoking related* | 632 | 1.008 | 0.995–1.022 | 0.238 | 1.002 | 0.988–1.017 | 0.74 |
| • not smoking related | 952 | 1.016 | 1.005–1.028 | 0.006 | 1.015 | 1.003–1.027 | 0.012 |
| • stomach (ICD 151) | 249 | 1.009 | 0.987–1.031 | 0.434 | 1.010 | 0.987–1.033 | 0.41 |
| • colorectal (ICD 153, 154) | 209 | 1.005 | 0.981–1.029 | 0.687 | 1.003 | 0.978–1.028 | 0.82 |
| • prostate (ICD 185) | 170 | 1.025 | 0.998–1.053 | 0.072 | 1.023 | 0.995–1.051 | 0.10 |
| • hematopoietic (ICD 200–8) | 120 | 1.038 | 1.006–1.072 | 0.019 | 1.041 | 1.008–1.075 | 0.015 |
| CHD | 757 | 1.007 | 0.994–1.020 | 0.291 | 1.003 | 0.991–1.017 | 0.60 |
| Respiratory disease | 389 | 0.999 | 0.981–1.016 | 0.877 | 1.000 | 0.982–1.018 | 0.99 |
| Stroke | 493 | 0.994 | 0.979–1.010 | 0.484 | 0.994 | 0.978–1.010 | 0.44 |
*Smoking-related cancer (showing ICD-9 code number in brackets): lip (140), tongue (141), mouth and pharynx (143–149), esophagus (150), pancreas (157), respiratory tract (160–163) and urinary tract (188–189).
**The following risk factors (RF) were adjusted for both baseline risk and age: age at baseline, major disease at baseline, systolic blood pressure, random serum glucose, serum uric acid, alcohol use, smoking, marital status, body mass index, and lived in Japan when young.
We used a simple regression model to examine the relation between fasting insulin level and height at HHP Exam 4 with fasting insulin level as the dependent variable (n = 3,458 subjects), adjusting for age at Exam 4. This yielded a regression coefficient of 0.26 (95% CI 0.10–0.42; P = 0.001), demonstrating that every 1 cm increase in height corresponded to an increase of 0.26 mIU/L in fasting insulin level.
An analysis of the HHP/HAAS subpopulation described above divided according to median height (161.4 cm) into shorter and taller categories, demonstrated higher prevalence of the T allele of the FOXO3 rs2802292 SNP in the taller subgroup (Table 5A). A phenotype-genotype analysis showed, moreover, that taller baseline height was associated with the T allele, TT subjects being 1.4 cm taller than GG/GT subjects (Table 5B).
Table 5. Comparison of FOXO3 rs2802292 genotype with height at Exam 4.
| A. Category vs. genotype | |||||||||
| GG | GT | TT | X2 | P | GG/GT | TT | X2 | P | |
| >161.4 cm* | 26 | 108 | 159 | 134 | 159 | ||||
| <161.4 cm† | 25 | 138 | 131 | 6.4 | 0.041 | 163 | 131 | 5.5 | 0.020 |
The subpopulation chosen for this analysis was from participants whose blood sample for genotyping was collected at HHP/HAAS follow-up Exam 4 (in 1991–1993). *The taller subgroup in the contingency table analysis comprised subjects whose height was greater than median height of all subjects (161.4 cm).
The shorter subgroup comprised subjects whose height was equal to or less than median height of all subjects.
P shown was result obtained from 1-way ANOVA.
A Cox regression model with time-dependent variables (as explained in the data analysis section of Methods) demonstrated an increase in relative risk of mortality for baseline height as the population aged (Table 6). Prior to age 70 years, relative risk of mortality for each cm increase in baseline height was 0.998, and increased to 1.007, 1.010 and 1.014 for age categories 71–80, 81–95, and >96 years, respectively (P = 0.16, 0.088 and 0.016, respectively), compared to RR prior to age 70 years.
Table 6. Comparison of RR of mortality for each cm increase in baseline height for different life stages estimated by a Cox regression model.
| Life stage (years) | ≤70 | 71–80 | 81–95 | >96 |
| RR for baseline height on mortality | 0.998 | 1.007 | 1.010 | 1.014 |
| P value compared to those aged ≤70 | N/A | 0.157 | 0.088 | 0.016 |
RR is shown for each specific life stage. N/A, not applicable.
Discussion
The present prospective study spanning more than 40 years demonstrates that height is positively associated with increased mortality. Taller study participants not only were at increased risk of death but also tended to have higher fasting insulin levels and lower frequency of the longevity-associated FOXO3 genotypes GG or GT of the rs2802292 SNP. This is among the largest and most detailed of studies to date to have examined this issue and the first to examine the relation of FOXO3 genotype to human body size, a phenotype of longevity in model organisms.
The relationship between mortality and adult height in humans is complicated. Mortality is related to socio-economic status early and later in life, and other factors such as quality of health care, accessibility to health care facilities and the healthy lifestyle choices of individuals. It is also related to various known and unknown biological processes. Height is both influenced by familial factors and is an indicator of socio-economic status and nutrition during childhood. Currently, the common wisdom in epidemiology is that height in humans in developed nations is a proxy for socio-economic status during early childhood (birth through age 5 years in particular) [27], [28]. Thus taller people are taller due to better medical care and nutrition during early childhood. Therefore, one might expect taller persons to have lower mortality rates.
In support of this hypothesis, several studies have found an inverse association between height and mortality, with the lowest mortality in the tallest individuals within various age groups [1]–[8]. Certain features of these studies are, however, of concern. The age range of participants at baseline was large, e.g., 40–64 years in some [1]–[3] and even greater in an another, consistent with possible cohort bias [4]. In many of the studies a large proportion of participants were in their forties, fifties and sixties at establishment of the cohort, but relatively few studies had large cohorts that were followed to very old age or extinction and still fewer had large numbers of study participants who lived to older age groups (i.e., octogenarians, nonagenarians and centenarians) where we detected a height-mortality effect. Another drawback of these studies was that many used follow-up time, not age, as the time scale in Cox regression models used for their analysis. It is well known that human height tends to decrease in the elderly. In general, all else being equal, we found that older persons at the baseline examination (aged in their 60 s) were shorter than younger persons (aged in their 40 s; see Table 2). The rate of all-cause mortality and CHD mortality both increase substantially in mid to later life. According to the Centers for Disease Control and Prevention mortality data for the US population from 1968 to 1998, the annual death rate of males in America males increases from <1% at ages 45–54 to ≥6% for ages 75–84 years. If follow-up time is used as the time scale, the combination of shorter height in older men and higher mortality in the older age group artificially biases results towards an inverse association between height and mortality. In fact, use of follow-up time as the time scale will not only overestimate the inverse association between height and mortality should an inverse association exist, but may produce an inverse association when this does not exist. This phenomenon could explain why the height-mortality association declined with length of follow-up in the Whitehall Study [3]. The authors of that study found that by 15 years of follow-up, the only appreciable effect of height was on mortality from respiratory disease. As follow-up time increased, the artificial effect produced by the incorrect time-scale decreased. Therefore, to avoid bias, when a Cox proportional hazard regression model is used for evaluation of human mortality, age, instead of follow-up time, should be used as the time scale [29], [30].
Interestingly, in earlier studies of the current cohort of aging men, we noted that taller men had lower prevalence of poor cognitive performance and Alzheimer’s disease suggesting that better nutrition and other favorable early life conditions might provide some later-life health advantages [31]. Stronger grip strength, another phenotype influenced by optimal early life nutrition, also predicts longevity in the HHP cohort and other populations [32]–[34]. Nevertheless, despite the influence of what may have been a healthier start in life, by late life, on average, shorter men outlived their taller counterparts.
Clinically, one might speculate that the slightly reduced overall lifespan of taller individuals on average could be an influence of the well-known increased prevalence of metabolic syndrome and cardiovascular disease in those born small for gestational age who undergo catch-up growth postpartum [14], [15]. Another influence could be from diets high in protein, particularly branched-chain amino acids in meat, that although helping increase linear growth early in life, are associated with reduced lifespan [35], [36]. High protein diets increased risk of cancer and premature death [2], [10], [16], [36]. We found that amongst nonsmoking Japanese American men from the HHP, those whose energy consumption was 15% below average lived longer [17].
The biological reason for an association of shorter stature in humans with longevity is not fully understood. Indeed, the developmental regulation of scaling of body size has been a key question in evolutionary biology for almost a century [37]–[39]. Interestingly, a recent study of Drosophila identified FOXO as an important genetic regulator of morphological scaling [40]. FOXO is a key regulatory gene in the IIS pathway, a nutrient- and energy-sensing biological pathway that is evolutionarily conserved from yeast to humans, and impacts model organism and human longevity [19]. According to this prior work, since nutrition is a primary regulator of body size in animals, and this effect appears to be regulated by FOXO, the evolutionary conservation of the insulin-signaling pathway suggests that FOXO may be a proximate evolutionary target of selection for animal morphological scaling. One nutritional cue for scaling for smaller body size could be a lack of calories. This may be a highly conserved stress response that evolved early in life’s history to increase an organism’s chance of surviving adversity [41]. The biological response appears to be altered FOXO expression during development, with resultant phenotypic changes including increased insulin sensitivity, smaller body size and longer lifespan [42], [43].
The present study supports this work, finding that the longevity-associated G allele of the FOXO3 SNP rs2802292 (genotypes GG and GT) [19] is associated with shorter stature. Since FOXO3 is a transcription factor involved in insulin/IGF-1 signaling and the longevity/short stature-associated G allele is associated with lower fasting insulin [19], a possible biological explanation is apparent.
Using the Cox regression model with age as the time scale, stratified by year of birth, effectively reduced bias, and allowed the true relation (estimated RR) of the effect of baseline height on mortality to become apparent. Since our study utilized a genetically homogenous population American men of Japanese ancestry [19], possible confounding from cultural differences were substantially ameliorated, thus reducing bias and strengthening the reliability of the findings.
Conclusions
The present longitudinal study involving a relatively homogenous population followed for more than 40 years has found height to be positively associated with mortality. FOXO3 genotype might be a contributing factor to this association. Replication is required in other settings in order to determine whether the present findings can be generalized to other racial groups and populations.
Supporting Information
Use of time-dependent covariates in Cox regression model to estimate relative risks of baseline height for different life stages (age ≤70, 71–80, 81–95, and >95 years).
(DOCX)
Funding Statement
This study was supported by contracts NO1-HC-05102 (Honolulu Heart Program) from the National Heart, Lung, and Blood Institute and NO1-AG-4-2149 (Honolulu-Asia Aging Study) from the National Institute on Aging (NIA); grants 2U01AG019349, 1R01AG038707, 2R01AG027060 from the NIA; and support from the Office for Research and Development, Department of Veterans Affairs. The views in this article do not necessarily reflect those of the federal government of the United States of America. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Marmot MG, Shipley MJ, Rose G (1984) Inequalities in death—specific explanations of a general pattern? Lancet i (8384) 1003–1006. [DOI] [PubMed] [Google Scholar]
- 2. Davey Smith G, Hart C, Upton M, Hole D, Gillis C, et al. (2000) Height and risk of death among men and women: aetiological implications of associations with cardiorespiratory disease and cancer mortality. J Epidemiol Community Health 54: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leon DA, Smith GD, Shipley M, Strachan D (1995) Adult height and mortality in London: early life, socioeconomic confounding, or shrinkage? J Epidemiol Community Health 49: 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peck AM, Vågerö DH (1989) Adult body height, self perceived health and mortality in the Swedish population. J Epidemiol Community Health 43: 380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allebeck P, Bergh C (1992) Height, body mass index and mortality: do social factors explain the association? Public Health 106: 375–382. [DOI] [PubMed] [Google Scholar]
- 6. Crimmins EM, Finch CE (2006) Infection, inflammation, height, and longevity. Proc Natl Acad Sci USA 103: 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kemkes-Grottenthaler A (2005) The short die young: the interrelationship between stature and longevity-evidence from skeletal remains. Am J Phys Anthropol 28: 340–347. [DOI] [PubMed] [Google Scholar]
- 8. Mizushima S, Yamori Y (1992) Nutritional improvement, cardiovascular diseases and longevity in Japan. Nutr Health 8: 97–105. [DOI] [PubMed] [Google Scholar]
- 9. Holzenberger M, Martín-Crespo RM, Vicent D, Ruiz-Torres A (1991) Decelerated growth and longevity in men. Arch Gerontol Geriatr 13: 89–101. [DOI] [PubMed] [Google Scholar]
- 10. Hebert PR, Ajani U, Cook NR, Lee IM, Chan KS, et al. (1997) Adult height and incidence of cancer in male physicians (United States). Cancer Causes Control 8: 591–597. [DOI] [PubMed] [Google Scholar]
- 11. van Heemst D, Beekman M, Mooijaart SP, Heijmans BT, Brandt BW, et al. (2005) Reduced insulin/IGF-1 signalling and human longevity. Aging Cell 4: 79–85. [DOI] [PubMed] [Google Scholar]
- 12. Salaris L, Poulain M, Samaras TT (2012) Height and survival at older ages among men born in an inland village in Sardinia (Italy), 1866–2006. Biodemography Soc Biol 58: 1–13. [DOI] [PubMed] [Google Scholar]
- 13. Samaras TT, Storms LH (1992) Impact of height and weight on life span. Bull World Health Organ 70: 259–267. [PMC free article] [PubMed] [Google Scholar]
- 14. Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME (1989) Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. Brit Med J 298: 564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barker DJ, Kajantie E, Osmond C, Thornburg KL, Eriksson JG (2011) How boys grow determines how long they live. Am J Hum Biol 23: 412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sue LY, Schairer C, Ma X, Williams C, Chang SC, et al. (2009) Energy intake and risk of postmenopausal breast cancer: an expanded analysis in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) cohort. Cancer Epidemiol Biomarkers Prev 18: 2842–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Willcox BJ, Yano K, Chen R, Willcox DC, Rodriguez BL, et al. (2004) How much should we eat? The association between energy intake and mortality in a 36-year follow-up study of Japanese-American men. J Gerontol A Biol Sci Med Sci 59: 789–795. [DOI] [PubMed] [Google Scholar]
- 18. Samaras TT, Elrick H, Storms LH (2003) Is height related to longevity? Life Sci 72: 1781–1802. [DOI] [PubMed] [Google Scholar]
- 19. Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, et al. (2008) FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci USA 105: 13987–13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Worth RM, Kagan A (1970) Ascertainment of men of Japanese ancestry in Hawaii through World War II Selective Service registration. J Chronic Dis 23: 389–397. [DOI] [PubMed] [Google Scholar]
- 21. Kagan A, Harris BR, Winkelstein W Jr, Johnson KG, Kato H, et al. (1974) Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: demographic, physical, dietary and biochemical characteristics. J Chronic Dis 27: 345–364. [DOI] [PubMed] [Google Scholar]
- 22.Kagan A, Ed. (1996) The Honolulu Heart Program: An Epidemiological Study of Coronary Heart Disease and Stroke. Harwood Academic Publishers, Amsterdam, The Netherlands. [Google Scholar]
- 23. Burchfiel CM, Curb JD, Arakaki R, Abbott RD, Sharp DS, et al. (1996) Cardiovascular risk factors and hyperinsulinemia in elderly men: the Honolulu Heart Program. Ann Epidemiol 6: 490–497. [DOI] [PubMed] [Google Scholar]
- 24. White L, Petrovitch H, Ross GW, Masaki KH, Abbott RD, et al. (1996) Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. JAMA 276: 955–960. [PubMed] [Google Scholar]
- 25. Reed DM, Foley DJ, White LR, Heimovitz H, Burchfiel CM, et al. (1998) Predictors of healthy aging in men with high life expectancies. Am J Public Health 88: 1463–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cox DR (1972) Regression models and life-tables. J Roy Statist Soc 34 (series B) 187–220. [Google Scholar]
- 27. Singh-Manoux A, Gourmelen J, Ferrie J, Silventoinen K, Guéguen A, et al. (2010) Trends in the association between height and socioeconomic indicators in France, 1970–2003. Econ Hum Biol 8: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kerr GR, Lee ES, Lorimor RJ, Mueller WH, Lam MM (1982) Height distributions of U.S. children: associations with race, poverty status and parental size. Growth 46: 135–149. [PubMed] [Google Scholar]
- 29. Thiébaut AC, Bénichou J (2004) Choice of time-scale in Cox's model analysis of epidemiologic cohort data: a simulation study. Stat Med 23: 3803–3820. [DOI] [PubMed] [Google Scholar]
- 30. Korn EL, Graubard BI, Midthune D (1997) Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol 145: 72–80. [DOI] [PubMed] [Google Scholar]
- 31. Abbott RD, White LR, Ross GW, Petrovitch H, Masaki KH, et al. (1998) Height as a marker of childhood development and late-life cognitive function: the Honolulu-Asia Aging Study. Pediatrics 102: 602–609. [DOI] [PubMed] [Google Scholar]
- 32. Rantanen T, Harris T, Leveille SG, Visser M, Foley D, et al. (2000) Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci 55: M168–M173. [DOI] [PubMed] [Google Scholar]
- 33. Rantanen T, Masaki K, He Q, Ross GW, Willcox BJ, et al. (2012) Midlife muscle strength and human longevity up to age 100 years: a 44-year prospective study among a decedent cohort. Age (Dordr) 34: 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuh D, Bassey J, Hardy R, Aihie Sayer A, Wadsworth M, et al. (2002) Birth weight, childhood size, and muscle strength in adult life: evidence from a birth cohort study. Am J Epidemiol 156: 627–633. [DOI] [PubMed] [Google Scholar]
- 35. Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, et al. (2014) The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 19: 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, et al. (2014) Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 19: 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonner JT (2006) Why Size Matters: From Bacteria to Blue Whales. Princeton University Press, Princeton, NJ. [Google Scholar]
- 38.Thompson DW (1917) On Growth and Form., Cambridge University Press, Cambridge, UK. [Google Scholar]
- 39.Huxley JS (1932) Problems of Relative Growth. Methuen & Co. Ltd, London, UK. [Google Scholar]
- 40. Tang H, Smith-Caldas MSR, Driscoll MV, Salhadar S, Shingleton AW (2011) FOXO regulates organ-specific phenotypic plasticity in Drosophila . PLoS Genet 7 (article e1002373) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sinclair DA (2005) Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Devel 126: 987–1002. [DOI] [PubMed] [Google Scholar]
- 42. Guarente L (2013) Calorie restriction and sirtuins revisited. Genes Dev 27: 2072–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bartke A, Sun LY, Longo V (2013) Somatotropin signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev 93: 571–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Use of time-dependent covariates in Cox regression model to estimate relative risks of baseline height for different life stages (age ≤70, 71–80, 81–95, and >95 years).
(DOCX)


